Published online Sep 14, 2017. doi: 10.3748/wjg.v23.i34.6365
Peer-review started: June 6, 2017
First decision: June 22, 2017
Revised: July 10, 2017
Accepted: August 2, 2017
Article in press: August 2, 2017
Published online: September 14, 2017
Processing time: 101 Days and 12.8 Hours
Heterotopic pancreas (HP) is a relatively uncommon entity that is defined as pancreatic tissue without a true anatomical or vascular connection to the pancreas. HP does not cause symptoms in most cases but can occasionally produce various manifestations, including nausea, vomiting, abdominal pain, and even heterotopic pancreatitis. Here, we report an unusual case in which heterotopic pancreatitis complicated by the formation of a pseudocyst that caused gastric outlet obstruction was diagnosed based on serum hyperamylasemia and findings from endoscopic ultrasonography (EUS)-guided fine needle aspiration (EUS-FNA) cytology. EUS-guided single pigtail stent insertion was successfully performed for recurrent gastric outlet obstruction. The patient has remained healthy and symptom-free during 4 years of surveillance. In the context of the relevant literature, the described case is a rare case of HP complicated by a pseudocyst treated via EUS-FNA and stent insertion.
Core tip: Heterotopic pancreas (HP), which is defined as the atypical presence of pancreatic tissue without an anatomical or vascular connection to the pancreas, is relatively rare. As an intra- or submucosal lesion, HP is typically asymptomatic and found incidentally. We report a case involving a 40-year-old man with gastric outlet obstruction secondary to pseudocyst formation in HP.
- Citation: Jin HB, Lu L, Yang JF, Lou QF, Yang J, Shen HZ, Tang XW, Zhang XF. Interventional endoscopic ultrasound for a symptomatic pseudocyst secondary to gastric heterotopic pancreas. World J Gastroenterol 2017; 23(34): 6365-6370
- URL: https://www.wjgnet.com/1007-9327/full/v23/i34/6365.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i34.6365
Heterotopic pancreas (HP) is defined as the presence of pancreatic tissue in an abnormal location without anatomical or vascular connection to the orthotopic pancreas. HP was first described by Jean-Schultz in 1729 and was confirmed histologically by Klobb[1]. Other terms for HP, such as ectopic pancreas, aberrant pancreas, and accessory pancreas, have been used in the literature[2]. In most cases, HP is asymptomatic and found incidentally[1], but it can occasionally produce non-specific symptoms that depend on its location, size, and types of complications. Here, we describe a case involving a 40-year-old man with gastric outlet obstruction secondary to heterotopic pancreatitis with pseudocyst formation.
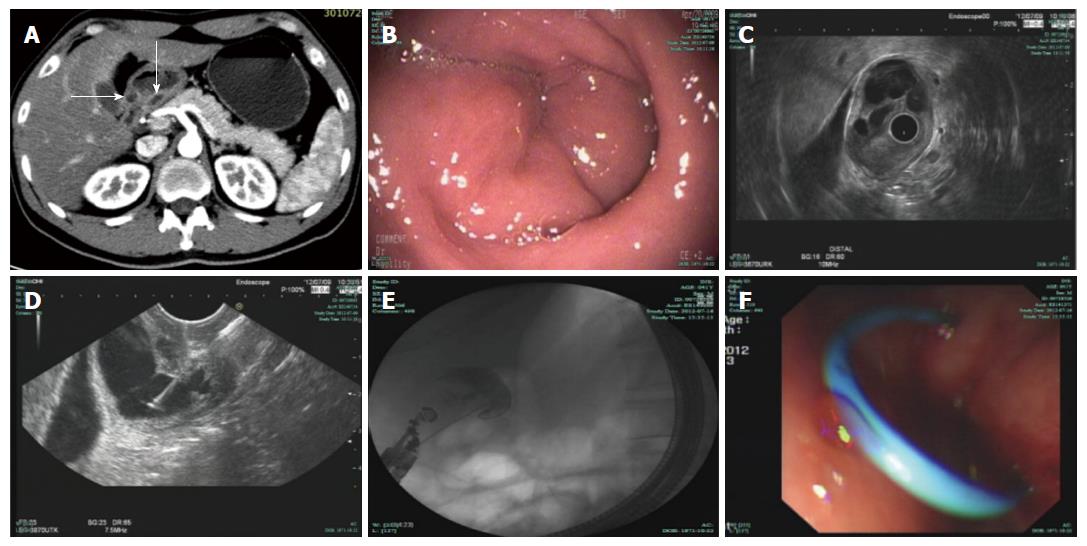
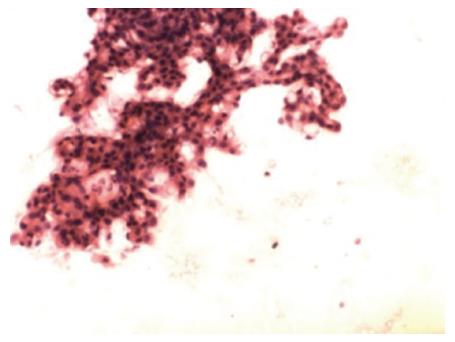
