Published online Sep 14, 2017. doi: 10.3748/wjg.v23.i34.6294
Peer-review started: May 19, 2017
First decision: June 22, 2017
Revised: July 5, 2017
Accepted: August 8, 2017
Article in press: August 8, 2017
Published online: September 14, 2017
Processing time: 118 Days and 13.4 Hours
To analyze access (availability, affordability and acceptability) to biologicals for Crohn’s disease (CD) in ten European countries and to explore the associations between these dimensions, the uptake of biologicals and economic development.
A questionnaire-based survey combined with desk research was carried out in May 2016. Gastroenterologists from the Czech Republic, France, Germany, Hungary, Latvia, Poland, Romania, Slovakia, Spain and Sweden were invited to participate and provide data on the availability of biologicals/biosimilars, reimbursement criteria, clinical practice and prices, and use of biologicals. An availability score was developed to evaluate the restrictiveness of eligibility and administrative criteria applied in the countries. Affordability was defined as the annual cost of treatment as a share of gross domestic product (GDP) per capita. Correlations with the uptake of biologicals, dimensions of access and GDP per capita were calculated.
At the time of the survey, infliximab and adalimumab were reimbursed in all ten countries, and vedolizumab was reimbursed in five countries (France, Germany, Latvia, Slovakia, Sweden). Reimbursement criteria were the least strict in Sweden and Germany, and the strictest in Hungary, Poland and Slovakia. Between countries, the annual cost of different biological treatments differed 1.6-3.3-fold. Treatments were the most affordable in Sweden (13%-37% of the GDP per capita) and the least affordable in the Central and Eastern European countries, especially in Hungary (87%-124%) and Romania (141%-277%). Biosimilars made treatments more affordable by driving down the annual costs. The number of patients with CD on biologicals per 100000 population was strongly correlated with GDP per capita (0.91), although substantial differences were found in the uptake among countries with similar economic development. Correlation between the number of patients with CD on biologicals per 100000 population and the availability and affordability was also strong (-0.75, -0.69 respectively).
Substantial inequalities in access to biologicals were largely associated with GDP. To explain differences in access among countries with similar development needs further research on acceptance.
Core tip: We carried out a questionnaire survey with gastroenterologists combined with desk research to analyze access to biologicals for Crohn’s disease in ten European countries. Regarding availability, reimbursement criteria were the least restrictive in Sweden and Germany, and the most restrictive in Hungary, Poland and Slovakia. Between countries, the annual cost of biological treatments differed 1.6-3.3-fold. Treatments were the most affordable in Sweden and the least affordable in Hungary and Romania. The number of patients on biologicals per 100000 population was strongly correlated with gross domestic product, although substantial differences were found in the uptake among countries with similar economic development.
- Citation: Péntek M, Lakatos PL, Oorsprong T, Gulácsi L, Pavlova M, Groot W, Rencz F, Brodszky V, Baji P, Crohn’s Disease Research Group. Access to biologicals in Crohn’s disease in ten European countries. World J Gastroenterol 2017; 23(34): 6294-6305
- URL: https://www.wjgnet.com/1007-9327/full/v23/i34/6294.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i34.6294
Crohn’s disease (CD) is a chronic inflammatory disorder of the gastrointestinal tract that is characterized by diarrhea, abdominal pain, rectal bleeding, fever and fatigue. The prevalence of CD in Europe varies from 1.5 to 213 cases per 100000 persons[1,2]. Due to the early onset and chronic character of the disease, patients have to deal with a considerable impairment in health-related quality of life[3,4] and lower work capacity throughout their lifetime, leading to substantial economic burden both on patients and society[5].
Biological drugs have revolutionized the treatment of inflammatory bowel diseases, like CD. Infliximab was the first biological drug that received its marketing authorization in Europe by the European Medicines Agency (EMA) in 2001 for adult patients with moderately to severely active CD[6]. This was followed by adalimumab, registered in 2003, vedolizumab, registered in 2014 and ustekinumab registered in 2016. Recently, biosimilars of infliximab (2013, 2016) and of adalimumab (2017) have been registered as well. These drugs are monoclonal antibodies with different mechanisms of action (infliximab and adalimumab are anti-tumor necrosis agents, vedolizumab is an anti-integrin drug and ustekinumab is an interleukin-12 and -23 inhibitor) but with similar safety profile and comparable efficacy[7-9]. Clinical evidence confirmed the efficacy, safety and effectiveness of these drugs for the treatment of CD, as they substantially improve the ability to achieve disease remission, slow disease progression, decrease the need of surgery and increase work participation and quality of life[6]. However, biologicals are more costly than standard treatments, annual cost of drug therapy is usually above €10000 per patient. Mainly due to the increase in the use of biological drugs, recent studies indicated that direct healthcare costs have shifted from hospitalization and surgery towards drug therapy[10-12].
Access to biologicals varies significantly between countries. This is largely driven by differences in budgetary constraints[3,13]. Due to the high price and budget impact of biologicals, most countries have regulated the access to reimbursed treatment. Differences in regulations lead to inequalities in access to biologicals even among European countries with a very similar economic situation[3,14]. Rencz et al[3] found an up to 96-fold difference in the uptake of biologicals for CD among nine selected countries. The reasons for this heterogeneity in access to biologicals among the Central and Eastern European countries (CEE countries) has not been clarified. However, according to the authors, it is not explained by differences in prevalence and incidence of inflammatory bowel diseases, prices of biologicals, total expenditure on health, geographical access, and cost-effectiveness.
The appearance of biosimilar drugs as potentially cost-effective alternatives is expected to lead to improvements in access to biological therapy. The first biosimilar infliximab drugs (brand names Remsima and Inflectra) were approved in 2013 by the EMA for the same indications as the original biological drug[10]. A couple prospective and a number of retrospective cohorts with biosimilar infliximab in inflammatory bowel diseases have confirmed that its efficacy and safety are comparable to those of the original biological product[3,10,14]. Biosimilars are substantially (20%-70%) cheaper than the originator. Budget impact analyses suggest that significant savings can be achieved with biosimilar infliximab in CD[14,15]. In Belgium, Germany, Italy, the Netherlands and the United Kingdom, the annual projected cost savings are estimated to be €11.95 million (10% price reduction) to €35.85 million (30% price reduction) in the case of CD patients[15]. Total cost savings achievable over three years in Bulgaria, the Czech Republic, Hungary, Poland, Romania and Slovakia are expected to be €8.0 million (switching not allowed) and €16.9 million (switching allowed in 80% of the patients)[16]. These savings can be used either to improve access to biological treatments (e.g., increase the number of patients with access to biologicals), or can be allocated to other areas of care[17].
