Published online Sep 14, 2017. doi: 10.3748/wjg.v23.i34.6252
Peer-review started: June 8, 2017
First decision: July 13, 2017
Revised: July 29, 2017
Accepted: August 15, 2017
Article in press: August 15, 2017
Published online: September 14, 2017
Processing time: 100 Days and 0.6 Hours
To investigate the potential effect of curcumin on hepatitis B virus (HBV) covalently closed circular DNA (cccDNA) and the underlying mechanism.
A HepG2.2.15 cell line stably transfected with HBV was treated with curcumin, and HBV surface antigen (HBsAg) and e antigen (HBeAg) expression levels were assessed by ELISA. Intracellular HBV DNA replication intermediates and cccDNA were detected by Southern blot and real-time PCR, respectively. The acetylation levels of histones H3 and H4 were measured by Western blot. H3/H4-bound cccDNA was detected by chromatin immunoprecipitation (ChIP) assays. The deacetylase inhibitors trichostatin A and sodium butyrate were used to study the mechanism of action for curcumin. Additionally, short interfering RNAs (siRNAs) targeting HBV were tested along with curcumin.
Curcumin treatment led to time- and dose-dependent reductions in HBsAg and HBeAg expression and significant reductions in intracellular HBV DNA replication intermediates and HBV cccDNA. After treatment with 20 μmol/L curcumin for 2 d, HBsAg and cccDNA levels in HepG2.2.15 cells were reduced by up to 57.7% (P < 0.01) and 75.5% (P < 0.01), respectively, compared with levels in non-treated cells. Meanwhile, time- and dose-dependent reductions in the histone H3 acetylation levels were also detected upon treatment with curcumin, accompanied by reductions in H3- and H4-bound cccDNA. Furthermore, the deacetylase inhibitors trichostatin A and sodium butyrate could block the effects of curcumin. Additionally, transfection of siRNAs targeting HBV enhanced the inhibitory effects of curcumin.
Curcumin inhibits HBV gene replication via down-regulation of cccDNA-bound histone acetylation and has the potential to be developed as a cccDNA-targeting antiviral agent for hepatitis B.
Core tip: We showed that curcumin inhibited hepatitis B virus (HBV) replication and expression via reductions in covalently closed circular DNA-bound histone acetylation. Furthermore, siRNAs targeting HBV acted synergistically with curcumin, resulting in enhanced inhibition of HBV.
- Citation: Wei ZQ, Zhang YH, Ke CZ, Chen HX, Ren P, He YL, Hu P, Ma DQ, Luo J, Meng ZJ. Curcumin inhibits hepatitis B virus infection by down-regulating cccDNA-bound histone acetylation. World J Gastroenterol 2017; 23(34): 6252-6260
- URL: https://www.wjgnet.com/1007-9327/full/v23/i34/6252.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i34.6252
Hepatitis B virus (HBV) is a species of the genus Orthohepadnavirus. HBV infection leads to severe diseases, including hepatitis, liver cirrhosis and hepatocellular carcinoma[1]. HBV has also been suggested to be involved in the development of pancreatic cancer[2]. Approximately 350 million individuals are infected with HBV, and more than 0.6 million people with HBV infection die every year as a result of end-stage liver disease and hepatocellular carcinoma worldwide[1,3]. The HBV genome is made of partially double-stranded relaxed circular DNA (rcDNA), 3020-3320 bp in size[4]. After the virus infects hepatocytes, rcDNA is released into the nucleus and converted to covalently closed circular DNA (cccDNA), which, along with histone binding, constitutes a minichromosome. The mean copies of cccDNA per HBV-infected hepatocyte range from 5 to 50[5,6]. Moreover, cccDNA serves as the critical template for viral replication and mRNA synthesis, which is the source of persistent and recurrent HBV infection. Nucleos(t)ide analogues (NAs) can efficiently inhibit HBV replication; however, they do not promote clearance of residual cccDNA[7].
Several treatment strategies are available to target cccDNA by inhibiting cccDNA minichromosome formation or inducing cccDNA degradation. Disubstituted sulfonamide (DSS) compounds have been reported to be specific inhibitors that prevent rcDNA conversion into cccDNA; however, DSS cannot promote cccDNA decay[8]. Lucifora et al[9] showed that inducing apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3A (APOBEC3A) and 3B (APOBEC3B) cytidine deaminase by interferon-α and lymphotoxin-β receptor activation induces cccDNA degradation via cytidine deamination and apurinic/apyrimidinic site formation. However, the absence of specificity of these cytidine deaminases results in genomic damage and cell-cycle arrest[10]. Recently, with the aid of DNA-cleaving enzymes, including zinc-finger nucleases (ZFN), TAL effector nucleases (TALENs), and CRISPR-associated system 9 (Cas9) proteins, specific targeting of HBV cccDNA was shown to cleave cccDNA[11-15]. Nevertheless, chronic expression of enzymes leads to off-target cleavage at homologous sequences in the human genome and represents a major limitation.
Furthermore, cccDNA-bound acetylated histones can modulate HBV replication and expression[16,17]. Hepatitis B virus X (HBx) protein can be recruited onto a cccDNA minichromosome to accelerate acetylation. Using a cccDNA chromatin immunoprecipitation (ChIP)-Seq assay, Tropberger et al[18] reported that low levels of histone posttranslational modifications (PTMs) were associated with transcriptional repression and promoter silencing.
Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] was isolated from the rhizome of Curcuma longa L. (Zingiberaceae family), which exhibits antimicrobial activities against various bacteria, viruses, fungi, and parasites[19-23]. Curcumin can inhibit HBV via down-regulation of the gluconeogenesis gene coactivator PGC-1α[24] or trans-activation of transcription and increased stability of p53[25].
Based on findings that curcumin can inhibit p300 histone acetyltransferase activity[26,27], we hypothesized that deacetylation of cccDNA-bound histones may contribute to the inhibitory activities of curcumin on HBV. Therefore, the effects of curcumin on cccDNA-bound histones and on steady-state levels of HBV cccDNA were investigated in detail in the present study.
HepG2.2.15 cells (an HBV stably transfected human hepatocarcinoma cell line) were maintained in DMEM medium (Gibco, Carlsbad, CA, United States) supplemented with 10% foetal bovine serum (Gibco), 1% GlutaMAX-I (Gibco) and 1% MEM Non-Essential Amino Acids Solution (Gibco). Transfection of siRNAs into HepG2.2.15 cells was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, United States). The sequences were 5’-GAAUCCUCACAAUACCGCAtt and 5’-UGAGAGUCCAAGAGUCCUCtt for HBx-siRNA and 5’-GAAUCCUCACAAUACCGCAtt and 5’-UGCGGUAUU GUGAGGAUUCtt for hepatitis B virus S antigen (HBs)-siRNA[28].
Cells were seeded at approximately 60%-80% confluence 24 h prior to treatment with different concentrations of curcumin (Sigma, St. Louis, MO, United States) dissolved in dimethyl sulphoxide (DMSO). For histone acetylation blocking assays, cells were co-treated with 20 μmol/L curcumin and either 5 mmol/L sodium butyrate (Sigma) or 1 μmol/L trichostatin A (TSA, Sigma).
Nucleoproteins were extracted using a Nucleoprotein Extraction Kit (Sangon Biotech, Shanghai, China) according to the manufacturer’s instructions. For Western blot analysis, proteins were subjected to SDS-polyacrylamide gel electrophoresis on 12.5% gels and were then electrophoretically transferred to nitrocellulose membranes (Millipore, Billerica, MA, United States). The membranes were blocked with 5% non-fat milk in Tris-buffered saline (TBS; pH 7.5) with 0.05% Tween 20 for 2 h at RT and were then probed with rabbit polyclonal anti-acetyl-histone H3 (Abnova, diluted 1:1000) overnight at 4 °C. Mouse monoclonal anti-histone H3 (Beyotime Biotechnology, diluted 1:1000) served as an internal control protein. Horseradish peroxidase-conjugated goat anti-mouse antibody (Biosharp, 1:5000) was used as a secondary antibody. Protein brands were visualized by enhanced chemiluminescence (ECL) using an ECL kit (Millipore).
HepG2.2.15 cells were lysed in 800 μL of lysis buffer [50 mmol/L Tris-HCl (pH 8.0), 10 mmol/L EDTA, 150 mmol/L NaCl and 1% SDS] and incubated for 30 min at 4 °C. Cell lysates were adjusted to 0.5 mol/L KCl and centrifuged for 1 min at 10000 g to precipitate protein-bound DNA. Supernatants were digested with 0.5 mg/mL proteinase K for 2 h at 55 °C. The cccDNA was purified by phenol/chloroform (1:1) extraction and isopropanol precipitation in the presence of 15 μg of tRNA and 200 mM NaAc (pH 5.2).
Purified DNA was digested with Plasmid-Safe ATP-Dependent DNase (Epicenter, Madison, WI, United States) to degrade contaminating HBV inserted in cellular genomic DNA and OC (open circular) species and was then subjected to PCR amplification to select HBV cccDNA forms, as previously described[15]. The cccDNA was later subjected to real-time-PCR using SYBR Green Real-time PCR Master Mix (Roche, Mannheim, Germany) and cccDNA-specific primers: 5’-TGCACTTCGCTTCACCT (forward) and 5’-AGGGGC ATTTGGTGGTC (reverse). PCR was performed using an Applied Biosystems StepOne Real-Time PCR System. cccDNA copy numbers were quantified according to a standard curve generated from an HBV plasmid in a concentration range of 102-108 copies.
Total RNA was extracted from HepG2.2.15 cells using TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions and was then subjected to real-time-PCR using primers for cccDNA quantification; β-actin mRNA transcript levels were used to normalize the expression of each RNA sample.
cccDNA ChIP assays were performed using EpiQuik Acetyl-Histone H3 ChIP and EpiQuik Acetyl-Histone H4 ChIP kits (EpiGentek, Farmingdale, NY, United States). Briefly, cells were collected and in vivo cross-linked in fresh culture medium containing 1% formaldehyde for 10 min at RT and were then lysed in 200 μL CP3A for 10 min at RT to isolate nuclear pellets. Chromatin solutions were sonicated for 4 pulses of 12 s each at level 5 using a Branson Microtip probe, followed by a 40-s rest on ice between each pulse to generate 200- to 1000-bp DNA fragments. Supernatants were diluted with CP4 at a 1:1 ratio, and 5 μL was removed as “input DNA”. Chromatin was then subjected to immunoprecipitation for 1 h at RT using strip wells pre-coated with antibodies specific to acetyl-histone H3, acetyl-histone H4 or normal mouse IgG. After six washes with CP1, immunoprecipitated chromatins and input DNA coated on the strip wells were digested with proteinase K and then purified using collection tubes. Purified DNA was subjected to Plasmid-Safe ATP-Dependent DNase digestion and real-time PCR amplification, as described above.
Culture supernatants of HepG2.2.15 cells were harvested and analysed for HBV surface antigen (HBsAg) and e antigen (HBeAg) levels using a Thermo Scientific Multiskan FC Microplate Photometer and ELISA kits (InTec, Xiamen, China).
HepG2.2.15 cells were washed twice with ice-cold PBS and lysed in 800 μL of lysis buffer [50 mmol/L Tris-HCl (pH 7.4), 1 mmol/L EDTA and 1% NP-40]. Cell lysates were centrifuged for 1 min at 10000 g to precipitate cell nuclei. Cellular genomic DNA and cccDNA were removed by the addition of 10 mM MgCl2 and 100 μg/mL DNase I, and the mixture was incubated for 30 min at 37 °C. Digestion was stopped by the addition of 25 mmol/L EDTA (pH 8.0). Proteins were digested with 0.5 mg/mL proteinase K and 1% SDS for 2 h at 55 °C. HBV DNA from intracellular core particles was purified by phenol/chloroform (1:1) extraction and isopropanol precipitation in the presence of 15 μg of tRNA and 200 mmol/L NaAc (pH 5.2)[28,29].
For Southern blot analysis, HBV DNA was subjected to agarose gel electrophoresis, followed by denaturation and transfer to nylon membranes using a Model 785 Vacuum Blotter (Bio-Rad, Hercules, CA, United States). DNA was fixed to membranes using an Ultraviolet Crosslinker (UVP, Upland, CA, United States). DNA hybridization and detection were performed using the DIG High Prime DNA Labelling and Detection Starter Kit II (Roche).
Statistical analyses were performed using unpaired t-tests. The results were evaluated with GraphPad Prism 5 and are expressed as the mean ± SD. P values < 0.05 (a) or < 0.01 (b) were set as the level of significance.
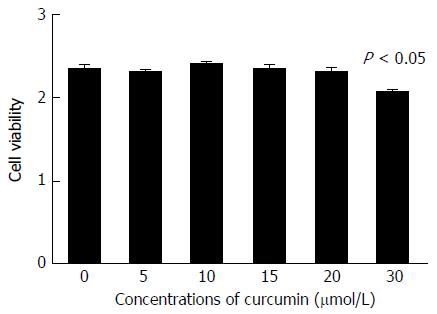
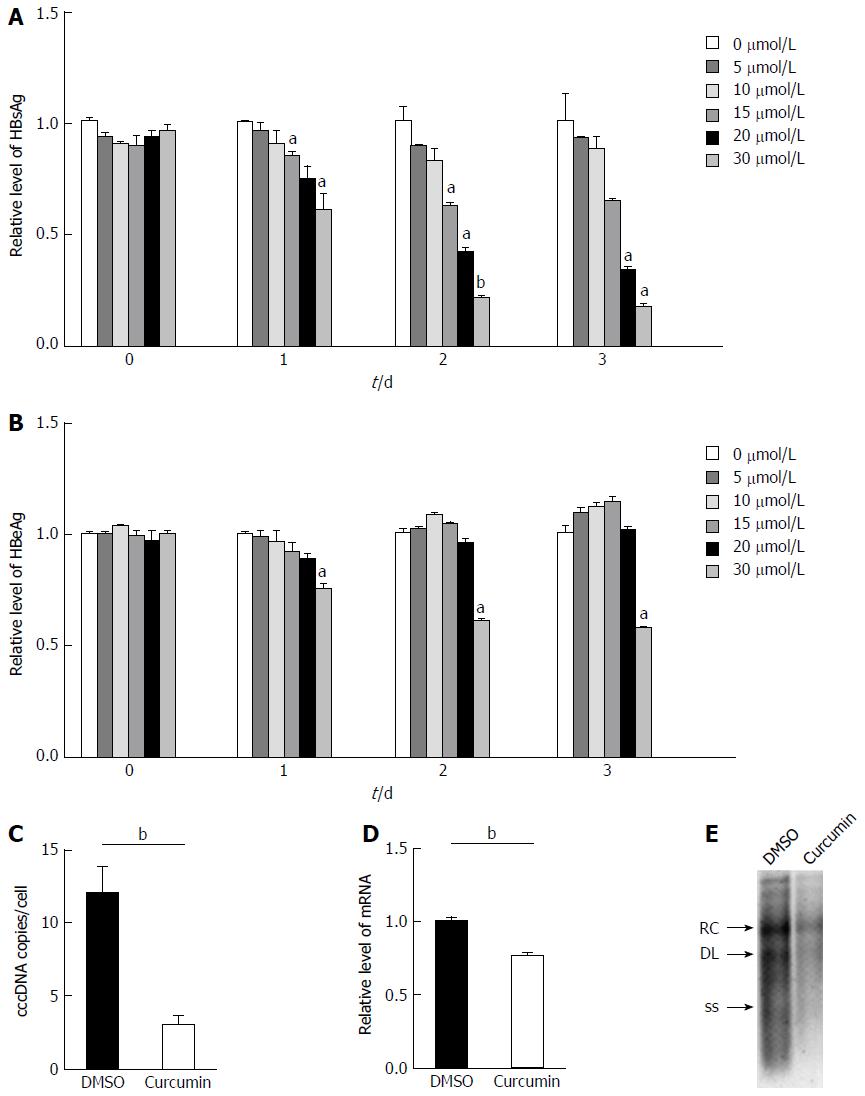
To confirm whether curcumin directly inhibits HBV expression, HepG2.2.15 cells were treated with various concentrations of curcumin for three consecutive days. Cell cytotoxicity was assessed using the CCK-8 assay, which revealed that there was no detectable toxic effect when cells were treated with less than 20 μmol/L curcumin (Figure 1). Cell culture supernatants from each day were collected and analysed for levels of HBsAg and HBeAg. Curcumin decreased HBsAg levels both dose- and time-dependently; HBsAg levels were reduced by up to 57.7%, 2 d after treatment with 20 μmol/L curcumin (Figure 2A). HBeAg was not reduced by 20 μmol/L of curcumin (Figure 2B). HBV cccDNA and HBV DNA from intracellular core particles were assayed 2 d after treatment with 20 μmol/L curcumin. RT-PCR and Southern blot experiments revealed strong reductions in cccDNA (75.5%; Figure 2C), mRNA (24.4%; Figure 2D) and HBV replication intermediates (Figure 2E).
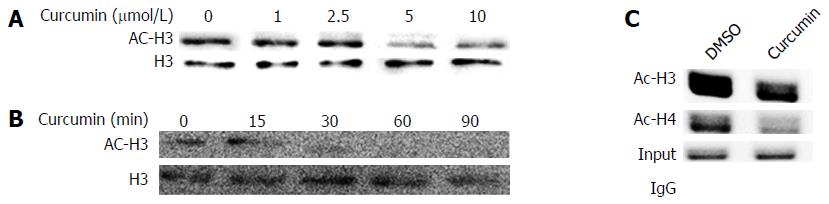
To investigate the effects of curcumin on chromosomal histone acetylation, equivalent numbers of HepG2.2.15 cells were treated with various concentrations of curcumin for 2 d or with 20 μmol/L curcumin for different durations. As shown in Figure 3A and B, curcumin decreased histone acetylation levels both dose- and time-dependently. We found that 5 μmol/L curcumin was sufficient to decrease histone acetylation. When cells were treated with 20 μmol/L curcumin, acetylated histone H3 was reduced 30 min after treatment. Using a cccDNA ChIP assay, we found that the acetylation levels of cccDNA-bound histone H3 and histone H4 were significantly reduced when cells were treated with 20 μmol/L curcumin (Figure 3C). This finding is similar to our observed reduction of chromosomal histone acetylation.
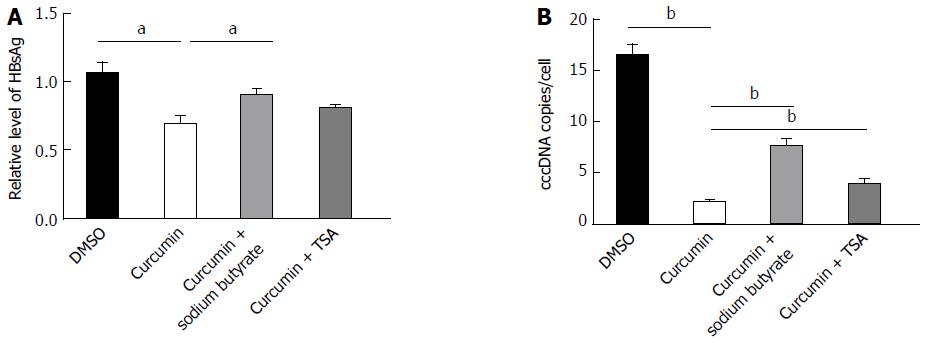
The histone deacetylase inhibitors sodium butyrate and TSA were used to test whether curcumin inhibits HBV by decreasing histone acetylation. HepG2.2.15 cells were treated with 20 μmol/L curcumin for 2 d, which resulted in significant reductions in the levels of HBsAg (Figure 4A) and cccDNA (Figure 4B). HBsAg and cccDNA levels increased by 31.3% and 2.4-fold, respectively, when cells were co-treated with curcumin and sodium butyrate compared with cells treated with curcumin alone. The histone deacetylase inhibitor TSA also partially blocked the inhibitory effect of curcumin on HBV, although the effect was much weaker than that of sodium butyrate. These findings suggest that curcumin inhibits HBV by inducing histone deacetylation.
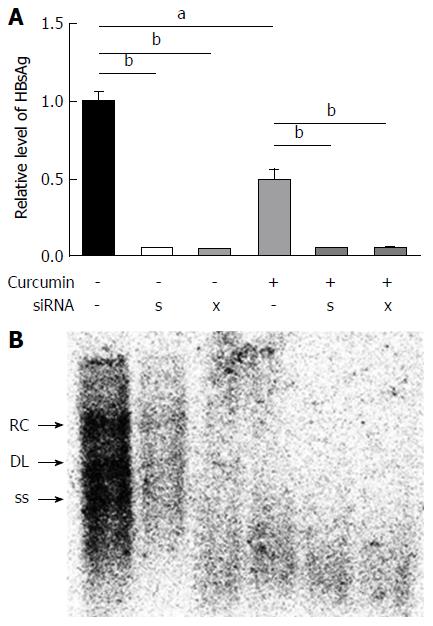
Because HBx also regulates epigenetic modifications of cccDNA-bound histones[16], HBx-siRNA and HBs-siRNA were used to enhance the inhibitory effects of curcumin. HepG2.2.15 cells were transfected with 20 nmol/L siRNAs and treated with 20 μmol/L curcumin for 2 subsequent days. Transfection with siRNAs alone significantly reduced HBsAg expression to a level below the limit of detection (Figure 5A). Moreover, a further reduction was observed in HBV DNA levels from intracellular core particles extracted from cells that received combined treatment with siRNAs and curcumin compared with cells treated with curcumin alone (Figure 5B).
The present study showed that curcumin not only inhibited intracellular HBV replication and HBsAg and HBeAg expression but also exhibited potent inhibitory effects on HBV cccDNA. Moreover, curcumin also reduced levels of both chromosomal and cccDNA-bound histones H3/H4, and addition of the histone deacetylase inhibitors sodium butyrate and TSA blocked the inhibitory effects of curcumin on HBV. These findings suggest that curcumin may induce the deacetylation of cccDNA-bound histones H3/H4, disrupt the steady state of HBV cccDNA and lead to the potent inhibition of HBV mRNA transcription and protein expression, along with a reduction in DNA replication. Furthermore, enhanced inhibition of intracellular HBV replication was revealed when curcumin was combined with siRNAs against HBV. Since HBx is pivotal for the steady state of cccDNA by regulating epigenetic modifiers of cccDNA-bound histones[16,30], the combination of curcumin with siRNAs, especially those targeting HBx, may lead to a synergistic effect in modulating the cccDNA steady state.
Several host-related factors, including histone proteins, regulate transcription and translation processes of cccDNA minichromosomes. Acetylation status changes of cccDNA-bound histones can regulate cccDNA transcription[16]. Previous studies have indicated that acetyltransferase inhibitors and deacetylase activators inhibit cccDNA transcription[16,31]. Curcumin has been reported to be an inhibitor of histone acetyltransferase (HAT), which specifically represses the activity of the p300/CREB-binding protein (CBP) HAT[26,27]. In the present study, curcumin mediated reductions in the levels of chromosomal and cccDNA-bound histone acetylation, which might result from the induction of histone deacetylation, because the deacetylase inhibitors TSA and sodium butyrate could block the inhibitory effects of curcumin on HBV.
HBV cccDNA is the primary template that allows for HBV gene expression and viral replication, and the steady state of cccDNA minichromosomes in the nuclei of hepatocytes contributes to persistent infection by HBV[32]. While there are no therapeutics currently available that target cccDNA, targeted therapeutics are attractive because the elimination of cccDNA results in the genomic cure of HBV infection[5]. Although genomic editing techniques, such as the CRISPR/CAS9 system, have shown promise in their capacity to edit HBV cccDNA, translating this finding into the clinic will be a challenge, mostly because of the need for the development of safe vectors for gene therapies.
Curcumin has been widely used as a dietary supplement for food colouring and flavouring, suggesting that it is safe for humans. However, poor bioavailability represents the biggest challenge for the clinical application of curcumin[33]. Poor absorption and rapid metabolism of curcumin in the body lead to low levels in the plasma and tissue. Fortunately, various efforts have been undertaken to promote its bioavailability. Removing the β-diketone moiety prevents curcumin from retro-aldol decomposition at physiological pH values[34]. Curcumin conjugated to two folic acid molecules (curcumin-2FA) increases water solubility and exhibits more efficient targeting of cancer cells that overexpress folic acid receptors[35]. Additionally, many other curcumin analogues can improve its bioavailability[36-40]. Moreover, curcumin-modified silver nanoparticles exhibited more efficient inhibition of respiratory syncytial virus[21]. Improving the bioavailability of curcumin will extend its clinical applications, especially in the treatment of HBV infection. Nevertheless, the long-term effects of curcumin on HBV cccDNA and its role in the elimination of cccDNA, along with combined effects of curcumin with other therapeutics, should be investigated in detail.
Taken together, the present study demonstrates that curcumin inhibits HBV by reducing cccDNA-bound histone acetylation and could potentially be developed as a cccDNA-targeting therapeutic for anti-HBV therapy.
Hepatitis B virus (HBV) covalently closed circular DNA (cccDNA) is the critical template for viral replication and mRNA synthesis. The elimination of cccDNA is indicative of complete cure of hepatitis B. Nucleos(t)ide analogues (NAs) can efficiently inhibit HBV replication but do not result in clearance of residual cccDNA. Curcumin can inhibit HBV; however, its mechanism is unclear. The potential role of curcumin in the regulation of the steady state of cccDNA is thus of interest.
The steady state of cccDNA minichromosomes can be regulated through deacetylation of cccDNA-bound histones. Hepatitis B virus X (HBx) protein is recruited onto cccDNA minichromosomes and plays a pivotal role in the steady state of cccDNA by regulating epigenetic modifiers of cccDNA-bound histones.
This is the first study indicating that curcumin inhibits HBV gene replication by reducing cccDNA-bound histone acetylation.
Curcumin has the potential to be developed as a cccDNA-targeting antiviral agent for hepatitis B.
HBV covalently closed circular DNA (cccDNA): After the HBV infects hepatocytes, virus genome is released into the nucleus and converted to cccDNA, which, along with histone binding, constitutes a minichromosome and serves as the critical template for viral replication and mRNA synthesis.
The authors have evaluated the effects of curcumin on HBV DNA replication and gene expression in the HepG2.2.15 cell line. Interestingly, curcumin showed robust inhibitory effects on HBV cccDNA. In addition, the authors have described the mechanism underlying the effect of curcumin on HBV cccDNA, which might be through histone deacetylation or reducing histone acetylation.
| 1. | Fourati S, Pawlotsky JM. Recent advances in understanding and diagnosing hepatitis B virus infection. F1000Res. 2016;5:pii: F1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Hassan MM, Li D, El-Deeb AS, Wolff RA, Bondy ML, Davila M, Abbruzzese JL. Association between hepatitis B virus and pancreatic cancer. J Clin Oncol. 2008;26:4557-4562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Kim DH, Kang HS, Kim KH. Roles of hepatocyte nuclear factors in hepatitis B virus infection. World J Gastroenterol. 2016;22:7017-7029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (3)] |
| 4. | Kay A, Zoulim F. Hepatitis B virus genetic variability and evolution. Virus Res. 2007;127:164-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 5. | Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64:1972-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 726] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 6. | Taranta A, Tien Sy B, Zacher BJ, Rogalska-Taranta M, Manns MP, Bock CT, Wursthorn K. Hepatitis B virus DNA quantification with the three-in-one (3io) method allows accurate single-step differentiation of total HBV DNA and cccDNA in biopsy-size liver samples. J Clin Virol. 2014;60:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | You CR, Lee SW, Jang JW, Yoon SK. Update on hepatitis B virus infection. World J Gastroenterol. 2014;20:13293-13305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (2)] |
| 8. | Cai D, Mills C, Yu W, Yan R, Aldrich CE, Saputelli JR, Mason WS, Xu X, Guo JT, Block TM. Identification of disubstituted sulfonamide compounds as specific inhibitors of hepatitis B virus covalently closed circular DNA formation. Antimicrob Agents Chemother. 2012;56:4277-4288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 9. | Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X, Sprinzl MF, Koppensteiner H, Makowska Z, Volz T. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343:1221-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 762] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 10. | Landry S, Narvaiza I, Linfesty DC, Weitzman MD. APOBEC3A can activate the DNA damage response and cause cell-cycle arrest. EMBO Rep. 2011;12:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 11. | Cradick TJ, Keck K, Bradshaw S, Jamieson AC, McCaffrey AP. Zinc-finger nucleases as a novel therapeutic strategy for targeting hepatitis B virus DNAs. Mol Ther. 2010;18:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Chen J, Zhang W, Lin J, Wang F, Wu M, Chen C, Zheng Y, Peng X, Li J, Yuan Z. An efficient antiviral strategy for targeting hepatitis B virus genome using transcription activator-like effector nucleases. Mol Ther. 2014;22:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 13. | Lin SR, Yang HC, Kuo YT, Liu CJ, Yang TY, Sung KC, Lin YY, Wang HY, Wang CC, Shen YC. The CRISPR/Cas9 System Facilitates Clearance of the Intrahepatic HBV Templates In Vivo. Mol Ther Nucleic Acids. 2014;3:e186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 307] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 14. | Seeger C, Sohn JA. Targeting Hepatitis B Virus With CRISPR/Cas9. Mol Ther Nucleic Acids. 2014;3:e216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 15. | Ramanan V, Shlomai A, Cox DB, Schwartz RE, Michailidis E, Bhatta A, Scott DA, Zhang F, Rice CM, Bhatia SN. CRISPR/Cas9 cleavage of viral DNA efficiently suppresses hepatitis B virus. Sci Rep. 2015;5:10833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 16. | Belloni L, Pollicino T, De Nicola F, Guerrieri F, Raffa G, Fanciulli M, Raimondo G, Levrero M. Nuclear HBx binds the HBV minichromosome and modifies the epigenetic regulation of cccDNA function. Proc Natl Acad Sci USA. 2009;106:19975-19979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 412] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 17. | Lee JY, Kim NA, Sanford A, Sullivan KE. Histone acetylation and chromatin conformation are regulated separately at the TNF-alpha promoter in monocytes and macrophages. J Leukoc Biol. 2003;73:862-871. [PubMed] |
| 18. | Tropberger P, Mercier A, Robinson M, Zhong W, Ganem DE, Holdorf M. Mapping of histone modifications in episomal HBV cccDNA uncovers an unusual chromatin organization amenable to epigenetic manipulation. Proc Natl Acad Sci USA. 2015;112:E5715-E5724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 194] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 19. | Moghadamtousi SZ, Kadir HA, Hassandarvish P, Tajik H, Abubakar S, Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int. 2014;2014:186864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 541] [Cited by in RCA: 542] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 20. | Ali A, Banerjea AC. Curcumin inhibits HIV-1 by promoting Tat protein degradation. Sci Rep. 2016;6:27539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 21. | Yang XX, Li CM, Huang CZ. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale. 2016;8:3040-3048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 187] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 22. | Pécheur EI. Curcumin against hepatitis C virus infection: spicing up antiviral therapies with ‘nutraceuticals’? Gut. 2014;63:1035-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Anggakusuma , Colpitts CC, Schang LM, Rachmawati H, Frentzen A, Pfaender S, Behrendt P, Brown RJ, Bankwitz D, Steinmann J. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut. 2014;63:1137-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 24. | Rechtman MM, Har-Noy O, Bar-Yishay I, Fishman S, Adamovich Y, Shaul Y, Halpern Z, Shlomai A. Curcumin inhibits hepatitis B virus via down-regulation of the metabolic coactivator PGC-1alpha. FEBS Lett. 2010;584:2485-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Kim HJ, Yoo HS, Kim JC, Park CS, Choi MS, Kim M, Choi H, Min JS, Kim YS, Yoon SW. Antiviral effect of Curcuma longa Linn extract against hepatitis B virus replication. J Ethnopharmacol. 2009;124:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Zhu X, Li Q, Chang R, Yang D, Song Z, Guo Q, Huang C. Curcumin alleviates neuropathic pain by inhibiting p300/CBP histone acetyltransferase activity-regulated expression of BDNF and cox-2 in a rat model. PLoS One. 2014;9:e91303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 27. | Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga U, Kundu TK. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem. 2004;279:51163-51171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 601] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 28. | Meng Z, Xu Y, Wu J, Tian Y, Kemper T, Bleekmann B, Roggendorf M, Yang D, Lu M. Inhibition of hepatitis B virus gene expression and replication by endoribonuclease-prepared siRNA. J Virol Methods. 2008;150:27-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Meng Z, Qiu S, Zhang X, Wu J, Schreiter T, Xu Y, Yang D, Roggendorf M, Schlaak J, Lu M. Inhibition of woodchuck hepatitis virus gene expression in primary hepatocytes by siRNA enhances the cellular gene expression. Virology. 2009;384:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Rivière L, Gerossier L, Ducroux A, Dion S, Deng Q, Michel ML, Buendia MA, Hantz O, Neuveut C. HBx relieves chromatin-mediated transcriptional repression of hepatitis B viral cccDNA involving SETDB1 histone methyltransferase. J Hepatol. 2015;63:1093-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 31. | Pollicino T, Belloni L, Raffa G, Pediconi N, Squadrito G, Raimondo G, Levrero M. Hepatitis B virus replication is regulated by the acetylation status of hepatitis B virus cccDNA-bound H3 and H4 histones. Gastroenterology. 2006;130:823-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 381] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 32. | Guidotti LG, Isogawa M, Chisari FV. Host-virus interactions in hepatitis B virus infection. Curr Opin Immunol. 2015;36:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 33. | Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3258] [Cited by in RCA: 3771] [Article Influence: 198.5] [Reference Citation Analysis (0)] |
| 34. | Kumari N, Kulkarni AA, Lin X, McLean C, Ammosova T, Ivanov A, Hipolito M, Nekhai S, Nwulia E. Inhibition of HIV-1 by curcumin A, a novel curcumin analog. Drug Des Devel Ther. 2015;9:5051-5060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Mishra A, Das BC. Curcumin as an anti-human papillomavirus and anti-cancer compound. Future Oncol. 2015;11:2487-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Bharitkar YP, Das M, Kumari N, Kumari MP, Hazra A, Bhayye SS, Natarajan R, Shah S, Chatterjee S, Mondal NB. Synthesis of Bis-pyrrolizidine-Fused Dispiro-oxindole Analogues of Curcumin via One-Pot Azomethine Ylide Cycloaddition: Experimental and Computational Approach toward Regio- and Diastereoselection. Org Lett. 2015;17:4440-4443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Ahsan N, Mishra S, Jain MK, Surolia A, Gupta S. Curcumin Pyrazole and its derivative (N-(3-Nitrophenylpyrazole) Curcumin inhibit aggregation, disrupt fibrils and modulate toxicity of Wild type and Mutant α-Synuclein. Sci Rep. 2015;5:9862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 38. | Gogoi B, Sen Sarma N. Curcumin-cysteine and curcumin-tryptophan conjugate as fluorescence turn on sensors for picric Acid in aqueous media. ACS Appl Mater Interfaces. 2015;7:11195-11202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Pan Y, Wang Y, Cai L, Cai Y, Hu J, Yu C, Li J, Feng Z, Yang S, Li X. Inhibition of high glucose-induced inflammatory response and macrophage infiltration by a novel curcumin derivative prevents renal injury in diabetic rats. Br J Pharmacol. 2012;166:1169-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 40. | Wang Y, Xiao J, Zhou H, Yang S, Wu X, Jiang C, Zhao Y, Liang D, Li X, Liang G. A novel monocarbonyl analogue of curcumin, (1E,4E)-1,5-bis(2,3-dimethoxyphenyl)penta-1,4-dien-3-one, induced cancer cell H460 apoptosis via activation of endoplasmic reticulum stress signaling pathway. J Med Chem. 2011;54:3768-3778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Farshadpour F, Inoue K, Jarcuska P, Kim K, Larrubia JR S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Zhang FF