Published online Sep 28, 2017. doi: 10.3748/wjg.v23.i36.6694
Peer-review started: December 15, 2016
First decision: February 23, 2017
Revised: March 12, 2017
Accepted: June 1, 2017
Article in press: June 1, 2017
Published online: September 28, 2017
Processing time: 284 Days and 19.3 Hours
To unravel relationships between gastrointestinal (GI) symptoms impairing quality of life (QOL) and clinical profiles of diabetes mellitus (DM) patients.
We enrolled 134 outpatients with type 2 DM. Mean age was 64.7 years, mean body mass index was 24.7 kg/m2, mean glycated hemoglobin was 7.1%, and mean DM duration was 13.7 years. GI symptom-related QOL was determined using the Izumo scale, based on five factors, i.e., heartburn, gastralgia, postprandial fullness, constipation and diarrhea. The sum of scores obtained for the three questions in each domain was calculated, and subjects with a score of 5 or higher were considered to be symptomatic with impaired QOL. JMP Clinical version 5.0 was used for all statistical analyses.
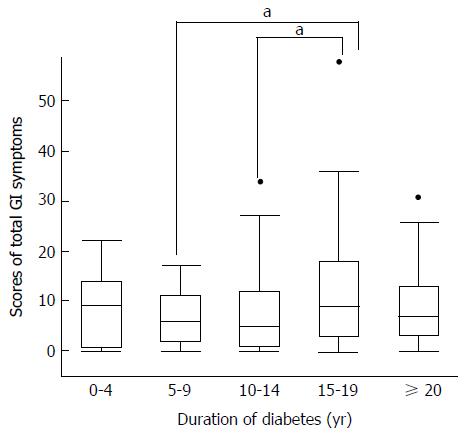
Lower abdominal symptoms were found to be more frequent than those affecting the upper abdomen. Diabetic duration and medications showed associations with GI symptoms. We identified differences in peak prevalences of the five symptoms. Gastralgia (P = 0.02 vs 10-14 years) and total GI symptoms (P = 0.01 and P = 0.02 vs 5-9 years and 10-14 years, respectively) peaked at a diabetes duration of 15-19 years. Heartburn (P = 0.004) and postprandial fullness (P = 0.03) tended to increase with disease duration. Constipation and diarrhea showed bimodal peaks, with the first early and the second late (e.g., P = 0.03 at 15-19 years vs 10-14 years for diarrhea) in the disease course. Finally, GI symptoms showed clustering that reflected the region of the GI tract affected, i.e., constipation and diarrhea had similar frequencies (P < 0.0001).
Our study highlights the importance of questioning patients about QOL impairment due to abdominal symptoms, especially in the early and the late periods of diabetes.
Core tip: We describe the results of a questionnaire survey of 134 type 2 diabetes mellitus outpatients experiencing gastrointestinal symptoms. The novel finding is that symptom frequencies differed among disease durations and according to affected gastrointestinal regions. Lower abdominal symptoms not only manifested during the late but also in the early stage of diabetes when there were no organ complications related to this disease. Our study highlights the importance of not underestimating gastrointestinal symptoms and of questioning patients about quality of life impairment due to abdominal symptoms, especially in both the early period and after a diabetes duration of 10 or even 15 years.
- Citation: Fujishiro M, Kushiyama A, Yamazaki H, Kaneko S, Koketsu Y, Yamamotoya T, Kikuchi T, Sakoda H, Suzuki R, Kadowaki T. Gastrointestinal symptom prevalence depends on disease duration and gastrointestinal region in type 2 diabetes mellitus. World J Gastroenterol 2017; 23(36): 6694-6704
- URL: https://www.wjgnet.com/1007-9327/full/v23/i36/6694.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i36.6694
The dramatic increase in the incidence of type (T)2 diabetes mellitus (DM) over the last century has become a major public health concern worldwide[1]. Both micro- and macrovascular complications of diabetes damage a variety of organs, leading to various symptoms, and impair quality of life (QOL) in patients with T2DM.
Among these symptoms, those affecting the gastrointestinal (GI) tract occur frequently in patients with diabetes[2]. Various GI symptoms are associated with this disease[3-5], especially diabetic neuropathy which develops with longstanding diabetes[6,7]. GI hyper- and hypofunction lead to delayed gastric or esophageal emptying, diabetic gastroparesis, constipation and diarrhea[6,8,9]. Other GI-related disorders and symptoms are also known to accompany diabetes, such as obesity with gastroesophageal reflux disease[10,11], and aging with gastric mucosal injury[12].
GI disorders in diabetes have thus far been poorly characterized, despite their high frequencies in both T1DM and T2DM patients[13]. Hyperglycemia-associated neuropathy was long considered to be a major mechanism underlying the pathogenesis of GI symptoms, probably via oxidative stress and inflammation, as with other microangiopathies. Levels of enteral hormones, such as incretin-related peptides [e.g., glucagon-like peptide-1 (GLP-1), GLP-2, pancreatic polypeptide (PPY)][14] and serotonin[15], and enteric neurotransmitters, such as vasoactive intestinal peptide (VIP)[16], are altered in patients with DM, and affect GI motility and neural fiber growth. Furthermore, smooth muscle cells, interstitial cells of Cajal[17], gut microbiota[18] and intestinal stem cells[19], may be altered in DM and these changes are potentially related to GI symptoms. The relevance of these mechanisms in the development of human GI symptoms in T2DM remains uncertain.
