Published online Sep 21, 2017. doi: 10.3748/wjg.v23.i35.6429
Peer-review started: March 20, 2017
First decision: June 8, 2017
Revised: July 16, 2017
Accepted: August 2, 2017
Article in press: August 2, 2017
Published online: September 21, 2017
Processing time: 186 Days and 22.7 Hours
To evaluate the feasibility and reliability of endoscopic transpapillary bile duct biopsy for the diagnosis of biliary strictures.
A total of 360 patients (241 men) who underwent endoscopic retrograde cholangiopancreatography for biliary strictures with biopsy from April 2012 to March 2016 at Tokyo Medical University Hospital were retrospectively reviewed. This study was approved by our Institutional Review Board (No. 3516). Informed consent was obtained from all individual participants included in this study. The biopsy specimens were obtained using a novel slim biopsy forceps (Radial Jaw 4P, Boston Scientific, Boston, MA, United States).
The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 69.6%, 100%, 100%, 59.1%, and 78.8%, respectively. The sensitivity was 75.6% in bile duct cancer, 64% in pancreatic cancer, 61.1% in gallbladder cancer, and 57.1% in metastasis. In bile duct cancer, a lower sensitivity was observed for perihilar bile duct stricture (68.7%) than for distal bile duct stricture (83.1%). In terms of the stricture lengths of pancreatic cancer, gallbladder cancer, and metastasis, a longer stenosis resulted in a better sensitivity. In particular, there was a significant difference between pancreatic cancer and gallbladder cancer (P < 0.05). One major complication was perforation of the extrahepatic bile duct with bile leakage.
Endoscopic transpapillary biopsy alone using novel slim biopsy forceps is feasible and reliable, but restrictive. Biopsy should be performed in consideration of the stricture level, stricture length, and cancer type.
Core tip: Various radiological imaging procedures have been established as first-line modalities for detecting biliary strictures. However, a definitive diagnosis of biliary strictures can only be established by histocytological examination. At present, there are several histocytological sampling techniques such as aspiration cytology, brush cytology, aspiration needle biopsy, and forceps biopsy. However, the optimal sampling technique remains controversial. In this study, we found that transpapillary biopsy was feasible and reliable for diagnosing biliary strictures and should be performed in consideration of the stricture level, stricture length, and cancer type.
- Citation: Yamamoto K, Tsuchiya T, Itoi T, Tsuji S, Tanaka R, Tonozuka R, Honjo M, Mukai S, Kamada K, Fujita M, Asai Y, Matsunami Y, Nagakawa Y, Yamaguchi H, Sofuni A. Evaluation of novel slim biopsy forceps for diagnosis of biliary strictures: Single-institutional study of consecutive 360 cases (with video). World J Gastroenterol 2017; 23(35): 6429-6436
- URL: https://www.wjgnet.com/1007-9327/full/v23/i35/6429.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i35.6429
To date, transabdominal ultrasonography (TAUS), computed tomography (CT), magnetic resonance imaging (MRI), and magnetic resonance cholangiopancreatography (MRCP) have been established as the first-line modalities for detecting biliary strictures, being minimally invasive modalities. However, a definitive diagnosis of biliary strictures can only be established by histocytological examination, which is crucial for making a decision regarding the further management of this disease entity.
Endoscopic retrograde cholangiopancreatography (ERCP) is the most widely used close-up examination method using direct cholangiography for evaluating biliary strictures which allows transpapillary histocytological sampling from the strictures by aspiration cytology, brush cytology, and endobiliary forceps biopsy. Several studies have examined whether combining tissue sampling techniques can improve the diagnostic accuracy for biliary strictures during ERCP[1-4]. Theoretically, core tissue sampling using biopsy forceps can provide more sufficient information for diagnosing biliary strictures than cytology. However, as conventional biopsy forceps has been used in gastrointestinal tract diseases and has a thick and hard shaft, transpapillary insertion via the accessory channel of the duodenoscope is difficult.
Recently, a novel biopsy forceps which has a thin and soft shaft has been developed. Herein, we evaluated this novel slim biopsy forceps for the diagnosis of biliary strictures.