This study explores three different dimensions of access to biologicals (originators and biosimilars) for CD, namely availability, affordability and acceptability in ten selected European countries (the Czech Republic, France, Germany, Hungary, Latvia, Poland, Romania, Slovakia, Spain and Sweden).
These countries differ not only regarding their economic development but also regarding the organization and financing of their health care system, which might also influence the access to biological treatments[3,14,18]. While Spain and Sweden have a tax-based health care system, France and Germany follow the Bismarkian model with social health insurance. In the CEE countries (except for the Czech Republic) the share of public financing is usually lower than in the Western-European countries. The source of public resources is mainly tax revenue in Sweden and Spain, while it is social health insurance contribution in the rest of the countries (see further in details[19]).
Thus, in our study, we also aim to explore whether differences in availability and affordability of biologicals are associated with the uptake of biologicals (in terms of number of patients on biologicals per 100000 population) and the economic situation of the country or the financing of the health care system.
A questionnaire was developed to collect information on access to biologicals and was sent in May 2016 to one expert (gastroenterologist) in each of the ten European countries included in the study, i.e., the Czech Republic, France, Germany, Hungary, Latvia, Poland, Romania, Slovakia, Spain and Sweden. The questionnaire was developed based on questionnaires used in prior studies in rheumatoid arthritis of Putrik et al[18,20]. The country experts who were invited to fill in the questionnaire, were selected based on the principle of non-probability convenience sampling, which resulted in a sample drawn through the professional network of the researchers.
The questionnaire was sent to the 10 experts who accepted to take part in the survey (contributors of the paper). The returned questionnaires were checked and in case of missing or incomplete answers, the collaborating experts were contacted to clarify the information. Finally, a preliminary report including the results was sent to all collaborating experts for a review and data check.
The questionnaire-based survey was combined with desk research, where relevant indicators, such as countries’ gross domestic product (GDP), population size and health care financing indicators were identified. The number of CD patients on biologicals was extracted from resources provided by the collaborating experts or was calculated from the total number of CD patients and the estimated share of CD patients on biologicals. Furthermore, drug prices and other data derived from the questionnaires, were also checked during the desk research.
The questionnaire included questions on (1) the availability, reimbursement status and prices of originator and biosimilar biologicals registered for CD at the time of the survey (Remicade, Remsima, Inflectra, Humira and Entyvio)[Stelara (ustekinumab) and Flexabi (biosimilar infliximab) were not in use at the time of the survey]; (2) the clinical and reimbursement guidelines and eligibility criteria for biological treatment of adults with luminal CD; (3) the number of adult CD patients in the given country and the use of biologicals; and (4) additional dimensions of access to biologicals. The collaborating experts were also asked to indicate the reference for the data they provided (i.e., for drug prices, prevalence data and use of biologicals).
The three dimensions of access - affordability, availability, acceptability were analyzed separately.
Availability: We identified the number of biologicals for CD registered and reimbursed in the ten countries based on data from the questionnaire and desk research. Based on these data, we also developed an availability score to assess the restrictiveness of clinical eligibility and administrative requirements to biologicals, based on the following items: (1) whether there is a required level of disease activity [such as Crohn’s Disease Activity Index (CDAI)] or disease severity for initiation of biological treatment: not specified (0 point), CDAI > 220 (1 point), CDAI > 300 (2 points); (2) required failure of /intolerance to non-biological treatment before a patient is eligible for a biological: not required (0 point), steroids (1 point), immunosuppressive (1 point), steroids OR immunosuppressive (1 point), steroids AND immunosuppressive (2 points); (3) whether there are other administrative procedures required after the indication of the need of a biological is given: no other procedures (0 point), other requirements (e.g., approval or authorization by the health insurance fund) (1 point); (4) whether only approved centers can administer biological treatment: no restriction to approved centers (0 point), restriction to approved centers (1 point); and (5) whether only specific specialists can indicate and prescribe biologicals for the treatment of CD in adults: gastroenterologist, immunologist and GP/other (0 point), gastroenterologist and immunologist only (1 point), gastroenterologist only (2 points).
For each country, the subscores were summed up to obtain the country availability score within the range 0-8. Higher score indicates more restrictive clinical eligibility criteria and administrative requirements.
Affordability: Based on data from the questionnaire and desk research, the annual drug cost per person (2016) was calculated for each drug available in a given country and was also presented as a percentage of the country GDP per capita, which was the affordability ratio in our study. The annual costs were calculated based on the prices provided by collaborating experts, which were verified in the desk-research. The sources of the acquisition cost of drugs were mainly state institutes for drug control, European drug databases, pharmaceutical companies and national health insurance funds. If prices found during the desk research were not matching with the answers given by the experts, clarification was asked from the respondents to identify the most accurate data. Information on drug dose and frequency of its administration for induction and maintenance therapy was taken from on EMA product information (http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002782/WC500168528.pdf).
Acceptability: The questionnaire contained a question on barriers to access to biologicals. The collaborating experts were asked to indicate which of the following items they considered as a barrier to access to biologicals: limited availability of the drugs due to financial reasons; too strict reimbursement criteria; strict monitoring requirements; physicians’ preferences; patients’ co-payments; patients’ preferences; limited access of patients to inflammatory bowel diseases centers (IBD centers); limited access of patients to health care in general. Experts were also asked to give an estimation on out of every 10 of their infliximab patients how many were treated with biosimilars.
We calculated Pearson’s correlations between the affordability (defined as the annual cost of treatment as a percentage of GDP), the availability score (explained above), the number of CD patients on biologicals per 100000 population the GDP per capita, and the share of public expenditure in the total health expenditure and the share of governmental expenditure in the total public health expenditure. Significance level of 5% was used.
Registration, reimbursement: At the time of the survey (May 2016), five biologicals were approved by the European Medicine Agency (Remicade: 01/2001, Humira: 09/2003, Remsima, Inflectra: 09/2013, Entyvio: 05/2014.). Infliximab and adalimumab were reimbursed in all the ten countries. In Latvia and Hungary, only biosimilar infliximab was reimbursed for new patients. Entyvio was only reimbursed in France, Germany, Slovakia, Spain and Sweden. Furthermore, according to the experts, in Slovakia and Spain, Entyvio could not be used as a first line biological therapy, only as a second line after a failure of the first biological.