A 40-year-old man was admitted to a community hospital for upper abdominal pain accompanied by nausea and vomiting. He also complained of abdominal distention and moderate fever (38.3 °C). Physical examination revealed minimal tenderness in the epigastric region without a palpable mass or peritoneal signs, and the patient’s vital signs were unremarkable. Routine blood tests showed a significant elevation of the leukocyte count and neutrophil ratio, and biochemical examinations revealed serum amylase of 460 U/L and urine amylase of 3500 U/L. Transabdominal ultrasound was initially performed when the patient was diagnosed with pancreatitis, but no gallbladder stones or common bile duct stones were detected. The patient was treated for acute pancreatitis; his treatments included fasting, injection of a proton pump inhibitor, and somatostatin. All symptoms disappeared 3 d later but recurred after the patient ingested fluids. Subsequently, the patient was transferred to our hospital for further diagnosis. A computed tomography (CT) scan of the abdomen revealed manifestations of a normal pancreas and an ill-defined enhancing mass at the gastric antrum that was causing circumferential narrowing of the pyloric channel (Figure 1A). In an esophagogastroduodenoscopy (EGD) examination, a sub-epithelial lesion without overlying umbilication or dimpling was found in the prepyloric region (Figure 1B). The pyloric channel was only wide enough to allow for the passage of an Olympus Q260 endoscope (OD 9.2 mm), but the duodenum was normal. Radial endoscopic ultrasonography (EUS), which was used as an additional diagnostic tool, confirmed the presence of a cystic lesion measuring 21 mm × 25 mm (Figure 1C) originating from the third layer with anechoic contents and debris. Subsequently, EUS-guided fine needle aspiration (EUS-FNA) was successfully performed (Figure 1D), and a total of 25 mL of clear fluid was aspirated to the greatest possible extent with negative pressure. Cystic fluid analysis revealed an amylase level of 83770 U/L and a carcinoembryonic antigen level of 3.7 μg/L; a cytologic examination of smears revealed clusters of benign-appearing ducts and small acini mixed with inflammatory cells (Figure 2).
The patient was allowed to ingest low-fat fluids 5 d after the procedure, but the aforementioned symptoms immediately re-emerged, and serum amylase increased to 512 U/L. A narrowed pyloric channel was observed in a second EGD examination. The patient refused surgical treatment. Balloon dilation, bougie dilation, and needle-knife puncture of the cyst were not initially selected. In accordance with an endoscopic drainage procedure used for pancreatic pseudocysts, we successfully performed cyst cavity (20 mm × 23 mm) puncture, wire exchange, and the gradual insertion of a single pigtail stent (5 Fr × 4 cm) using a 19-gauge needle (EchoTip Ultra, ECHO-19; Cook Endoscopy, Winston-Salem, NC, United States) (Figure 1E and F). Informed consent was obtained from the patient and his family prior to this endoscopic procedure. No abnormal signs or symptoms were noted during or immediately after the procedure. Shortly thereafter, the patient’s abdominal pain and vomiting subsided, and his leukocyte count and serum amylase returned to normal. The patient was discharged on the sixth day after the second drainage procedure.