As for the pathophysiological mechanisms underlying the development of complications, hyperglycemia and the resultant glycation products[20], oxidative stress[21] and inflammation[22], are the main candidates. GI symptoms in DM are associated with both poor glycemic control and diabetic complications, as shown in a large number of subjects[23], although we found no relationships between GI symptoms and glycemic control, probably due to the long and varied disease durations of our subjects.
There has been little systematic and comprehensive research focusing on GI conditions in patients with T2DM. The Izumo Scale, a validated and useful tool for QOL assessment, is a scale for assessing GI symptoms[13,14]. Applying this scale, we investigated actual GI symptoms employing a questionnaire and determined whether the symptoms identified are related to the clinical profiles of patients with T2DM.
Patients who visited the department of diabetes and metabolic diseases in our hospital, including those with T1DM and T2DM, from December 2011 to March 2014, were enrolled in this study. We asked 200 consecutive patients to answer the questionnaire. Of the 170 patients who gave informed consent and responded to the questionnaire, data from 134 were analyzed after exclusion of 31 non-T2DM patients and 5 with a past history of GI tract surgery. This protocol was approved by our institutional ethics review committees (approval number 3643). Patient QOL was assessed by asking the subjects if they suffered from GI symptoms and then scoring the symptoms according to the Izumo scale, as described below.
Patient profiles, including diabetic microangiopathy, were collected from medical records. We considered subjects to have “diabetic autonomic neuropathy (DAN)” if they had any autonomic signs or symptoms. Distal symmetric polyneuropathy (DSP) includes symmetric neurosensory signs and symptoms, such as tingling, numbness or cramps in the legs, based on the attending physician’s assessment. Diabetic retinopathy (DR) includes non-proliferative DR diagnosed by the presence of microaneurysms and retinal hemorrhages[24], and all DR cases were confirmed by an ophthalmologist. Diabetic nephropathy was clinically diagnosed by attending physicians based on microalbuminuria or overt proteinuria with no evidence of other kidney or urological disease[25].
The Izumo Scale was developed and validated by Furuta et al[26]. The Izumo Scale is a self-administered questionnaire designed to assess the effects of abdominal symptoms on QOL and includes 15 items in five domains with three items in each domain: heartburn (questions 1-3), gastralgia (questions 4-6), postprandial fullness (questions 7-9), constipation (questions 10-12), and diarrhea (questions 13-15)[27]. Each question is rated on a 6-point Likert scale from 0 to 5, with higher values indicating more severe symptoms. This scale has been shown to have good internal consistency, reproducibility, as well as good correlations with the visual analogue scale of abdominal symptoms and the Gastrointestinal Symptoms Rating Scale (GSRS), and is thus widely utilized in Japan[28-31]. The sum of scores obtained for the three questions in each domain was calculated, and subjects with a score of 5 or higher were considered to be symptomatic with impaired QOL[30]. When the five symptoms were classified by GI region, heartburn was considered to involve the esophagus, gastralgia and postprandial fullness the upper GI tract, and constipation and diarrhea the lower GI tract.
JMP Clinical version 5.0 (SAS Institute, Cary, NC, United States) was used for all statistical analyses. ANOVA was used to compare scores among different groups. Frequencies were compared using Fisher’s exact test. Trend comparisons for frequencies among disease duration, age, body mass index (BMI), and glycated hemoglobin (HbA1c) were performed using a Cochran-Armitage test. Hierarchical cluster analysis was performed to assess the relationships among GI symptoms. All P values are two-sided, and P < 0.05 was considered to indicate a statistically significant difference.
The characteristics of enrolled patients are listed in Table 1. We enrolled 134 patients with T2DM (87 males and 47 females, mean age: 64.7 years, range: 29-88 years). Mean diabetes duration was 13.7 (0.3-33.0) years, BMI was 24.7 (16.5-42.5) kg/m2 and HbA1c was 7.1 (5.2-11.6)%. As to incidences of diabetic microangiopathy, 32 patients (24%) had DAN, 20 (15%) had DSP, 31 (23%) had DR and 64 (48%) had nephropathy. As to antidiabetic drugs, 8 patients (6%) received no medications, 34 (25%) used various forms of insulin including 22 (16%) also taking oral antidiabetic drugs (OADs), the details of which are presented in Table 1.