A total of 360 patients (241 men) who underwent ERCP for biliary strictures with biopsy from April 2012 to March 2016 at Tokyo Medical University Hospital were retrospectively reviewed.
Patients were excluded in the following cases: (1) prior histological confirmation of malignancy; (2) postoperative biliary strictures; (3) ampullary tumor; and (4) less than 6 mo of follow-up in patients with negative malignant results. The final diagnosis was established by histopathological examination of tissues obtained endoscopically or surgically. If the histopathological diagnosis was negative for carcinoma, a clinical diagnosis was made from the clinical course for over 6 mo or more and several radiological image findings from various imaging modalities such as TAUS, CT, MRI, MRCP, and endoscopic ultrasonography (EUS). If biopsy forceps failed to achieve a definitive diagnosis and biliary strictures or filling defects were still indeterminate even with the use of various radiological modalities, peroral cholangioscopy was performed for a definitive diagnosis.
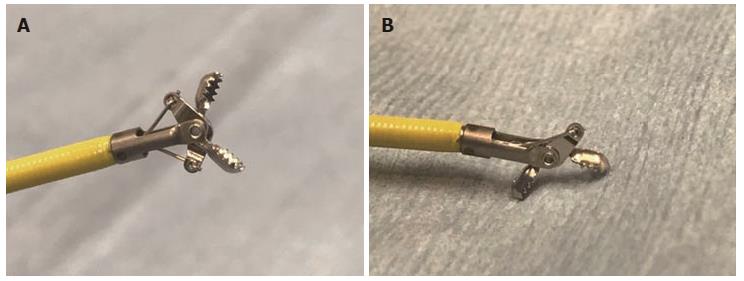
ERCP was performed using the standard technique with a duodenoscope. When biliary strictures were identified under radiographic guidance, endoscopic sphincterotomy (EST) was performed before tissue sampling. The biopsy specimens were obtained using a novel slim biopsy forceps (Radial Jaw 4P, Boston Scientific, Boston, MA, United States) (Figure 1A, video 1). The forceps was transpapillary advanced to the distal end of the stricture alongside the guidewire. The obtained specimen was immediately fixed in 10% formalin. All procedures were performed by operators with experience of more than 100 ERCP cases per year.
This study was approved by our Institutional Review Board (No. 3516) and informed consent was obtained from all individual participants included in this study.
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall accuracy were calculated. Statistical analyses were performed using StatMate III (ATMS, Tokyo, Japan). A P-value < 0.05 was considered to indicate a statistically significant difference.
The characteristics of the patients are shown in Table 1. Their median age was 71 ± 11.8 years (range: 17-95 years). The diseases were bile duct cancer in 132 patients, pancreatic cancer in 86, gallbladder cancer in 18, metastasis of other cancers in 14, and benign biliary stricture in 110. Among the patients with metastases, the primary sites were as follows: colon cancer in 3 patients, gastric cancer in 2, pancreatic cancer in 2, lung cancer in 2, breast cancer in 2, ovarian cancer in 2, and cholangiocellular carcinoma in 1.
| Patient characteristics | |
| Number of patients | 360 |
| Age [mean ± SD (range), yr] | 71 ± 11.8 (17-95) |
| Sex, male/female | 241/119 |
| Disease (number of patients) | |
| Bile duct cancer | 132 |
| Pancreatic cancer | 86 |
| Gallbladder cancer | 18 |
| Metastasis | 14 |
| Benign stricture | 110 |
The biopsy forceps could be inserted via the papilla after EST, and the specimens evaluated were collected from all 360 patients (technical success: 100%), with 820 bites in total (range: 1-6 bites). The mean number of biopsies was 2.28 ± 0.91 (Table 2).
