Published online Sep 14, 2017. doi: 10.3748/wjg.v23.i34.6339
Peer-review started: April 1, 2017
First decision: April 26, 2017
Revised: May 15, 2017
Accepted: June 19, 2017
Article in press: June 19, 2017
Published online: September 14, 2017
Processing time: 169 Days and 18.3 Hours
To evaluate the role of P2Y1R in visceral hypersensitivity in rats with experimental irritable bowel syndrome.
A rat model of irritable bowel syndrome was generated by intra-colonic administration of acetic acid (AA) and assessed by histology and myeloperoxidase (MPO) activity assay. Then P2Y1R expression in the colonic tissue was detected by Western blot. In order to explore the regulatory role of P2Y1R in visceral hypersensitivity, an agonist (MRS2365) and an antagonist (MRS2179) of P2Y1R were intra-colonically administered and effects were tested through a colorectal distension test. The abdominal withdrawal reflex and abdominal electromyography were tested during the course.
Model assessment tests showed an obvious inflammatory reaction that appeared on the 2nd d after the AA injection, and the inflammatory reaction gradually recovered and almost disappeared on the 7th d. The model finished on day 8 and showed a clear feature of IBS that had no organic lesion. The average expression of P2Y1R was significantly higher in the AA group than in the naïve group (0.319 ± 0.02 vs 0.094 ± 0.016, P < 0.001). MRS2365 could effectively raise the colonic hypersensitivity status at intervention doses of 10 (AUC value from 0.30 ± 0.089 to 1.973 ± 0.127 mv·s, P < 0.01) and 100 μmol/L (AUC value from 0.290 ± 0.079 to 1.983 ± 0.195 mv·s, P < 0.01); MRS2179 could effectively reduce the hypersensitivity status at intervention dose of 100 μmol/L (from a mean baseline AUC value of 1.587 ± 0.099 mv·s to 0.140 ± 0.089 mv·s, P < 0.0001). Differences between the MRS2179 group (1.88 ± 1.45) and either the MRS2365 group (3.96 ± 0.19) or the combined treatment (MRS2179 and MRS2365) group (3.28 ± 0.11) were significant (P < 0.01).
P2Y1R plays a regulatory role in visceral hypersensitivity in rats with experimental IBS. Specific antagonists of P2Y1R may have potential therapeutic value in treating abdominal pain in IBS.
Core tip: P2Y1R is believed to be part of a high-sensitivity response to mechanical stimulation but whether it is involved in visceral hypersensitivity of irritable bowel syndrome is discussing. We detected its expression in the colon of a rat model of irritable bowel syndrome, and explored its regulatory role in visceral hypersensitivity with a specific agonist and an antagonist. The abdominal withdrawal reflex and electromyography tests showed effective changes after intervention. It was interesting to note that in case of combined treatment of the two drugs, the antagonist showed higher effects, which indicated that other P2 receptors might be involved in the hypersensitivity of irritable bowel syndrome.
- Citation: Wu J, Cheng Y, Zhang R, Liu D, Luo YM, Chen KL, Ren S, Zhang J. P2Y1R is involved in visceral hypersensitivity in rats with experimental irritable bowel syndrome. World J Gastroenterol 2017; 23(34): 6339-6349
- URL: https://www.wjgnet.com/1007-9327/full/v23/i34/6339.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i34.6339
Irritable bowel syndrome (IBS) is characterized by hypersensitivity and pain before defecation in the relative absence of colon inflammation or structural changes[1]. Studies have reported that visceral hypersensitivity could be mediated by purinergic signaling pathways, which participate in pain transmission[2-4] and possibly induce the pain and discomfort experienced by patients with IBS[5,6].
Nucleotides contribute to the sensation of pain in somatic and visceral organs[5-7]. Cells release adenosine triphosphate (ATP) and other nucleotides in response to a variety of stimuli via the mediation of a class of receptors termed P2[6]. P2 includes the ionotropic (P2XRs) and metabotropic (P2YRs) subtypes. P2XRs have been identified to contribute to pain transmission and be involved in many organic and functional gastrointestinal disorders including IBS[7,8]. The mammalian P2Y1R cloned thus far is widely expressed in animal tissues[6]. Accumulating evidence has shown that P2Y1R can mediate nociceptive signaling[9] and studies[10-12] have suggested that P2Y1R may provide a high-sensitivity system to mechanical stimulation in functional gastrointestinal disorders. However, whether P2Y1R participates in the generation and regulation of visceral hypersensitivity in IBS remains unknown.
In this study, we produced a rat model of post-infectious IBS by intra-colonic injection of acetic acid (AA), and measured the changes of P2Y1R expression in the colon of this model. Moreover, to further explore the role of P2Y1R in the development of visceral hypersensitivity, a specific agonist (MRS2365) and an antagonist (MRS2179) of P2Y1R were used to alter the functional status of P2Y1R, and the abdominal responses were detected after this alteration.
Adult male Sprague-Dawley rats weighing 180-250 g were used. Breeding, maintenance and euthanization of the animals followed the ethical guidelines for the investigations of experimental pain in conscious animals[13], and the study was approved by the Ethical Committee of Xi’an Jiaotong University. All animals were obtained from the standard animal laboratory at the Medical College of Xi’an Jiaotong University and were maintained at a consistent temperature of 20 ± 2 °C under a 12 h light/dark cycle with free access to food and water.
A rat model of post-infectious IBS (AA group) was produced in this study. After an overnight fast, the rats were anesthetized with 10% chloral hydrate by intraperitoneal injection at a dose of 1 mL/kg. On day 1, colitis was induced by intracolonic injection of 1 mL of 4% AA at the site 8 cm proximal to the anus for 60 s. Then the rats were allowed to recover for 7 d from the colitis and the modeling finished on day 8. A PBS group was processed in the same manner except that 1 mL of saline was injected instead of 4% AA. A naïve control group comprised rats that were untreated.