| Variable | n (%) | mean (95%CI) |
| Sex, male/female | 87/47 (65/35) | |
| Age of 65 yr or older | 72 (54) | 64.7 (62.8-66.6) |
| BMI of 25 kg/m2 or more | 56 (42) | 24.7 (24.0-25.4) |
| HbA1c of 7% or more | 69 (51) | 7.1 (6.9-7.3) |
| Duration of diabetes, yr | 13.7 (12.4-14.9) | |
| ≤ 4 | 12 (9) | |
| 5-9 | 27 (20) | |
| 10-14 | 35 (26) | |
| 15-19 | 31 (23) | |
| ≥ 20 | 29 (22) | |
| Incidence of diabetic microangiopathy | ||
| Autonomic neuropathy | 32 (24) | |
| Distal symmetric polyneuropathy | 20 (15) | |
| Retinopathy | 31 (23) | |
| Nephropathy | 64 (48) | |
| Use of antidiabetic drugs | ||
| None | 8 (6) | |
| Insulins | 34 (25) | |
| Insulins with OAD | 22 (16) | |
| OAD only | 86 (64) | |
| 1 OAD | 63 (47) | |
| 2 OAD | 30 (22) | |
| 3 OAD | 22 (16) | |
| ≥ 4 OAD | 11 (8) | |
| GLP-1 | 6 (4) | |
| GLP-1 with OAD | 3 (2) | |
| Insulins | 34 (25) | |
| Range of injections per day | 1-5 | |
| Range of total daily insulin doses, IU | 2-114 | |
| Insulin glargine | 12 | |
| Insulin detemir | 7 | |
| Human NPH insulin | 14 | |
| Insulin aspart | 2 | |
| Insulin glulisine | 4 | |
| Insulin lispro | 2 | |
| Premixed human insulin 30/70 | 9 | |
| Biphasic insulin aspart 30/70 | 1 | |
| α-Glucosidase inhibitors | 38 (28) | |
| Acarbose (150/200/300 mg) | 3 (1/1/1) | |
| Miglitol (50/75/100/150 mg) | 22 (1/12/18) | |
| Voglibose (0.6/0.9 mg) | 13 (5/8) | |
| Biguanides | 67 (50) | |
| Buformin 150 mg | 1 | |
| Metformin (500/750/1000/1500/2250 mg) | 66 (12/20/14/19/1) | |
| Thiazolidinediones | 26 (19) | |
| Pioglitazone (15/30 mg) | 26 (18/8) | |
| Sulfonylureas | 54 (40) | |
| Glimepiride (0.5/1/2/3/4 mg) | 26 (10/10/2/1/3/) | |
| Gliclazide (20/40/120 mg) | 21 (15/5/1) | |
| Glibenclamide (1.25/2.5/5 mg) | 7 (1/4/2) | |
| Glinides | 3 (2) | |
| Repaglinide 0.75 mg | 1 | |
| Mitiglinide (10/30 mg) | 2 (1/1) | |
| Dipeptidyl peptidase-4 inhibitors | 53 (40) | |
| Alogliptin 25 mg | 12 | |
| Sitagliptin (50/100 mg) | 17 (15/2) | |
| Vildagliptin (50/100 mg) | 24 (5/19) | |
| Glucagon-like peptide-1 agonists | 6 (4) | |
| Liraglutide (0.6/0.9 mg) | 4 (1/3) | |
| Exenatide 20 mg | 2 | |
| Dietary information | ||
| Receiving dietary therapy | 62 (46) | |
| With salt restriction to < 6 g per day | 51 (38) | |
| Range of total energy intake, kcal | 1200-1800 | |
| Range of total energy intake per IBW, kcal/IBW | 21-29 | |
| Use of antithrombotic or anti-inflammatory agents | ||
| Antiplatelet agents | 29 (22) | |
| Anticoagulants | 7 (5) | |
| Steroids/NSAIDs | 8 (6) | |
| Others1 | 7 (5) | |
| Use of GI agents | ||
| Antacids | 29 (22) | |
| Mucosal protectives | 13 (10) | |
| Antimicrobials | 11 (8) | |
| GI stimulants | 18 (13) | |
| Anti-diarrheals | 0 (0) | |
| Baseline blood parameters, mean ± SD | ||
| WBC, × 1000/μL | 6.1 ± 1.9 | |
| RBC, × 10000/μL | 439 ± 78.1 | |
| Hb, g/dL | 13.4 ± 2.4 | |
| Hct, % | 40.8 ± 6.9 | |
| Plt, × 10000/μL | 21.9 ± 6.6 | |
| TP, g/dL | 7.1 ± 0.5 | |
| Alb, g/dL | 3.8 ± 0.3 | |
| AST(GOT), U/L | 24.4 ± 13.4 | |
| ALT(GPT), U/L | 24.5 ± 17.7 | |
| γ-GTP, U/L | 34.1 ± 24.3 | |
| CK, U/L | 113 ± 63.6 | |
| T-Cho, mg/dL | 180 ± 39.2 | |
| HDL-C, mg/dL | 60.3 ± 18.3 | |
| TG, mg/dL | 128 ± 67.2 | |
| cLDL, mg/dL | 95.4 ± 26.2 | |
| BUN, mg/dL | 17.6 ± 11.9 | |
| Cre, mg/dL | 0.9 ± 0.8 | |
| eGFR, mL/min per 1.73 m2 | 66.6 ± 22.8 | |
| UA, mg/dL | 5.5 ± 1.4 |
Eighty-six (64%) patients received OAD only, including 63 (47%) with one OAD, 30 (22%) with 2 OADs, 22 (16%) with three OADs, and 11 (8%) with four or more OADs. In detail, 38 patients (28%) used α-glucosidase inhibitors, 67 (50%) used biguanides, 26 (19%) used thiazolidinediones, 54 (40%) used sulfonylureas, 3 (2%) used glinides and 53 (40%) used dipeptidyl peptidase-4 (DPP4) inhibitors, at various daily doses, as indicated in Table 1. Six patients (4%) used GLP-1 agonists, 3 of whom were also taking an OAD. Sixty-two patients (46%) were receiving diet therapy with daily intakes ranging from 1200-1800 kcal (21-29 kcal/IBW) and, in 51 patients (38%), salt was restricted to less than 6 g/d.
As to antithrombotic or anti-inflammatory agents, 29 patients (22%) used antiplatelet agents, 7 (5%) used anticoagulants, 8 (6%) used steroids or nonsteroidal anti-inflammatory drugs and 7 (5%) used other antithrombotic agents such as prostaglandin E1 derivatives, prostaglandin I2 derivatives, or ethyl esters of eicosapentaenoic acid. As to GI agents, 29 patients (22%) used antacids, 13 (10%) used mucosal protectives, 11 (8%) used antimicrobials, and 18 (13%) used GI stimulants, but none were taking anti-diarrheal agents. Baseline blood parameter findings were unremarkable.