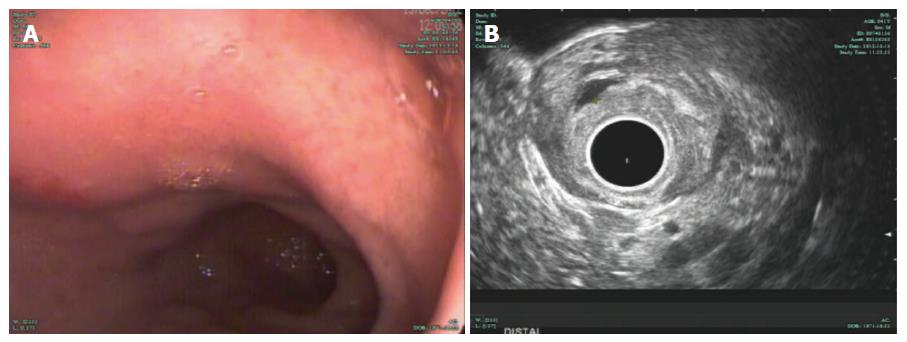
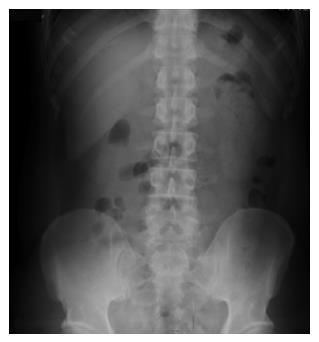
Five months later, the patient returned for a follow-up examination without any complaints. EGD surveillance revealed no stent at the original position, and radial EUS confirmed the presence of a smaller anechoic cystic lesion measuring 3 mm × 5 mm (Figure 3A and B). The stent had completely passed, as confirmed by abdominal radiography (Figure 4). The patient has remained healthy and symptom-free during 4 years of follow-up.
In autopsy studies, HP has generally had an incidence ranging from 0.5%-13.0% and been more common in 30- to 50-year-old subjects, with male predominance[3]. HP is most frequently located in the stomach (25%-38%), followed by the duodenum (17%-36%) and the jejunum (15%-21.7%); it is rarely found in the esophagus, common bile duct, spleen, mesentery, mediastinum or fallopian tube[4]. In the gastrointestinal tract, HP is most frequently located only in the submucosa (54%) and is less frequently found in both the submucosa and muscularis propria (23%), only the muscularis propria (8%), the subserosa (11%), and the whole wall (4%)[1].
HP has been classified into four types by Heinrich and Gaspar-Fuentes[5]. Type I HP shows the presence of ducts, acini, and islets of Langerhans cells; type II HP shows only ducts and acini; type III HP has only ducts; and type IV HP has only islets. Most cases of HP are type II. The pathogenesis of HP remains unclear, although the two most widely accepted hypotheses are the misplacement and metaplasia theories. The misplacement theory proposes that ectopic pancreatic tissue develops from misplacement during foregut rotation, whereas the metaplasia theory suggests that heterotopia arises from a pancreatic metaplasia of the endoderm that migrates to the submucosa during embryogenesis[6].
HP is typically asymptomatic; however, depending on its location and size, HP can occasionally cause clinical symptoms, the most common of which are vomiting and abdominal pain. Peptic ulceration and gastrointestinal bleeding are rare presentations, as are malignant transformation, pancreatitis, pseudocyst formation, gastric outlet obstruction, jejunal intussusception, and ileus[3,7-10]. A thorough search of the English literature revealed five cases of gastric heterotopic pancreatitis in the last 20 years, including our case[11-14] (Table 1). These cases involved three male patients and two female patients. The median age of the patients was 32 years. Epigastric pain was reported in four cases, and vomiting occurred in three cases. In our case, the patient presented with recurrent epigastric pain and vomiting, which were mainly induced by gastric outlet obstruction induced by heterotopic pancreatitis. The symptoms of pain and fever in our case might have been attributable to inflammation or chemical irritation of the involved tissue and enzyme secretion.
