Published online Sep 7, 2017. doi: 10.3748/wjg.v23.i33.6100
Peer-review started: December 29, 2016
First decision: March 18, 2017
Revised: April 13, 2017
Accepted: July 22, 2017
Article in press: July 24, 2017
Published online: September 7, 2017
Processing time: 256 Days and 20.3 Hours
To clarify the mechanisms of HOX transcript antisense intergenic RNA (HOTAIR) in gastric cancer (GC) migration and invasion.
Quantitative real-time polymerase chain reaction (qPCR) was used to detect the expression level of HOTAIR in GC tissues. The correlation of its expression with clinicopathological features was analyzed. Area under receiver operating characteristic curve (AUCROC) was constructed to evaluate the diagnostic value of HOTAIR. Wound-healing assay and Transwell assay were performed to detect the biological effects of HOTAIR in GC cells. qPCR, western blot and immunohistochemistry were used to evaluate the mRNA and protein expression of E-cadherin. RNA-binding protein immunoprecipitation was used for the analysis of EZH2 interactions with HOTAIR. Chromatin immunoprecipitation assay was performed to investigate direct interactions between EZH2 and E-cadherin.
The expression of HOTAIR was up-regulated in GC tumorous tissues compared with the para-tumorous tissues (P < 0.001). Its over-expression was correlated with tumor-node-metastasis (TNM) stage (P = 0.024), tumor invasion (P = 0.018), lymph node metastasis (P = 0.023), and poor prognosis (P < 0.001). Multivariate Cox regression analysis confirmed expression of HOTAIR as an independent predictor of overall survival (P = 0.033), together with TNM stage (P = 0.002) and lymph node metastasis (P = 0.002). The AUCROC was up to 0.709 (95%CI: 0.623-0.785, P < 0.001). Knockdown of HOTAIR by siRNA in GC cells suppressed the migration and invasion of GC cells. Significantly negative correlation between HOTAIR and E-cadherin was found in GC tissues and cell lines, and HOTAIR contributed to the regulation of E-cadherin through binding to EZH2 with the E-cadherin promoter.
HOTAIR may play a pivotal role in tumor cell migration and invasion. It can be used as a potential diagnostic and prognostic biomarker for GC.
Core tip: In this study, we found that HOX transcript antisense intergenic RNA (HOTAIR) expression was up-regulated in gastric cancer (GC) tissues. High expression of HOTAIR was associated with clinicopathological characteristics and poor prognosis in GC patients. Additional experiments revealed that HOTAIR knockdown significantly inhibited the invasion and migration of GC cells. We also tested whether HOTAIR recruited EZH2 to promote tumor cell migration and invasion by repressing E-cadherin in GC. The findings from our study will help clarify the role of HOTAIR in GC progression and its potential as a therapeutic target.
- Citation: Chen WM, Chen WD, Jiang XM, Jia XF, Wang HM, Zhang QJ, Shu YQ, Zhao HB. HOX transcript antisense intergenic RNA represses E-cadherin expression by binding to EZH2 in gastric cancer. World J Gastroenterol 2017; 23(33): 6100-6110
- URL: https://www.wjgnet.com/1007-9327/full/v23/i33/6100.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i33.6100
Gastric cancer (GC) is one of the most common malignancies worldwide, having high rates of both incidence and mortality[1]. Even though remarkable progress has been made in surgical resection and chemotherapy, which are the main therapeutic approaches for GC, the prognosis continues to be dismal[2]. The main cause of treatment failure is cancer recurrence and metastasis[3,4].
Tumor metastasis is a complex process, in which cancer cells leave the primary tumor site and migrate to distant secondary organs. Metastatic cancer cells fractionally retain their epithelial properties and obtain mesenchymal characteristics that give them the ability to invade or distract[5]. This process is called epithelial-to-mesenchymal transition (EMT). EMT is characterized by E-cadherin expression loss and N-cadherin and vimentin up-regulation[6]. It is regulated by many signaling pathways, transcriptional factors and post-transcriptional factors[7]. Post-transcriptional regulatory networks include the miRNA and long noncoding (lnc)RNA families[8]. Emerging evidence shows that lncRNAs have an important role in regulating EMT and cancer metastasis[5,9]. Several important lncRNAs are reported to induce EMT, including highly upregulated in liver cancer (HULC)[10], metastasis-associated lung adenocarcinoma transcript (MALAT)-1[11], H19[12], and HOX transcript antisense intergenic RNA (HOTAIR)[13].