| Sample collection rate | |
| Total number of biopsies | 820 |
| Mean number of biopsies, ± SD (range) | 2.28 ± 0.91 (1-6) |
| Overall results | |
| Sensitivity | 69.6% |
| Specificity | 100% |
| PPV | 100% |
| NPV | 59.1% |
| Accuracy | 78.8% |
The sensitivity, specificity, PPV, NPV, and accuracy were 69.6%, 100%, 100%, 59.1%, and 78.8%, respectively (Table 2). The sensitivity was 75.6% in bile duct cancer, 64% in pancreatic cancer, 61.1% in gallbladder cancer, and 57.1% in metastasis (Table 3).
| Diagnosis | n | Sensitivity |
| Bile duct cancer | 100/132 | 75.6% |
| Pancreatic cancer | 55/86 | 64.0% |
| Gallbladder cancer | 11/18 | 61.1% |
| Metastasis | 8/14 | 57.1% |
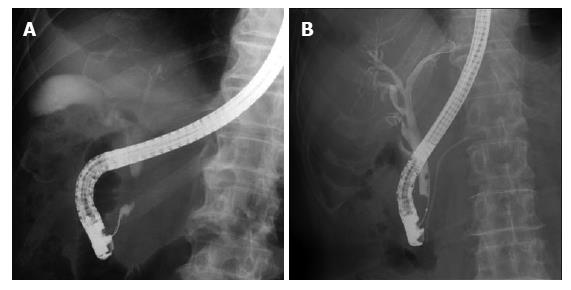
The examination results for bile duct cancer are shown in Table 4. According to the stricture level of bile duct cancer, the sensitivity was 68.7% in the perihilar bile duct and 83.1% in the distal bile duct; 95% in the middle and 76.7% in the lower. Furthermore, comparing the type of stricture with space that the biopsy forceps could open with the type of stricture without space in the lower bile duct (Figure 2), the sensitivity with an open space was 84.6% and that without an open space was 64.7%.
| n | Sensitivity | |
| Total | 100/132 | 75.60% |
| Site of stricture | ||
| Perihilar bile duct | 46/67 | 68.7% |
| Distal bile duct | 54/65 | 83.1% |
| Middle | 21/22 | 95% |
| Lower | 33/43 | 76.7% |
| (Open space + | 22/26 | 84.6% |
| (Open space − | 11/17 | 64.7% |
The examination results for extrinsic neoplasms are shown in Table 5. As for pancreatic cancer according to the stricture level, the sensitivity was 45.5% in the middle bile duct and 66.7% in the lower bile duct. In addition, according to the localization of the primary tumor, the sensitivity was 68.4% in pancreatic head cancer and 30% in pancreatic body cancer. As for gallbladder cancer according to the stricture level, the sensitivity was 54.5% in the perihilar bile duct and 71.4% in the distal bile duct. As for metastasis according to the stricture level, the sensitivity was 66.7% in the perihilar bile duct and 50% in the distal bile duct.
| n | Sensitivity | |
| Pancreatic cancer | 55/86 | 64.0% |
| Site of stricture | ||
| Middle bile duct | 5/11 | 45.5% |
| Lower bile duct | 50/75 | 66.7% |
| Localization of tumor | ||
| Head | 52/76 | 68.4% |
| Body | 3/10 | 30.0% |
| Gallbladder cancer | 11/18 | 61.1% |
| Site of stricture | ||
| Perihilar bile duct | 6/11 | 54.5% |
| Distal bile duct | 5/7 | 71.4% |
| Metastasis | 8/14 | 57.1% |
| Site of stricture | ||
| Perihilar bile duct | 4/6 | 66.7% |
| Distal bile duct | 4/8 | 50.0% |
Apart from the stricture level, the mean stricture lengths were 16.9 mm, 22 mm, and 17.1 mm in the biopsy-positive group and 13.5 mm, 13.4 mm, and 13.2 mm in the biopsy-negative group for pancreatic cancer, gallbladder cancer, and metastasis, respectively (Table 6). The biopsy-positive group tended to have a longer stricture length than the biopsy-negative group. In particular, there was a significant difference between pancreatic cancer and gallbladder cancer (P < 0.05).
| Positive (mm) | Negative (mm) | P value | |
| Pancreatic cancer | 16.9 | 13.5 | < 0.05 |
| Gallbladder cancer | 22 | 13.4 | < 0.05 |
| Metastasis | 17.1 | 13.2 | NS |
There were no procedure-related adverse events, although there was 1 patient with perihilar bile duct cancer in whom perforation of the distal bile duct with bile leakage was observed. The condition was improved only by temporary endoscopic nasobiliary drainage tube placement.