| Author | Year | Age/gender | Symptom(s) | Size (mm) | Location | Serum amylase (IU/L) | Preoperative diagnosis | Diagnostic method | Therapy |
| Matsushita | 1997 | 33/M | Epigastric pain | 20 | N/A | 59 | SMT | Surgery | Enucleation |
| Hirasaki | 2005 | 32/M | Epigastric pain | 35 | Angulus | 262 | SMT | Surgery | Partial resection |
| Matissek | 2012 | 15/F | Vomiting | 30 | Pylorus | 144 | SMT | Surgery | Gastroduodenostomy |
| Matsumoto | 2012 | 21/F | Epigastric pain and vomiting | 26 | Antrum | 122 | HP | EUS-FNA | Laparoscopic partial resection |
| Our case | 2017 | 40/M | Epigastric pain and vomiting | 25 | Pylorus | 460 | HP | EUS-FNA | EUS-guided stenting |
It is typically complex and challenging to preoperatively diagnose HP due to its varied presentations. Gastric HP is difficult to radiologically diagnose; however, the double contrast barium meal technique may characteristically show a focally raised mucosal area with associated superficial ulceration within the gastric antrum[15]. CT can localize lesions with normal pancreatic tissue but cannot distinguish HP from other submucosal tumors[16]. Upper endoscopy generally reveals a broad-based, umbilicated, submucosal lesion, with superficial biopsies being non-diagnostic in the vast majority of cases[17]. EUS has been reported to be helpful in diagnosing HP; EUS-FNA in particular is especially effective for confirming a diagnosis of HP, with a reported sensitivity of 80%-100%[18,19]. A novel sampling method using submucosal endoscopy with a mucosal flap was recently reported by Kobara et al[20]. Submucosal dissection allows for the collection of tissue samples even from small lesions (< 2 cm in diameter) under direct endoscopic view. This method was used to eventually prove that a gastrointestinal submucosal tumor (SMT) was a gastric duplication cyst with HP and an ectopic submucosal gland[21]. This approach can be used for preoperative diagnosis of an SMT, albeit with certain limitations.
Symptomatic HP should be respected. However, the optimal management of asymptomatic HP remains debatable. Surgical resection has been reported in most HP cases[11-14]. In the aforementioned cases (Table 1), four patients underwent partial resection of the stomach because this approach was preferable for the preservation of gastric function. The prognosis after surgery was generally favorable, and complete symptom remission was achieved in these cases. Endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR) have also been reported for symptomatic HP; these approaches offer the possibility of localized treatment for HP with relatively low mortality and morbidity rates[22]. Zhong et al[23] reported 60 cases of gastric HP that were treated using ESD or EMR; in particular, 14 patients underwent EMR, and 46 patients underwent ESD. Complications occurred in 12 (20%) patients, including arterial bleeding in nine patients and pneumoperitoneum in three patients; all of these patients were treated conservatively. However, it has been reported that in total, 23% of gastric HP cases are located in the muscularis propria, the subserosa, or the whole wall; the technical difficulty and complication rates of endoscopic procedures may be significantly increased for HP at these locations[1]. Recently, EUS has been increasingly used for pancreatic fluid collection, biliary and pancreatic duct drainage, gallbladder drainage, and other applications[24]. The roles of interventional EUS continue to expand, and growing global experience supports the feasibility and efficacy of this approach[25,26]. In our case, an endoscopic view of the lesion did not reveal the typical umbilicated indentation that has been recognized as characteristic of a central ductal orifice. The absence of communication between the glandular epithelium and the gastric lumen can be attributed to the retention of exocrine secretions that subsequently led to recurrent episodes of acute obstructive pancreatitis, the formation of a retention pseudocyst, and eventually recurrent gastric outlet obstruction. EUS-guided stenting was used to relieve the gastric outlet obstruction by creating an artificial fistula in the pseudocyst. Consequently, this stenting not only rapidly relieved clinical symptoms but also resulted in long-term conversion of symptomatic HP to asymptomatic HP that no longer required surgical treatment. To our knowledge, no previous reports have described heterotopic pancreatitis treated using interventional EUS.