HOTAIR is identified as an oncogene involved in many kinds of cancers, including breast cancer[14], esophageal squamous cell carcinoma[15], colorectal cancer[16], and GC[13]. HOTAIR acts mainly through the polycomb repressive complex (PRC)2 (EZH2, RbAp46, RbAp48 and SUZ12)[17], which trimethylates histone H3 lysine-27 (H3K27) of the HOXD locus and repress HOXD gene expression[14]. EZH2 which is the core catalytic component of the PRC2, can affect cancer progression by altering H3K27 trimethylation and silencing transcription[17].
A previous study showed that silencing of HOTAIR inhibits GC cell migration, invasion and metastasis, and reverses the EMT in GC cells[13]. HOTAIR in combination with PRC2 could epigenetically inhibit miR34a, and control C-Met (HGF/C-Met/Snail pathway) and Snail, thus promoting the EMT process of GC cells and tumor metastasis[13]. However, the overall clinical role of HOTAIR in GC and the molecular mechanisms of HOTAIR involved in GC cell metastasis have not yet been fully investigated.
Up-regulation of HOTAIR expression in GC tissues was found in the present study. We also found that over-expression of HOTAIR was related to the clinicopathological characteristics of and poor prognosis in GC patients. Additional experiments revealed that HOTAIR knockdown significantly repressed migration and invasion. Finally, we tested whether HOTAIR recruited EZH2 to promote tumor cell migration and invasion by repressing E-cadherin in GC. These studies will help clarify the role of HOTAIR in GC progression and its potential as a therapeutic target.
A cohort of 65 primary GC patients was enrolled in this study, each of who underwent surgery at the First Affiliated Hospital of Nanjing Medical University and Jining NO.1 People’s Hospital between 2008 and 2009. None received chemotherapy or radiotherapy prior to surgery. This study was approved by the University Ethics Committee. All patients participated after providing informed consent. Tumor stage was evaluated in accordance with the tumor-node-metastasis (TNM) classification system (UICC/AJCC 2002). Clinical characteristics are shown in Table 1. Patients discharged from hospital were followed up routinely and according to a scheduled program, at least once a year.
| Characteristic | Expression of HOTAIR | P value | |
| Low n = 32 | High n = 33 | ||
| Sex | 0.408 | ||
| Female | 7 | 11 | |
| Male | 25 | 22 | |
| Age in yr | 0.455 | ||
| ≤ 60 | 17 | 21 | |
| > 60 | 15 | 12 | |
| Histological grade | 1.000 | ||
| Well/moderate | 14 | 15 | |
| Other | 18 | 18 | |
| Tumor invasion depth, T | 0.018 | ||
| Tis, T1,T2 | 15 | 6 | |
| T3 or above | 17 | 27 | |
| Lymph node metastasis, N | 0.023 | ||
| N0 | 12 | 4 | |
| N1 or above | 20 | 29 | |
| TNM stage | 0.024 | ||
| I/II | 23 | 14 | |
| III/IV | 9 | 19 | |
| Tumor location | 0.102 | ||
| Antrum | 8 | 15 | |
| Cardia | 10 | 9 | |
| Angulus | 13 | 5 | |
| Body | 1 | 3 | |
| Full stomach | 0 | 1 | |
| Laure’s classification | 0.373 | ||
| Intestinal | 17 | 15 | |
| Diffuse | 12 | 17 | |
| Mixed | 3 | 1 | |
Human GC cell lines SGC-7901and BGC-823 were obtained from the Chinese Academy of Sciences Committee on Type Culture Collection Cell Bank. Cells were cultured in RPMI 1640 or Dulbecco’s modified Eagle’s medium (Gibco-BRL, Shanghai, China) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Shanghai, China), 100 U/mL penicillin, and 100 mg/mL streptomycin (Invitrogen) in an incubator at 37 °C with 5% CO2.
Total RNA was extracted from the frozen tissues using TRIzol reagent (Invitrogen). RNA concentrations were estimated by spectrophotometer absorbance readings of 260 nm. One microgram of total RNA was used for reverse transcription to cDNA with a Reverse Transcription Kit (TaKaRa, Shiga, Japan). An ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA, United States) was used to quantify HOTAIR. Ten microliters of SYBR Premix ExTaq (TaKaRa) was mixed. Primer sequences are listed in Supplementary Table 1. The relative expression was calculated using the equation: ΔCt = Ct (target gene) – Ct (GAPDH); Fold change = 2–[ΔCt(tumour) – ΔCt(nomal)][18].
HOTAIR small interfering (si)RNAs and negative control siRNA (si-NC) were purchased from Invitrogen. The siRNA sequences are listed in Supplementary Table 1. BGC-823 or SGC-7901 cells were grown in 6-well plates until confluent, then transfected with Lipofectamine 2000 (Invitrogen). At 48 h after transfection, cells were harvested for qPCR or western blot analysis.
Cells were seeded in 6-well plates in normal cell growth medium and incubated to confluence. A 20-μL tip was used to make a straight scratch, simulating a wound. The medium was changed to medium containing 1% FBS. At different time points, images of the plates were acquired using a microscope.
For the migration assays, at 48 h after transfection, 5 × 104 cells in serum-free medium were placed into the upper chamber of an insert (8-μm pore size; Millipore, Bedford, MA, United States). For the invasion assays, 105 cells in serum-free medium were placed into the upper chamber of an insert coated with Matrigel (Sigma-Aldrich, St. Louis, MO, United States). Medium containing 10% FBS was added to the lower chamber. After incubation for 24 h, the cells remaining on the upper membrane were removed with cotton wool. Cells that had migrated or invaded through the membrane were stained with methanol and 0.1% crystal violet, imaged and counted in five random fields per well using an IX71 inverted microscope (Olympus, Tokyo, Japan). Experiments were repeated independently three times.
Cell protein lysates were separated by 15% SDS-PAGE, transferred to 0.22-μm nitrocellulose membranes (Sigma-Aldrich) and incubated with specific antibodies. GAPDH antibody was used as a control. Autoradiograms were quantified by densitometry (Quantity One software; Bio-Rad, Hercules, CA, United States). Anti-E-cadherin was purchased from Abcam (Cambridge, United Kingdom). Anti-GAPDH was purchased from Cell Signaling Technology (Danvers, MA, United States).
Binding protein immunoprecipitation assay (RIP) experiments were performed using a Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore). Antibody for EZH2 RIP assays was from Abcam.
Chromatin immunoprecipitation assay (ChIP) assays were performed using an EZ-CHIP KIT (Millipore). EZH2 antibody was obtained from Abcam. H3 trimethyl Lys 27 antibody was from Millipore. The ChIP primer sequences are listed in Supplementary Table 1. Quantification of immunoprecipitated DNA was performed using qPCR with SYBR Green Mix (TaKaRa). ChIP data were calculated as a percentage relative to the input DNA by the equation of 2(Input Ct - Target Ct) × 0.1 × 100.
The immunohistochemical analysis of E-cadherin was performed as described previously[19]. To quantify E-cadherin protein expression, both the intensity and extent of immunoreactivity were evaluated and scored. In the present study, staining intensity was scored as follows: 0, negative staining; 1, weak staining; 2, moderate staining; and 3, strong staining. The scores of the extent of immunoreactivity ranged from 0 to 3, and were determined according to the percentage of cells that showed positive staining in each microscopic field of view (0, < 25%; 1, 25%-50%; 2, 50%-75%; 3, 75%-100%). A final score ranging from 0 to 9 was achieved by multiplying the scores for intensity and extent.
SPSS version 21.0 software was used for all statistical analysis. Significance of the differences between groups was estimated by Student’s t-test, χ2 test, or Mann-Whitney test. Survival curves were estimated by the Kaplan-Meier method. The log-rank test was used to estimate the statistical difference between survival curves. Cox proportional hazards analysis was performed to calculate the hazard ratio (HR) and the 95% confidence interval (CI) to evaluate the association between HOTAIR expression and overall survival (OS) time. Multivariate Cox regression was performed to adjust for other covariates. Spearman correlation analysis was performed to investigate the correlation between HOTAIR and E-cadherin mRNA expression. Receiver operating characteristic (ROC) curve was produced to evaluate the diagnostic value for differentiating between GC and adjacent non-tumor tissues. A two-tailed P value of ≤ 0.05 was considered statistically significant. All the graphs were plotted using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, United States).
