Published online Sep 14, 2017. doi: 10.3748/wjg.v23.i34.6357
Peer-review started: February 10, 2017
First decision: March 7, 2017
Revised: April 4, 2017
Accepted: May 4, 2017
Article in press: May 5, 2017
Published online: September 14, 2017
Processing time: 216 Days and 6.8 Hours
To investigate potential biomarkers for predicting postoperative pancreatic fistula (POPF) after pancreaticoduodenectomy (PD).
We prospectively recruited 83 patients to this study. All patients underwent PD (Child’s procedure) at the Division of Hepatobiliary and Pancreas Surgery at the First Bethune Hospital of Jilin University between June 2011 and April 2015. Data pertaining to demographic variables, clinical characteristics, texture of pancreas, surgical approach, histopathological results, white blood cell count, amylase and choline levels in the serum, pancreatic/gastric drainage fluid, and choline and amylase levels in abdominal drainage fluid were included in the analysis. Potential correlations between these parameters and postoperative complications such as, POPF, acute pancreatitis, hemorrhage, delayed gastric emptying, and biliary fistula, were assessed.
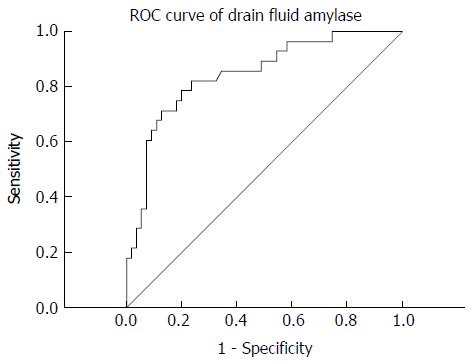
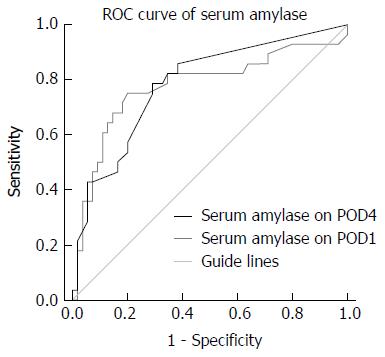
Twenty-eight out of the 83 (33.7%) patients developed POPF. The severity of POPF was classified as Grade A in 8 (28%) patients, grade B in 16 (58%), and grade C in 4 (14%), according to the pancreatic fistula criteria. On univariate and multivariate logistic regression analyses, higher amylase level in the abdominal drainage fluid on postoperative day (POD)1 and higher serum amylase levels on POD4 showed a significant correlation with POPF (P < 0.05). On receiver operating characteristic curve analysis, amylase cut-off level of 2365.5 U/L in the abdominal drainage fluid was associated with a 78.6% sensitivity and 80% specificity [area under the curve (AUC): 0.844; P = 0.009]. A cut-off serum amylase level of 44.2 U/L was associated with a 78.6% sensitivity and 70.9% specificity (AUC: 0.784; P = 0.05).
Amylase level in the abdominal drainage fluid on POD1 and serum amylase level on POD4 represent novel biomarkers associated with POPF development.
Core tip: In this study, we sought to identify biomarkers that could help predict the risk of postoperative pancreatic fistula (POPF) after pancreaticoduodenectomy. Diagnosis of POPF was based on the International Study Group of Pancreatic Fistula criteria. Association between POPF and various clinical and biochemical parameters was assessed. Amylase level in the abdominal drainage fluid on postoperative day 1 and serum amylase level on postoperative day 4 showed a significant association with POPF and represent novel biomarkers associated with POPF development.