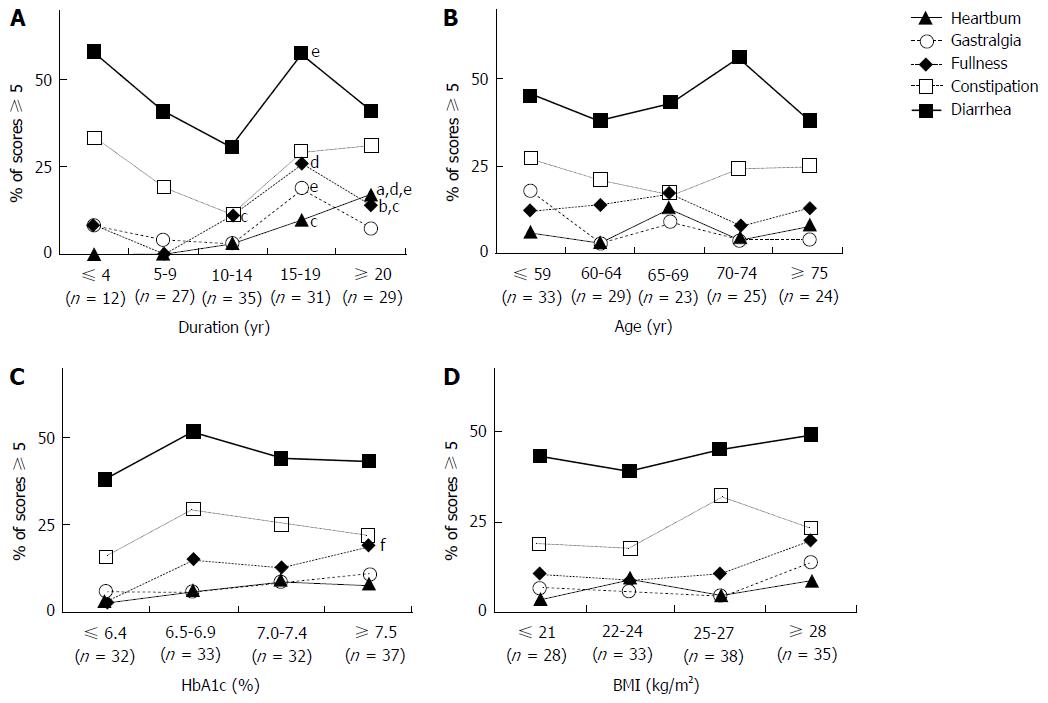
In the QOL assessment employing the Izumo Scale, the scores of total GI symptoms peaked during the 15- to 19-year diabetes duration period (Figure 1). When GI symptoms were analyzed separately, lower GI symptoms were found to be most frequent. Prevalence patterns differed between the esophagus/upper GI tract and lower GI tract regions. Scores for heartburn (P = 0.004) and postprandial fullness (P = 0.03) consistently and significantly rose as disease duration increased (Figure 2A).
Scores for gastralgia, like total GI symptoms, peaked during the 15- to 19-year diabetes duration period. Interestingly, only the scores for constipation and diarrhea showed bimodal peaks, the first in the early period of diabetes and the second late in the disease course. Age showed a similar but weaker relationship to diabetes duration. There were no apparent relationships of GI symptoms with age or BMI, but postprandial fullness, alone among the five symptoms, was slightly associated with high HbA1c levels (Figure 2B-D).
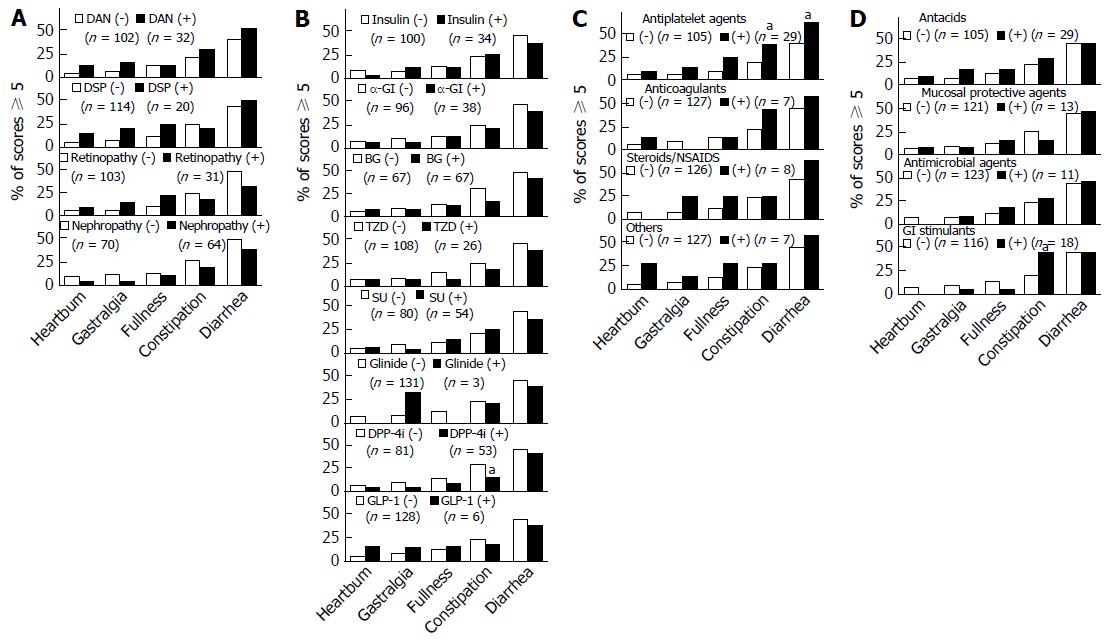
Next, we examined whether there were any relationships between GI symptoms and patient characteristics such as diabetic microangiopathy and the drugs administered (Figure 3A-D). None of the GI symptoms showed significant relationships with any of the forms of diabetic microangiopathy (Figure 3A), though symptoms tended to be more frequent in patients with than in those without DAN and/or DSP. As shown in Figure 3B, the rate of DPP4 inhibitor use was lower in patients suffering from constipation (P = 0.04). Insulin therapy and oral hypoglycemic agents other than DPP4 inhibitors were not related to GI symptoms. The numbers of patients using glinides or GLP-1 agonists were too small to allow statistically meaningful evaluation. As shown in Figure 3C, of the 29 patients taking antiplatelet agents, 38% (n = 11) had constipation (P = 0.046) and 62% (n = 18) had diarrhea (P = 0.03). Of the 18 patients using GI stimulants, 44% (n = 8) had constipation (P = 0.03) (Figure 3D).