In summary, this case demonstrates the use of a novel endoscopic approach to successfully manage a gastric outlet obstruction secondary to heterotopic pancreatitis with pseudocyst formation. Based on this case, we suggest that HP should be considered in the differential diagnosis of a submucosal gastric mass. EUS-guided intervention can be beneficial for both diagnosing and providing symptomatic relief of heterotopic pancreatitis with pseudocyst formation, and endoscopic treatment should be recommended in selected cases.
A 40-year-old male complained of upper abdominal pain, nausea, and vomiting.
Heterotopic pancreatitis with pseudocyst formation located in the prepyloric region causing gastric outlet obstruction.
Hyperplastic polyp, gastric adenoma, gastrointestinal stromal tumor, and lymphoma.
Laboratory workup revealed a significant elevation of the leukocyte count and neutrophil ratio, serum amylase of 460 U/L, and urine amylase of 3500 U/L.
The abdominal computed tomography scan showed an ill-defined enhancing mass at the gastric antrum. Endoscopic ultrasound (EUS) revealed a cystic lesion measuring 21 mm × 25 mm originating from the third layer with anechoic contents and debris.
Histology revealed heterotopic pancreatic tissue in the prepyloric mucosa with clusters of benign-appearing ducts and small acini mixed with inflammatory cells.
EUS-guided stenting.
A total of four cases of gastric heterotopic pancreatitis have been reported in the literature. However, this is the first report of heterotopic pancreatitis with pseudocyst formation treated using interventional EUS.
Heterotopic pancreas (HP) is a rare entity, and should be considered in the differential diagnosis of a submucosal gastric mass. Although surgical resection has been reported in most symptomatic HP cases, interventional EUS can sometimes be an effective minimally invasive treatment procedure for HP.
The authors describe an interesting and rare case of HP complicated with pseudocyst. Heterotopic pancreatitis with pseudocyst formation has been reported previously, but this case is unique in that endoscopic drainage was performed, which has not been previously described to our knowledge.
| 1. | Ulrych J, Fryba V, Skalova H, Krska Z, Krechler T, Zogala D. Premalignant and malignant lesions of the heterotopic pancreas in the esophagus: a case report and review of the literature. J Gastrointestin Liver Dis. 2015;24:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Jiang LX, Xu J, Wang XW, Zhou FR, Gao W, Yu GH, Lv ZC, Zheng HT. Gastric outlet obstruction caused by heterotopic pancreas: A case report and a quick review. World J Gastroenterol. 2008;14:6757-6759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Mulholland KC, Wallace WD, Epanomeritakis E, Hall SR. Pseudocyst formation in gastric ectopic pancreas. JOP. 2004;5:498-501. [PubMed] |
| 4. | Christodoulidis G, Zacharoulis D, Barbanis S, Katsogridakis E, Hatzitheofilou K. Heterotopic pancreas in the stomach: a case report and literature review. World J Gastroenterol. 2007;13:6098-6100. [PubMed] [DOI] [Full Text] |
| 5. | Gaspar Fuentes A, Campos Tarrech JM, Fernández Burgui JL, Castells Tejón E, Ruíz Rossello J, Gómez Pérez J, Armengol Miró J. [Pancreatic ectopias]. Rev Esp Enferm Apar Dig. 1973;39:255-268. [PubMed] |
| 6. | Rodriguez AA, Berquist W, Bingham D. Gastric outlet obstruction caused by heterotopic pancreas in an adolescent. Dig Dis Sci. 2015;60:835-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Ruan M, Liu M, Cheng L, Xie W, Chen L. Increased 18F-FDG uptake of heterotopic pancreatitis in the small intestine: A CARE-compliant case report. Medicine (Baltimore). 2016;95:e4465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Haj M, Shiller M, Loberant N, Cohen I, Kerner H. Obstructing gastric heterotopic pancreas: case report and literature review. Clin Imaging. 2002;26:267-269. [PubMed] |
| 9. | Chung JP, Lee SI, Kim KW, Chi HS, Jeong HJ, Moon YM, Kang JK, Park IS. Duodenal ectopic pancreas complicated by chronic pancreatitis and pseudocyst formation--a case report. J Korean Med Sci. 1994;9:351-356. [PubMed] |