The myeloperoxidase (MPO) activity assay was performed and histological sections of the distal colon were stained to determine the inflammatory condition and ensure the recovery from colitis on the 7th d. Then, abdominal responses and stool features of model rats were detected to assess the hypersensitivity and abdominal features.
Colorectal distension procedure and detection of visceral hypersensitivity: Colorectal distension (CRD) was conducted to assess the visceral perception thresholds in the gut[10,14], which was reported to be the best method to elicit visceral hypersensitivity[15]. Conscious rats of the AA, PBS and naïve groups (n = 8) were studied on the 8th d of modeling. They were fixed in Bollman cages (steel tubes; length, 18 cm; diameter, 6 cm) where they could not move freely, turn around or escape. After 2 d of adaptation, a disposable silicon balloon-urethral catheter (8F, B. Braun Medical Industries, Malaysia) lubricated with liquid paraffin oil was inserted intrarectally 8 cm from the anus and was then fixed to the tail with adhesive tape. The balloon was distended with pre-warmed (37 °C) water. Ascending-limit phasic distension (0.2, 0.4, 0.6, 0.8, 1.0 and 1.2 mL) was applied for 30 s. Each volume was repeated three times at intervals of 5 min to obtain an accurate estimate.
During the CRD, abdominal hypersensitivity was measured by the abdominal withdrawal reflex (AWR) and electromyography (EMG). AWR is a semi-quantitative scored test for IBS models to assess hypersensitivity[16]. The score was assigned as follows: 0 = no behavioral response to distension; 1 = brief head movements followed by immobility; 2 = contraction of abdominal muscle without lifting of abdomen; 3 = lifting of abdomen; and 4 = body arching and lifting of pelvic structure.
EMG was also used to quantitatively measure visceral motor responses of conscious rats[17] during the CRD. The rats were first briefly anesthetized by diethyl ether inhalation and then fixed in the Bollman cages. A prepared bipolar silver electrode (diameter = 0.8 mm; SBT Co., Ltd., China) was used to link the rats to the biology function laboratory system. One end of the electrode was sunk into the external oblique muscle 0.5 cm above the inguinal ligament. The other end was connected to a BL-420F biology function laboratory system (Chengdu Taimeng Technology Co., Ltd., China) such that activation of the muscle could be recorded and analyzed on a computer.
Total stool weight: Stools were collected every day after the rats were placed into the Bollman cage. Total stool weight (weight of fresh defecation) and dry weight (weight of feces air-dried for 24 h) were measured. Water content of the stool was calculated by the equation: Water content = (dry weight/net weight) × 100%.
MPO activity assay: The MPO activity assay was performed to quantify the inflammation in the distal colon according to the model preparation procedure described previously[17]. Six rats from each group (the AA group, PBS group and naïve group) were randomly chosen to harvest distal colonic tissues on the 2nd and 7th d (3 rats per day for each group) after the injection of 4% AA to produce the model. These two time points were selected according to a previous study[1] to represent the inflammatory phase and the subsiding phase, respectively. Briefly, an 8-cm segment of distal colon was collected via laparotomy, minced in 1 mL of 50 mmol/L potassium phosphate buffer (pH 6.0) containing 14 mmol/L hexadecyltrimethylammonium bromide, homogenized and sonicated. The lysates were frozen and thawed three times and then centrifuged for 2 min at 4 °C at 15000 rpm. Aliquots of the supernatants were mixed with potassium phosphate buffer containing o-dianisidine-HCL (Sigma-Aldrich, St. Louis, MO, United States) and 0.0005% H2O2. The change in absorbance at 460 nm was spectrophotometrically determined. The MPO activity was expressed as units/g of wet tissue. An enzyme unit was defined as the conversion rate of 1 μmol of H2O2 per min at 25 °C.
Histological examination of colonic inflammation: To examine the extent of colonic inflammation, histological tissues were collected at the selected time points (the 2nd and 7th d as mentioned above). The tissues were fixed in formalin for 12 h, and pathological sections were sliced at a thickness of 5 μm and processed for hematoxylin-eosin staining. The slides were observed under an optical microscope, and inflammatory cells were observed by a pathologist blinded to the treatments and time points.
Protein expression of P2Y1R in the distal colon of rats (n = 8) in the AA, PBS and naïve groups were examined by Western blot. Distal colonic tissue was collected under anesthesia by intraperitoneal injection of 10% chloral hydrate. The tissues were either used immediately or stored at -80 °C for later use. P2Y1R antibody was purchased from Santa Cruz Biotechnology (catalog sc-377324).
Briefly, frozen sections of colonic tissue were trimmed and homogenized in RIPA buffer containing 50 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 1% NA-40, 0.25% sodium-deoxycholate, 1 mmol/L EDTA, 1 mmol/L PMSF, 1 mg/mL aprotinin, 1 mg/mL leupeptin, 1 mg/mL pepstatin, 1 mmol/L Na3VO4, and 1 mmol/L NaF. A total of 20 μg of protein from the intestinal homogenate was mixed with gel-loading buffer, boiled for 5 min, and loaded onto a 12% polyacrylamide gel. Electrophoresis was then conducted, and the protein was transferred to a nitrocellulose membrane. Then, the membrane was pre-incubated in 1 × Tris-buffered saline containing 5% skim milk at room temperature for 2 h. Subsequently, the membrane was incubated at 4 °C overnight with rabbit primary antibody against either P2Y1R (1:500) or β-actin (1:1000, control) in 1 × Tris-buffered saline containing 5% skim milk. After washing, the membranes were incubated with anti-rabbit IgG (1:3000) at room temperature for 2 h. Bands were visualized with a SuperSignal Substrate Chemiluminescence Kit (Pierce 34077).
Drug preparation: The P2Y1R agonist MRS2365 (2157/1, R&D Systems China Co., Ltd., China) and the antagonist MRS2179 (ab120414, ABCAM Corporation) were dissolved in phosphate-buffered saline at concentrations of 0.1 to 100 μmol/L, respectively, which had been proven effective in other studies[18,19]. The drugs were administered by intra-colonic instillation using a 0.2 cm diameter tube that was inserted into the colon 8 cm from the anus and held still for 10 min per day. All the rats were shortly narcotized by inhalation with ether before instillation of drugs and CRD was carried out when they recovered consciousness.