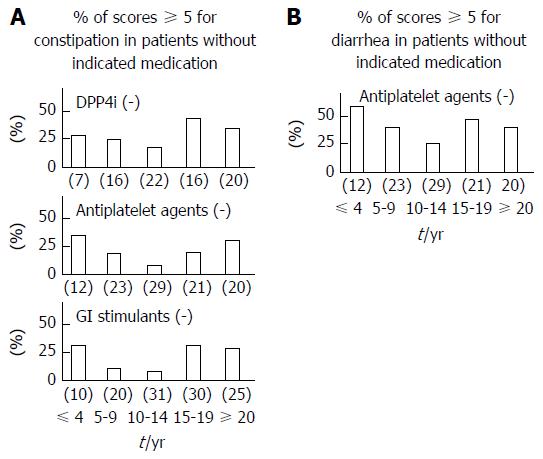
We next investigated the effect of diabetic duration on constipation, excluding users of DPP4 inhibitors, antiplatelet agents or GI stimulants. We focused on the association of diabetes duration with constipation (Figure 4A), and on that with diarrhea, except in antiplatelet agent users (Figure 4B). When users of these medications were excluded, the early and late peaks in constipation and diarrhea remained.
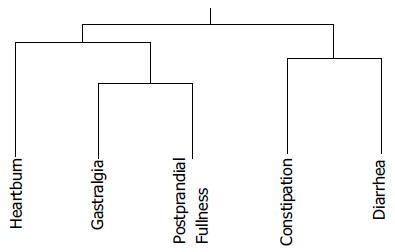
A cluster analysis tree is presented in Figure 5. Gastralgia and postprandial fullness are more similar to each other than to constipation and diarrhea. Heartburn is closer to upper than to lower GI symptoms. The relationship between constipation and diarrhea was found to be closer than the other associations identified in this study. The overlaps between two different symptoms on the Izumo Scale are shown in Table 2. Significant differences, determined by Fisher’s exact test, between the presence and absence of two distinct symptoms, were demonstrated.
As in the cluster analysis, heartburn was associated with gastralgia (P = 0.03) or postprandial fullness (P = 0.002). Gastralgia and postprandial fullness (P < 0.0001) were strongly related to each other, as were constipation and diarrhea (P < 0.0001). Postprandial fullness was found to be related to all GI tract regions, showing modest interactions with both constipation (P = 0.004) and diarrhea (P = 0.001). Furthermore, all 31 patients with constipation also had episodes of diarrhea, while only 52.5% of those complaining mainly of diarrhea also had constipation (Table 2).
We investigated whether outpatients with T2DM actually have GI symptoms and, if present, the clinical severity of such symptoms. However, patients do not usually complain of GI symptoms. Overall, 10%-20% of adults have functional GI disorders[32-35]. In patients with diabetes, symptoms involving all portions of the GI tract are reportedly more common than in the general population[36]. However, we found that total symptom scores did not increase linearly with disease duration. In fact, rates peaked during different periods, according to GI regions. Lower abdominal symptoms were prominent early in the disease course, while diabetic complications were still mild, as well as in the late stage.
Significant confounding factors include aging, glycemic control and BMI reduction[5,8-10,37]. Japanese patients have become increasingly obese over the past 20 years and those in the younger generation have poor glycemic control at the diagnosis of T2DM, independent of hyperglycemic symptoms[38]. Therefore, symptom prevalence may change in the future.
We found no significant relationships between GI symptoms and either DAN or DSP. There might, however, be a detection bias or low sensitivity for DAN and DSP, since a previous report noted that symptoms tend to be related to lifestyle factors, rather than to either glycemic control or peripheral neuropathy[36]. Another study found no association of GI symptoms with either diabetic neuropathy or psychiatric illnesses[39]. Early symptoms might well be related to psychiatric illnesses and/or lifestyle factors but we did not obtain data pertaining to such factors in the present study.