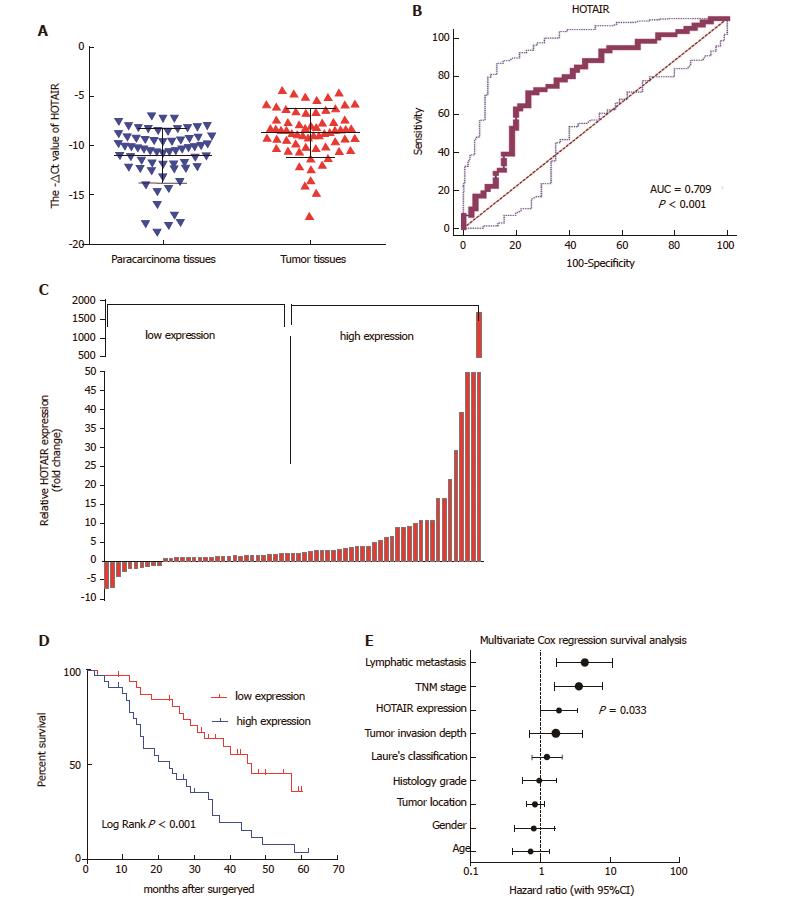
HOTAIR showed markedly higher expression in GC tissues as compared with matched adjacent non-tumor tissues (P < 0.001; Figure 1A).
An ROC curve was generated by comparing HOTAIR expression in GC tissues to expression in matched adjacent non-tumor tissues. With a cut-off value of 9.31, the area under the ROC curve (AUCROC) reached 0.709 (95%CI: 0.623-0.785, P < 0.001; Figure 1B). The sensitivity was 64.62%, the specificity was 75.38%, and Youden's index was 0.4.
Patients with GC were divided into two groups based on the cut-off ratio of HOTAIR expression (2.35-fold) in tumor tissues: high-expression group (n = 33) and low-expression group (n = 32) (Figure 1C). Table 1 showed that HOTAIR over-expression was significantly correlated with tumor invasion (P = 0.018), lymph node metastasis (P = 0.023) and higher TNM stage (P = 0.024). However, there was no correlation between HOTAIR expression and age, sex and histological grade (P > 0.05).
To assess the impact of HOTAIR expression on OS of GC patients, Kaplan-Meier analysis and log rank test were used. The results revealed that patients in the high-expression group had a shorter OS (median OS: 25.9 mo) than those in the low-expression group (median OS: 42.5 mo, P < 0.001; Figure 1D). Univariate analyses of clinical variables considered as potential predictors of survival are shown in Table 2. Further analysis in a multivariate Cox proportional hazards model showed that HOTAIR expression (P = 0.033), along with TNM stage (P = 0.002) and lymph node metastasis (P = 0.002), were strongly associated with OS. These results revealed that HOTAIR expression was an independent prognostic indicator of OS.
| Factor | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| HOTAIR expression | 2.943 | 1.580-5.483 | 0.001 | 1.998 | 1.058-3.772 | 0.033 |
| Tumor invasion depth, T1, T2/above | 2.651 | 1.316-5.341 | 0.006 | 1.676 | 0.691-4.067 | 0.254 |
| Lymphatic metastasis, absent/present | 3.470 | 1.459-8.250 | 0.005 | 4.324 | 1.701-10.993 | 0.002 |
| TNM stage, I/II, III/IV | 4.494 | 2.351-8.592 | < 0.001 | 3.598 | 1.624-7.975 | 0.002 |
| Histology grade, well, moderate/others | 0.967 | 0.535-1.750 | 0.913 | |||
| Age, < 60/> 60 | 0.730 | 0.396-1.344 | 0.312 | |||
| Sex, male/female | 0.822 | 0.428-1.581 | 0.557 | |||
| Tumor location | 0.839 | 0.623-1.131 | 0.250 | |||
| Lauren’s classification | 1.256 | 0.757-2.084 | 0.378 | |||
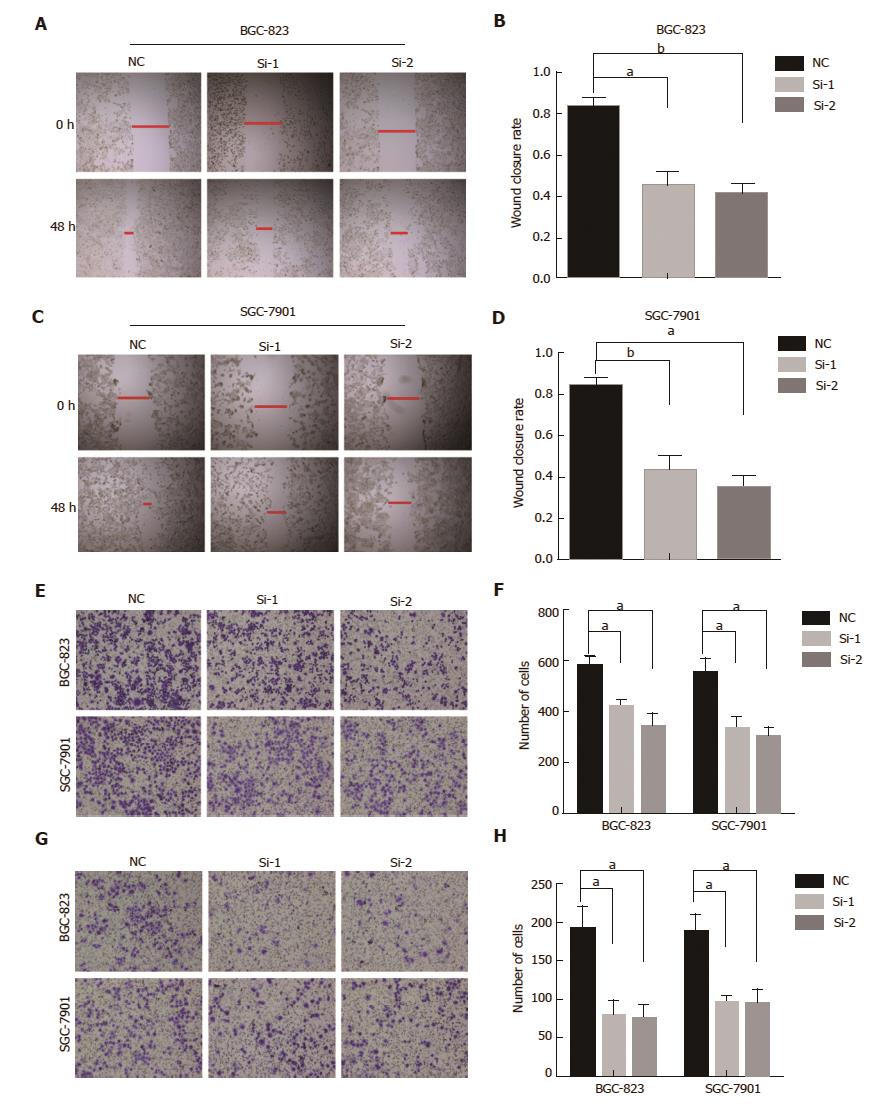
The wound-healing assay showed that HOTAIR knockdown cells were significantly slower than control cells (Figure 2A-D). Transwell assay and invasion assay demonstrated knockdown of HOTAIR notably reduced the number of BGC-823 or SGC-7901 cells migrating across the membrane (Figure 2E-H). These results confirmed that knockdown of HOTAIR inhibited the migration and invasion of GC cells.
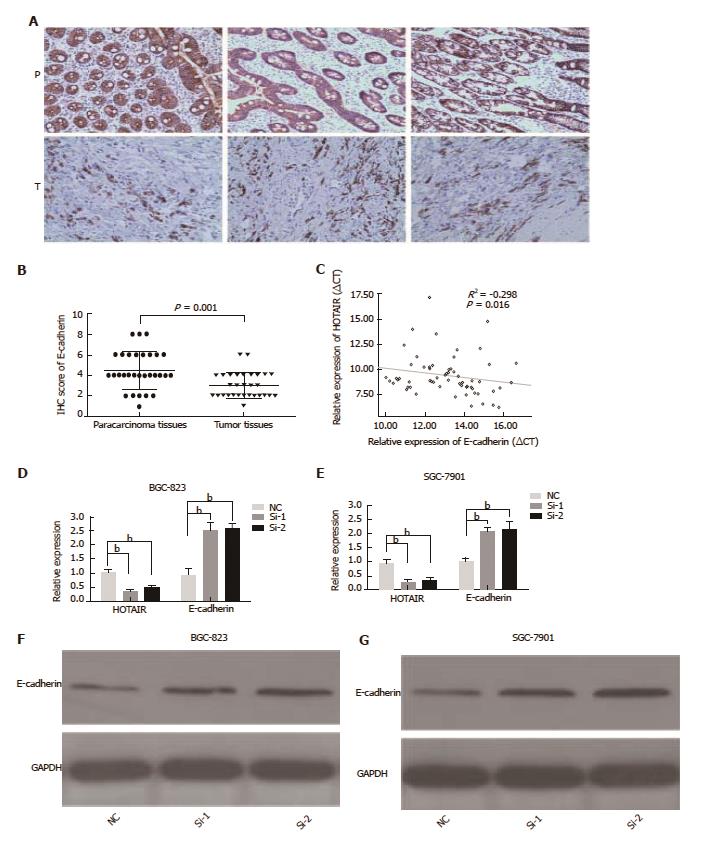
The HOTAIR and E-cadherin expression levels in GC tissues were detected by qPCR and immunohistochemistry. E-cadherin expression was significantly down-regulated in tumor tissues (P = 0.001), as compared with the para-tumorous tissues (Figure 3A and B). E-cadherin mRNA levels were negatively associated with HOTAIR expression in GC tissues (r2 = -0.298, P = 0.016) (Figure 3C). HOTAIR silencing strikingly enhanced the expression of E-cadherin at transcript (Figure 3D and E) and protein (Figure 3F and G) levels in BGC-823 and SGC-7901 cells. These results demonstrated that HOTAIR promoted EMT in GC tissues and cell lines, and that HOTAIR over-expression was significantly associated with migration and invasion.
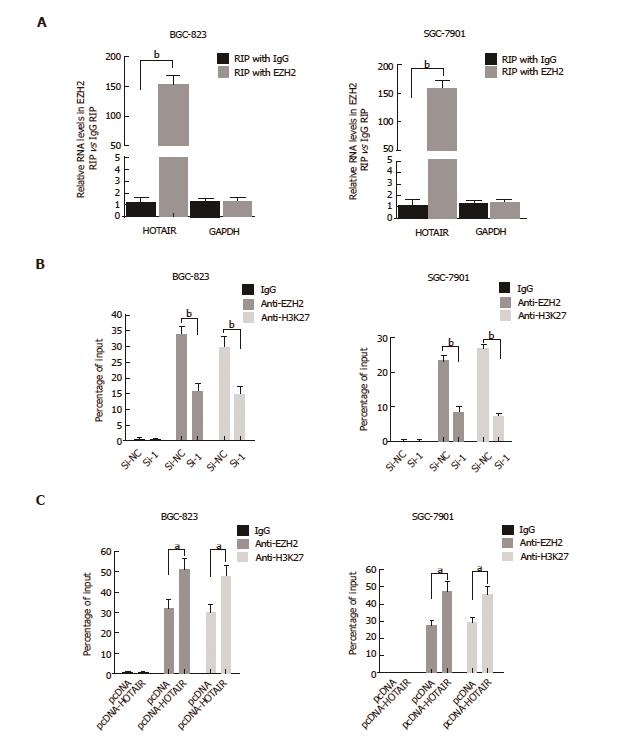
HOTAIR plays its role in the epigenetic regulation of gene expression mainly by binding to PRC2 complex. We firstly used RIP assay to investigate whether HOTAIR could bind to EZH2, which is the core catalytic component of the PRC2. The results showed that endogenous HOTAIR was abundant in the anti-EZH2 RIP fraction (Figure 4A), validating that HOTAIR can bind to EZH2 in GC cells. Then we conducted ChIP assay to determine whether HOTAIR regulates E-adherin via recruiting EZH2 in GC cell lines. ChIP assay demonstrated that knockdown of HOTAIR significantly reduced the binding of EZH2 and H3K27me3 with the E-cadherin promoter in GC cells (Figure 4B). The rescue experiments showed that up-regulation of HOTAIR increased the binding of EZH2 and H3K27me3 with the E-cadherin promoter in GC cells (Figure 4C). These results indicated that HOTAIR inhibits E-cadherin expression partly through combining with EZH2.
LncRNAs are non-protein-coding transcripts > 200 nucleotides. Dysregulation of lncRNAs has been found in GC cells and their aberrant expression is correlated with tumorigenesis, metastasis, prognosis or diagnosis[20,21].
We found that HOTAIR was markedly up-regulated in GC tissues, which was correlated with the invasion of primary tumor, lymph node metastasis, and TNM stage. The ROC curve used for distinguishing between GC tissues and normal tissues showed that HOTAIR is a promising cancer biomarker. Univariate and multivariate Cox regression analyses demonstrated that HOTAIR is a valuable prognostic factor independent of major clinicopathological features. We also found that decreased HOTAIR expression inhibited migration and invasion of tumor cells.
To clarify the molecular mechanism of HOTAIR action contributing to GC metastasis, we also investigated potential target genes involved in cell migration and invasion. Previous studies have revealed that the expression of epithelial markers (such as E-cadherin) are increased following HOTAIR knockdown in GC cells[13,22,23]. Further mechanistic studies have shown that by recruiting and binding to PRC2, HOTAIR could epigenetically silence miR34a expression to promote GC cell EMT and metastasis[13]. However, there are still some issues that need to be clarified. For one thing, E-cadherin is a target gene of EZH2. Because EZH2 can mediate transcriptional silencing of E-cadherin through trimethylation of H3 lysine 27[24,25], and HOTAIR binds to EZH2[14]. Furthermore, a significantly negative correlation was found between HOTAIR and E-cadherin in GC tissues in the present study, and knockdown of EZH2 also up-regulated E-cadherin expression in GC cells as shown by another study[26]. So, why does HOTAIR not regulate expression of E-cadherin directly by binding to EZH2? We speculate that HOTAIR promotes tumor cell migration and invasion by repressing E-cadherin via EZH2 directly. To confirm this, we performed ChIP analysis in HOTAIR-silenced GC cell lines. Not unexpectedly, HOTAIR contributed to the regulation of E-cadherin via recruiting EZH2 and H3K27me3 to the E-cadherin promoter. Our findings provide additional insight into the mechanisms by which HOTAIR promotes GC migration and invasion.
In conclusion, we demonstrated that HOTAIR was significantly up-regulated in GC tissues, and its overexpression was correlated with tumor progression and poor prognosis. We can distinguish between cancerous and non-cancerous lesions based on the expression of HOTAIR. HOTAIR may regulate the invasive ability of GC cells, partially via EMT regulation. Our findings have provided new insight into the GC pathogenesis, which benefits diagnosis and therapy in cancer.
Gastric cancer (GC) is one of the most aggressive malignancies with high morbidity and mortality worldwide. Treatment options are limited due to the lack of knowledge of the molecular and genetic bases of gastric carcinogenesis. A deeper understanding of the molecular mechanisms of GC will shed light on its pathogenesis, and identification of new biomarkers for diagnosis and prognosis may improve individualized treatment strategies in the future.
Emerging evidence suggest that long non-coding (lnc)RNA may play a role in gastric carcinogenesis. HOX transcript antisense intergenic RNA (HOTAIR) is one of the well-documented lncRNAs, which is aberrantly expressed in several tumors and plays a crucial role in cancer development. However, the overall clinical role of HOTAIR in GC and the molecular mechanisms of HOTAIR involved in GC cell metastasis has not yet been well investigated.
The authors demonstrated that HOTAIR promotes tumor cell migration and invasion by repressing E-cadherin via EZH2 directly. Their findings have provided additional insight into the mechanisms of gastric carcinogenesis.
The results from this study provide novel clues for further investigation of HOTAIR as a potential biomarker and therapeutic target for GC.
RNA-binding protein immunoprecipitation assay was used for the analysis of EZH2 interactions with HOTAIR. ChIP assay was performed to investigate direct interactions between EZH2 and E-cadherin.
The study aimed to investigate the clinical significance and the mechanism behind lncRNA HOTAIR in GC. The overall study is solid and well designed. The results are consistent with the proposed hypothesis.
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21462] [Article Influence: 1951.1] [Reference Citation Analysis (6)] |
| 2. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1504] [Article Influence: 150.4] [Reference Citation Analysis (1)] |
| 3. | Lordick F, Allum W, Carneiro F, Mitry E, Tabernero J, Tan P, Van Cutsem E, van de Velde C, Cervantes A. Unmet needs and challenges in gastric cancer: the way forward. Cancer Treat Rev. 2014;40:692-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 4. | Yang K, Choi YY, Zhang WH, Chen XZ, Song MK, Lee J, Zhang B, Chen ZX, Kim HI, Chen JP. Strategies to improve treatment outcome in gastric cancer: a retrospective analysis of patients from two high-volume hospitals in Korea and China. Oncotarget. 2016;7:44660-44675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Dhamija S, Diederichs S. From junk to master regulators of invasion: lncRNA functions in migration, EMT and metastasis. Int J Cancer. 2016;139:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 225] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 6. | Kang Y, Massagué J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 1193] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 7. | Serrano-Gomez SJ, Maziveyi M, Alahari SK. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol Cancer. 2016;15:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 328] [Cited by in RCA: 642] [Article Influence: 64.2] [Reference Citation Analysis (1)] |
| 8. | Guo F, Parker Kerrigan BC, Yang D, Hu L, Shmulevich I, Sood AK, Xue F, Zhang W. Post-transcriptional regulatory network of epithelial-to-mesenchymal and mesenchymal-to-epithelial transitions. J Hematol Oncol. 2014;7:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 9. | Xu Q, Deng F, Qin Y, Zhao Z, Wu Z, Xing Z, Ji A, Wang QJ. Long non-coding RNA regulation of epithelial-mesenchymal transition in cancer metastasis. Cell Death Dis. 2016;7:e2254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Li SP, Xu HX, Yu Y, He JD, Wang Z, Xu YJ, Wang CY, Zhang HM, Zhang RX, Zhang JJ. LncRNA HULC enhances epithelial-mesenchymal transition to promote tumorigenesis and metastasis of hepatocellular carcinoma via the miR-200a-3p/ZEB1 signaling pathway. Oncotarget. 2016;7:42431-42446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 219] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 11. | Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F, Liu Y. TGF-β-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin Cancer Res. 2014;20:1531-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 352] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 12. | Liang WC, Fu WM, Wong CW, Wang Y, Wang WM, Hu GX, Zhang L, Xiao LJ, Wan DC, Zhang JF. The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget. 2015;6:22513-22525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 508] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 13. | Liu YW, Sun M, Xia R, Zhang EB, Liu XH, Zhang ZH, Xu TP, De W, Liu BR, Wang ZX. LincHOTAIR epigenetically silences miR34a by binding to PRC2 to promote the epithelial-to-mesenchymal transition in human gastric cancer. Cell Death Dis. 2015;6:e1802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 14. | Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4202] [Cited by in RCA: 4302] [Article Influence: 268.9] [Reference Citation Analysis (0)] |
| 15. | Chen FJ, Sun M, Li SQ, Wu QQ, Ji L, Liu ZL, Zhou GZ, Cao G, Jin L, Xie HW. Upregulation of the long non-coding RNA HOTAIR promotes esophageal squamous cell carcinoma metastasis and poor prognosis. Mol Carcinog. 2013;52:908-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 16. | Luo ZF, Zhao D, Li XQ, Cui YX, Ma N, Lu CX, Liu MY, Zhou Y. Clinical significance of HOTAIR expression in colon cancer. World J Gastroenterol. 2016;22:5254-5259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2806] [Cited by in RCA: 2945] [Article Influence: 122.7] [Reference Citation Analysis (5)] |
| 18. | Chang JT, Chen IH, Liao CT, Wang HM, Hsu YM, Hung KF, Lin CJ, Hsieh LL, Cheng AJ. A reverse transcription comparative real-time PCR method for quantitative detection of angiogenic growth factors in head and neck cancer patients. Clin Biochem. 2002;35:591-596. [PubMed] |
| 19. | Sun M, Liu XH, Li JH, Yang JS, Zhang EB, Yin DD, Liu ZL, Zhou J, Ding Y, Li SQ. MiR-196a is upregulated in gastric cancer and promotes cell proliferation by downregulating p27(kip1). Mol Cancer Ther. 2012;11:842-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Riquelme I, Ili C, Roa JC, Brebi P. Long non-coding RNAs in gastric cancer: mechanisms and potential applications. Oncotarget. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Li T, Mo X, Fu L, Xiao B, Guo J. Molecular mechanisms of long noncoding RNAs on gastric cancer. Oncotarget. 2016;7:8601-8612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 251] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 22. | Xu ZY, Yu QM, Du YA, Yang LT, Dong RZ, Huang L, Yu PF, Cheng XD. Knockdown of long non-coding RNA HOTAIR suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int J Biol Sci. 2013;9:587-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 23. | Lee NK, Lee JH, Park CH, Yu D, Lee YC, Cheong JH, Noh SH, Lee SK. Long non-coding RNA HOTAIR promotes carcinogenesis and invasion of gastric adenocarcinoma. Biochem Biophys Res Commun. 2014;451:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Han T, Jiao F, Hu H, Yuan C, Wang L, Jin ZL, Song WF, Wang LW. EZH2 promotes cell migration and invasion but not alters cell proliferation by suppressing E-cadherin, partly through association with MALAT-1 in pancreatic cancer. Oncotarget. 2016;7:11194-11207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 25. | Liu L, Xu Z, Zhong L, Wang H, Jiang S, Long Q, Xu J, Guo J. Enhancer of zeste homolog 2 (EZH2) promotes tumour cell migration and invasion via epigenetic repression of E-cadherin in renal cell carcinoma. BJU Int. 2016;117:351-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Wang YJ, Liu JZ, Lv P, Dang Y, Gao JY, Wang Y. Long non-coding RNA CCAT2 promotes gastric cancer proliferation and invasion by regulating the E-cadherin and LATS2. Am J Cancer Res. 2016;6:2651-2660. [PubMed] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Wei D, Testini M S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Xu XR