Drug concentration selection: Five groups of AA rats, three groups of PBS rats and three groups of naïve rats (n = 8 for each) were set for MRS2365 or MRS2179 treatment each. AA rats were randomly divided into an untreated control group and four treated groups (0.1, 1, 10 and 100 μmol/L). The PBS and naïve groups were divided into an untreated control group and two treated groups (10 and 100 μmol/L). The drugs were administered for 7 d at 10 mL/kg body weight every day. CRD and EMG were carried out at 2, 12, 24, 48, 72 and 168 h from the initiation of treatment to select the most effective concentrations. Our preliminary experimental results showed that distension volumes could cause abdominal responses beginning at 0.8 mL and get the highest response at 1.2 mL. Therefore, we chose 1.2 mL to examine the down-regulatory role of MRS2179 in CRD course, and 0.8 mL for the up-regulatory role of MRS2365.
Drug intervention: AA rats were treated with MRS2365 and/or MRS2179 at their selected concentrations. AA rats were divided into four groups (n = 8 each): no treatment (PBS), MRS2179, MRS2365 and combined treatment (first MRS2365, then MRS2179). The regimen lasted 7 d, and drugs were administered at a dose of 10 mL/kg body weight every day. CRD was carried out under the distension volume of 1.2 mL. The AWR and EMG were observed at 2, 12, 24, 48, 72 and 168 h from the initiation of treatment.
All examinations were performed in duplicate. Statistical analyses were performed using SPSS 17.0 software. Quantitative data are expressed as the mean ± SE. The one-way ANOVA approach was used to compare two groups. Two-way ANOVA was used for multiple group comparisons. A value of P < 0.05 was regarded as statistically significant.
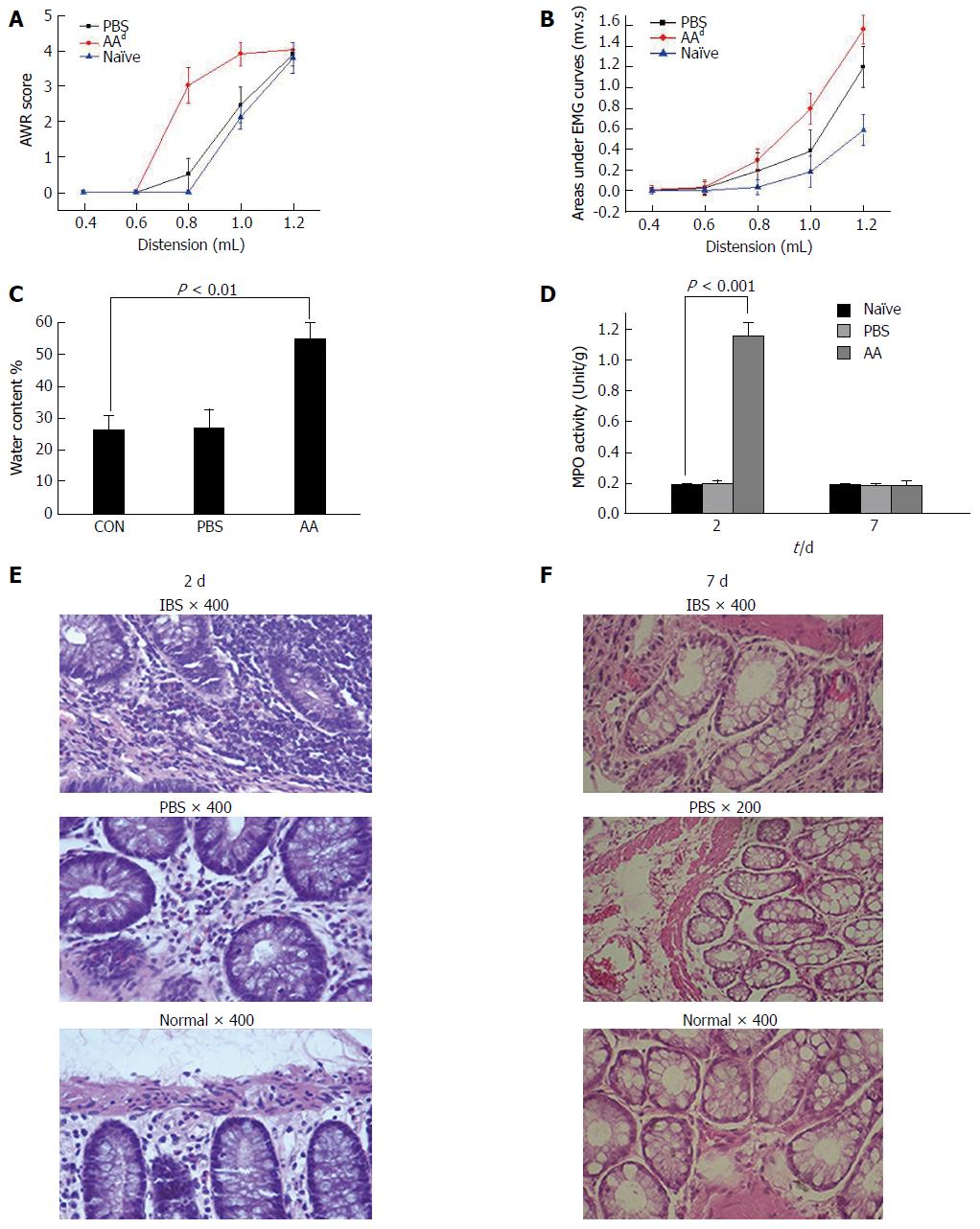
Because IBS characteristically appears with no inflammation or other organic problems in the colon, and a post-inflammatory model has been proven to demonstrate the major features of IBS after acute inflammation in the colon subsides[17], we chose to use this procedure to create a post-inflammatory animal model. The AWR score was significantly increased from distension volumes of 0.8 (3.0 ± 0.54) mL in the AA model compared with the two control groups and overlapped at 1.2 (4.0 ± 0.02) mL on a highest score of 4 which is the highest score that AWR defined (two-way ANOVA, P < 0.001, Figure 1A). The areas under the EMG curves (AUC) were significantly increased from volume of 0.8 (0.31 ± 0.11) to 1.2 ml (1.55 ± 0.15) in the AA rats compared with the control groups (P < 0.001, Figure 1B). The responses in PBS rats were also increased compared with naïve rats although there was no significant change (P = 0.224).