Among anti-hyperglycemic medications, only the relationship between DPP4 inhibitors and constipation was significant. Constipation is a well-known side effect of these drugs[40], and physicians often discontinue or do not start DPP4 inhibitors due to this adverse effect[41]. Oral medications are generally prescribed to patients with poor glycemic control[42] and are frequently used to manage longstanding diabetes[43]. Administration of anti-platelet agents was associated with a variety of symptoms, as expected. These agents were still preferentially used despite apparently being harmful to the upper, middle and lower GI tracts[44,45], since the treatment of cardiovascular diseases has the highest priority. However, the bimodal peaks for constipation and diarrhea late and early in the course of diabetes were maintained and were independent of medication use.
The mechanisms underlying rapid development of lower GI complications, such as diarrhea and constipation, are unknown. In our cluster analysis, the affected GI tract region was found to be an important factor. Furthermore, the mechanisms underlying the bimodal early and late disturbances are suggested to not be the same. Early diabetic neuropathy is reportedly multifactorial, being triggered by impairment of insulin signaling, abnormal blood flow, and oxidative stress including N-acetylglucosamine, activation of protein kinase C, activation of the polyol sugar pathway and glucose autoxidation, as well as non-enzymatic protein glycation[46]. Insulin growth factors (IGFs) possess multiple neurotrophic functions, including neuronal survival, neurite outgrowth and regeneration. C-peptide also exerts IGF-like activity, such that endogenous proinsulin production might be related to the pathogenesis of GI symptoms[47]. One possible explanation of mechanistic differences between early lower GI symptoms and upper GI symptoms might involve cellular composition. The lower GI tract contains colonic stem cells and their functions are disrupted in states of diabetic enteropathy[19].
Lower GI tract symptoms were found to be most frequent and cluster analysis demonstrated these symptoms to be related more to the affected region of the GI tract than to its functions, such as acid secretion and motility. In fact, a high concordance between constipation and diarrhea is unusual in general populations[48]. Upper and lower GI symptoms reportedly overlap and early satiety more frequently overlaps with constipation than diarrhea in general populations[49,50].
This study has several limitations. First, it is difficult to determine the causes of symptoms with a cross-sectional study design. There is diagnostic uncertainty regarding the GI disorders studied, because the reporting of symptoms was mainly subjective. Endoscopy can be used to exclude organic diseases with symptoms similar to those of diabetic GI disorders (e.g., inflammatory bowel disease, colonic polyposis, and celiac disease)[51]. Few therapeutic or diagnostic approaches are available to assess the GI disorders possibly associated with diabetes. Organic diseases not requiring surgery and functional disorders could not be precisely distinguished because we did not perform upper and lower GI endoscopy or gastric emptying scintigraphy at the time of this study, since it was retrospective. Our results might have low sensitivity due to the small sample sizes in some groups.
In conclusion, our study highlights the importance of questioning patients about QOL impairment due to abdominal symptoms, especially lower GI symptoms in the early period and both lower and upper GI symptoms in the late period of diabetes. The heterogeneous nature of the underlying pathophysiological mechanisms underlying GI symptoms, including medication usage, should be taken into consideration when managing patients with T2DM.
Type 2 diabetes mellitus (T2DM) incidence is dramatically increasing, and both micro- and macrovascular complications of diabetes lead to various symptoms and impair quality of life (QOL) in T2DM patients. Among these symptoms, those affecting the gastrointestinal (GI) tract are frequent in T2DM patients. GI disorders in diabetes have been poorly characterized. Hyperglycemia-associated neuropathy was considered to be related to GI symptoms, probably via oxidative stress and inflammation. Levels of enteral hormones are altered in patients with DM, affecting both GI motility and neural fiber growth. Furthermore, smooth muscle cells, interstitial cells of Cajal, gut microbiota and intestinal stem cells may also be altered in DM and these changes might be related to GI symptoms. The relevance of these mechanisms in human GI symptoms affecting T2DM patients have yet to be clarified. The Izumo Scale, a validated and useful tool for QOL assessment, is a scale for assessing GI symptoms. Applying this scale, we investigated actual GI symptoms employing a questionnaire and determined whether the symptoms identified are related to the clinical profiles of patients with T2DM.
This study highlights the importance of not underestimating gastrointestinal symptoms and of questioning patients about QOL impairment due to abdominal symptoms, especially in both the early period and after a diabetes duration of 10 or even 15 years.
The novel finding of this study is that symptom frequencies differed among disease durations and according to affected gastrointestinal regions. Lower abdominal symptoms not only manifested during the late but also in the early stage of diabetes when there were no organ complications related to this disease. This results are apparently inconsistent with the previously suggested mechanisms of GI symptoms related to DM, especially diabetic neuropathy which develops with longstanding diabetes.
This study highlights the importance of questioning patients about QOL impairment due to abdominal symptoms, especially lower GI symptoms in the early period and both lower and upper GI symptoms in the late period of diabetes, while underscoring the need for systematic and comprehensive research focusing on GI conditions in patients with T2DM.
Izumo Scale: This self-administered questionnaire designed to assess the effects of abdominal symptoms on QOL includes 15 items in five domains with three items in each domain: heartburn, gastralgia, postprandial fullness, constipation, and diarrhea. This scale was developed and validated by Furuta et al. Each question is rated on a 6-point Likert scale from 0 to 5, with higher values indicating more severe symptoms.
