Published online Sep 7, 2017. doi: 10.3748/wjg.v23.i33.6155
Peer-review started: May 22, 2017
First decision: June 22, 2017
Revised: July 21, 2017
Accepted: August 8, 2017
Article in press: August 8, 2017
Published online: September 7, 2017
Processing time: 110 Days and 11.6 Hours
To identify the clinical features of gastric mucosa-associated lymphoid tissue (MALT) lymphoma with extra copies of MALT1.
This is a multi-centered, retrospective study. We reviewed 146 patients with MALT lymphoma in the stomach who underwent fluorescence in situ hybridization analysis for t(11;18) translocation. Patients were subdivided into patients without t(11;18) translocation or extra copies of MALT1 (Group A, n = 88), patients with t(11;18) translocation (Group B, n = 27), and patients with extra copies of MALT1 (Group C, n = 31). The clinical background, treatment, and outcomes of each group were investigated.
Groups A and C showed slight female predominance, whereas Group B showed slight male predominance. Mean ages and clinical stages at lymphoma diagnosis were not different between groups. Complete response was obtained in 61 patients in Group A (69.3%), 22 in Group B (81.5%), and 21 in Group C (67.7%). Helicobacter pylori (H. pylori) eradication alone resulted in complete remission in 44 patients in Group A and 13 in Group C. In Group B, 14 patients underwent radiotherapy alone, which resulted in lymphoma disappearance. Although the difference was not statistically significant, event-free survival in Group C tended to be inferior to that in Group A (P = 0.10).
Patients with t(11;18) translocation should be treated differently from others. Patients with extra copies of MALT1 could be initially treated with H. pylori eradication, similar to patients without t(11;18) translocation or extra copies of MALT1.
Core tip: We subdivided and retrospectively reviewed 146 patients with gastric MALT lymphoma into patients without t(11;18) translocation or extra copies of MALT1 (Group A, n = 88, 60.3%), patients with t(11;18) translocation (Group B, n = 27, 18.5%), and patients with extra copies of MALT1 (Group C, n = 31, 21.2%). Groups A and C exhibited similar clinical characteristics. Helicobacter pylori eradication alone resulted in complete remission in approximately half the patients in Group A and Group C. Consequently, patients with extra copies of MALT1 could be treated similar to patients without t(11;18) translocation or extra copies of MALT1.
- Citation: Iwamuro M, Takenaka R, Nakagawa M, Moritou Y, Saito S, Hori S, Inaba T, Kawai Y, Toyokawa T, Tanaka T, Yoshino T, Okada H. Management of gastric mucosa-associated lymphoid tissue lymphoma in patients with extra copies of the MALT1 gene. World J Gastroenterol 2017; 23(33): 6155-6163
- URL: https://www.wjgnet.com/1007-9327/full/v23/i33/6155.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i33.6155
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) is one of the non-Hodgkin lymphomas, originating in tissues or organs outside of the lymph nodes. Typically, this neoplasm involves the gastrointestinal tract, thyroid, ocular adnexa, lungs, salivary glands, liver, and skin[1,2]. Of these organs, the stomach is the most frequently identified primary site. Approximately two-thirds of patients with gastric MALT lymphoma have chronic Helicobacter pylori (H. pylori) infection in the stomach, which is believed to be the causative organism. Clinically, eradication of H. pylori is the first-line management of choice, resulting in regression of lymphoma in 75% to 80% of patients[3]. Meanwhile, cases with chromosomal abnormalities of t(11;18)(q21;q21)/API2-MALT1 translocation have been shown to be resistant to H. pylori eradication. Consequently, evaluation of t(11;18)(q21;q21) translocation at the initial workup is valuable to forecast the response to antibiotic therapy against H. pylori[4].
Previously, we reported a case of gastric MALT lymphoma with trisomy 18[5]. In that case, although there were no fusion genes of API2-MALT1, trisomy 18 was identified as extra copies of MALT1, using a fluorescence in situ hybridization (FISH) analysis for t(11;18)(q21;q21)/API2-MALT1 translocation, since MALT1 is located on chromosome 18q21. The patient required radiation therapy to treat the gastric lymphoma lesions, because the gastric lesions only partially improved after eradication therapy for H. pylori. Though radiation therapy resulted in complete remission, MALT lymphoma recurred in the stomach 16 mo later. Previous studies investigating chromosome aneuploidy in MALT lymphomas also reported that trisomy 18 might be indicative of progression or relapse in patients with gastric MALT lymphoma[6-8]. Based on our experience and previous reports, we hypothesized that gastric MALT lymphoma with extra copies of the MALT1 gene may be resistant to H. pylori eradication and may show more frequent progression or relapse than those without chromosomal aberrations. However, no studies have described the initial treatment and response in such patients. The purpose of this study is to reveal the clinical characteristics and outcomes of gastric MALT lymphoma in patients with t(11;18)(q21;q21)/API2-MALT1 translocation or extra copies of MALT1, in order to determine initial treatment strategies for cases presenting with chromosomal aneuploidy.