Eligibility criteria for biological treatment and administrative requirements: Eligibility criteria for biological treatment were based on national clinical guidelines in France, Germany, Poland, Spain and Sweden (Table 1). In Latvia and Slovakia, the eligibility criteria were based on reimbursement guidelines since there are no national clinical guidelines available. In Hungary, Romania and the Czech Republic, both clinical and reimbursement guideline existed. While in the Czech Republic both clinical and reimbursement guidelines were followed, in Hungary and Romania clinical practice followed the reimbursement guideline when differences of clinical and reimbursement guidelines occur. The references for the guidelines for each country are shown in Table 1.
| Country | Guideline | Source, organization, last update, web-link |
| The Czech Republic | Clinical | Bortlík et al[25] 2016 by IBD Working Group of the Czech Society of Gastroenterology |
| Reimbursement | Reimbursement criteria of the SUKL1; Edited by: SUKL, http://www.sukl.cz | |
| France | Clinical | ECCO guidelines, Crohn's Disease Guidelines (2010), Check list ANTI TNF, Check list VEDOLIZUMAB by GETAID2https://www.getaid.org/recommandations.html |
| Reimbursement | No | |
| Germany | Clinical | German Guidelines on Crohn’s disease; DGVS German Society of Gastroenterology (2014) |
| Reimbursement | No | |
| Hungary | Clinical | Miheller et al[26] 2009 |
| Reimbursement | The diagnostic and treatment of Crohn’s disease] by NHIFA3 (2013) http://www.oep.hu/data/cms989735/0626_a_felnottkori_crohn_betegseg_diagnosztikajanak_es_kezelesenek_finanszirozasi_protokollja.pdf | |
| Latvia | Clinical | No national guideline, but following the ECCO guideline |
| Reimbursement | National Health Service of Latvia. No specific document, but part of the general regulations on medication reimbursement.(2016) | |
| Poland | Clinical | [The treatment of Crohn’s Disease (ICD-10 K 50)], National Health Fund, (2014) http://onkologia-online.pl/upload/obwieszczenie/2015.10.28/b/b.32.pdf |
| Reimbursement | No | |
| Romania | Clinical | National Insurance Fund Protocol (2013) http://www.cnas.ro/default/index/index/lang/EN |
| Reimbursement | National Insurance Fund protocol (2013) http://www.cnas.ro/default/index/index/lang/EN | |
| Slovakia | Clinical | No national guideline, but following the ECCO guideline |
| Reimbursement | Protocol for starting and continuing the biological treatment. Date first approvals: infliximab 2005, adalimumab 2008, vedolizumab 2016; The Slovakian Gastroenterology Association and The Union of Health Insurance Companies. | |
| Spain | Clinical | Guidelines for biologics by GETECCU4 (2013) http://geteccu.org/formacion/guias-y-documentos-de-consenso; Cabriada et al[27] 2013 |
| Reimbursement | No | |
| Sweden | Clinical | (1) National Guidelines for the treatment of Crohn’s disease; The Swedish Society of Gastroenterology (2017) http://www.svenskgastroenterologi.se/sites/default/files/pagefiles/Riktlinjer_Lakemedelsbehandling_vid_Crohns_2012.pdf |
| (2) The use of IFX biosimilar in patients with IBD; Swedish Society of Gastroenterology (2017) http://www.svenskgastroenterologi.se/sites/default/files/pagefiles/SGF_riktlinjer_Biosimilarer_150903.pdf | ||
| (3) The Medical Product Agency: Drug treatment of IBD, novel recommendations by the Medical Product Agency, Sweden (2012) https://lakemedelsverket.se/upload/halso-och-sjukvard/behandlingsrekommendationer/L%C3%A4kemedelsbehandling%20vid%20inflammatorisk%20tarmsjukdom%20-%20ny%20rekommendation_bokm%C3%A4rken.pdf | ||
| Reimbursement | No |
An overview of the clinical criteria for eligibility for the initiation of biological treatment and further administration requirements are presented in Table 2. According to the respondents, none of the countries had requirements on disease duration to initiate a biological treatment. In six countries CDAI scale was required to be used for the assessment the disease severity. In the Czech Republic, Romania and Slovakia, a CDAI score ≥ 220 was required to start biological treatment, while in France, Hungary and Poland, only patients with CDAI score > 300 were entitled for treatment. (There are some exemptions, for example the contributing experts from Poland and Slovakia also mentioned that patients with severe fistulising CD did not have to fulfill the CDAI score requirement.) In the Czech Republic, France, Germany, Romania, Spain and Sweden a failure of one non-biological treatment (steroid OR immunosuppressant) was required to start biological treatment, while in Hungary, Latvia, Poland and Slovakia patients had to fail both steroids and immunosuppressant treatment. In most of the countries there were no specific criteria to satisfy for maintaining biological therapy, but maintenance was based on the clinicians’ judgement. Only in Hungary and in Sweden, it was recommended to evaluate maintenance on the CDAI scale.
| Cz | Fr | D | Hu | Lv | Pl | Ro | Sk | Es | Se | |
| Required level of disease activity (such as CDAI) or disease severity required for initiation of biological treatment | ||||||||||
| Not specified (0 point) | x | x | x | x | x | |||||
| CDAI > 220 (1 point) | x | x | ||||||||
| CDAI > 300 (2 points) | x | x | x | |||||||
| Required failure of /intolerance to non-biological treatment before a patient is eligible for a biological | ||||||||||
| Steroids (1 point) | x | |||||||||
| Immunosuppressive (1 point) | x | |||||||||
| Steroids OR immunosuppressive (1 point) | x | x | x | x | ||||||
| Steroids AND Immunosuppressive (2 points) | x | x | x | x | ||||||
| Other procedures required after the indication of a biological treatment | ||||||||||
| No other procedures (0 point) | x | x | x | x | x | x | x | x | ||
| Other requirements (e.g., approval or authorization by the health insurance fund) (1 point) | x | x | ||||||||
| Approved centers necessary for a biological treatment | ||||||||||
| No restriction to approved centers (0 point) | x | x | x | |||||||
| Restriction to approved centers (1 point) | x | x | x | x | x | x | x | |||
| Specialists who may indicate and prescribe biologicals for the treatment of CD in adults | ||||||||||
| Gastroenterologist, immunologist and GP/other (0 point) | x | x | ||||||||
| Gastroenterologist and immunologist (1 point) | x | |||||||||
| Only gastroenterologist (2 points) | x | x | x | x | x | x | x | |||
| Total availability score (min 0 to max 8) | 4 | 4 | 1 | 7 | 5 | 7 | 6 | 7 | 4 | 1 |
In six countries (the Czech Republic, Hungary, Poland, Romania, Slovakia and Spain), only approved centers could use biologicals. In Latvia, the treatment could only be started in a center where three different gastroenterologists gave approval. In most countries, treatment could be started immediately after indication. However, in Slovakia and Romania, the treatment could only start after the authorization process was completed, including a written application to the health insurance company, and/or the purchase and delivery of the medication by the company. In six countries, only gastroenterologists had the permission to indicate and prescribe biologicals to patients with CD. In Germany and France, immunologists could also indicate and prescribe biologicals. In Germany and Sweden, other specialties such as internists, surgeons or GPs were similarly entitled to prescribe and indicate biologicals.