| 10. | Rimal D, Thapa SR, Munasinghe N, Chitre VV. Symptomatic gastric heterotopic pancreas: clinical presentation and review of the literature. Int J Surg. 2008;6:e52-e54. [PubMed] |
| 11. | Matsushita M, Hajiro K, Takakuwa H. Acute pancreatitis occurring in gastric aberrant pancreas accompanied by paralytic ileus. Am J Gastroenterol. 1997;92:2121-2122. [PubMed] |
| 12. | Hirasaki S, Tanimizu M, Moriwaki T, Nasu J. Acute pancreatitis occurring in gastric aberrant pancreas treated with surgery and proved by histological examination. Intern Med. 2005;44:1169-1173. [PubMed] |
| 13. | Matissek C, Grundhuber H, Steinborn M, Hosie S. Gastric outlet obstruction caused by heterotopic pancreatitis. Eur J Pediatr Surg. 2012;22:165-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Matsumoto T, Tanaka N, Nagai M, Koike D, Sakuraoka Y, Kubota K. A case of gastric heterotopic pancreatitis resected by laparoscopic surgery. Int Surg. 2015;100:678-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Kilman WJ, Berk RN. The spectrum of radiographic features of aberrant pancreatic rests involving the stomach. Radiology. 1977;123:291-296. [PubMed] |
| 16. | Cho JS, Shin KS, Kwon ST, Kim JW, Song CJ, Noh SM, Kang DY, Kim HY, Kang HK. Heterotopic pancreas in the stomach: CT findings. Radiology. 2000;217:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Filip R, Walczak E, Huk J, Radzki RP, Bieńko M. Heterotopic pancreatic tissue in the gastric cardia: a case report and literature review. World J Gastroenterol. 2014;20:16779-16781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Rodriguez FJ, Abraham SC, Allen MS, Sebo TJ. Fine-needle aspiration cytology findings from a case of pancreatic heterotopia at the gastroesophageal junction. Diagn Cytopathol. 2004;31:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Attwell A, Sams S, Fukami N. Diagnosis of ectopic pancreas by endoscopic ultrasound with fine-needle aspiration. World J Gastroenterol. 2015;21:2367-2373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Kobara H, Mori H, Fujihara S, Nishiyama N, Kobayashi M, Kamata H, Masaki T. Bloc biopsy by using submucosal endoscopy with a mucosal flap method for gastric subepithelial tumor tissue sampling (with video). Gastrointest Endosc. 2013;77:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Kobara H, Mori H, Masaki T. Gastric Duplication Cyst with Heterotopic Pancreas and Ectopic Submucosal Gland on Submucosal Endoscopy. Dig Endosc. 2016;28:223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Ryu DY, Kim GH, Park DY, Lee BE, Cheong JH, Kim DU, Woo HY, Heo J, Song GA. Endoscopic removal of gastric ectopic pancreas: an initial experience with endoscopic submucosal dissection. World J Gastroenterol. 2010;16:4589-4593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Zhong YS, Shi Q, Yao LQ, Zhou PH, Xu MD, Wang P. Endoscopic mucosal resection/endoscopic submucosal dissection for gastric heterotopic pancreas. Turk J Gastroenterol. 2013;24:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Ryozawa S, Fujita N, Irisawa A, Hirooka Y, Mine T. Current status of interventional endoscopic ultrasound. Dig Endosc. 2017;29:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Siddiqui AA, Adler DG, Nieto J, Shah JN, Binmoeller KF, Kane S, Yan L, Laique SN, Kowalski T, Loren DE. EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: a large retrospective, multicenter U.S. experience (with videos). Gastrointest Endosc. 2016;83:699-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 218] [Article Influence: 21.8] [Reference Citation Analysis (1)] |
| 26. | Teoh AY, Ho LK, Dhir VK, Jin ZD, Kida M, Seo DW, Wang HP, Yang AM, Binmoeller KF, Varadarajulu S. A multi-institutional survey on the practice of endoscopic ultrasound (EUS) guided pseudocyst drainage in the Asian EUS group. Endosc Int Open. 2015;3:E130-E133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Attwell A, Fiori E, Kobara H, Patne SC S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Zhang FF