Considering the paucity of data available in the literature on this topic, this study may reinforce the clinical relevance of assessing intestinal disorders in diabetes and increase the interest of the scientific community in this topic. Overall, this report describes relevant novel findings that may be further addressed and confirmed with detailed experimental studies.
| 1. | Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 2999] [Article Influence: 249.9] [Reference Citation Analysis (2)] |
| 2. | Talley NJ, Young L, Bytzer P, Hammer J, Leemon M, Jones M, Horowitz M. Impact of chronic gastrointestinal symptoms in diabetes mellitus on health-related quality of life. Am J Gastroenterol. 2001;96:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 150] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Phillips LK, Deane AM, Jones KL, Rayner CK, Horowitz M. Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol. 2015;11:112-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 4. | Bharucha AE, Batey-Schaefer B, Cleary PA, Murray JA, Cowie C, Lorenzi G, Driscoll M, Harth J, Larkin M, Christofi M. Delayed Gastric Emptying Is Associated With Early and Long-term Hyperglycemia in Type 1 Diabetes Mellitus. Gastroenterology. 2015;149:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Ko GT, Chan WB, Chan JC, Tsang LW, Cockram CS. Gastrointestinal symptoms in Chinese patients with Type 2 diabetes mellitus. Diabet Med. 1999;16:670-674. [PubMed] |
| 6. | Azpiroz F, Malagelada C. Diabetic neuropathy in the gut: pathogenesis and diagnosis. Diabetologia. 2016;59:404-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553-1579. [PubMed] |
| 8. | Horowitz M, Harding PE, Maddox AF, Wishart JM, Akkermans LM, Chatterton BE, Shearman DJ. Gastric and oesophageal emptying in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32:151-159. [PubMed] |
| 9. | Chang J, Rayner CK, Jones KL, Horowitz M. Diabetic gastroparesis-backwards and forwards. J Gastroenterol Hepatol. 2011;26 Suppl 1:46-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Akyüz F, Uyanıkoglu A, Ermis F, Arıcı S, Akyüz Ü, Baran B, Pinarbasi B, Gul N. Gastroesophageal reflux in asymptomatic obese subjects: An esophageal impedance-pH study. World J Gastroenterol. 2015;21:3030-3034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Matsuura B, Nunoi H, Miyake T, Hiasa Y, Onji M. Obesity and gastrointestinal liver disorders in Japan. J Gastroenterol Hepatol. 2013;28 Suppl 4:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Tarnawski AS, Ahluwalia A, Jones MK. Increased susceptibility of aging gastric mucosa to injury: the mechanisms and clinical implications. World J Gastroenterol. 2014;20:4467-4482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (4)] |
| 13. | D’Addio F, Fiorina P. Type 1 Diabetes and Dysfunctional Intestinal Homeostasis. Trends Endocrinol Metab. 2016;27:493-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Latorre R, Sternini C, De Giorgio R, Greenwood-Van Meerveld B. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol Motil. 2016;28:620-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 260] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 15. | Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 855] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 16. | Adeghate E, Ponery AS, Sharma AK, El-Sharkawy T, Donáth T. Diabetes mellitus is associated with a decrease in vasoactive intestinal polypeptide content of gastrointestinal tract of rat. Arch Physiol Biochem. 2001;109:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Ordög T. Interstitial cells of Cajal in diabetic gastroenteropathy. Neurogastroenterol Motil. 2008;20:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Vaarala O, Atkinson MA, Neu J. The “perfect storm” for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57:2555-2562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 377] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 19. | D’Addio F, La Rosa S, Maestroni A, Jung P, Orsenigo E, Ben Nasr M, Tezza S, Bassi R, Finzi G, Marando A. Circulating IGF-I and IGFBP3 Levels Control Human Colonic Stem Cell Function and Are Disrupted in Diabetic Enteropathy. Cell Stem Cell. 2015;17:486-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Brownlee M. Glycation products and the pathogenesis of diabetic complications. Diabetes Care. 1992;15:1835-1843. [PubMed] |
| 21. | Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3956] [Cited by in RCA: 3776] [Article Influence: 236.0] [Reference Citation Analysis (11)] |
| 22. | Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1360] [Cited by in RCA: 1885] [Article Influence: 145.0] [Reference Citation Analysis (1)] |
| 23. | Bytzer P, Talley NJ, Hammer J, Young LJ, Jones MP, Horowitz M. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97:604-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 140] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 24. | Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 611] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 25. | Tanaka K, Hara S, Hattori M, Sakai K, Onishi Y, Yoshida Y, Kawazu S, Kushiyama A. Role of elevated serum uric acid levels at the onset of overt nephropathy in the risk for renal function decline in patients with type 2 diabetes. J Diabetes Investig. 2015;6:98-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Furuta K, Ishihara S, Sato S, Miyake T, Ishimura N, Koshino K, Tobita H, Moriyama I, Amano Y, Adachi K. [Development and verification of the Izumo Scale, new questionnaire for quality of life assessment of patients with gastrointestinal symptoms]. Nihon Shokakibyo Gakkai Zasshi. 2009;106:1478-1487. [PubMed] |
| 27. | Kakuta E, Yamashita N, Katsube T, Kushiyama Y, Suetsugu H, Furuta K, Kinoshita Y. Abdominal symptom-related QOL in individuals visiting an outpatient clinic and those attending an annual health check. Intern Med. 2011;50:1517-1522. [PubMed] |