Letters of inquiry regarding patients with gastric MALT lymphoma were sent from the Department of Gastroenterology and Hepatology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, to ten collaborating institutions. The inclusion criteria were: (1) patients with pathologically diagnosed MALT lymphoma in the stomach, and (2) patients who underwent fluorescence in situ hybridization analysis for t(11;18)(q21;q21)/API2-MALT1 translocation on biopsy samples from the gastric MALT lymphoma lesions. FISH analysis was performed for all patients on fresh biopsy samples using dual-color, dual-fusion translocation probes for API2/MALT1. MALT lymphoma with a large-cell component was excluded from this study. Finally, a total of 146 patients diagnosed with gastric MALT lymphoma between October 1997 and November 2015 were identified. Thus, these patients were retrospectively registered in this study.
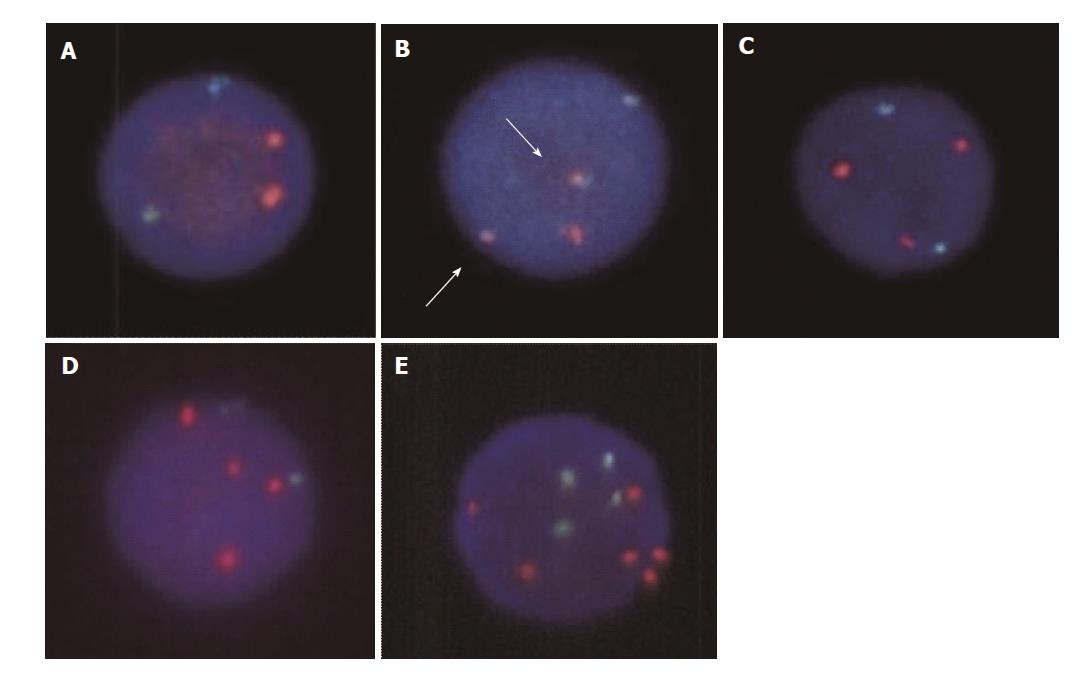
According to the FISH result for t(11;18) translocation, patients were subdivided into three groups as follows: (1) Group A: patients without t(11;18)(q21;q21)/API2-MALT1 translocation or extra copies of MALT1 (Figure 1A), (2) Group B: patients with t(11;18) translocation (Figure 1B), and (3) Group C: patients with extra copies of MALT1 (Figure 1C). To determine the patient characteristics of gastric MALT lymphoma with or without chromosomal aberrations, we retrospectively examined the gender, age at diagnosis, endoscopic features, clinical stages according to Lugano classification[9], H. pylori infection status, treatments, response to treatments, and outcomes. H. pylori infection status was examined by urea breath tests, rapid urease tests, microscopic observations or culture tests on endoscopically biopsied specimens, stool antigen tests, serum or urine antibody tests, or a combination of these methods. Success of H. pylori eradication was confirmed by urea breath tests, rapid urease tests, microscopic observations or culture tests on endoscopically biopsied specimens, or stool antigen tests. The follow-up period was defined as the period from the lymphoma diagnosis to death from any cause or to the patient’s last hospital visit.
Macroscopic features of gastric lymphoma lesions observed during esophagogastroduodenoscopy were classified into the following six subtypes[10]: (1) erosions/ulcers; (2) early gastric cancer-like lesion that formed a slightly depressed area; (3) whitish mucosa; (4) cobblestone appearance; (5) submucosal tumor-like lesion; and (6) mixed (a combination of these five subtypes). All cases were reviewed and their subtypes were classified by board certified endoscopists.
Event-free survival was measured from diagnosis until documented progression/relapse, death from primary disease, or commencement of the second treatment for any reason. For the comparisons of the two groups, statistical analyses including ttests, χ2 tests, and Ftests were performed using JMP 8.0.1 software (SAS Institute, Cary, NC, United States). Cumulative event-free probabilities were calculated by Kaplan-Meier analysis and log-rank tests were performed using the JMP 8.0.1 software. P < 0.05 was considered to indicate a statistically significant difference. The present study was approved by the Ethical Committee of the Okayama University Hospital and adhered to the Declaration of Helsinki.
In this study, clinical data of 146 patients (76 women and 70 men) were collected from nine institutions (Table 1). FISH analysis for t(11;18)(q21;q21)/API2-MALT1 revealed that t(11;18) translocation and extra copies of MALT1 were not identified in 88 patients (Figure 1A). Fusion genes of API2-MALT1 indicating t(11;18)(q21;q21)/API2-MALT1 translocation were detected in 27 patients (10 women and 17 men) (Figure 1B). Extra copies of MALT1 were found in 31 patients; the ratio of MALT1 signals to API2 signals was 3:2 in 28 patients, suggesting trisomy 18 (Figure 1C), and 4:2 in one patient, suggesting tetrasomy 18, (Figure 1D). The remaining two patients had a combination of lymphoma cells with either trisomy 18 or tetrasomy 18. In one patient with extra copies of MALT1, several lymphoma cells had six MALT1 signals with four API2 signals, which is considered as trisomy 18 with tetraploidy (Figure 1E).
| Total | Group A: | Group B: | Group C: | Group A vs B, | Group A vs C, | |
| No chromosome aberration1 | t(11;18) positive | Extra copies of MALT1 | p value | p value | ||
| n | 146 | 88 | 27 | 31 | ||
| Sex | ||||||
| Male | 70 (47.9) | 40 (45.5) | 17 (63.0) | 13 (41.9) | 0.11 | 0.73 |
| Female | 76 (52.1) | 48 (54.5) | 10 (37.0) | 18 (58.1) | ||
| Age (mean ± SD, yr) | 65.4 ± 12.6 | 65.9 ± 12.3 | 64.2 ± 11.2 | 65.0 ± 14.9 | 0.53 | 0.75 |
| Stage (Lugano system) | ||||||
| I | 131 | 81 | 24 | 26 | 0.922 | 0.292 |
| II1 | 3 | 1 | 1 | 1 | ||
| II2 | 1 | 1 | 0 | 0 | ||
| IV | 11 | 5 | 2 | 4 | ||
| H. pylori | ||||||
| Positive | 89 (61.0) | 66 (75.0) | 2 (7.4) | 21 (67.7) | < 0.01 | 0.439 |
| Negative | 57 (39.0) | 22 (25.0) | 25 (92.6) | 10 (32.3) | ||
Patients without t(11;18) translocation or extra copies of MALT1 (Group A) showed slight female predominance (48 women and 40 men), while patients with t(11;18)(q21;q21) translocation (Group B) showed slight male predominance (10 women and 17 men). However, there was no statistically significant difference between Groups A and B regarding sex ratio (P = 0.11). Patients with extra copies of MALT1 (Group C) showed slight female predominance (18 females and 13 males) compared to Group A (P = 0.73). The mean ages at lymphoma diagnosis was 65.9 ± 12.3 years in Group A, 64.2 ± 11.2 years in Group B, and 65.0 ± 14.9 years in Group C and was not different between groups. Lymphoma lesions were localized in the stomach without lymph node or other organ involvement in most of the patients (131/146, 89.7%), thus they were classified as stage I according to the Lugano system of staging[10]. H. pylori infection status was examined in all patients. In Group A, 75.0% of the patients were positive for H. pylori. Group C had similar positivity for H. pylori (67.7%). In contrast, only 7.4% of the patients in Group B had H. pylori infection (P < 0.01, vs Group A). Endoscopic features are summarized in Table 2. Overall, gross findings of the gastric lymphoma lesions were as follows: erosions/ulcers in 44 patients (30.1%), whitish mucosa in 42 patients (28.8%), submucosal tumor-like lesions in 29 patients (19.9%), cobblestone appearance in 17 patients (11.6%), early gastric cancer-like lesions in 10 patients (6.8%), and mixed lesions in four patients (2.7%). The ratio of each macroscopic morphology was similar between Groups A and C. Moreover, no patients in Group B presented with submucosal tumor-like lesions.
| Total | Group A: No chromosome aberration1 | Group B: t(11;18) positive | Group C: Extra copies of MALT1 | |
| Macroscopic feature | ||||
| Erosions/ulcers | 44 (30.1) | 23 (26.1) | 9 (33.3) | 12 (38.7) |
| Early gastric cancer-like | 10 (6.8) | 7 (8.0) | 2 (7.4) | 1 (3.2) |
| Whitish mucosa | 42 (28.8) | 25 (28.4) | 12 (44.4) | 5 (16.1) |
| Cobblestone appearance | 17 (11.6) | 12 (13.6) | 3 (11.1) | 2 (6.5) |
| Submucosal tumor | 29 (19.9) | 19 (21.6) | 0 | 10 (32.3) |
| Mixed | 4 (2.7) | 2 (2.3) | 1 (3.7) | 1 (3.2) |
Treatments and outcomes are shown in Table 3. Follow-up periods were 3.9 ± 3.1 years in Group A, 5.1 ± 4.5 years in Group B, and 2.9 ± 2.2 years in Group C. Only four patients died during the follow-up period: One patient in Group A died 2.4 years after lymphoma diagnosis because of concomitant advanced lung cancer, although complete response was obtained for gastric MALT lymphoma after H. pylori eradication. Two patients in Group C died of pneumonia 1.1 years and 2.0 years after lymphoma diagnosis, respectively. The other patient in Group A had stage IV lymphoma involving the stomach, ileum, colon, rectum, and lymphadenopathies of the neck, supraclavicular, subphrenic, and retroperitoneum. The patient underwent chemotherapy with cyclophosphamide, vincristine sulfate, and prednisone, followed by rituximab monotherapy and yttrium-90 ibritumomab tiuxetan monotherapy. However, the patient died of refractory lymphoma 1.4 years after the initial diagnosis due to intestinal perforation. Since pathological analysis was waived because of the patient’s deteriorated condition, it was unknown whether histologic transformation occurred in the terminal stage. All patients, except for the four mortality cases, were alive at the patient’s last visit to each institution. Five patients in Group A and one in Group B did not undergo esophagogastroduodenoscopy during the follow-up period, thus their lymphoma status, whether remitted or unchanged, was unknown. Complete response was obtained in 61 patients in Group A (69.3%), 22 in Group B (81.5%), and 21 in Group C (67.7%). On the other hand, lymphoma lesions were partially remitted or unchanged in 20 patients in Group A (22.7%), four in Group B (14.8%), and eight in Group C (25.8%).
| Total | Group A:No chromosome aberration1 | Group B:t(11;18) positive | Group C:Extra copies of MALT1 | |
| Treatment | ||||
| Eradication alone | 85 | 62 | 3 | 20 |
| RT alone | 23 | 8 | 15 | 0 |
| Chemotherapy alone | 8 | 3 | 2 | 3 |
| RT and chemotherapy | 2 | 1 | 0 | 1 |
| Eradication RT | 17 | 6 | 5 | 6 |
| Eradication and chemotherapy | 3 | 2 | 0 | 1 |
| Eradication and RT and chemotherapy | 2 | 1 | 1 | 0 |
| None | 6 | 5 | 1 | 0 |
| Outcome | ||||
| Live without disease | 103 | 61 | 21 | 21 |
| Live with disease | 33 | 20 | 5 | 8 |
| Live, unknown disease status | 6 | 5 | 1 | 0 |
| Dead by other cause | 3 | 1 | 0 | 2 |
| Dead by MALT lymphoma | 1 | 1 | 0 | 0 |
| Follow-up period (mean ± SD, yr) | 3.9 ± 3.3 | 3.9 ± 3.1 | 5.1 ± 4.5 | 2.9 ± 2.2 |
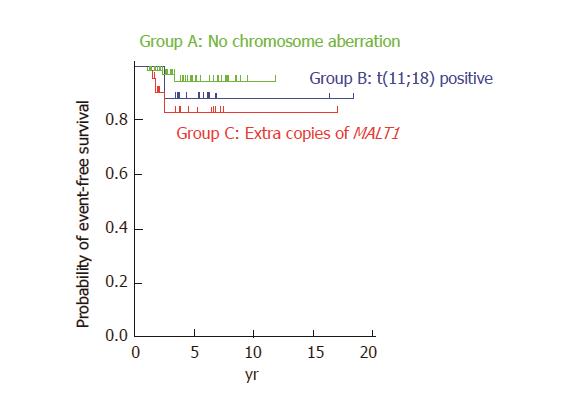
With regard to treatment regimens, most of the patients without t(11;18) translocation or extra copies of MALT1 (Group A) were treated with H. pylori eradication alone (n = 62, 70.5%). More than half of the patients with t(11;18)(q21;q21) translocation (Group B) were treated with radiotherapy alone (n = 15, 55.6%). Approximately two-thirds of the patients with extra copies of MALT1 (Group C) were treated with H. pylori eradication alone (n = 20, 64.5%). Table 4 shows treatment regimens that resulted in complete remission of MALT lymphoma lesions. Complete remission of lymphoma was observed in 44 (72.1%) and 13 (61.9%) patients in Groups A and C, respectively, by H. pylori eradication alone. In contrast, only one (4.5%) patient achieved complete remission after H. pylori eradication alone, whereas 14 (63.6%) patients achieved complete remission after radiotherapy alone in Group B. Figure 2 shows cumulative event-free probabilities between the three groups. Although event-free survival of Group C patients seems to be inferior than that of the Group A and Group B patients, log-rank test revealed that the difference between Groups A and C was not statistically significant (P = 0.10).
| Total | Group A: No chromosome aberration1 | Group B:t(11;18) positive | Group C:Extra copies of MALT1 | |
| n | 103 | 61 | 21 | 21 |
| Treatment | ||||
| Eradication alone | 58 | 44 | 1 | 13 |
| RT alone | 20 | 6 | 14 | 0 |
| Chemotherapy alone | 4 | 1 | 1 | 2 |
| RT and chemotherapy | 2 | 1 | 0 | 1 |
| Eradication and RT | 13 | 5 | 4 | 4 |
| Eradication and chemotherapy | 3 | 2 | 0 | 1 |
| Eradication, RT and chemotherapy | 1 | 0 | 1 | 0 |
| None | 2 | 2 | 0 | 0 |
To our knowledge, the present report is the largest retrospective study investigating chromosome aberrations in gastric MALT lymphoma. Our review of 146 patients revealed that 31 patients (21.2%) had extra copies of MALT1. Prevalence of trisomy 18 in MALT lymphomas has been reported to range from 5% to 39%[11-17]. Other chromosomal abnormalities involved in MALT lymphomas are trisomies 3, 7 and 12. The incidence varies between reports in the literature: trisomy 3 can be detected in 6% to 24%, trisomy 7 in 3% to 15%, and trisomy 12 in 3% to 38%[17].
The clinical significance of these chromosomal numerical changes has been investigated in several studies[6-8,18-20]. Tanimoto et al[19] investigated 34 patients with primary ocular adnexal MALT lymphoma and found that disease recurrence was documented in five cases, all of which had trisomy 18. Statistical analysis revealed that the time to recurrence was significantly shorter in patients with trisomy 18 than in those without trisomy 18 (P = 0.05). We also reported a patient with gastric MALT lymphoma with trisomy 18 in whom only partial improvement was documented after eradication therapy for H. pylori, and lymphoma recurred in the stomach 16 months after radiotherapy[5]. On the other hand, Taji et al[17] retrospectively reviewed 13 patients with localized MALT lymphoma in the stomach, and reported that all patients with unresponsive or progressive disease had t(11;18) translocation or trisomy 3. Conversely, one patient with complete response had trisomy 18, and another patient with complete response had both trisomies 12 and 18. Based on these findings, the authors speculate that trisomy 3 is related to resistance to H. pylori eradication therapy and disease recurrence in gastric MALT lymphoma, whereas trisomies 12 and 18 are not. Nakamura and colleagues reviewed 90 cases of gastric MALT lymphoma and reported that extra copies of MALT1 were found in 18 of 71 (25%) cases[6]. Although overall survival was not associated with the presence of extra copies of MALT1, disease progression or relapse of lymphoma was more frequently observed in patients with extra copies of MALT1. Possible correlations between extra copies of MALT1 and progression or relapse of lymphoma have been described in other studies as well[7,8]. In the present study, although the difference was not statistically significant, log-rank test revealed similar tendencies (Figure 2). Consequently, patients with additional copies of MALT1 may require more frequent clinical follow-up.
In the present study, the sex ratio of the patients with extra copies of MALT1 (Group C) was similar to that of the patients without t(11;18) translocation or extra copies of MALT1 (Group A), with the number of females being greater than males. On the contrary, although the difference was not statistically significant, patients with t(11;18) translocation (Group B) included more males than females. It was also noteworthy that Group C patients had similar prevalence of H. pylori infection to Group A. Other patient characteristics, such as the age at lymphoma diagnosis, clinical stages, and macroscopic morphology were not different between Groups A and C. The majority of Group A and Group C patients were treated with H. pylori eradication alone. Moreover, H. pylori eradication alone resulted in complete remission of lymphoma in 44 patients in Group A and 13 in Group C. Overall, the results observed in this study suggest that patients with trisomy and tetrasomy 18 require no distinct treatment from patients without aberrations. To our knowledge, the present study is the first to reveal clinical responsiveness to H. pylori eradication in gastric MALT lymphoma patients with extra copies of MALT1. In addition, in the present study, FISH analysis was performed by using fresh biopsy samples, whereas previous reports primarily involved analyses of archival pathologic specimens with the aim of clarifying the etiology of chromosomal aberrations. We believe that our results would be more readily interpreted in actual clinical settings for physicians responsible for gastric MALT lymphoma patient therapy.
The present study included 27 patients (18.5%) with t(11;18)(q21;q21)/API2-MALT1 translocation. t(11;18)(q21;q21)/API2-MALT1 is the most frequent translocation among gastric, pulmonary, intestinal, and cutaneous MALT lymphomas, accounting for 13% to 35% of cases[3,21,22]. Other chromosomal aberrations detected in MALT lymphomas include t(1;14)(p22;q32), t(14;18)(q32;q21), t(3;14)(q27;q32), and t(3;14)(p14.1;q32)[3]. Several of such karyotypic alterations have been known to activate the nuclear pathway, which is considered to lead to lymphomagenesis. Thus, these translocation-positive MALT lymphomas arise independent of H. pylori infection and are unresponsive to antibiotic treatment[3]. Moreover, these patients often show a late response and lymphoma relapse during follow-up[18,23]. Regardless of the adverse clinical features, t(11;18) translocation is infrequently identified in transformed MALT lymphoma or diffuse large B-cell lymphoma, indicating that high-grade transformation is less likely to occur in translocation-positive MALT lymphomas than in those without chromosome aberration[3,8,18,24]. In this context, polymerase chain reaction or FISH analysis for t(11;18) translocation is essential during the initial workup to determine the appropriate treatment strategy[25]. Of additional clinical interest is that trisomy and tetrasomy 18 can be detected as extra copies of MALT1 by FISH analysis, since MALT1 is located on chromosome 18[18,20].
In our study, all patients with t(11;18) translocation (Group B), except two, were negative for H. pylori infection. With regard to the macroscopic morphology of the gastric lesions, no patients in Group B presented with submucosal tumor-like lesions (Table 2). We speculate that this result reflects the lower proliferative potential of MALT lymphoma cells with t(11;18) translocation, compared to that of cells without t(11;18) translocation or extra copies of MALT1. Since almost no patients in Group B had H. pylori infection, most of the patients were treated with radiotherapy and/or chemotherapy. These clinical features were concordant with previously reported characteristics of MALT lymphoma with t(11;18) (q21;q21)/API2-MALT1 translocation[8,22-24].
There were several limitations associated with this study. First, the presence of extra copies of MALT1 is often suggestive of partial or complete trisomy/tetrasomy 18; both are different. Trisomy/tetrasomy 18 should be determined only when extra copies are identified by FISH using centromere-specific probes for chromosome 18, or when gains of chromosome 18 are confirmed by array comparative genomic hybridization or by cytogenetic analysis (G-banding). However, these assays could not be performed because of the retrospective nature of this study. Second, not all chromosome alterations were investigated. As described above, trisomies 3, 7 and 12 and t(14;18) and t(3;14) translocations can be involved in MALT lymphomas[26,27]. Therefore, Group A may have been heterogeneous and included patients with chromosomal aberrations other than t(11;18) translocation and trisomy 18. Third, only patients who underwent FISH analysis for t(11;18) translocation were enrolled in this study. FISH analysis may be waived for patients in which H. pylori was eradicated immediately following the diagnosis of MALT lymphoma and lymphoma regression was achieved. Thus, selection bias is a possibility in this study. Fourth, because this is a retrospective, multi-centered study, treatment strategies of each patient may have varied according to the attending physician’s preference and the availability of chemotherapy and/or radiotherapy at each institution. Fifth, methods to examine H. pylori infection status varied among the different institutions participating in this study. Prospective studies utilizing uniform protocols are required to definitively compare treatment responses and prognosis in each group.
In conclusion, we reviewed 146 patients, including 31 (21.2%) with extra copies of MALT1 and 27 (18.5%) with t(11;18)(q21;q21)/API2-MALT1 translocation. Patients with t(11;18) translocation should be treated differently from others. However, patients with extra copies of MALT1 have similar clinical characteristics to cases without t(11;18) translocation or extra copies of MALT1. We propose that these patients can be initially managed in the same way as cases without chromosomal aberrations. However, since progression or relapse tended to be more frequently seen in patients with extra copies of MALT1, these patients may require closer clinical follow-up.
Previous studies investigating chromosome aneuploidy in extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) reported that trisomy 18 might be indicative of progression or relapse in patients with gastric MALT lymphoma.
No studies have described the initial treatment and response in patients with extra copies of MALT1.
Patients with extra copies of MALT1 have similar clinical characteristics to cases without t(11;18) translocation or extra copies of MALT1. The present study is the first to reveal clinical responsiveness to Helicobacter pylori eradication in gastric MALT lymphoma patients with extra copies of MALT1.
Patients with extra copies of MALT1 can be initially managed in the same way as cases without chromosomal aberrations.
MALT lymphoma is one of the non-Hodgkin lymphomas, originating in tissues or organs outside of the lymph nodes. Typically, this neoplasm involves the gastrointestinal tract, thyroid, ocular adnexa, lungs, salivary glands, liver, and skin. Of these organs, the stomach is the most frequently identified primary site.
This is a well-designed multicenter study on the role of extra copies of MALT1 in gastric MALT lymphoma. The conclusion can be supported by the results. It can add something interest to current knowledge.
| 1. | Nakamura S, Matsumoto T. Helicobacter pylori and gastric mucosa-associated lymphoid tissue lymphoma: recent progress in pathogenesis and management. World J Gastroenterol. 2013;19:8181-8187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Isaacson PG, Chott A, Nakamura S, Muller-Hermelink HK, Harris NL, Swerdlow SH. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. IARC, Lyon 2008; 214-217. |
| 3. | Raderer M, Kiesewetter B, Ferreri AJ. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA Cancer J Clin. 2016;66:153-171. [PubMed] |
| 4. | Nakamura T, Nakamura S, Yonezumi M, Seto M, Yokoi T. The t(11; 18)(q21; q21) translocation in H. pylori-negative low-grade gastric MALT lymphoma. Am J Gastroenterol. 2000;95:3314-3315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Ishikawa H, Iwamuro M, Okada H, Hori K, Kita M, Kawano S, Kawahara Y, Tanaka T, Kondo E, Yoshino T. Recurrence after radiotherapy for gastric mucosa-associated lymphoid tissue (MALT) lymphoma with trisomy 18. Intern Med. 2015;54:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Nakamura S, Ye H, Bacon CM, Goatly A, Liu H, Banham AH, Ventura R, Matsumoto T, Iida M, Ohji Y. Clinical impact of genetic aberrations in gastric MALT lymphoma: a comprehensive analysis using interphase fluorescence in situ hybridisation. Gut. 2007;56:1358-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Krugmann J, Tzankov A, Dirnhofer S, Fend F, Greil R, Siebert R, Erdel M. Unfavourable prognosis of patients with trisomy 18q21 detected by fluorescence in situ hybridisation in t(11;18) negative, surgically resected, gastrointestinal B cell lymphomas. J Clin Pathol. 2004;57:360-364. [PubMed] |
| 8. | Remstein ED, Kurtin PJ, James CD, Wang XY, Meyer RG, Dewald GW. Mucosa-associated lymphoid tissue lymphomas with t(11;18)(q21;q21) and mucosa-associated lymphoid tissue lymphomas with aneuploidy develop along different pathogenetic pathways. Am J Pathol. 2002;161:63-71. [PubMed] |
| 9. | Rohatiner A, d'Amore F, Coiffier B, Crowther D, Gospodarowicz M, Isaacson P, Lister TA, Norton A, Salem P, Shipp M. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol. 1994;5:397-400. [PubMed] |
| 10. | Nakamura T, Nakamura S. Current review and reappraisal of gastrointestinal MALT lymphoma with special reference to endoscopic appearances and histopathological diagnoses. Gastroenterol Endoscopy. 2000;42:1163-1176 (in Japanese with English abstract). |
| 11. | Wotherspoon AC, Finn TM, Isaacson PG. Trisomy 3 in low-grade B-cell lymphomas of mucosa-associated lymphoid tissue. Blood. 1995;85:2000-2004. [PubMed] |
| 12. | Dierlamm J, Pittaluga S, Wlodarska I, Stul M, Thomas J, Boogaerts M, Michaux L, Driessen A, Mecucci C, Cassiman JJ. Marginal zone B-cell lymphomas of different sites share similar cytogenetic and morphologic features. Blood. 1996;87:299-307. [PubMed] |
| 13. | Ott G, Kalla J, Steinhoff A, Rosenwald A, Katzenberger T, Roblick U, Ott MM, Müller-Hermelink HK. Trisomy 3 is not a common feature in malignant lymphomas of mucosa-associated lymphoid tissue type. Am J Pathol. 1998;153:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Blanco R, Lyda M, Davis B, Kraus M, Fenoglio-Preiser C. Trisomy 3 in gastric lymphomas of extranodal marginal zone B-cell (mucosa-associated lymphoid tissue) origin demonstrated by FISH in intact paraffin tissue sections. Hum Pathol. 1999;30:706-711. [PubMed] |
| 15. | Brynes RK, Almaguer PD, Leathery KE, McCourty A, Arber DA, Medeiros LJ, Nathwani BN. Numerical cytogenetic abnormalities of chromosomes 3, 7, and 12 in marginal zone B-cell lymphomas. Mod Pathol. 1996;9:995-1000. [PubMed] |
| 16. | Dierlamm J, Michaux L, Wlodarska I, Pittaluga S, Zeller W, Stul M, Criel A, Thomas J, Boogaerts M, Delaere P. Trisomy 3 in marginal zone B-cell lymphoma: a study based on cytogenetic analysis and fluorescence in situ hybridization. Br J Haematol. 1996;93:242-249. [PubMed] |
| 17. | Taji S, Nomura K, Matsumoto Y, Sakabe H, Yoshida N, Mitsufuji S, Nishida K, Horiike S, Nakamura S, Morita M. Trisomy 3 may predict a poor response of gastric MALT lymphoma to Helicobacter pylori eradication therapy. World J Gastroenterol. 2005;11:89-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Ruskoné-Fourmestraux A, Fischbach W, Aleman BM, Boot H, Du MQ, Megraud F, Montalban C, Raderer M, Savio A, Wotherspoon A; EGILS group. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut. 2011;60:747-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 19. | Tanimoto K, Sekiguchi N, Yokota Y, Kaneko A, Watanabe T, Maeshima AM, Matsuno Y, Harada M, Tobinai K, Kobayashi Y. Fluorescence in situ hybridization (FISH) analysis of primary ocular adnexal MALT lymphoma. BMC Cancer. 2006;6:249. [PubMed] |
| 20. | Xia H, Nakayama T, Sakuma H, Yamada S, Sato F, Takino H, Okabe M, Fujiyoshi Y, Hattori H, Inagaki H. Analysis of API2-MALT1 fusion, trisomies, and immunoglobulin VH genes in pulmonary mucosa-associated lymphoid tissue lymphoma. Hum Pathol. 2011;42:1297-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Nakamura S, Matsumoto T. Treatment Strategy for Gastric Mucosa-Associated Lymphoid Tissue Lymphoma. Gastroenterol Clin North Am. 2015;44:649-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Auer IA, Gascoyne RD, Connors JM, Cotter FE, Greiner TC, Sanger WG, Horsman DE. t(11;18)(q21;q21) is the most common translocation in MALT lymphomas. Ann Oncol. 1997;8:979-985. [PubMed] |
| 23. | Yeh KH, Kuo SH, Chen LT, Mao TL, Doong SL, Wu MS, Hsu HC, Tzeng YS, Chen CL, Lin JT. Nuclear expression of BCL10 or nuclear factor kappa B helps predict Helicobacter pylori-independent status of low-grade gastric mucosa-associated lymphoid tissue lymphomas with or without t(11;18)(q21;q21). Blood. 2005;106:1037-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Tan SY, Ye H, Liu H, Lim KH, Toh HC, Ng CF, Chung YF, Wotherspoon AC, Du MQ. t(11;18)(q21;q21)-positive transformed MALT lymphoma. Histopathology. 2008;52:777-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Extranodal marginal zone B-cell lymphoma, gastric MALT lymphoma. In: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Non-Hodgkin's Lymphomas, version 4. 2014. Available from: https://www.nccn.org/about/nhl.pdf.. |
| 26. | Streubel B, Simonitsch-Klupp I, Müllauer L, Lamprecht A, Huber D, Siebert R, Stolte M, Trautinger F, Lukas J, Püspök A. Variable frequencies of MALT lymphoma-associated genetic aberrations in MALT lymphomas of different sites. Leukemia. 2004;18:1722-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 269] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 27. | Remstein ED, Dogan A, Einerson RR, Paternoster SF, Fink SR, Law M, Dewald GW, Kurtin PJ. The incidence and anatomic site specificity of chromosomal translocations in primary extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) in North America. Am J Surg Pathol. 2006;30:1546-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Abdel-Salam OM, Garcia-Olmo D, Koch TR, Milone M S- Editor: Ma YJ L- Editor: A E- Editor: Xu XR