In this study, we found that transpapillary biopsy using a novel slim biopsy forceps could be easily and safely performed and was effective for diagnosing biliary strictures.
At present, there are several cytological and histological sampling techniques such as aspiration cytology, brush cytology, aspiration needle biopsy, and forceps biopsy. However, the optimal sampling technique remains controversial. The ideal technique for obtaining tissue for a definitive diagnosis of malignant biliary strictures should be simple, safe, and effective with a high sensitivity and specificity.
Although bile cytology is easy, its accuracy range is very low (6%-32%)[5,6]. Mohandas et al[7]. described that bile cytology with dilation of biliary strictures up to 10-Fr during ERCP enhanced its sensitivity by 63% compared with the 27% sensitivity of bile cytology without dilation. Regarding brush cytology, although this can be undertaken relatively easily and safely in a short time, its sensitivity is still considered to be low (30%-57%)[1-3]. Farrell et al[8] reported that the sensitivity of brush cytology increased by 85% after stricture dilation and endoscopic needle aspiration compared with the 57% sensitivity of brush cytology alone. In contrast, de Bellis et al[9] suggested that stricture dilation did not improve the sensitivity of brush cytology for cancer detection. Hence, the efficacy of stricture dilation for diagnosis is still controversial.
On the other hand, transpapillary forceps biopsy has not been widely used because it is thought to be technically difficult, resulting in a waste of time. Moreover, its sensitivity is very variable (15%-81%)[1-3,10-14] (Table 7). In 2002, de Bellis et al[9] summarized 502 previously reported cases and found that the sensitivity and specificity of transpapillary forceps biopsy were 56% (43%-81%) and 97% (90%-100%), respectively. Their report showed that the diagnostic performance of forceps biopsy was higher than that of other tissue sampling methods[15,16]. Furthermore, forceps biopsy is useful not only for the diagnosis of malignant/benign tumors but also for the selection of therapeutic methods (e.g., chemotherapy) based on the histological types of cancer, and the judgment of the superficial intraductal spread of bile duct cancer.
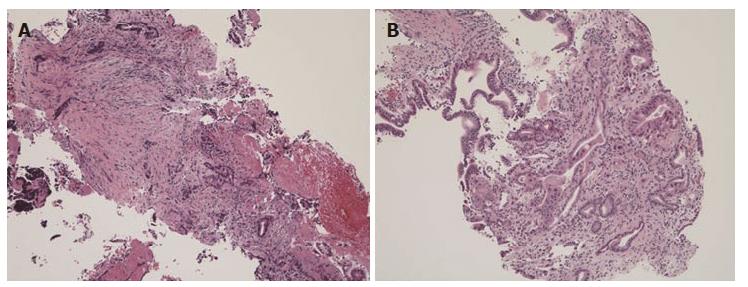
In the present study, we used a novel slim biopsy forceps (Radial Jaw 4P, Boston Scientific) (Figure 1A). The Radial Jaw series are the world’s first disposable biopsy forceps released in 1991. The first type of Radial Jaw had a diameter of 2.2 mm and had tiny teeth around the cup. After repeated improvements, the present Radial Jaw 4P was released in 2011. Radial Jaw 4P has a thinner shaft of 1.8 mm, a wire with a sheath to pass through the scope smoothly, and an open width/angle of 5.4 mm/150°. In addition, its thinner cup increases sampling capacity and its swing function helps achieve tangential biopsy (Figure 1B). Sufficient tissue samples for a definitive diagnosis, which include the mucosal cancer or invasive cancer below the bile duct epithelium, can be obtained using this forceps (Figure 3). Hence, the present Radial Jaw 4P may be more appropriate for performing optimal biopsy. In fact, in the present study, this novel forceps could collect adequate specimens for evaluation from all of the patients. Thus, our data showed a higher sensitivity than previous studies of bile duct biopsies (Table 7), suggesting that this novel slim biopsy forceps appears suitable as a tissue sampling device for the diagnosis of biliary strictures.
Regarding bile duct cancer, a lower sensitivity was observed for the perihilar bile duct stricture than for the distal bile duct stricture. This may be due to the narrow and smooth stricture and the distance from the papilla, preventing the biopsy forceps from being able to sufficiently open the stricture and precisely hitting the tumor. In contrast, a better sensitivity was observed for the distal bile duct stricture, particularly in the middle bile duct. For the lower bile duct, a better sensitivity was observed for the stricture with an open space than for the stricture without an open space. In the stricture without an open space for a biopsy forceps to open sufficiently, adequate and accurate biopsy tissue samplings from the target site may be slightly difficult.
Thus far, some reports have shown that the combination of brush cytology and forceps biopsy improved sensitivity[3,8,10]. However, a recent systematic review and a meta-analysis have suggested that the combination of brush cytology and forceps biopsy only modestly increased sensitivity[17]. These combination techniques may be ideal because the tumor is usually exposed to the bile duct surface, allowing brush cytology to obtain cells from the entire lumen surface in bile duct cancer, particularly in severe strictures of the perihilar bile duct and lower bile duct without an open space. On the other hand, the sensitivity of biopsy in pancreatic cancer was lower than that in bile duct cancer similarly to previous reports[1,3,11,18]. Theoretically, tumor cells from the surface of the bile duct in pancreatic cancer appear to be fewer than tumor cells in bile duct cancer. Thus, sensitivity in pancreatic cancer as well as in gallbladder cancer and metastases, namely, “extrinsic neoplasms”, appears to be limited even with the use of additional brush cytology in combination with biopsy forceps. Interestingly, some reports[3,19] have also demonstrated that improvement of sensitivity cannot be expected with the combination of aspiration cytology and brush cytology for cancers with a bile duct stricture by extrinsic exclusion.
Furthermore, the sensitivity for a stricture in pancreatic body cancer was lower than that for a stricture in pancreatic head cancer. This is the reason why the bile duct stricture in pancreatic body cancer, which even shows a biliary stricture that is far from the bile duct, occurs not by direct invasive reaction but as a secondary fibrous cicatricial stricture by stromal reaction. In addition, many pancreatic body cancers occur in the middle bile duct stricture, resulting in a lower sensitivity for the middle bile duct stricture.
In the present study, we showed that a longer stricture had a better sensitivity for diagnosis. This suggests an increase in the positive diagnostic rate of forceps biopsy because the contact area with the bile duct of extrinsic cancer becomes wider according to the stricture length. Hence, to increase the diagnostic accuracy, multiple biopsies appear necessary for the diagnosis of a shorter biliary stricture due to extrinsic cancers.
Recently, EUS-FNA has been introduced for evaluating biliary strictures[12,20]. EUS-FNA may be a preferable method for tissue sampling of neoplasms of extrinsic origin such as pancreatic cancer, gallbladder cancer, and metastasis after obtaining negative or nondiagnostic ERCP tissue sampling results. On the other hand, ERCP-based tissue sampling may be better for bile duct cancer of biliary epithelial origin.
Pancreatitis and cholangitis are generally observed, although their incidence is low and their severity is mild in most cases requiring only conservative treatment[2,21]. Although rare, bile duct perforation can occur during forceps biopsy[1]. Repeated biopsies at the same site may account for the perforation. In the present study, 1 case of perforation of the extrahepatic bile duct with bile leakage occurred during forceps biopsy in a patient with perihilar bile duct cancer. Although Radial Jaw 4P is a soft biopsy forceps, care should be taken during its usage.
Various tips in performing forceps biopsy have been reported. Of interest is the study of Tamada et al[22] which suggested factors for a successful bile duct biopsy under percutaneous transhepatic cholangioscopy (PTCS) from the viewpoint of the growth type of bile duct cancers. They recommended that 2 biopsy samples should be obtained from the mass in polypoid-type bile duct cancers and from the margin in stenotic-type bile duct cancers with a dilated vessel. Moreover, 3 biopsy samples should be obtained from the margin of the stricture in stenotic-type bile duct cancers without a dilated vessel, and biopsy specimens should be obtained from within the stricture in pancreatic cancer. Hence, transpapillary bile duct biopsy is performed under fluoroscopic guidance and is not similar to the biopsy performed under direct vision by PTCS. Thus, more than 3 biopsy specimens may be necessary and the biopsy sites, such as the margin of the stricture or within the stricture, should be considered according to the types of cancers such as cancers of biliary epithelial or extrinsic origin.
The limitations of this study were its retrospective nature, the lack of a control group, and the inclusion of a single-center experience.
In conclusion, endoscopic transpapillary biopsy using a novel slim biopsy forceps is feasible and reliable, but is a restrictive method for tissue sampling of malignant biliary strictures. When performing the biopsy, it is necessary to consider the stricture levels, stricture lengths, and types of cancers such as cancers of biliary epithelial or extrinsic origin to improve the diagnosis of malignant biliary strictures.
There are several cytological and histological sampling techniques for a definitive diagnosis of malignant biliary strictures. However, the optimal sampling technique remains controversial. The ideal technique should be simple, safe, and effective with a high sensitivity and specificity.
Transpapillary forceps biopsy has not been widely used because it is thought to be technically difficult, resulting in a waste of time. Moreover, its sensitivity is very variable (15%-81%). In the present study, a novel slim biopsy forceps could collect adequate specimens for evaluation from all of the patients and our data showed a higher sensitivity (70%) than previous studies of bile duct biopsies.
It is necessary to consider the stricture levels, stricture lengths, and types of cancers such as cancers of biliary epithelial or extrinsic origin to improve the diagnosis of malignant biliary strictures.
Endoscopic transpapillary biopsy using a novel slim biopsy forceps is feasible and reliable.
Endoscopic transpapillary biopsy for diagnosis of biliary strictures should be performed in consideration of the stricture level, stricture length, and cancer type.
The paper deals with the problem of obtaining reliable biopsies from lesions in the biliary tree. Evidently the methods described here yields a progress in this field. The paper is well written. This is good study exploring the efficacy and diagnostic value of a new slim forceps by Boston. The manuscript is generally interesting and offers useful clinical information to those who carry out endoscopic retrograde cholangiopancreatography.
| 1. | Pugliese V, Conio M, Nicolò G, Saccomanno S, Gatteschi B. Endoscopic retrograde forceps biopsy and brush cytology of biliary strictures: a prospective study. Gastrointest Endosc. 1995;42:520-526. [PubMed] |
| 2. | Jailwala J, Fogel EL, Sherman S, Gottlieb K, Flueckiger J, Bucksot LG, Lehman GA. Triple-tissue sampling at ERCP in malignant biliary obstruction. Gastrointest Endosc. 2000;51:383-390. [PubMed] |
| 3. | Ponchon T, Gagnon P, Berger F, Labadie M, Liaras A, Chavaillon A, Bory R. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: results of a prospective study. Gastrointest Endosc. 1995;42:565-572. [PubMed] |
| 4. | Umeda J, Itoi T. Current status of preoperative biliary drainage. J Gastroenterol. 2015;50:940-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Davidson B, Varsamidakis N, Dooley J, Deery A, Dick R, Kurzawinski T, Hobbs K. Value of exfoliative cytology for investigating bile duct strictures. Gut. 1992;33:1408-1411. [PubMed] |