Overall, Sweden and Germany had the lowest availability scores among the ten countries (1 out of 8), while Hungary, Poland and Slovakia had the highest scores (7 out of 8), indicating the most restrictive eligibility criteria and administration requirements (Table 2).
Regarding the availability of the biosimilars, Inflectra and/or Remsima were reimbursed in all of the ten countries at the time of the analysis. Three countries (the Czech Republic, Hungary, Spain) had specific criteria on switching to biosimilars. In Hungary, new infliximab patients had to be treated with a biosimilar, and in Poland, patients receiving the original biological drug were obliged to switch to the biosimilars of infliximab as maintenance therapy once the biological infliximab was used. In Spain switching was mandatory only in some hospitals/regions.
The annual cost of treatment per patient ranged from €10638 (Poland) to €29081 Euro (Germany) for Remicade; from €9157 (Sweden) to €23915 (Germany) for Remsima; from €6841 (Sweden) to €22213 (Germany) for Inflectra; from €10625 (France) to €24402 (Germany) for Humira; and from €19243 (Sweden) to €30218 (Spain) for Entyvio. Between countries, the annual therapeutic cost of Remicade showed a 2.7-fold variation, while the difference for the biosimilar Inflectra and Remsima showed a 2.6 and 3.3-fold variation respectively. For Humira and Entyvio, these were 2.3 and 1.6 respectively. According to data provided by the respondents, the appearance of the two biosimilars led to a price reduction for Remicade in some countries, which resulted in the same annual cost of originator and biosimilar infliximab products in five countries (the Czech Republic, France, Latvia, Poland and Slovakia) (Table 3).
| Cz | Fr | D | Hu | Lv | Pl | Ro | Sk | Es | Se | |
| Annual total drug cost per patient (€) | ||||||||||
| Remicade | 11925 | 13439 | 29081 | 15204 | 11202 | 10638 | 15469 | 12020 | 16591 | 16169 |
| Remsima | 11925 | 13439 | 23915 | 13694 | 11202 | 10638 | 12375 | 12020 | 12443 | 9157 |
| Inflectra | 11925 | 13439 | 22213 | 10674 | 11201 | 10638 | 12375 | 12020 | 12443 | 6841 |
| Humira | 11131 | 10625 | 24402 | 12326 | 14050 | 14800 | 24360 | 13697 | 12209 | 15286 |
| Entyvio | - | - | 24651 | - | - | - | 22275 | 20207 | 30218 | 19243 |
| Annual cost, % of GDP (Affordability ratio) | ||||||||||
| Remicade | 69% | 36% | 69% | 124% | 80% | 84% | 176% | 74% | 73% | 31% |
| Remsima | 69% | 36% | 57% | 111% | 80% | 84% | 141% | 74% | 55% | 18% |
| Inflectra | 69% | 36% | 53% | 87% | 80% | 84% | 141% | 74% | 55% | 13% |
| Humira | 65% | 28% | 58% | 100% | 101% | 117% | 277% | 84% | 54% | 30% |
| Entyvio | - | - | 59% | - | - | - | 253% | 124% | 133% | 37% |
| Average, without Entyvio | 68% | 34% | 59% | 106% | 85% | 92% | 184% | 77% | 59% | 23% |
| Average, all drugs | 68% | 34% | 59% | 106% | 85% | 92% | 198% | 86% | 74% | 26% |
Large differences can be seen in the cost of treatment as a percentage of GDP per capita across countries (Table 3). Based on these indicators, treatments are most affordable is Sweden (13%-37% of the GDP) and least affordable in the CEE countries, especially in Hungary (87%-124%) and Romania (141%-277%). Biosimilars made infliximab treatment more affordable, as the cost of the cheapest biosimilar treatment was lower than the GDP per capita (except for Romania).
In half of the countries, all five biologicals were covered at 100% by the health insurance system. Although in two countries, patient co-payments existed. In Germany a 10% copayment was required, in Latvia this was 25%.
Different barriers were selected from a list by the respondents with regard to the access to biologicals, such as “limited availability of the drugs due to financial reasons” (the Czech Republic, Poland and Sweden) and “physicians’ preferences” (Germany, Poland and Sweden) being the ones most frequently mentioned. Other barriers selected were “too strict reimbursement criteria” (the Czech Republic and Poland), “limited access to IBD centers” (Germany and Poland), “limited access to healthcare in general” (Poland and Romania), “patients’ co-payments” (Latvia) and “patients’ preferences” (Germany). Other barriers mentioned were time consuming and lengthy authorization process (Slovakia), non-referral of patients to specialist (Germany). According to the experts, after the introduction of biosimilars the access to biologicals became easier/much easier (the Czech Republic, Poland, Slovakia and Sweden) or stayed the same (Germany, Latvia and Romania). No clear opinion was reported for the other three countries.