| 28. | Kinoshita Y, Chiba T. Characteristics of Japanese patients with chronic gastritis and comparison with functional dyspepsia defined by ROME III criteria: based on the large-scale survey, FUTURE study. Intern Med. 2011;50:2269-2276. [PubMed] |
| 29. | Yoshioka T, Okimoto N, Okamoto K, Sakai A. A comparative study of the effects of daily minodronate and weekly alendronate on upper gastrointestinal symptoms, bone resorption, and back pain in postmenopausal osteoporosis patients. J Bone Miner Metab. 2013;31:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Okimoto E, Ishimura N, Morito Y, Mikami H, Shimura S, Uno G, Tamagawa Y, Aimi M, Oshima N, Kawashima K. Prevalence of gastroesophageal reflux disease in children, adults, and elderly in the same community. J Gastroenterol Hepatol. 2015;30:1140-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Kinoshita Y, Chiba T. Therapeutic effects of famotidine on chronic symptomatic gastritis: subgroup analysis from FUTURE study. J Gastroenterol. 2012;47:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-721.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1456] [Article Influence: 104.0] [Reference Citation Analysis (2)] |
| 33. | Galmiche JP, Clouse RE, Bálint A, Cook IJ, Kahrilas PJ, Paterson WG, Smout AJ. Functional esophageal disorders. Gastroenterology. 2006;130:1459-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 318] [Article Influence: 15.9] [Reference Citation Analysis (1)] |
| 34. | Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1243] [Cited by in RCA: 1207] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 35. | Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3413] [Cited by in RCA: 3410] [Article Influence: 170.5] [Reference Citation Analysis (4)] |
| 36. | Mjörnheim AC, Finizia C, Blohmé G, Attvall S, Lundell L, Ruth M. Gastrointestinal symptoms in type 1 diabetic patients, as compared to a general population. A questionnaire-based study. Digestion. 2003;68:102-108. [PubMed] |
| 37. | Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989-1996. [PubMed] |
| 38. | Kushiyama A, Yoshida Y, Kikuchi T, Suzawa N, Yamamoto M, Tanaka K, Okayasu M, Tahara T, Takao T, Onishi Y. Twenty-year trend of increasing obesity in young patients with poorly controlled type 2 diabetes at first diagnosis in urban Japan. J Diabetes Investig. 2013;4:540-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Clouse RE, Lustman PJ. Gastrointestinal symptoms in diabetic patients: lack of association with neuropathy. Am J Gastroenterol. 1989;84:868-872. [PubMed] |
| 40. | Williams-Herman D, Engel SS, Round E, Johnson J, Golm GT, Guo H, Musser BJ, Davies MJ, Kaufman KD, Goldstein BJ. Safety and tolerability of sitagliptin in clinical studies: a pooled analysis of data from 10,246 patients with type 2 diabetes. BMC Endocr Disord. 2010;10:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 200] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 41. | Otsuki H, Kosaka T, Nakamura K, Shimomura F, Kuwahara Y, Tsukamoto T. Safety and efficacy of teneligliptin: a novel DPP-4 inhibitor for hemodialysis patients with type 2 diabetes. Int Urol Nephrol. 2014;46:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Kobayashi M, Yamazaki K, Hirao K, Oishi M, Kanatsuka A, Yamauchi M, Takagi H, Kawai K. The status of diabetes control and antidiabetic drug therapy in Japan--a cross-sectional survey of 17,000 patients with diabetes mellitus (JDDM 1). Diabetes Res Clin Pract. 2006;73:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 43. | Kosachunhanun N, Benjasuratwong Y, Mongkolsomlit S, Rawdaree P, Plengvidhya N, Leelawatana R, Bunnag P, Pratipanawatr T, Krittiyawong S, Suwanwalaikorn S. Thailand diabetes registry project: glycemic control in Thai type 2 diabetes and its relation to hypoglycemic agent usage. J Med Assoc Thai. 2006;89 Suppl 1:S66-S71. [PubMed] |
| 44. | Scheiman JM. Prevention of damage induced by aspirin in the GI tract. Best Pract Res Clin Gastroenterol. 2012;26:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Nadatani Y, Watanabe T, Tanigawa T, Sogawa M, Yamagami H, Shiba M, Watanabe K, Tominaga K, Fujiwara Y, Yoshiyama M. Incidence and risk factors of gastrointestinal bleeding in patients on low-dose aspirin therapy after percutaneous coronary intervention in Japan. Scand J Gastroenterol. 2013;48:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Dobretsov M, Romanovsky D, Stimers JR. Early diabetic neuropathy: triggers and mechanisms. World J Gastroenterol. 2007;13:175-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 47. | Ekberg K, Brismar T, Johansson BL, Jonsson B, Lindström P, Wahren J. Amelioration of sensory nerve dysfunction by C-Peptide in patients with type 1 diabetes. Diabetes. 2003;52:536-541. [PubMed] |
| 48. | Talley NJ, Holtmann G, Agréus L, Jones M. Gastrointestinal symptoms and subjects cluster into distinct upper and lower groupings in the community: a four nations study. Am J Gastroenterol. 2000;95:1439-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Talley NJ, Dennis EH, Schettler-Duncan VA, Lacy BE, Olden KW, Crowell MD. Overlapping upper and lower gastrointestinal symptoms in irritable bowel syndrome patients with constipation or diarrhea. Am J Gastroenterol. 2003;98:2454-2459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 179] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 50. | de Bortoli N, Martinucci I, Bellini M, Savarino E, Savarino V, Blandizzi C, Marchi S. Overlap of functional heartburn and gastroesophageal reflux disease with irritable bowel syndrome. World J Gastroenterol. 2013;19:5787-5797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Yantiss RK, Odze RD. Optimal approach to obtaining mucosal biopsies for assessment of inflammatory disorders of the gastrointestinal tract. Am J Gastroenterol. 2009;104:774-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Bulc M, Fiorina P S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Li D