| 6. | Kurzawinski TR, Deery A, Dooley JS, Dick R, Hobbs KE, Davidson BR. A prospective study of biliary cytology in 100 patients with bile duct strictures. Hepatology. 1993;18:1399-1403. [PubMed] |
| 7. | Mohandas KM, Swaroop VS, Gullar SU, Dave UR, Jagannath P, DeSouza LJ. Diagnosis of malignant obstructive jaundice by bile cytology: results improved by dilating the bile duct strictures. Gastrointest Endosc. 1994;40:150-154. [PubMed] |
| 8. | Farrell RJ, Jain AK, Brandwein SL, Wang H, Chuttani R, Pleskow DK. The combination of stricture dilation, endoscopic needle aspiration, and biliary brushings significantly improves diagnostic yield from malignant bile duct strictures. Gastrointest Endosc. 2001;54:587-594. [PubMed] |
| 9. | de Bellis M, Fogel EL, Sherman S, Watkins JL, Chappo J, Younger C, Cramer H, Lehman GA. Influence of stricture dilation and repeat brushing on the cancer detection rate of brush cytology in the evaluation of malignant biliary obstruction. Gastrointest Endosc. 2003;58:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Howell DA, Parsons WG, Jones MA, Bosco JJ, Hanson BL. Complete tissue sampling of biliary strictures at ERCP using a new device. Gastrointest Endosc. 1996;43:498-502. [PubMed] |
| 11. | Sugiyama M, Atomi Y, Wada N, Kuroda A, Muto T. Endoscopic transpapillary bile duct biopsy without sphincterotomy for diagnosing biliary strictures: a prospective comparative study with bile and brush cytology. Am J Gastroenterol. 1996;91:465-467. [PubMed] |
| 12. | Rösch T, Hofrichter K, Frimberger E, Meining A, Born P, Weigert N, Allescher HD, Classen M, Barbur M, Schenck U. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc. 2004;60:390-396. [PubMed] |
| 13. | Kitajima Y, Ohara H, Nakazawa T, Ando T, Hayashi K, Takada H, Tanaka H, Ogawa K, Sano H, Togawa S. Usefulness of transpapillary bile duct brushing cytology and forceps biopsy for improved diagnosis in patients with biliary strictures. J Gastroenterol Hepatol. 2007;22:1615-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Weber A, von Weyhern C, Fend F, Schneider J, Neu B, Meining A, Weidenbach H, Schmid RM, Prinz C. Endoscopic transpapillary brush cytology and forceps biopsy in patients with hilar cholangiocarcinoma. World J Gastroenterol. 2008;14:1097-1101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 15. | De Bellis M, Sherman S, Fogel EL, Cramer H, Chappo J, McHenry L Jr, Watkins JL, Lehman GA. Tissue sampling at ERCP in suspected malignant biliary strictures (Part 1). Gastrointest Endosc. 2002;56:552-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | de Bellis M, Sherman S, Fogel EL, Cramer H, Chappo J, McHenry L Jr, Watkins JL, Lehman GA. Tissue sampling at ERCP in suspected malignant biliary strictures (Part 2). Gastrointest Endosc. 2002;56:720-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2015;81:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 358] [Article Influence: 32.5] [Reference Citation Analysis (1)] |
| 18. | Kubota Y, Takaoka M, Tani K, Ogura M, Kin H, Fujimura K, Mizuno T, Inoue K. Endoscopic transpapillary biopsy for diagnosis of patients with pancreaticobiliary ductal strictures. Am J Gastroenterol. 1993;88:1700-1704. [PubMed] |
| 19. | Lee SJ, Lee YS, Lee MG, Lee SH, Shin E, Hwang JH. Triple-tissue sampling during endoscopic retrograde cholangiopancreatography increases the overall diagnostic sensitivity for cholangiocarcinoma. Gut Liver. 2014;8:669-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | DeWitt J, Misra VL, Leblanc JK, McHenry L, Sherman S. EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointest Endosc. 2006;64:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Ryan ME. Cytologic brushings of ductal lesions during ERCP. Gastrointest Endosc. 1991;37:139-142. [PubMed] |
| 22. | Tamada K, Kurihara K, Tomiyama T, Ohashi A, Wada S, Satoh Y, Miyata T, Ido K, Sugano K. How many biopsies should be performed during percutaneous transhepatic cholangioscopy to diagnose biliary tract cancer? Gastrointest Endosc. 1999;50:653-658. [PubMed] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Bramhall S, Hussain A, Kreisel W, Lee HC, Neri V, Garcia-Olmo D S- Editor: Ma YJ L- Editor: A
E- Editor: Huang Y