The water content of stools (Figure 1C) from naïve rats, PBS rats and AA rats were weighed and calculated to determine the stool features. Compared with naïve rats, there was an increase in the stool water content in the AA rats (55.1% ± 4.8% vs 25.6% ± 4.6%, one-way ANOVA, P < 0.01, n = 8), indicating much looser stools from the AA rats. No such effect was observed in the naïve rats and PBS rats (25.9% ± 5.4%).
The results of MPO activity assay (Figure 1D) of colonic tissue in the AA group showed that the enzyme activity was dramatically increased (1.153 ± 0.09 vs 0.190 ± 0.01 compared with naïve rats) on the 2nd d of modeling (one-way ANOVA, P < 0.001, n = 3); however, no significant difference was found between the PBS and naïve groups on the 2nd d of modeling and among the three groups on the 7th d of modeling (one-way ANOVA, P = 0.861, n = 3). These two examinations indicated that an obvious inflammatory reaction appeared on the 2nd d after the AA injection, and the inflammatory reaction gradually recovered and showed almost no inflammation on the 7th d. The model finished on day 8 and showed a clear feature of IBS that had no organic lesion.
Histological examination (Figure 1E and F) showed that many inflammatory cells such as neutrophils and lymphocytes were found in the lamina propria of distal colonic tissues on the 2nd d of modeling. In contrast, no remarkable inflammatory features were observed in the two control groups on the 2nd d of modeling and in all the three groups (AA, PBS and naïve rats) on the 7th d of modeling.
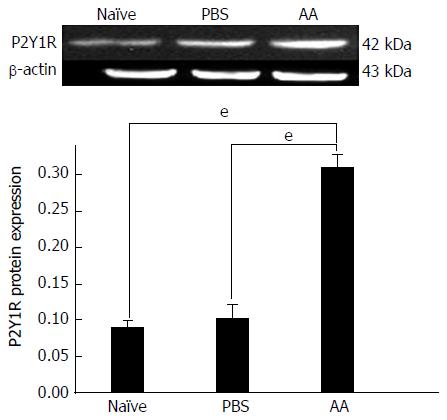
Compared with naïve rats (0.094 ± 0.016) and PBS rats (0.105 ± 0.013), the protein levels of P2Y1R in the distal colon of AA rats (0.319 ± 0.02) were significantly higher (one-way ANOVA, P < 0.001, Figure 2). The P2Y1R expression in PBS rats was higher than that in naïve rats although there was no significant change (P = 0.398).
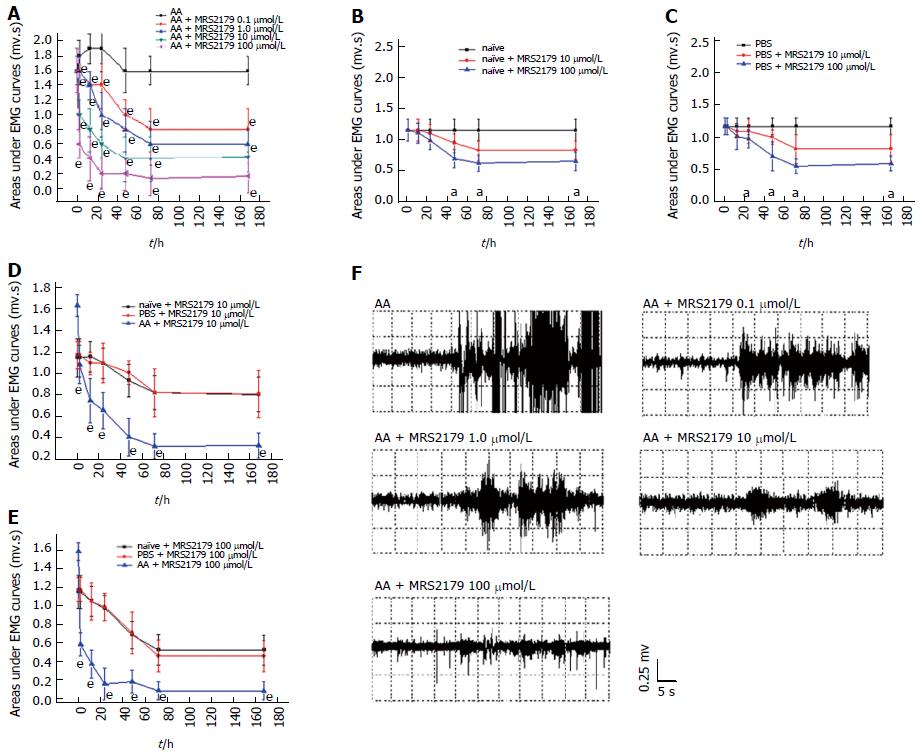
The AUC was detected by two-way ANOVA, in which the dose of the drug ranging from 0 to 100 μmol/L was used as the first factor and the time as the second factor. MRS2179 produced a dose-dependent analgesic effect in the CRD test on AA rats (P < 0.0001, Figure 3A). A significant decrease was observed at concentrations of 0.1 to 100 μmol/L at 2 to 168 h time points. Maximal effects of MRS2179 were observed at 100 μmol/L at 72 h after administration (from a mean baseline AUC value of 1.587 ± 0.099 mv·s to 0.140 ± 0.089 mv·s).