Use of biologicals and its associations with the affordability availability and the economic development: The estimated number of CD patients, and the number of CD patients on biologicals (with references to the data sources) are presented in Table 4. The estimated number of CD patients treated with biologicals per 1000 patients showed a large variance between countries, ranging from 1.8 in Latvia to 312.6 in France. The number of patients on biologicals per 100000 in the population was the highest in Sweden (53.5), and the lowest in Latvia (0.2).
| Country | Estimated number of CD patients/source | Number of patients on biologicals1/source | Patients on biologicals per 100000 population (calculated) | Patients on biologicals per 1000 patients (calculated) | ||
| Cz | 8768 | Rencz et al[3], 2015 (based on estimation) | 990 | Rencz et al[3], 2015 | 9.4 | 112.9 |
| Fr | 72522 | Kirchgesner et al[28], 2017 (administrative database) | 22671 | Estimation based on Kirchgesner et al[28], 2017 | 34.0 | 312.6 |
| D | 180000 | Estimate by the collaborating expert based on CD incidence and prevalence in two regional cohort studies from the 90ties. | 27000 | Estimation (based on the estimated % of patients on biologicals and the total number of CD patients) | 32.9 | 150.0 |
| Hu | 9775 | Rencz et al[3], 2015 (based on epidemiology study) | 1870 | Rencz et al[3], 2015 | 19.0 | 191.3 |
| Lv | 1695 | Rencz et al[3], 2015 (based on estimation) | 3 | Rencz et al[3], 2015 | 0.2 | 1.8 |
| Pl | 32049 | Rencz et al[3], 2015 (based on estimation) | 888 | Rencz et al[3], 2015 | 2.3 | 27.7 |
| Ro | 11000 | Estimate for 2016 by the collaborating expert based on National database including 13 IBD centers | 253 | Rencz et al[3], 2015 | 1.3 | 23.0 |
| Sk | 3687 | Rencz et al[3], 2015 (epidemiology study) | 690 | Rencz et al[3], 2015 | 12.7 | 187.1 |
| Es | 60000 | Arin Letamendia et al[29], 2008 (prospective, population-based study) | 15000 | Estimation (based on the estimated % of patients on biologicals from the ENEIDA database2 and the total number of CD patients) | 32.3 | 250.0 |
| Se | 34318 | SWIBREG3 combined with the Swedish National Patient Register | 5270 | SWIBREG3 combined with The Prescribed Drug Register | 53.5 | 153.6 |
Experts gave an estimation on how many out of every 10 of their infliximab patients were treated with biosimilars. There were large differences between countries. At the time of the survey, in Romania, less than 1 out of 10 infliximab patients was treated with biosimilar, while in Latvia all infliximab patients were treated with biosimilars. The remaining countries had a treatment rate between 2/10 and 4/10, except for Poland with a treatment rate of 7/10.
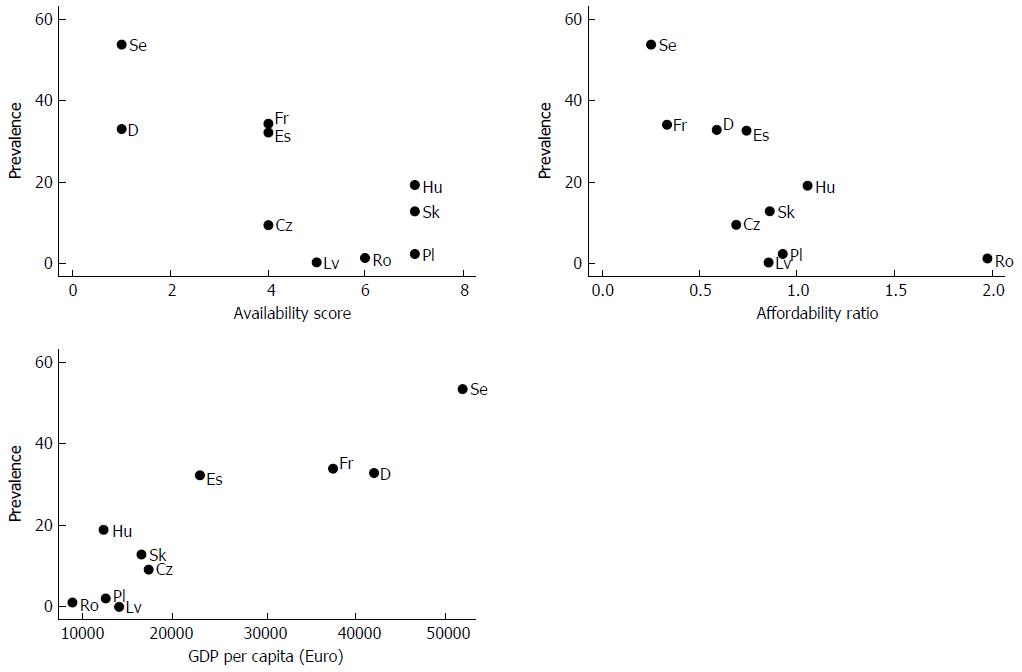
In Table 5, we present the correlation matrix of the variables of interest. Correlation between the number of CD patients on biologicals per 100000 population and the availability score and affordability was strong (-0.75, -0.69 respectively). GDP per capita was strongly associated with both prevalence of biologicals in CD population (0.91), availability score (-0.88) and affordability measure (-0.75). According to the results, we found no significant correlations between health care financing indicators and access. Thus, we can conclude that it is rather the wealth of the country than the organization or financing of the health care system, which influences access. The association between prevalence, GDP and the measures of access are also presented as graphs (Figure 1).
| No. of patients on biologicals per 100000 population | Availability score | Affordability ratio | GDP per capita | % of public health expenditure in the total health expenditure | % of general government expenditure in public health expenditure | |
| No. of patients on biologicals per 100000 population | 1.0000 | - | - | - | - | - |
| -0.7497 | 1.0000 | - | - | - | - | |
| Availability score | (P = 0.0125) | - | - | - | - | - |
| Affordability ratio | -0.6920 | 0.5989 | 1.0000 | - | - | - |
| (P = 0.0266) | (P = 0.0673) | - | - | |||
| GDP per capita | 0.9077 | -0.8810 | -0.7464 | 1.0000 | - | - |
| (P = 0.0003) | (P = 0.0008) | (P = 0.0132) | - | - | - | |
| % of public health expenditure in the total health expenditure | 0.3879 | -0.5338 | -0.1553 | 0.4907 | 1.0000 | - |
| (P = 0.2680) | (P = 0.1120) | (P = 0.6683) | (P = 0.149) | - | - | |
| % of general government expenditure in public health expenditure | 0.6661 | -0.4384 | -0.3741 | 0.4233 | 0.1547 | 1.0000 |
| (P = 0.0713) | (P = 0.2772) | (P = 0.3612) | (P = 0.296) | (P = 0.7146) | - |
In this study, we have analyzed three dimensions of access to biological therapy for CD, namely availability, affordability and acceptability in ten European countries. We have also explored the associations between these dimensions and the uptake of biologicals (in terms of number of patients on biologicals per 100000 population) as well as the economic development of the countries.
Regarding availability, there is a wide European consensus on clinical guidelines based on the best available evidence. For instance, the European Crohn’s and Colitis Organization regularly publishes their recommendations on the management of CD. However, besides the uniformity in drug registration and international professional guidelines, we found that treatment practices and access to biological treatment are still highly diverse in Europe. We found the least restrictive eligibility criteria and administration requirements in Sweden and Germany, and the most restrictive criteria in the CEE countries, namely in Hungary, Poland and Slovakia. In most of the CEE countries examined, there are separate reimbursement guidelines followed in the clinical practice. According to these, the eligibility criterion of treatment is usually ≥ 300 CDAI score, which is higher than the recommendations of national and international clinical guidelines (≥ 220 CDAI score). Furthermore, in these CEE countries, biological therapy is recommended only if patients fail both corticosteroid and immunosuppressive therapy. Regarding other requirements, in most of the countries, biologicals may only be indicated and prescribed by gastroenterologist and only approved centers may treat patients with biologicals.
We found large (1.6-3.3 times) differences regarding the annual cost of biological treatments across the countries. Treatments are most affordable in Sweden and Germany, and least affordable in the CEE countries, considering a higher economic burden of the biologicals in these countries. The annual cost of adalimumab treatment exceeds the GDP per capita in four CEE countries (Hungary, Latvia, Poland and Romania). The cheapest available infliximab treatment exceeds the GDP per capita only in Romania. Thus, biosimilars improve the affordability of biologicals. In countries where vedolizumab was available at the time of the survey, the yearly cost of treatment was lower than the GDP per capita only in Sweden and Germany.
The number of patients treated with biologicals per 100000 population varied greatly between the countries (0.2-53.5). The prevalence is the highest in Sweden, followed by Germany, France and Spain and the lowest in Poland, Romania and Latvia. We found that the uptake is strongly correlated with the GDP per capita of the country. However, we can also see large differences between countries with similar economic situation as it was also found by previous papers[3,14]. In the Czech Republic, Hungary and Slovakia, the CD prevalence (9.4-19) is much higher than in Poland and Latvia (2.3 and < 1 respectively). Reimbursement criteria do not necessarily explain the differences in the uptake of biologicals either, as availability scores are the same in Hungary, Poland and Slovakia. Furthermore, in Poland the treatments are slightly more affordable than in Hungary. In Poland, limited access to IBD centers and to healthcare in general were indicated by the collaborating expert as barriers to access in addition to strict reimbursement criteria. Furthermore, Poland is the only country where maximum duration of maintenance treatment is limited to 12 mo. In Latvia, substantial patient co-payments (25%) can also contribute to the low uptake of biologicals.
In a previous study on access to biologicals in CD, Rencz et al[3] found that access to biologicals in inflammatory bowel diseases varied greatly (up to 96-fold differences were found) even in some selected CEE countries. We found even higher inequalities in access among Western European and CEE countries. In rheumatology, many more patients are treated with biologicals than in inflammatory bowel diseases, at least in the CEE countries[21]. Similarly to our findings for CD, Putrik et al[18,20], Orlewska et al[22], and Hoebert et al[23] also found that macro-economic indicators (such as GDP or total health expenditure) largely explained the differences in access to biological treatment in rheumatoid arthritis. However, Gulácsi et al[14] highlighted that GDP cannot always explain the intercountry differences, which we also showed in our analysis.
We found that the number of patients treated with biologicals per 100000 population also correlates with availability and affordability, but the correlations among these items are lower than with the GDP per capita. As mentioned before, even though Hungary, Poland and Slovakia have the same availability scores, the uptake of biologicals is much lower in Poland as pointed out by Gulácsi (2016) as well[14]. The same stands for Spain and Latvia. Nevertheless, we observed that the availability and affordability dimensions are also correlated. Thus, in countries where biological therapy is less affordable, reimbursement conditions (eligibility criteria and administrative requirements) are more restrictive.
Acceptability of biologicals, including attitudes of physicians and patients, appears to be an important determinant of the uptake of biologicals and most likely to explain differences among countries with similar economic development, availability and affordability. Thus, this factor needs further research.
We found that biosimilars improved the affordability of biologicals, and drove down the cost of infliximab treatment under the GDP per capita in most of the countries. The decrease in cost leads to budget saving in most of the countries, which could be reinvested to treat more patients and improve access to therapy. The use of biosimilars was the most frequent in Poland (7/10) due to a mandatory switch of all infliximab patients to biosimilar. Furthermore, recently in 2017, as an incentive, the maximum infliximab treatment was prolonged to 24 mo while adalimumab is still limited to 12 mo. In Latvia all infliximab patients received biosimilar. In Hungary, new infliximab patients had to be treated with biosimilar, and also in Spain switching was mandatory depending on the center. For biosimilars, the acceptability of these drugs by health care actors is even more crucial to realized budget savings. According to previous studies, a negative attitude was primarily due to the lack of efficacy and safety data in inflammatory bowel diseases[17]. In a study carried out in 2014 among 51 Hungarian gastroenterologists, 20% had no concerns and 65% some concerns about biosimilars to treat CD[17]. Nevertheless, in a discrete choice experiment, physicians were more willing to use biosimilars when some benefits regarding the access to treatment was offered for patients in return[17,24].
Finally, we also acknowledge some limitations of this study. Only 10 countries participated in the study, although the sample is diverse, as countries with different economic development were selected, which enabled a comparison between Western-European and CEE countries. Country-specific data were provided by a single gastroenterologist in each country. Nevertheless, these data were verified by the desk research. To calculate annual cost of treatments, publicly available official list prices were used as real prices are not known and can vary even within countries. There are uncertainties regarding the epidemiology data used for the analysis. For example, it is also difficult to provide reliable data on the total number of CD patients in the countries, as in most countries registers exist only for patients treated with biologicals. There are considerable differences in the prevalence of CD across the European countries. These differences can show real diversities across countries, but this can also be the result of different methodological approach or time of the epidemiological studies as well as of the different prevalence of undiagnosed CD patients. Thus, we used the number of patients on biologicals per 100000 population in the correlation analysis to disregard the differences in prevalence across the 10 countries. Nevertheless, in some cases we also had to rely on estimations of experts regarding the total number of patients on biologicals, which results in uncertainties in the number of patients on biologicals per 100000 population as well. Also, we carried out a macro level analysis, and did not consider the determinants of access at individual level, such as socio-demographic characteristics of patients. Finally, in this study, we did not aim to explore whether worse access to treatments impacts the patient’s health status.
We found substantial inequalities in the access to biologicals for CD among European countries. Access was strongly determined by the economic development of the country. However we revealed large differences even among countries with a similar economic development. These differences cannot be entirely explained by the availability (eligibility criteria) or the affordability of biologicals, thus acceptance of and attitude to biologicals should be explored further.
We thank for the contribution of Crohn’s Disease Research Group, which included Laurent Peyrin Biroulet, MD, PhD, Inserm U954, Department of Gastroenterology, Nancy University Hospital, Lorraine University, France; Martin Bortlik, IBD Clinical and Research Centre, ISCARE a.s., 1st Faculty of Medicine, Charles University, 170004 Prague, Czech Republic; Mihai M Diculescu, Department of Gastroenterology and Hepatology, Carol Davila University, 020022 Bucharest, Romania; Axel Dignass, Department of Medicine I, Agaplesion Markus Hospital, Wilhelm-Epstein-Str. 4 60431 Frankfurt, Germany; Fernando Gomollón, Gastroenterology Unit, Clinical Universitary Hospital “Lozano Blesa”, CIBEREHD, Avenida San Juan Bosco 15, Zaragoza 50009, Spain; Jonas Halfvarson. Department of Gastroenterology, Faculty of Medicine and Health, Örebro University, SE 70182 Örebro, Sweden; Tibor Hlavaty, Gastroenterology Unit, Department of Internal Medicine V, University Hospital Bratislava, SK-82606 Bratislava, Slovakia; Juris Pokrotnieks, Stradins University, Riga, Latvia; Edyta Zagorowicz, The Maria Skłodowska-Curie Memorial Cancer Centre and Institute of Oncology, Department of Oncological Gastroenterology, 5 Roentgen Street, 02-781 Warsaw, Poland; Medical Centre for Postgraduate Education, Department of Gastroenterology and Hepatology and Clinical Oncology, 99/103 Marymoncka Street, 01-813 Warsaw, Poland.
Access to biologicals in Crohn’s disease (CD) varies significantly between countries, largely driven by differences in budgetary constraints. The aim of our study is to analyze access (availability, affordability and acceptability) to biologicals for CD in ten European countries and to explore the associations between these dimensions, the uptake of biologicals and economic development.
Affordability of biological treatments greatly varies across countries. Due to the high price and budget impact of biologicals, most countries have regulated the access to reimbursed treatment. Differences in regulations lead to inequalities in access to biologicals even among European countries with a very similar economic situation. Nevertheless, the appearance of biosimilar drugs as potentially cost-effective alternatives is expected to lead to improvements in access to biological therapy.
The authors compared access to biologicals for CD among ten European countries, and we found substantial inequalities. Reimbursement criteria were the least strict in Sweden and Germany, and the strictest in Hungary, Poland and Slovakia. Treatments were the most affordable in Sweden (13%-37% of the GDP per capita) and the least affordable in the Central and Eastern European countries, especially in Hungary (87%-124%) and Romania (141%-277%).
The authors concluded that access was strongly determined by the economic development of the country. However large differences were revealed even among countries with a similar economic development, which cannot be entirely explained by the availability (eligibility criteria) or the affordability of biologicals. Thus, other factors such as acceptability and attitudes, also strongly determine the use of biologicals in a given country.
CD is a chronic inflammatory disorder of the gastrointestinal tract that is characterized by diarrhea, abdominal pain, rectal bleeding, fever and fatigue. Biological drugs have revolutionized the treatment of inflammatory bowel diseases. These drugs are monoclonal antibodies with different mechanisms of action (infliximab and adalimumab are anti-tumor necrosis agents, vedolizumab is an anti-integrin drug and ustekinumab is an interleukin-12 and -23 inhibitor) but with similar safety profile and comparable efficacy. Clinical evidence confirmed the efficacy, safety and effectiveness of these drugs for the treatment of CD, as they substantially improve the ability to achieve disease remission, slow disease progression, decrease the need of surgery and increase work participation and quality of life.
The authors present that the difference of accessibility to biologicals, namely availability, affordability, and acceptability, for CD in ten selected European countries and the associations between these dimensions with the uptake of biologicals and economic development. Because limited data exist describing the accessibility to biologics according to economic development, I regard this to be an important study. The results are thoroughly analyzed and discussed. The manuscript is well-written.
| 1. | Burisch J, Jess T, Martinato M, Lakatos PL; ECCO -EpiCom. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7:322-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 763] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 2. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3595] [Article Influence: 256.8] [Reference Citation Analysis (6)] |
| 3. | Rencz F, Péntek M, Bortlik M, Zagorowicz E, Hlavaty T, Śliwczyński A, Diculescu MM, Kupcinskas L, Gecse KB, Gulácsi L. Biological therapy in inflammatory bowel diseases: access in Central and Eastern Europe. World J Gastroenterol. 2015;21:1728-1737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Hoivik ML, Bernklev T, Moum B. Need for standardization in population-based quality of life studies: a review of the current literature. Inflamm Bowel Dis. 2010;16:525-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Mandel MD, Bálint A, Lovász BD, Gulácsi L, Strbák B, Golovics PA, Farkas K, Kürti Z, Szilágyi BK, Mohás A. Work disability and productivity loss in patients with inflammatory bowel diseases in Hungary in the era of biologics. Eur J Health Econ. 2014;15 Suppl 1:S121-S128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (7)] |
| 6. | Gulácsi L. Biological and biosimilar therapies in inflammatory conditions: challenges for the Central and Eastern European countries. Eur J Health Econ. 2014;15 Suppl 1:S1-S4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Baji P, Gulácsi L, Péntek MV, Hevér N, Brodszky V. Systematic review and analysis of evidences on clinical efficacy and cost-effectiveness of biological drugs for the treatment of adult Crohn’s Disease. In: Baji P, editor. Budapest: Budapesti Corvinus Egyetem Egészségügyi Közgazdaságtan Tanszék, 2013. . |
| 8. | Moćko P, Kawalec P, Pilc A. Safety profile of biologic drugs in the therapy of Crohn disease: A systematic review and network meta-analysis. Pharmacol Rep. 2016;68:1237-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Cholapranee A, Hazlewood GS, Kaplan GG, Peyrin-Biroulet L, Ananthakrishnan AN. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther. 2017;45:1291-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 253] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 10. | Gulácsi L, Brodszky V, Baji P, Kim H, Kim SY, Cho YY, Péntek M. Biosimilars for the management of rheumatoid arthritis: economic considerations. Expert Rev Clin Immunol. 2015;11 Suppl 1:S43-S52. [PubMed] |
| 11. | Rocchi A, Benchimol EI, Bernstein CN, Bitton A, Feagan B, Panaccione R, Glasgow KW, Fernandes A, Ghosh S. Inflammatory bowel disease: a Canadian burden of illness review. Can J Gastroenterol. 2012;26:811-817. [PubMed] |
| 12. | van der Valk ME, Mangen MJ, Leenders M, Dijkstra G, van Bodegraven AA, Fidder HH, de Jong DJ, Pierik M, van der Woude CJ, Romberg-Camps MJ. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut. 2014;63:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 426] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 13. | Boncz I, Sebestyén A. Financial deficits in the health services of the UK and Hungary. Lancet. 2006;368:917-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Gulácsi L, Rencz F, Poór G, Szekanecz Z, Brodszky V, Baji P, Péntek M. Patients’ access to biological therapy in chronic inflammatory conditions; per capita GDP does not explain the intercountry differences. Ann Rheum Dis. 2016;75:942-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Jha A, Upton A, Dunlop WC, Akehurst R. The Budget Impact of Biosimilar Infliximab (Remsima®) for the Treatment of Autoimmune Diseases in Five European Countries. Adv Ther. 2015;32:742-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 16. | Brodszky V, Rencz F, Péntek M, Baji P, Lakatos PL, Gulácsi L. A budget impact model for biosimilar infliximab in Crohn’s disease in Bulgaria, the Czech Republic, Hungary, Poland, Romania, and Slovakia. Expert Rev Pharmacoecon Outcomes Res. 2016;16:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Baji P, Gulácsi L, Lovász BD, Golovics PA, Brodszky V, Péntek M, Rencz F, Lakatos PL. Treatment preferences of originator versus biosimilar drugs in Crohn’s disease; discrete choice experiment among gastroenterologists. Scand J Gastroenterol. 2016;51:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Putrik P, Ramiro S, Kvien TK, Sokka T, Pavlova M, Uhlig T, Boonen A; Working Group ‘Equity in access to treatment of rheumatoid arthritis in Europe’. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis. 2014;73:198-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 276] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 19. | OECD. Health Care Systems: Efficiency and Policy settings. OECD Publishing, 2010. . |
| 20. | Putrik P, Ramiro S, Kvien TK, Sokka T, Uhlig T, Boonen A; Equity in Clinical Eligibility Criteria for RA treatment Working Group. Variations in criteria regulating treatment with reimbursed biologic DMARDs across European countries. Are differences related to country’s wealth? Ann Rheum Dis. 2014;73:2010-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Péntek M, Poór G, Wiland P, Olejárová M, Brzosko M, Codreanu C, Brodszky N, Gulácsi L. Biological therapy in inflammatory rheumatic diseases: issues in Central and Eastern European countries. Eur J Health Econ. 2014;15 Suppl 1:S35-S43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Orlewska E, Ancuta I, Anic B, Codrenau C, Damjanov N, Djukic P, Ionescu R, Marinchev L, Nasonov EL, Peets T. Access to biologic treatment for rheumatoid arthritis in Central and Eastern European (CEE) countries. Med Sci Monit. 2011;17:SR1-S13. [PubMed] |
| 23. | Hoebert JM, Mantel-Teeuwisse AK, van Dijk L, Bijlsma JW, Leufkens HG. Do rheumatoid arthritis patients have equal access to treatment with new medicines?: tumour necrosis factor-alpha inhibitors use in four European countries. Health Policy. 2012;104:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Baji P, Gulácsi L, Golovics PA, Lovász BD, Péntek M, Brodszky V, Rencz F, Lakatos PL. Perceived Risks Contra Benefits of Using Biosimilar Drugs in Ulcerative Colitis: Discrete Choice Experiment among Gastroenterologists. Value Health Reg Issues. 2016;10:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Bortlík M, Ďuricová D, Kohout P, Konečný M, Koželuhová J, Novotný A, Zbořil V, Prokopová L, Douda T, Stehlík J. Doporučení pro podávání biologické terapie u idiopatických střevních zánětů: třetí, aktualizované vydání. [Guidelines for the administration of biological therapy in patients with IBD: third, updated edition.]. Gastroent Hepatol. 2016;70:11-27. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Miheller P, Nagy F, Lakatos L, Molnar T, Bene L, Lakatos P, Horvath G, Ujszaszy L, Hunyadi B, Banai J. [Anti-TNF-alpha treatment in adult IBD-Guideline of the Hungarian College of Gastroenterology]. LAM (Lege Artis Medicinæ). 2009;19:515-522. |
| 27. | Cabriada JL, Vera I, Domènech E, Barreiro-de Acosta M, Esteve M, Gisbert JP, Panés J, Gomollón F; Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU). [Recommendations of the Spanish Working Group on Crohn’s Disease and Ulcerative Colitis on the use of anti-tumor necrosis factor drugs in inflammatory bowel disease]. Gastroenterol Hepatol. 2013;36:127-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Kirchgesner J, Lemaitre M, Rudnichi A, Racine A, Zureik M, Carbonnel F, Dray-Spira R. Therapeutic management of inflammatory bowel disease in real-life practice in the current era of anti-TNF agents: analysis of the French administrative health databases 2009-2014. Aliment Pharmacol Ther. 2017;45:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 29. | Arin Letamendia A, Borda Celaya F, Burusco Paternain MJ, Prieto Martínez C, Martínez Echeverría A, Elizalde Apestegui I, Laiglesia Izquierdo M, Macias Mendizábal E, Tamburri Moso P, Sánchez Valverde F. [High incidence rates of inflammatory bowel disease in Navarra (Spain). Results of a prospective, population-based study]. Gastroenterol Hepatol. 2008;31:111-116. [PubMed] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Hungary
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Beltowski J, Yang SK S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF