Published online Aug 28, 2017. doi: 10.3748/wjg.v23.i32.5962
Peer-review started: February 28, 2017
First decision: April 11, 2017
Revised: May 10, 2017
Accepted: June 9, 2017
Article in press: July 9, 2017
Published online: August 28, 2017
Processing time: 182 Days and 22.7 Hours
To evaluate the accuracy of the elastography score combined to the strain ratio in the diagnosis of solid pancreatic lesions (SPL).
A total of 172 patients with SPL identified by endoscopic ultrasound were enrolled in the study to evaluate the efficacy of elastography and strain ratio in differentiating malignant from benign lesions. The semi quantitative score of elastography was represented by the strain ratio method. Two areas were selected, area (A) representing the region of interest and area (B) representing the normal area. Area (B) was then divided by area (A). Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated by comparing diagnoses made by elastography, strain ratio and final diagnoses.
SPL were shown to be benign in 49 patients and malignant in 123 patients. Elastography alone had a sensitivity of 99%, a specificity of 63%, and an accuracy of 88%, a PPV of 87% and an NPV of 96%. The best cut-off level of strain ratio to obtain the maximal area under the curve was 7.8 with a sensitivity of 92%, specificity of 77%, PPV of 91%, NPV of 80% and an accuracy of 88%. Another estimated cut off strain ratio level of 3.8 had a higher sensitivity of 99% and NPV of 96%, but with less specificity, PPV and accuracy 53%, 84% and 86%, respectively. Adding both elastography to strain ratio resulted in a sensitivity of 98%, specificity of 77%, PPV of 91%, NPV of 95% and accuracy of 92% for the diagnosis of SPL.
Combining elastography to strain ratio increases the accuracy of the differentiation of benign from malignant SPL.
Core tip: This prospective study included 172 patients with solid pancreatic lesions (SPL) to evaluate the value of combining the elastography score to strain ratio for differentiating benign from malignant lesions. Adding both elastography to strain ratio resulted in a sensitivity of 98%, specificity of 77%, positive predictive value (PPV) of 91%, negative predictive value (NPV) of 95% and accuracy of 92% for the diagnosis of SPL. The best cut-off level of strain ratio was 7.8 with a sensitivity of 92%, specificity of 77%, PPV of 91%, NPV of 80% and an accuracy of 88%. So, adding both diagnostic tools increases the yielding of diagnosis.
- Citation: Okasha H, Elkholy S, El-Sayed R, Wifi MN, El-Nady M, El-Nabawi W, El-Dayem WA, Radwan MI, Farag A, El-sherif Y, Al-Gemeie E, Salman A, El-Sherbiny M, El-Mazny A, Mahdy RE. Real time endoscopic ultrasound elastography and strain ratio in the diagnosis of solid pancreatic lesions. World J Gastroenterol 2017; 23(32): 5962-5968
- URL: https://www.wjgnet.com/1007-9327/full/v23/i32/5962.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i32.5962
Solid pancreatic lesions (SPL) are mostly malignant with 5-year survival rates of less than 5%[1]. Endoscopic ultrasound guided fine needle aspiration (EUS-FNA) is a very good tool for the diagnosis of malignant SPL, with sensitivity and specificity rates of 91% and 94%, respectively[2,3], but it shows false negative results in approximately 15% of cases[4]. Strict follow up for negative FNA lesions is mandatory and may necessitate the use of invasive techniques to reach a full diagnosis, such as diagnostic laparoscopy[5].
The elastic properties of the tissues were used to assist in diagnosis by comparing color images in the B mode before and after compression[6,7]. This was used in endosonography to calculate the elastography of the lesion without using other invasive techniques[8,9]. A 5-scored system was developed by Giovannini et al[10] and colleagues to distinguish between benign and malignant lesions, yet it was very subjective. Then, the strain ratio was developed as a semi quantitative method by dividing the area of interest by the normal tissue to improve objectivity and reach a better diagnosis[11].
In this prospective study, we investigate the efficacy of endosonographic elastography and strain ratio for the differentiation of benign from malignant lesions.
Patients with SPL identified by EUS were enrolled in this prospective study. It included patients that were referred to the endoscopy units of both Cairo and Zagazig University Hospitals for endosonographic evaluation. The inclusion criteria were as follows: patients with identified SPL from prior radiological imaging; patients with extrahepatic biliary obstruction showing negative imaging results and referred for EUS; and patients above 18 years old. The exclusion criteria included: patients who declined to participate in the study, patients with a contraindication to the procedure, such as patients unfit for propofol sedation or coagulopathy, and patients lost to follow up or in whom the final diagnosis could not be reached. The ethical committee approved the study protocol and informed consents were obtained from all patients prior to the procedure.
The study was designed as a prospective study to evaluate the efficacy of elastography and strain ratio in diagnosing SPL. Eligible patients who agreed to participate in the study were appointed to the endoscopy room on the day of the procedure for EUS examination under conscious sedation with IV propofol administration. An EUS examination was performed on all patients with a linear Echoendoscope Pentax EG3830UT (HOYA Corporation, PENTAX Lifecare Division, Showanomori Technology Center, Tokyo, Japan) connected to a Hitachi EUB-7000 HV ultrasound unit (Hitachi Medical Systems, Tokyo, Japan). All examinations were performed by one endosonographer. For EUS-FNA biopsies, we used the Cook needle 22G (Echotip®; Wilson-Cook, Winston Salem, NC, United States). Elastography was applied to evaluate the SPL. Elastography is the sound wave technique to measure tissue deformation in response to compression. Theoretically, malignant lesions are harder than inflammatory ones. The hardness of the lesion is reflected by the degree of deformation represented by a color map (red-green-blue colors represent soft to hard tissue, respectively). Quantitative scores and strain ratios were determined during the procedure. EUS-FNA was performed after the elastography.
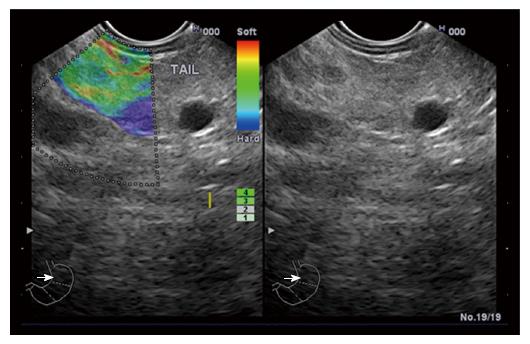
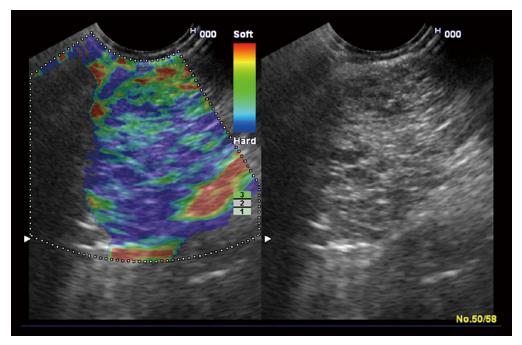
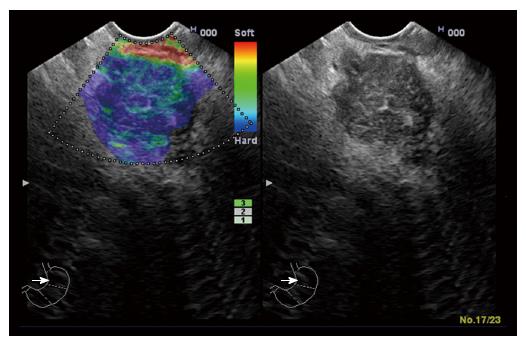
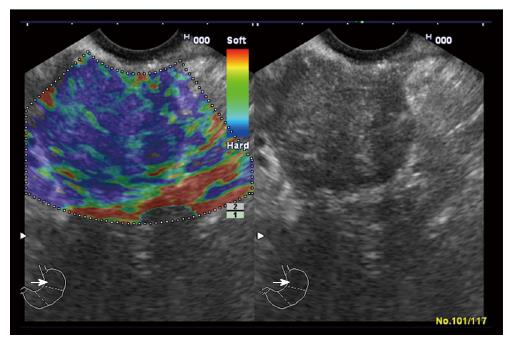
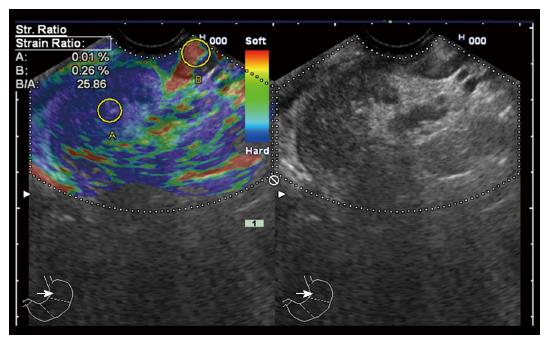
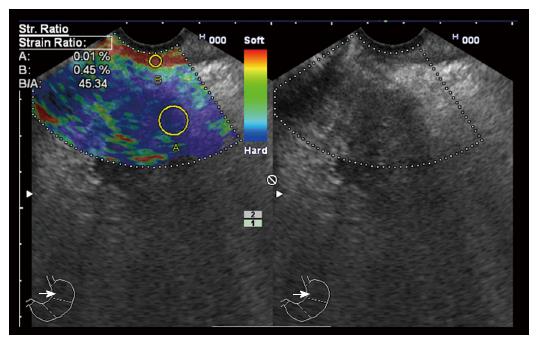
‘‘Elastic score’’ reported by Giovannini et al[10] was used. A score of 1 was defined as homogeneous soft tissue (green) and interpreted as normal tissue. A score of 2 was given to heterogeneous soft tissue (green, yellow, and red), and interpreted as fibrosis or inflammation as shown in Figure 1. A score of 3 represented mixed hard and soft tissues (mixed colors) or a honeycombed elastography pattern, interpreted as indeterminate for malignancy as shown in Figures 2 and 3. A score of 4 was given for hard (blue) lesions with a soft (green) central area, interpreted as malignant, hypervascularized lesions. Finally, a score of 5 represents predominantly hard (blue) lesions with dispersed heterogenic soft (green, red) areas, interpreted as advanced malignant lesions with necrotic areas as shown in Figure 4.