- Citation: Jin S, Shi XJ, Wang SY, Zhang P, Lv GY, Du XH, Wang GY. Drainage fluid and serum amylase levels accurately predict development of postoperative pancreatic fistula. World J Gastroenterol 2017; 23(34): 6357-6364
- URL: https://www.wjgnet.com/1007-9327/full/v23/i34/6357.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i34.6357
Pancreaticoduodenectomy (PD) is a common operative approach for treatment of various malignant diseases of the distal biliary duct, the head of pancreas, and the peri-ampullar region. It is also widely acknowledged as one of the most challenging surgical procedures. PD is associated with substantial perioperative mortality (2%-5% even in high-volume centers) and morbidity (30%-50%)[1,2]. Postoperative pancreatic fistula (POPF) is one of the most important complications of PD; reported incidence rates range from 2% to 25%[3]. POPF often leads to further complications, such as hemorrhage and abdominal abscess, and is associated with increased length of hospital stay[3,4]. POPF and other PD-associated complications have necessitated the development of new surgical modalities, like a combination of pancreaticojejunostomy with duct-mucosa pancreaticojejunostomy; however, the incidence of POPF following PD continues to be a concern[5].
Various pre-operative and intra-operative factors, such as preoperative jaundice and diameter of the pancreatic duct, are associated with POPF; however, these factors are not reliable predictors of POPF[6,7]. Biochemical markers in serum and drainage fluid may reflect the disease progression, and it is of translational significance to investigate their correlation with clinical characteristics and their potential value as predictors of POPF. In this study, we sought to identify potential predictors of POPF development, which may help optimize the treatment of these patients in clinical practice.
We prospectively recruited 83 patients to this study. All patients underwent PD at the Department of Hepatobiliary and Pancreatic Surgery at the First Bethune Hospital of Jilin University between June 2011 and April 2015. Preoperative, intraoperative and postoperative data were collected for each patient. Preoperative variables of interest included age, sex, history of diabetes, jaundice, plasma protein levels and pre-operative intervention for jaundice (if any). Intraoperative variables included pancreatic consistency, diameter of pancreatic duct, and the technique used for pancreatic anastomosis. Postoperative data included the results of pathological examination, pancreatic and gastric drainage volume, and complications after operation, such as POPF, hemorrhage, acute pancreatitis, delayed gastric emptying (DGE), and biliary fistula (Table 1).
| Complication | Definition |
| POPF | Drainage fluid amylase activity on or after postoperative day 3 is at least three times the upper limit in normal serum |
| Ascites | Ultrasound evidence of ascites depth > 5 cm |
| Hemorrhage | Requires postoperative transfusion of ≥ 2 U isogenic red blood cells |
| Biliary fistula | Abdominal drainage produces bilious fluid at 50 mL/d after surgery |
| DGE | Indwelling stomach tube for > 10 d |
Severity of POPF was classified as grade A, B or C, as defined by the International Study Group of Pancreatic Fistula[8]. Association between surgical outcomes and results of routine serological and biochemical investigations was assessed. The study protocol complied with the principles of the Declaration of Helsinki, and was approved by the Institutional Ethics Committee at the First Hospital of Jilin University. All patients had provided informed consent prior to their enrolment.
Forty-three patients received PD with a pancreatic drainage tube (size: 6, 7 or 8) anastomosed to the pancreatic parenchyma during the pancreaticojejunostomy; the tube was then drawn out through the distal bowel. In the remaining 40 patients, the standard Child’s procedure was performed with placement of external pancreatic drainage tube (size: 6, 7 or 8), which was fixed to the pancreatic parenchyma and left in the bowel. All patients were treated with cefoperazone and sulbactam (3.0 g/Q12 h) for the first 4 postoperative days (PODs) to prevent infection. Postoperatively, prophylactic intravenous octreotide was administered at a dose of 0.6 mg/24 h for 3 d to reduce the amount of pancreatic secretion.
Statistical analyses were conducted using SPSS software (version 19.0; SPSS Inc., IBM, Armonk, NY, United States). Univariate and multivariate analyses were performed to identify factors significantly related to POPF. Receiver operating characteristic (ROC) curve analysis was performed to assess the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the identified variables. A P value of less than 0.05 was considered indicative of statistical significance.
Out of 83 patients, 28 (33.7%) eventually developed POPF. The severity of POPF was classified as grade A in 8 (28%) patients, grade B in 16 (58%) and grade C in 4 (14%) (Table 2).
| Complication | Cases, n = 30 | Incidence, % |
| POPF | 28 | 33.7 |
| Type A | 8 | 28 |
| Type B | 16 | 58 |
| Type C | 4 | 14 |
| Ascites | 4 | 10 |
| Hemorrhage | 7 | 17.5 |
| Biliary leakage | 1 | 2.5 |
| DGE | 1 | 2.5 |
| Postoperative acute pancreatitis | 1 | 2.5 |
We first categorized the patients as POPF and non-POPF groups, and then compared the levels of different parameters between the two groups (Table 3). On univariate analysis, amylase level in the abdominal drainage fluid on POD1 and serum amylase level on PODs 1 and 4 were correlated with POPF development (P < 0.05) (Table 4).
| Variable | POPF (n = 14) | Non-POPF (n = 26) | t/z/χ2 | P value |
| Age in yr | 54.18 ± 6.945 | 54.04 ± 10.447 | 0.065 | 0.948 |
| Sex | ||||
| Male | 20 (71.4) | 32 (58.2) | 1.391 | 0.3371 |
| Female | 8 (28.6) | 23 (41.8) | ||
| Blood loss in mL | 300 (200, 575) | 200 (200, 600) | -1.266 | 0.205 |
| Pancreatic duct diameter in mm | ||||
| > 3 | 11 (39.3) | 33 (60.0) | 3.196 | 0.1041 |
| < 3 | 17 (60.7) | 22 (40.0) | ||
| Preoperative low plasma protein | ||||
| Yes | 7 (25.0) | 12 (21.8) | 0.106 | 0.7861 |
| No | 21 (75.0) | 43 (78.2) | ||
| Preoperative relief of jaundice | ||||
| Yes | 4 (14.3) | 4 (7.3) | 1.048 | 0.4331 |
| No | 24(85.7) | 51 (92.7) | ||
| Preoperative jaundice | ||||
| Yes | 18 (64.3) | 34 (61.8) | 0.048 | 1.000 |
| No | 10 (35.7) | 21 (38.2) | ||
| Surgical type | ||||
| External drainage of PD | 12 (42.9) | 27 (49.1) | 0.289 | 0.6471 |
| Internal drainage of PD | 16 (57.1) | 28 (50.9) | ||
| Diabetes | ||||
| Yes | 2 (7.1) | 0 (0.0) | 4.026 | 0.1111 |
| No | 26 (92.9) | 55 (100.0) | ||
| Duration of surgery in min | 267.5 (240.0, 327.5) | 280.0 (235.0, 335.0) | -0.082 | 0.935 |
| Hospitalization period in days | 15.00 (13.00, 18.00) | 21.50 (18.00, 27.75) | -4.385 | < 0.001 |
| Drainage fluid amylase level on POD1 in U/L | 6017.5 (2494.5, 11752.5) | 890.0 (350.0, 1500.0) | -5.110 | < 0.001 |
| Serum amylase level in U/L | ||||
| POD1 | 379.5 (157.5, 627.5) | 124.0 (80.0, 165.0) | -4.091 | < 0.001 |
| POD4 | 30.0 (30.0, 50.0) | 61.5 (45.5, 150.0) | -4.435 | < 0.001 |
| Serum albumin | ||||
| POD1 | 29.5 (25.3, 34.3) | 30.7 (26.6, 33.0) | -0.125 | 0.900 |
| POD3 | 30.1 (25.7, 34.6) | 30.4 (27.1, 33.2) | -0.111 | 0.912 |
| POD5 | 30.8 (27.8, 34.4) | 31.4 (28.4, 36.9) | -0.718 | 0.473 |
| Choline | ||||
| POD1 | 4957 (4022, 4957) | 5242 (4048, 6715) | -0.876 | 0.381 |
| POD3 | 3481 (2626, 4518) | 4056 (3345, 5020) | -1.425 | 0.154 |
| POD5 | 3268 (2696, 4537) | 4024 (3248, 5069) | -1.580 | 0.114 |
| WBC count | ||||
| POD1 | 14.73 (10.84, 18.75) | 15.49 (12.42, 18.41) | -0.751 | 0.452 |
| POD3 | 14.90 (11.74, 18.64) | 13.54 (10.34, 16.58) | -1.112 | 0.266 |
| POD5 | 11.99 (9.65, 15.56) | 11.24 (9.17, 14.79) | -0.510 | 0.610 |
| Pathology examination result | 2.445 | 0.485 | ||
| Cholangiocarcinoma | 12 (42.9) | 29 (52.7) | ||
| Pancreatic carcinoma | 3 (10.7) | 8 (14.5) | ||
| Ampullary carcinoma | 3 (10.7) | 7 (12.7) | ||
| Other | 10 (35.7) | 11 (20.0) |
| Factor | β | SE | Waldχ2 | OR (95%CI) | P value |
| Age in yr | 0.023 | 0.037 | 0.397 | 1.024 (0.952-1.101) | 0.529 |
| Sex, female/male | -0.586 | 0.500 | 1.375 | 0.557 (0.209-1.482) | 0.241 |
| Blood loss in mL | 0.000 | 0.001 | 0.257 | 1.000 (0.999-1.001) | 0.612 |
| Pancreatic duct diameter, | -0.841 | 0.475 | 3.135 | 0.431 (0.170-1.094) | 0.077 |
| > 3 mm/< 3 mm | |||||
| Preoperative low plasma protein, yes/no | 0.178 | 0.545 | 0.106 | 1.194 (0.410-3.476) | 0.744 |
| Preoperative relief of jaundice, yes/no | 0.754 | 0.749 | 1.012 | 2.125 (0.489-9.227) | 0.314 |
| Preoperative jaundice, yes/no | 0.106 | 0.482 | 0.048 | 1.112 (0.432-2.861) | 0.826 |
| Internal or External drainage of PD | 0.251 | 0.468 | 0.289 | 1.286 (0.514-3.214) | 0.591 |
| Diabetes, yes/no | 21.952 | 28420.722 | 0.000 | 3.417E9 (0.000) | 0.999 |
| Pathology examination result, ampullary carcinoma/pancreatic carcinoma/cholangiocarcinoma | 0.241 | 0.182 | 1.757 | 1.273 (0.891-1.819) | 0.185 |
| Duration of surgery in min | 0.000 | 0.003 | 0.008 | 1.000 (0.995-1.005) | 0.928 |
| Drainage fluid amylase level, POD1 > 5000 U/L | 0.000 | 0.000 | 10.293 | 1.000 (1.000--1.000) | 0.001 |
| Serum amylase level | |||||
| POD1 > 140 U/L | 0.004 | 0.001 | 9.982 | 1.004 (1.001-1.006) | 0.002 |
| POD4 > 140 U/L | 0.013 | 0.005 | 7.752 | 1.013 (1.004-1.022) | 0.005 |
| Serum albumin | |||||
| POD1 | 0.000 | 0.045 | 0.000 | 1.000 (0.916, 1.092) | 0.994 |
| POD3 | -0.006 | 0.044 | 0.020 | 0.994 (0.911, 1.084) | 0.887 |
| POD5 | -0.023 | 0.044 | 0.277 | 0.977 (0.897, 1.064) | 0.599 |
| Choline | |||||
| POD1 | -0.016 | 0.041 | 0.149 | 0.984 (0.908, 1.067) | 0.700 |
| POD3 | 0.033 | 0.046 | 0.521 | 1.034 (0.945, 1.131) | 0.470 |
| POD5 | 0.007 | 0.053 | 0.016 | 1.007 (0.908, 1.117) | 0.898 |
| WBC count | |||||
| POD1 | 0.000 | 0.000 | 1.153 | 1.000 (1.000, 1.000) | 0.283 |
| POD3 | 0.000 | 0.000 | 1.298 | 1.000 (0.999, 1.000) | 0.255 |
| POD5 | 0.000 | 0.000 | 2.026 | 1.000 (0.999, 1.000) | 0.155 |
Multivariate logistic regression analysis confirmed that amylase level in abdominal drainage fluid on POD1 and serum amylase level on POD4 were independent predictors of POPF (Table 5). On ROC curve analysis, a cut-off amylase level of 2365.5 U/L in the abdominal drainage fluid on POD1 as predictor of POPF was associated with 78.6% sensitivity, 80% specificity, 66.7% PPV and 88% NPV [area under the curve (AUC): 0.844; P = 0.009] (Figure 1). Similarly, a cut-off serum amylase level of 44.2 U/L on POD4 was associated with a 78.6% sensitivity and 70.9% specificity (AUC: 0.784; P = 0.05; Figure 2). The specificity of these associations was further highlighted by the observation that neither white blood cell (WBC) counts nor choline and albumin levels correlated with POPF development.
| Predictor | β | SE | Waldχ2 | OR (95%CI) | P value |
| Amylase level, POD1 > 5000 U/L | 0.000 | 0.000 | 6.728 | 1.000 (1.000--1.000) | 0.009 |
| Serum amylase, level POD1 > 140 U/L | 0.001 | 0.002 | 0.243 | 1.001 (0.998-1.004) | 0.622 |
| Serum amylase level, POD4 > 140 U/L | 0.009 | 0.004 | 3.826 | 1.009 (1.000-1.017) | 0.050 |
The Whipple procedure for PD for the treatment of benign and malignant tumors in the head of pancreas and the peri-ampullar region is one of the most technically challenging surgical procedures, with long duration of surgery and a high rate of associated complications[9,10]. The reported rates of postoperative complications following PD range from 30%-70%, and postoperative mortality rates have remained approximately 5% even in high-volume centers[11]. POPF accounts for 2%-25% of all complications of PD[12]. The advent of novel surgical modalities for pancreaticojejunostomy, such as the duct-mucosa pancreaticojejunostomy or pancreatic duct stent implantation, has not resulted in major improvement. Therefore, early detection of POPF development and adequate timely intervention is important to improve surgical outcomes in these patients[13-17]. There is a paucity of tools to identify patients who are at risk of development of POPF. Here, we report our prospective study to identify potential predictors of POPF. The results suggest that monitoring of amylase levels in drainage fluid and serum as biomarkers is quite promising.
Various factors have been linked to POPF in previous studies. These include pancreatic consistency, pancreatic duct diameter, and the results of assessment by the pathologist[18-22]; however, their predictive value has been shown to be relatively poor and not intuitive. In the present study, we observed an obvious correlation between amylase levels in the ascitic fluid on POD1 and the serum amylase level on POD4 with the occurrence of POPF. Cloyd et al[20] reported that serum amylase level on POD1 (> 140 U/L) predicts POPF with 81.5% sensitivity, 55.5% specificity, 29.3% PPV, and 93% NPV[23]. Likewise, some researchers demonstrated that drainage fluid amylase level on POD1 (> 350 U/L) can predict POPF with a 79% specificity, 100% sensitivity, 41% PPV, and 100% NPV. Furthermore, Popiela et al[24] showed that the drainage fluid amylase level (> 5000 U/L) on POD1 is a reliable predictor of POPF. Kawai et al[25] also found that the ratio of total amylase in drainage fluid could predict POPF. Our results are consistent with these previous publications, which indicate that amylase level in ascitic fluid on POD1 and blood amylase on POD4 could be used to identify patients who are most likely to suffer from this complication after PD.
Of note, we did not observe a significant association of albumin and WBC counts with POPF. Kawai et al[25] reported that serum albumin levels (< 3.0 g/d) and serum WBC count (> 9800 mm3) on POD4 predicted grades B and C POPF with a 69% sensitivity, a 96% specificity, an 88% PPV, and an 85% NPV. Relles et al[26] found that serum albumin (< 2.5 mg/L) and blood urea nitrogen (> 10 mg/dL) on POD1 were important predictors of perioperative morbidity following PD[27]. However, in our study, the level of serum albumin through POD1, POD3 and POD5 did not correlate with the development of POPF. Considering that acute stress can affect serum albumin level and WBC counts[28], these may not be appropriate measures to gauge the risk of POPF. A prospective trial showed that postoperative albumin levels were not associated with risk during abdominal operation[29]. Welsch et al[29] also reported poor specificity of serum WBC as a predictor of POPF. Our results are consistent with these reports. In addition, no significant association of POPF with cholinesterase level was observed on POD1, POD3 and POD5, which suggests that POPF may not have an obvious relationship with the postoperative change in hepatic reserve.
In our study, a cut-off amylase level of 2365.5 U/L in ascitic fluid on POD1 predicted POPF with 78.6% sensitivity, 80% specificity, 66.7% PPV, and 88% NPV. We hypothesize that in patients with POPF, the higher level of amylase in drainage fluid on POD1 derives from the failed pancreaticojejunal anastomosis, which leads to POPF.
Serum amylase has been used for diagnosis of acute pancreatitis for > 70 years; however, increased serum amylase levels are also observed in other diseases of salivary glands, oviduct epithelium and proximal duodenum, which limits its specificity for the diagnosis of pancreatitis. Intuitively, this should be less of a concern with respect to drainage fluid or generally following PD[30]. Indeed, in our study, serum amylase on POD4 showed a significant correlation with POPF, although serum amylase on POD1 did not effectively predict POPF. We found that serum amylase on POD4 of 44.2 U/L was an effective maker; however, this value is within the normal range of serum amylase, and thus serum amylase level on POD4 higher than 100 U/L is probably more appropriate. Serum amylase on POD4 with a discriminatory threshold of 100 U/L can predict POPF with 42.9% sensitivity, 55% specificity, 75% PPV, and 76.1% NPV. In the early postoperative stage, ischemic injury to pancreatic tissues caused by surgical damage to blood vessels, intraoperative and postoperative hypovolemia, and surgical stress leads to elevation of serum amylase[31]. However, no statistically significant correlation was observed between serum amylase level on POD1 and pancreatic leakage, and no correlation was found between serum amylase and POPF in theory, whereas absorption of exudates from pancreatic anastomosis cannot explain such a phenomenon. On the other hand, we believe that elevation in serum amylase level on POD4 reflects the preliminary establishment of a collateral pancreatic anastomosis, and that correction of hypovolumia may reflect the condition of pancreatic juice derived from accessory pancreatic duct or residual pancreatic section instead of the obstructed main pancreatic duct. Moreover, the erosion effect of amylopsin and trypsin on blood vessel can also increase the absorption speed and the amount of amylopsin into the blood, which reflects the increased serum amylase. Thus, blood amylase level on POD4 is of certain predictive value for the occurrence of POPF.
In our study, we found that amylase activity in drainage fluid on POD1 and serum amylase activity on POD4 could accurately predict POPF, whereas serum albumin and prealbumin did not show a significant predictive value. Measurement of amylase level in drainage fluid on POD1 and the serum amylase level on POD4 is a relatively easy and economical method. Our results support the use of amylase to predict POPF.
This may allow for timely interventions, such as increased duration of antibiotic and octreotide therapies, that may help prevent POPF and allow for timely risk-communication to the patient. It is also another procedure in the processing carried out in our medical center.
Identification of patients who are at high risk of postoperative pancreatic fistula (POPF) in the immediate postoperative period after is a key imperative to improve surgical outcomes of pancreaticoduodenectomy (PD). Biochemical markers in serum and drainage fluid may reflect disease progression, and it is of translational significance to investigate their correlation with clinical characteristics and their potential use to predict the risk of POPF. In this study, we sought to identify potential predictors of POPF development, which may help optimize the treatment of such patients in clinical practice.
Although anastomosis techniques used for PD significantly improves, the incidence of PD-associated POPF remains relatively high. POPF is a major threat to patients who undergo PD. Thus, early and accurate prediction of POPF is essential to achieve optimal surgical outcomes.
Previous studies have found several biochemical factors, such as serum amylase level on postoperative day (POD)1, drainage fluid amylase level on POD1, and a combination of serum albumin and leukocyte count as predictors of POPF. However, some of these markers have low positive predictive value. We demonstrate that amylase level in drainage fluid on POD1 and serum amylase level on POD4 are better predictors of POPF than those proposed earlier.
Measurement of amylase level in serum and drainage fluid is relatively straightforward and inexpensive. These two investigations can help identify patients who are at an increased risk of POPF. This may allow for timely interventions, such as increased duration of antibiotic and octreotide therapies, that may help prevent POPF. It is also another procedure in the processing carried out in our medical center.
Although several biochemical factors have been shown to predict POPF in previous studies, we found that just amylase levels in drainage fluid on POD1 and serum amylase level on POD4 could accurately predict POPF, whereas serum albumin and prealbumin were found to have negligible predictive value.
In this manuscript, the authors correlate the postoperative clinical factors with POPF rate, including prospectively 83 patients with PD, and analyzed the potential correlation between biomarkers and postoperative complications such a pancreatic fistula.
| 1. | Kastenberg ZJ, Morton JM, Visser BC, Norton JA, Poultsides GA. Hospital readmission after a pancreaticoduodenectomy: an emerging quality metric? HPB (Oxford). 2013;15:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Vollmer CM Jr, Sanchez N, Gondek S, McAuliffe J, Kent TS, Christein JD, Callery MP; Pancreatic Surgery Mortality Study Group. A root-cause analysis of mortality following major pancreatectomy. J Gastrointest Surg. 2012;16:89-102; discussion 102-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 3. | Ahmad SA, Edwards MJ, Sutton JM, Grewal SS, Hanseman DJ, Maithel SK, Patel SH, Bentram DJ, Weber SM, Cho CS. Factors influencing readmission after pancreaticoduodenectomy: a multi-institutional study of 1302 patients. Ann Surg. 2012;256:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 4. | Gulbinas A, Barauskas G, Pundzius J. Preoperative stratification of pancreas-related morbidity after the Whipple procedure. Int Surg. 2004;89:39-45. [PubMed] |
| 5. | Büchler MW, Friess H, Wagner M, Kulli C, Wagener V, Z’Graggen K. Pancreatic fistula after pancreatic head resection. Br J Surg. 2000;87:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 297] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 6. | Kingsnorth AN. Duct to mucosa isolated Roux loop pancreaticojejunostomy as an improved anastomosis after resection of the pancreas. Surg Gynecol Obstet. 1989;169:451-453. [PubMed] |
| 7. | Yeo CJ, Cameron JL, Maher MM, Sauter PK, Zahurak ML, Talamini MA, Lillemoe KD, Pitt HA. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580-588; discussion 588-592. [PubMed] |
| 8. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3551] [Article Influence: 169.1] [Reference Citation Analysis (35)] |
| 9. | Wellner UF, Kayser G, Lapshyn H, Sick O, Makowiec F, Höppner J, Hopt UT, Keck T. A simple scoring system based on clinical factors related to pancreatic texture predicts postoperative pancreatic fistula preoperatively. HPB (Oxford). 2010;12:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 10. | Kleespies A, Albertsmeier M, Obeidat F, Seeliger H, Jauch KW, Bruns CJ. The challenge of pancreatic anastomosis. Langenbecks Arch Surg. 2008;393:459-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Bassi C, Falconi M, Molinari E, Mantovani W, Butturini G, Gumbs AA, Salvia R, Pederzoli P. Duct-to-mucosa versus end-to-side pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: results of a prospective randomized trial. Surgery. 2003;134:766-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 12. | Whipple AO, Parsons WB, Mullins CR. TREATMENT OF CARCINOMA OF THE AMPULLA OF VATER. Ann Surg. 1935;102:763-779. [PubMed] |
| 13. | Xiong JJ, Altaf K, Mukherjee R, Huang W, Hu WM, Li A, Ke NW, Liu XB. Systematic review and meta-analysis of outcomes after intraoperative pancreatic duct stent placement during pancreaticoduodenectomy. Br J Surg. 2012;99:1050-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Lillemoe KD, Cameron JL, Kim MP, Campbell KA, Sauter PK, Coleman JA, Yeo CJ. Does fibrin glue sealant decrease the rate of pancreatic fistula after pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg. 2004;8:766-772; discussion 772-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 175] [Article Influence: 8.0] [Reference Citation Analysis (2)] |
| 15. | Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248-257; discussion 257-260. [PubMed] |
| 16. | DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, Yeo CJ, Clavien PA. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931-937; discussion 937-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 630] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 17. | Lee SE, Yang SH, Jang JY, Kim SW. Pancreatic fistula after pancreaticoduodenectomy: a comparison between the two pancreaticojejunostomy methods for approximating the pancreatic parenchyma to the jejunal seromuscular layer: interrupted vs continuous stitches. World J Gastroenterol. 2007;13:5351-5356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Kazanjian KK, Hines OJ, Eibl G, Reber HA. Management of pancreatic fistulas after pancreaticoduodenectomy: results in 437 consecutive patients. Arch Surg. 2005;140:849-854; discussion 854-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Veillette G, Dominguez I, Ferrone C, Thayer SP, McGrath D, Warshaw AL, Fernández-del Castillo C. Implications and management of pancreatic fistulas following pancreaticoduodenectomy: the Massachusetts General Hospital experience. Arch Surg. 2008;143:476-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Cloyd JM, Kastenberg ZJ, Visser BC, Poultsides GA, Norton JA. Postoperative serum amylase predicts pancreatic fistula formation following pancreaticoduodenectomy. J Gastrointest Surg. 2014;18:348-353. [PubMed] |
| 21. | van Berge Henegouwen MI, De Wit LT, Van Gulik TM, Obertop H, Gouma DJ. Incidence, risk factors, and treatment of pancreatic leakage after pancreaticoduodenectomy: drainage versus resection of the pancreatic remnant. J Am Coll Surg. 1997;185:18-24. [PubMed] |
| 22. | Bassi C, Butturini G, Molinari E, Mascetta G, Salvia R, Falconi M, Gumbs A, Pederzoli P. Pancreatic fistula rate after pancreatic resection. The importance of definitions. Dig Surg. 2004;21:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 231] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 23. | Sato N, Yamaguchi K, Chijiiwa K, Tanaka M. Risk analysis of pancreatic fistula after pancreatic head resection. Arch Surg. 1998;133:1094-1098. [PubMed] |
| 24. | Popiela T, Kedra B, Sierzega M, Gurda A. Risk factors of pancreatic fistula following pancreaticoduodenectomy for periampullary cancer. Hepatogastroenterology. 2004;51:1484-1488. [PubMed] |
| 25. | Kawai M, Tani M, Hirono S, Ina S, Miyazawa M, Yamaue H. How do we predict the clinically relevant pancreatic fistula after pancreaticoduodenectomy?--an analysis in 244 consecutive patients. World J Surg. 2009;33:2670-2678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Relles DM, Richards NG, Bloom JP, Kennedy EP, Sauter PK, Leiby BE, Rosato EL, Yeo CJ, Berger AC. Serum blood urea nitrogen and serum albumin on the first postoperative day predict pancreatic fistula and major complications after pancreaticoduodenectomy. J Gastrointest Surg. 2013;17:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Ryan AM, Hearty A, Prichard RS, Cunningham A, Rowley SP, Reynolds JV. Association of hypoalbuminemia on the first postoperative day and complications following esophagectomy. J Gastrointest Surg. 2007;11:1355-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Mahkovic-Hergouth K, Kompan L. Is replacement of albumin in major abdominal surgery useful? J Clin Anesth. 2011;23:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Welsch T, Frommhold K, Hinz U, Weigand MA, Kleeff J, Friess H, Büchler MW, Schmidt J. Persisting elevation of C-reactive protein after pancreatic resections can indicate developing inflammatory complications. Surgery. 2008;143:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | van den Bos R, Taris R, Scheppink B, de Haan L, Verster JC. Salivary cortisol and alpha-amylase levels during an assessment procedure correlate differently with risk-taking measures in male and female police recruits. Front Behav Neurosci. 2014;7:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Okano K, Kakinoki K, Suto H, Oshima M, Kashiwagi H, Yamamoto N, Akamoto S, Fujiwara M, Takama T, Usuki H. Persisting ratio of total amylase output in drain fluid can predict postoperative clinical pancreatic fistula. J Hepatobiliary Pancreat Sci. 2011;18:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Espinel J, Negoi I S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Zhang FF