While on naïve and PBS rats, MRS2179 at doses of 10 and 100 μmol/L was found to be effective after 48 h and 24 h (P < 0.05, Figure 3B and C). One-way ANOVA showed significant effects between AA rats and naïve/PBS rats at doses of 10 and 100 μmol/L (P < 0.0001, Figure 3D and E), and effects between naïve and PBS rats were not significant for the two doses (P = 0.872 and P = 0.959, respectively, Figure 3D and E).
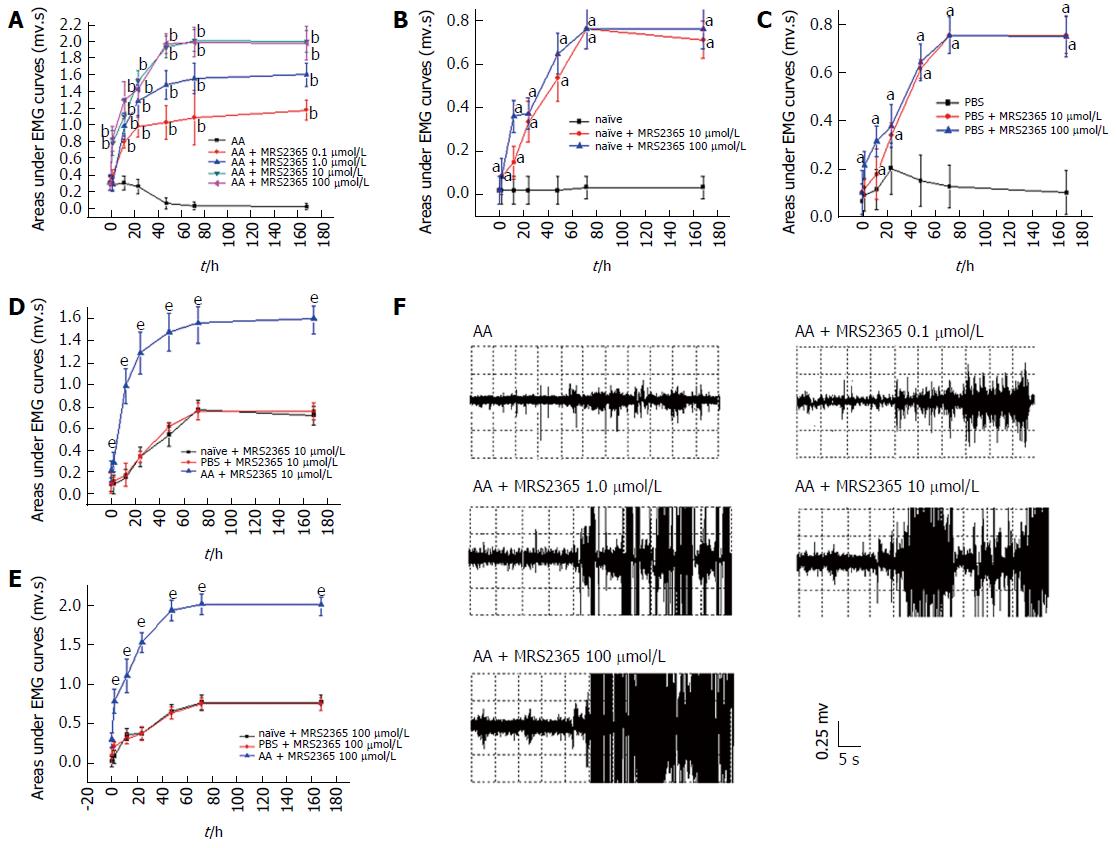
Two-way ANOVA indicated that MRS2365 produced a dose-dependent analgesic effect in the CRD test on AA rats (P < 0.0001, Figure 4A). MRS2365 significantly increased AUC at concentrations of 0.1 to 100 μmol/L at 2 to 168 h time points. MRS2365 at 10 and 100 μmol/L showed the most effective increase. Maximal effects of MRS2365 were observed at 10 (AUC value from 0.30 ± 0.089 to 1.973 ± 0.127 mv·s, P < 0.01) and 100 μmol/L at 72 h (AUC value from 0.290 ± 0.079 to 1.983 ± 0.195 mv·s, P < 0.01).
On naïve and PBS rats, MRS2365 at doses of 10 and 100 μmol/L was found to be effective at 2 to 168 h time points (P < 0.05, Figure 4B and C). Significant effects were found between AA and naïve/PBS rats at doses of 10 and 100 μmol/L (one-way ANOVA, P < 0.0001, Figure 4D and E), while effects between naïve and PBS rats were not significant for the two doses (P = 0.792 and P = 0.826, respectively).
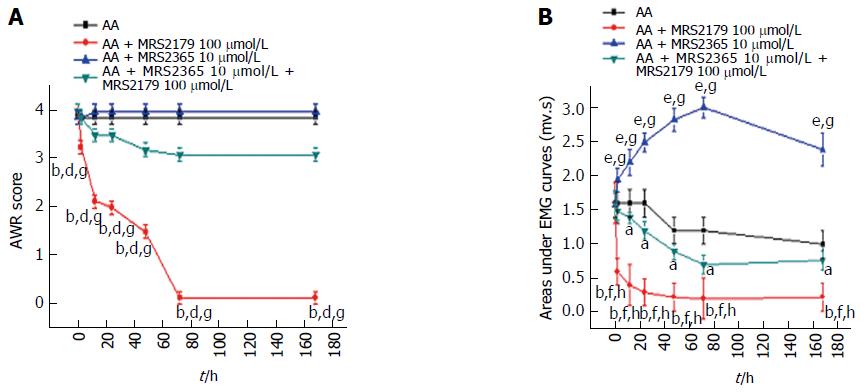
The most effective concentrations above (MRS2179 at 100 μmol/L and MRS2365 at 10 μmol/L) were applied in the drug intervention experiments for the four groups. AWR scoring (Figure 5A) showed that MRS2179 could significantly decrease the abdominal responses (two-way ANOVA, P < 0.0001). Differences between the MRS2179 group (1.88 ± 1.45) and the untreated AA rats (3.88 ± 0.33), MRS2365 group (3.96 ± 0.19) or the combined treatment group (3.28 ± 0.11) were significant (P < 0.01). No difference was detected among untreated AA rats, MRS2365-treated rats and rats in the combination group (P = 0.224).
Significant differences of AUC were also detected (Figure 5B). Compared with the untreated group, a significant decrease was detected in the MRS2179 group (two-way ANOVA, P < 0.001), which started at a baseline value of 1.52 ± 0.22 mv·s at time 0 h, peaked to 0.21 ± 0.15 mv·s at 72 h and continued to persist to 168 h. A significant increase was detected in the MRS2365 group (P < 0.001) which started at time 0 h (1.60 ± 0.20 mv·s), peaked at 72 h (2.98 ± 0.16 mv·s) and persisted to 168 h. Compared with the MRS2179 group, the difference for the MRS2365 groups and combination group was significant (P < 0.001). Compared with the combination group, significant differences were detected for untreated AA rats (P < 0.05) and MRS2365-treated rats (P < 0.01).
Colonic hypersensitivity in patients with IBS has been repeatedly confirmed[15,20,21], and lower colonic pain thresholds exist in most IBS patients[22]. This study investigated the possible role of P2Y1R in visceral hypersensitivity in a rat model of experimental IBS. Our results provided evidence that P2Y1R is involved in generation of visceral hypersensitivity in IBS.
This study was conducted in a post-inflammatory IBS rat model that has been proven to demonstrate the major features of IBS after subsidence of the acute inflammation in the colon and thus can be used to measure abdominal behaviors in conscious rats[17]. The MPO activity assay and histological examinations in this study proved that inflammation appeared on the 2nd d of model induction but was totally absent on the 7th d. However, the examination of abdominal behaviors and visceral hypersensitivity showed stronger AWR and abdominal EMG responses during the colorectal distention procedure. The AA rats also had a higher stool water content. However, although the differences between naïve rats and PBS rats were not significant, there was also a tiny increase of EMG and AWR scores in PBS rats, which was not expected. These may be caused by epithelial injury resulting from intubation because no intubation was done here for naïve rats, but PBS rats need to be treated by PBS in the anus of the colon. The differences almost disappeared when they were both treated with drugs by intubation in the following part of experiment. Overall, these results showed reserved hypersensitivity and abnormal fecal habits in this post-inflammatory IBS model, and these observations were similar with the reported visceral hypersensitivity in IBS patients.
P2YRs have been claimed to be expressed in intrinsic sensory nerves in the gut[23,24], where sensory neurons can evoke enteric reflex activities and mediate visceral pain via the spinal cord and brainstem[25]. Up to now it remains unclear whether P2Y1R is involved in generation and/or modulation of the lower pain thresholds of IBS. In this study, the higher expression of P2Y1R in the colon of AA rats emphasized their relationship with an elevated abdominal response towards mechanical stimuli. This occurrence may be related with excitation of sensory neurons located in submucosal and myenteric ganglia. Related studies[26,27] have suggested a possible mechanism that ATP might indirectly act on TRPV1Rs (which has received much attention in IBS) mediated by the P2Y1R in pain models of chronic ischemia. To further determine the role of P2Y1R, we studied changes of abdominal responses by functional regulation.
Both agonists and antagonists of the P2Y1R have been shown to reduce pain and hypersensitivity. Andó et al[18] demonstrated that MRS2365 had an analgesic effect on thermal pain threshold of neuropathic, acute pain and inflammatory pain. Chen et al[19] showed that MRS2179 could reverse tactile allodynia and spontaneous pain in a rat model of cancer-induced bone pain. They have indicated a bi-directional regulation of P2Y1R in pain. However, in our study, MRS2365 could effectively increase the colonic hypersensitivity status of AA rats, whereas MRS2179 led to a decrease in response to CRD test evoked visceral pain. The results showed an active role of P2Y1R in the generation/modulation of visceral hypersensitivity in the AA rats. This is in accordance with some other reports which indicated that P2Y1R is involved in the generation/modulation of nociceptive signals such as hypersensitivity in the gut[28-30]. It is not clear what exact role of P2Y1R plays in other pain events, but it will leads to high sensitivity in this IBS rat model. Moreover, specific antagonists of P2Y1R may have potential therapeutic value in treating abdominal pain in IBS.
Combined treatment of MRS2365 and MRS2179 showed a down-regulatory effect in AA rats. MRS2179 could not only effectively antagonize the elevated abdominal responses led by MRS2365, but also cause some more decrease. As a result, MRS2365 is a specific agonist of P2Y1R, whereas MRS2179 is not just a competitive antagonist of P2Y1R, but also selectively antagonizes other receptors, like P2X3R and P2Y2R[31,32]. Although we did not detect the expression of P2X3R and P2Y2R, our data suggested that besides P2Y1R, other receptors (which could be inhibited by MRS2179) might also play active roles in visceral hypersensitivity in the AA rats.
Studies on pain events have suggested interesting relationship between P2Y1R and P2X3R. P2Y1R was co-expressed with P2X3R and TRPV1Rs[31,33] in small diameter neurons, and was indicated to be an “ATP counterbalance” following mutual activation of P2Y1R and P2X3R. In the colon, enhanced expression of P2X3R in colon-specific sensory neurons has been found and chronic functional visceral hyperalgesia was associated with potentiation of ATP-evoked responses[34]. However, P2Y1R in this study showed an active role in causing visceral hypersensitivity, but not acted as a “counterbalance”. It might be in line with an explanation[10-12] that P2Y1R may provide a high-sensitive system to respond to minor touch-induced fluctuations in extracellular ATP mediated by P2X3R subjected to mechanical stimuli. It may fit with a recent study[35], suggesting that activation of P2Y1R could exert inhibitory control on P2X3R expression and increased P2X3R-mediated nociceptive flinch behaviors by promoting activity of P2Y1R downstream of p38. More research will be needed to clarify the mechanism of P2Y1R and P2X3R in pain of IBS.
P2Y2R is another subtype which has been demonstrated to dedicate to nociceptive events. Studies[30,36] have shown that P2Y1R and P2Y2R transcripts were detected in 80% and 49%-56% of retrogradely labeled colonic neurons, respectively, and agonists of P2Y1R and P2Y2R can evoke comparable sensitization of sensory neurons. However, one study on IBS-D patients[37] suggested that P2Y2R was more likely related to IBS pain. And another study on rat lumbar dorsal root ganglion neurons[38] showed that TRPV1R was co-expressed with P2Y2R. It is controversial whether P2Y1R or P2Y2R is more important for IBS pain. Our data suggested that both receptors may be involved in pain of the AA rats, but more research needs to be done to confirm this speculation.
Comorbidities or extra-intestinal symptoms are frequently in a sub-type of IBS patients. Some pain and hypersensitivity events in IBS patients, like chronic pelvic pain[39], primary headache[40] and higher reactivity to food antigens[41], might be also related to purinergic signaling. More research needs to be done to figure out whether there are connections between P2 receptors and these events, and it might help to understand the mechanism of P2Y1R involved in visceral pain in IBS.
Previous studies[29,42] indicated that P2Y1R may be responsible not only for post-infectious IBS, but also inflammation, like IBD. This study was conducted in a post-infectious IBS rat model and it was a question whether the higher expression of P2Y1R in AA rats was caused by inflammation? The model has been proven to demonstrate the major features of IBS after subsidence of the acute inflammation in the colon and thus can be used to measure abdominal behaviors in conscious rats[17]. The remained high visceral sensitivity without inflammation was a precondition of the study. However, P2Y1R could not be discounted as a potential mechanism contributing to anti-inflammation. It has been indicated in previous studies[36,43] that P2YRs could sensitize visceral afferents to evoke increased firing of colonic sensory of neurons to gut distension which is typically during, or following, an inflammatory insult. To clarify the possible anti-inflammatory role of P2Y1 in this post-inflammatory IBS model, further investigation needs to be done in other IBS models which are not post infectious.
In conclusion, we provided the evidence that P2Y1R is involved in generation and modulation of visceral hypersensitivity in IBS, and specific antagonists of P2Y1R may have potential therapeutic value in treating abdominal pain in IBS. A limitation of the study is that the localization of P2Y1R in the colon of IBS-like rats was not detected, which could help to better understand its role in visceral hypersensitivity. Moreover, studies on other P2 receptors, as well as their connections with the pain and hypersensitivity events in IBS, would help to strengthen our results.
Visceral hypersensitivity is considered to be a major feature of irritable bowel syndrome (IBS), the mechanism of which is not yet clear. P2Y1R is part of a high-sensitivity response to mechanical stimulation and is believed to dedicate to visceral hypersensitivity in IBS. In this study, the expression of P2Y1R was detected in a post-infectious IBS rat model, and the role of P2Y1R in the development of visceral hypersensitivity was detected by regulating its function.
P2Y1R has been shown to mediate nociceptive signaling and suggested to provide a high-sensitivity system to mechanical stimulation in functional gastrointestinal disorders, including IBS. However, whether P2Y1R participates in the generation and regulation of visceral hypersensitivity in IBS remains unclear. The results of this study provide the evidence of P2Y1R in IBS.
Using a post-inflammatory IBS rat model, we determined that P2Y1R is involved in the generation and modulation of visceral hypersensitivity in IBS. Moreover, specific antagonists of P2Y1R may have potential therapeutic value in treating abdominal pain in IBS.
P2Y1R may be positively involve in the generation and modulation of visceral hypersensitivity in IBS. Its specific antagonists may be used for treating abdominal pain and other relative discomfort in IBS.
IBS is a kind of functional gastrointestinal disorder, which is characterized by hypersensitivity and pain before defecation in the relative absence of colon inflammation or structural changes.
In the manuscript, the authors demonstrated that P2Y1R is involved in the generation and modulation of visceral hypersensitivity in IBS. This study is interesting in its scientific question, and it contributes to the knowledge of the IBS.
| 1. | Nozu T, Okumura T. Visceral sensation and irritable bowel syndrome; with special reference to comparison with functional abdominal pain syndrome. J Gastroenterol Hepatol. 2011;26 Suppl 3:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Lazarowski ER, Sesma JI, Seminario-Vidal L, Kreda SM. Molecular mechanisms of purine and pyrimidine nucleotide release. Adv Pharmacol. 2011;61:221-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Lazarowski ER. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012;8:359-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 4. | Burnstock G, Verkhratsky A. Purinergic signalling and the nervous system. Springer: Heidelberg New York Dordrecht London, 2015. ISBN: 978-3-642-28862-3 (Print) 978-3-642-28863-0 (Online). . |
| 5. | Burnstock G, Krügel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95:229-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 322] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 6. | Burnstock G. Purinergic signalling: from discovery to current developments. Exp Physiol. 2014;99:16-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 7. | Burnstock G. Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signal. 2014;10:3-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Deiteren A, van der Linden L, de Wit A, Ceuleers H, Buckinx R, Timmermans JP, Moreels TG, Pelckmans PA, De Man JG, De Winter BY. P2X3 receptors mediate visceral hypersensitivity during acute chemically-induced colitis and in the post-inflammatory phase via different mechanisms of sensitization. PLoS One. 2015;10:e0123810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Erb L, Weisman GA. Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdiscip Rev Membr Transp Signal. 2012;1:789-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Nakamura F, Strittmatter SM. P2Y1 purinergic receptors in sensory neurons: contribution to touch-induced impulse generation. Proc Natl Acad Sci USA. 1996;93:10465-10470. [PubMed] |
| 11. | Cooke HJ, Wunderlich J, Christofi FL. “The force be with you”: ATP in gut mechanosensory transduction. News Physiol Sci. 2003;18:43-49. [PubMed] |
| 12. | Gallego D, Gil V, Martínez-Cutillas M, Mañé N, Martín MT, Jiménez M. Purinergic neuromuscular transmission is absent in the colon of P2Y(1) knocked out mice. J Physiol. 2012;590:1943-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109-110. [PubMed] |
| 14. | Kanazawa M, Palsson OS, van Tilburg MA, Gangarosa LM, Fukudo S, Whitehead WE. Motility response to colonic distention is increased in postinfectious irritable bowel syndrome (PI-IBS). Neurogastroenterol Motil. 2014;26:696-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Delvaux M. Role of visceral sensitivity in the pathophysiology of irritable bowel syndrome. Gut. 2002;51 Suppl 1:i67-i71. [PubMed] |
| 16. | Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276-1285. [PubMed] |
| 17. | La JH, Kim TW, Sung TS, Kang JW, Kim HJ, Yang IS. Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis. World J Gastroenterol. 2003;9:2791-2795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Andó RD, Méhész B, Gyires K, Illes P, Sperlágh B. A comparative analysis of the activity of ligands acting at P2X and P2Y receptor subtypes in models of neuropathic, acute and inflammatory pain. Br J Pharmacol. 2010;159:1106-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Chen J, Wang L, Zhang Y, Yang J. P2Y1 purinoceptor inhibition reduces extracellular signal-regulated protein kinase 1/2 phosphorylation in spinal cord and dorsal root ganglia: implications for cancer-induced bone pain. Acta Biochim Biophys Sin (Shanghai). 2012;44:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Quigley EM, Craig OF. Irritable bowel syndrome; update on pathophysiology and management. Turk J Gastroenterol. 2012;23:313-322. [PubMed] |
| 21. | Chang JY, Talley NJ. Current and emerging therapies in irritable bowel syndrome: from pathophysiology to treatment. Trends Pharmacol Sci. 2010;31:326-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganière M, Verrier P, Poitras P. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771-1777. [PubMed] |
| 23. | Bertrand PP. ATP and sensory transduction in the enteric nervous system. Neuroscientist. 2003;9:243-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Bertrand PP. Bursts of recurrent excitation in the activation of intrinsic sensory neurons of the intestine. Neuroscience. 2004;128:51-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 26. | Kwon SG, Roh DH, Yoon SY, Moon JY, Choi SR, Choi HS, Kang SY, Han HJ, Beitz AJ, Oh SB. Acid evoked thermal hyperalgesia involves peripheral P2Y1 receptor mediated TRPV1 phosphorylation in a rodent model of thrombus induced ischemic pain. Mol Pain. 2014;10:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Kwon SG, Roh DH, Yoon SY, Moon JY, Choi SR, Choi HS, Kang SY, Han HJ, Beitz AJ, Lee JH. Blockade of peripheral P2Y1 receptors prevents the induction of thermal hyperalgesia via modulation of TRPV1 expression in carrageenan-induced inflammatory pain rats: involvement of p38 MAPK phosphorylation in DRGs. Neuropharmacology. 2014;79:368-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 640] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 29. | Malin SA, Molliver DC. Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Mol Pain. 2010;6:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Yousuf A, Klinger F, Schicker K, Boehm S. Nucleotides control the excitability of sensory neurons via two P2Y receptors and a bifurcated signaling cascade. Pain. 2011;152:1899-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1224] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 32. | Liu R, Ma S, Lu Z, Shen H, Sun L, Wei M. The ADP antagonist MRS2179 regulates the phenotype of smooth muscle cells to limit intimal hyperplasia. Cardiovasc Drugs Ther. 2015;29:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Gerevich Z, Illes P. P2Y receptors and pain transmission. Purinergic Signal. 2004;1:3-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Xu GY, Shenoy M, Winston JH, Mittal S, Pasricha PJ. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut. 2008;57:1230-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Chen Y, Li G, Huang LY. p38 MAPK mediates glial P2X7R-neuronal P2Y1R inhibitory control of P2X3R expression in dorsal root ganglion neurons. Mol Pain. 2015;11:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Hockley JR, Tranter MM, McGuire C, Boundouki G, Cibert-Goton V, Thaha MA, Blackshaw LA, Michael GJ, Baker MD, Knowles CH. P2Y Receptors Sensitize Mouse and Human Colonic Nociceptors. J Neurosci. 2016;36:2364-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Luo Y, Feng C, Wu J, Wu Y, Liu D, Wu J, Dai F, Zhang J. P2Y1, P2Y2, and TRPV1 Receptors Are Increased in Diarrhea-Predominant Irritable Bowel Syndrome and P2Y2 Correlates with Abdominal Pain. Dig Dis Sci. 2016;61:2878-2886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23:6058-6062. [PubMed] |
| 39. | Liao CH, Lin HC, Huang CY. Chronic Prostatitis/Chronic Pelvic Pain Syndrome is associated with Irritable Bowel Syndrome: A Population-based Study. Sci Rep. 2016;6:26939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 40. | Soares RL, Moreira-Filho PF, Maneschy CP, Breijão JF, Schmidte NM. The prevalence and clinical characteristics of primary headache in irritable bowel syndrome: a subgroup of the functional somatic syndromes. Arq Gastroenterol. 2013;50:281-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Soares RL, Figueiredo HN, Maneschy CP, Rocha VR, Santos JM. Correlation between symptoms of the irritable bowel syndrome and the response to the food extract skin prick test. Braz J Med Biol Res. 2004;37:659-662. [PubMed] |
| 42. | Grover M, Herfarth H, Drossman DA. The functional-organic dichotomy: postinfectious irritable bowel syndrome and inflammatory bowel disease-irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 43. | Knowles CH, Aziz Q. Basic and clinical aspects of gastrointestinal pain. Pain. 2009;141:191-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Soares RL, Stasi C S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Zhang FF