The semi quantitative score of elastography was represented by the strain ratio method. Two areas were selected, area (A) representing the region of interest and area (B) representing the normal area. Area (B) was then divided by area (A). For pancreatic lesions with a homogeneous pattern of elasticity, area A was chosen from any region, but in heterogeneous regions, area A was chosen to cover as much heterogeneous area as possible. Both areas were manually selected by these criteria. The means of strain ratios were calculated and used as final results for each patient as shown in Figures 5 and 6. Subsequently, the best cut-off value was selected from the receiver operating characteristic (ROC) curve and was used for the calculation of diagnostic value. The best cut-off value of strain ratio was also combined with the results of elastography for the calculation of diagnostic value.
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated by comparing diagnoses made by elastography, strain ratio and final diagnoses.
The final diagnosis of the SPL was obtained from the positive cytopathological examination of aspirate taken by EUS-FNA, the excisional biopsy of surgically removed tumors, and the presence of metastases or the follow up of benign lesions for at least one year.
From January 2013 to April 2016, 172 patients with pancreatic lesions were enrolled in this study. There were 120 males and 52 females with mean age of 55.7 years. The site, final diagnosis of pancreatic lesions, and elastography score are presented in Tables 1-3.
| Location of pancreatic lesions | Number of cases = 172 |
| Head of the pancreas | 118 |
| Uncinate process | 7 |
| Body of the pancreas | 22 |
| Tail of the pancreas | 4 |
| Diffuse involvement (pan-pancreatic lesion) | 21 |
| Nature of the lesion | Final diagnosis | Number of cases = 172 |
| Benign lesions | Pancreatitis | 49 |
| (49 cases) | -Chronic pancreatitis | 40 |
| -Autoimmune pancreatitis | 9 | |
| Malignant lesions | Ductal adenocarcinoma | 97 |
| (123 cases) | Mucinous neoplasm | 22 |
| Neuroendocrine tumors | 2 | |
| Lymphoma | 1 | |
| Metastasis | 1 |
| Diagnosis (n = 172) | Score 1 | Score 2 | Score 3 | Score 4 | Score 5 |
| Pancreatitis | 6 | 25 | 12 | - | 6 |
| Chronic pancreatitis | 6 | 21 | 8 | - | 5 |
| Autoimmune pancreatitis | - | 4 | 4 | - | 1 |
| Ductal adenocarcinoma | - | - | 28 | - | 69 |
| Mucinous neoplasm | - | - | 3 | - | 19 |
| Neuroendocrine tumors | - | - | 1 | - | 1 |
| Lymphoma | - | - | 1 | - | |
| Metastasis | - | - | - | - | 1 |
Scores 1 and 2 were considered benign while scores 3 to 5 were considered malignant. Elastography alone had a sensitivity of 99%, specificity of 63%, PPV of 87%, NPV of 96%, and accuracy of 88% (Table 4).
| Elasticity score | SR 7.8 | SR 3.8 | Elasticity score and SR 7.8 | |
| Sensitivity | 99% | 92% | 99% | 98% |
| Specificity | 63% | 77% | 53% | 77% |
| PPV | 87% | 91% | 84% | 91% |
| NPV | 96% | 80% | 96% | 95% |
| Accuracy | 88% | 88% | 86% | 92% |
The mean value of the strain ratio for benign lesions is 5.58 while the mean value for malignancy is 31.25; this difference was statistically significant at a p value of 0.01.
Based on the results of the ROC curve that was used for analysis, the best cut-off level of strain ratio to obtain the maximal area under the curve was 7.8 with a sensitivity of 92%, specificity of 77%, PPV of 91%, NPV of 80% and accuracy of 88%. Another cut off level of strain ratio was calculated at a level of 3.8 and demonstrated very high sensitivity (99%) and NPV (96%), but less specificity (53%), PPV (84%), and accuracy (86%). Adding elastography to strain ratio resulted in a sensitivity of 98%, specificity of 77%, PPV of 91%, NPV of 95% and accuracy of 92% for the diagnosis of SPL (Table 4).
The percentage of benign SPL in our study is 28%, which is similar to a study carried out by Pradermchai Kongkam and colleagues[12] that reported a percentage of 23 and is similar to a meta-analysis that presented a close figure of 26.5%[13].
The diagnostic value of EUS-FNA has always been questioned due to the high false negative rates encountered; these rates can reach up to 15%-17%[2,5]. These false negative findings are manifested mostly in focal lesions in patients with chronic pancreatitis due to a similar hypoechoic pattern when compared to the surrounding area[14].
EUS-FNA also has many drawbacks, including the need for multiple needle passes to obtain an adequate sample, iatrogenic complications[15], a learning curve and the need to evaluate many cases to obtain better efficacy.
These drawbacks raised the need to develop other techniques for the diagnosis of SPL with fewer complications and better efficacy. Dawwas and colleagues reported a sensitivity of 100% for EUS elastography but with a very low specificity of 16.7%[16]. This was in contrast to previous published studies[17,18] and was not in concordance with our study that showed a specificity of 63%. Still, a problem appeared when using the elastic score due to its subjectivity. In our study, 36.7% (18/49) of patients with chronic pancreatitis had scores of 3 and 5 which is supposed to indicate malignancy. This may be attributed to the presence of calcifications and fibrous strands, which increases the score. Additionally, 6 patients out of 40 with chronic pancreatitis scored 1 although this score is supposed to reflect normal pancreatic tissue. Considering that chronic pancreatitis is a well-known and established risk factor for the development of pancreatic cancer[19], SPL in patients with chronic pancreatitis is a worrisome feature that may indicate the development of malignancy on top of a chronic inflammatory condition. In a study of 373 patients with chronic pancreatitis, 4 of them developed pancreatic malignancy after a follow up period of 2 years[20]. Fifty percent of neuroendocrine tumors scored 2 instead of 4 and 71% of ductal adenocarcinomas had scores of 4 instead of 3 according to the scale. This was similar to a study published by Itokawa and colleagues in which only 33% of neuroendocrine tumors were scored 4 and 22% had a score of 1[9]. In our study, the 2 cases with neuroendocrine tumors were scored as 3 and 5 and not 4, which may explain why none of our cases had an elasticity score of 4. In a study done by Giovannini et al[21]. Sixteen point one of the lesions that had scores of 1 or 2 were adenocarcinoma. This renders elastography less specific although it has high sensitivity in our study sensitivity was 99% despite low specificity (63%).
As an elastography score is a very subjective tool and depends on the operator in most of the cases, another tool was added to increase its specificity to reach a better diagnosis[22-24]. The strain ratio with different cut off levels was mentioned in many studies[16,17,21]. We had a cut off level of 3.8 that had a sensitivity, specificity, PPV, NPV and accuracy of 99%, 53%, 84%, 86% and 96%, respectively. This was similar to the study done by Pradermchai Kongkam and colleagues[12] that identified a cut off value of 3.17 that gave a better specificity of 66.7%, but lower values in sensitivity, PPV, NPV, and accuracy 86.2%, 89.3%, 60%, and 81.6%, respectively. In our study, the best cut off value to differentiate benign from malignant SPL was 7.8, it has a sensitivity of 92%, specificity of 77%, PPV of 91%, NPV of 80% and accuracy of 88%.
Other studies have analyzed the usefulness of quantitative EUS-elastography. Iglesias-Garcia et al[25] published the strain ratio results of 86 consecutive patients with pancreatic solid lesions (49 adenocarcinomas, 27 inflammatory masses, 6 malignant neuroendocrine tumors, 2 metastatic oat cell lung cancers, 1 pancreatic lymphoma, and 1 pancreatic solid pseudopapillary tumor) and 20 controls. The strain ratio was significantly higher among patients with malignant pancreatic tumors than those with inflammatory masses. Normal pancreatic tissue showed a mean strain ratio of 1.68 (95%CI: 1.59-1.78). Inflammatory masses exhibited a strain ratio (mean 3.28; 95%CI: 2.61-3.96) that was significantly higher than that of the normal pancreas (P < 0.001), but lower than that of pancreatic adenocarcinoma (mean 18.12; 95%CI: 16.03-20.21) (P < 0.001). The highest strain ratio was found among endocrine tumors (mean 52.34; 95%CI: 33.96-70.71). The sensitivity and specificity of the strain ratio for the detection of pancreatic malignancies with a cut-off value of 6.04 were 100% and 92.9%, respectively, exceeding the accuracy obtained with qualitative elastography. Another publication retrospectively evaluated 109 patients with solid pancreatic masses using the same methodology. A total of 20 patients were diagnosed with chronic pancreatitis (6 without and 7 with focal inflammatory masses, and 7 with autoimmune pancreatitis), 72 were diagnosis with pancreatic cancer, 9 with pancreatic neuroendocrine tumors, and 8 with a normal pancreas. In the qualitative evaluation, all pancreatic cancers showed an intense blue coloration, whereas the inflammatory masses presented mixed colorations (green, yellow, and low-intensity blue). The mean strain ratio was 23.66 ± 12.65 for the inflammatory masses and 39.08 ± 20.54 for pancreatic cancer (P < 0.05)[9].
To increase the efficacy of the diagnosis of SPL, we combined elastography with the strain ratio level of 7.8 to have a sensitivity of 98%, a specificity of 77%, an accuracy of 92%, a PPV of 91% and an NPV of 95% and increased the accuracy compared to the use of each tool alone.
SPL should be investigated thoroughly to identify their type. The use of elastography combined with strain ratio increases the accuracy of differentiation between malignant and benign SPL.
Different real time elasticity scores were developed to distinguish between benign and malignant lesions, yet they are very subjective, which is an important drawback. Strain ratio is a semi-quantitative method developed by dividing the area of interest by the normal tissue to improve objectivity and reach a better diagnosis.
Accurate diagnosis of the nature of pancreatic masses aids a lot in the proper management. In this study, there is a suggestion that adding strain ratio to elastography increase the accuracy of diagnosis.
The literature suggests that adding strain ration to elastography score would add to proper diagnosis and differentiation of pancreatic masses. This study suggests a new cut off value for strain ratio to differentiate between benign and malignant pancreatic lesions being 7.8.
The study adds additional evidence of using two non-invasive techniques being elastography score and strain ration for diagnosis solid pancreatic masses.
Strain ratio: a quantitative method for proper diagnosing of the nature of lesions, calculated by dividing the area of interest by the normal tissue.
The authors have performed a good study, the manuscript is interesting.
| 1. | Gong Z, Holly EA, Bracci PM. Survival in population-based pancreatic cancer patients: San Francisco Bay area, 1995-1999. Am J Epidemiol. 2011;174:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 514] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 3. | Bhutani MS, Koduru P, Joshi V, Saxena P, Suzuki R, Irisawa A, Yamao K. The role of endoscopic ultrasound in pancreatic cancer screening. Endosc Ultrasound. 2016;5:8-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Hartwig W, Schneider L, Diener MK, Bergmann F, Büchler MW, Werner J. Preoperative tissue diagnosis for tumours of the pancreas. Br J Surg. 2009;96:5-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Yamabe A, Irisawa A, Bhutani MS, Shibukawa G, Fujisawa M, Sato A, Yoshida Y, Arakawa N, Ikeda T, Igarashi R. Efforts to improve the diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic tumors. Endosc Ultrasound. 2016;5:225-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Frey H. [Realtime elastography. A new ultrasound procedure for the reconstruction of tissue elasticity]. Radiologe. 2003;43:850-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Dietrich CF, Jenssen C, Herth FJ. Endobronchial ultrasound elastography. Endosc Ultrasound. 2016;5:233-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Guzman A, Valle RD, Bravo G, Robles-Medranda C. Endoscopic ultrasound elastography in the diagnosis of incidental malignant mesothelioma. Endosc Ultrasound. 2016;5:63-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Itokawa F, Itoi T, Sofuni A, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Umeda J, Tanaka R. EUS elastography combined with the strain ratio of tissue elasticity for diagnosis of solid pancreatic masses. J Gastroenterol. 2011;46:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Giovannini M, Hookey LC, Bories E, Pesenti C, Monges G, Delpero JR. Endoscopic ultrasound elastography: the first step towards virtual biopsy? Preliminary results in 49 patients. Endoscopy. 2006;38:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 216] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Nemakayala D, Patel P, Rahimi E, Fallon MB, Thosani N. Use of quantitative endoscopic ultrasound elastography for diagnosis of pancreatic neuroendocrine tumors. Endosc Ultrasound. 2016;5:342-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Kongkam P, Lakananurak N, Navicharern P, Chantarojanasiri T, Aye K, Ridtitid W, Kritisin K, Angsuwatcharakon P, Aniwan S, Pittayanon R. Combination of EUS-FNA and elastography (strain ratio) to exclude malignant solid pancreatic lesions: A prospective single-blinded study. J Gastroenterol Hepatol. 2015;30:1683-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Mei M, Ni J, Liu D, Jin P, Sun L. EUS elastography for diagnosis of solid pancreatic masses: a meta-analysis. Gastrointest Endosc. 2013;77:578-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Fritscher-Ravens A, Brand L, Knöfel WT, Bobrowski C, Topalidis T, Thonke F, de Werth A, Soehendra N. Comparison of endoscopic ultrasound-guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. Am J Gastroenterol. 2002;97:2768-2775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 169] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Eloubeidi MA, Tamhane A, Varadarajulu S, Wilcox CM. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc. 2006;63:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 16. | Dawwas MF, Taha H, Leeds JS, Nayar MK, Oppong KW. Diagnostic accuracy of quantitative EUS elastography for discriminating malignant from benign solid pancreatic masses: a prospective, single-center study. Gastrointest Endosc. 2012;76:953-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. Quantitative endoscopic ultrasound elastography: an accurate method for the differentiation of solid pancreatic masses. Gastroenterology. 2010;139:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 206] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 18. | Giovannini M. Endoscopic ultrasound elastography. Pancreatology. 2011;11 Suppl 2:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 439] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 20. | Malka D, Hammel P, Maire F, Rufat P, Madeira I, Pessione F, Lévy P, Ruszniewski P. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002;51:849-852. [PubMed] |
| 21. | Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, Geneviève M, Paolo A, Pierre D, Robert Y. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587-1593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 204] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 22. | Rahimi E, Younes M, Zhang S, Zhang S, Thosani N. Endoscopic ultrasound elastography to diagnose sarcoidosis. Endosc Ultrasound. 2016;5:212-214. |
| 23. | Soares JB, Iglesias-Garcia J, Goncalves B, Lindkvist B, Lariño-Noia J, Bastos P, Caetano AC, Ferreira A, Pimentel-Nunes P, Lopes L. Interobserver agreement of EUS elastography in the evaluation of solid pancreatic lesions. Endosc Ultrasound. 2015;4:244-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Dietrich CF, Jenssen C, Arcidiacono PG, Cui XW, Giovannini M, Hocke M, Iglesias-Garcia J, Saftoiu A, Sun S, Chiorean L. Endoscopic ultrasound: Elastographic lymph node evaluation. Endosc Ultrasound. 2015;4:176-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Iglesias-Garcia J, Lindkvist B, Lariño-Noia J, Domínguez-Muñoz JE. Endoscopic ultrasound elastography. Endosc Ultrasound. 2012;1:8-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Egypt
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Sato H, Raisch KP S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF