Published online Aug 21, 2017. doi: 10.3748/wjg.v23.i31.5700
Peer-review started: February 22, 2017
First decision: March 3, 2017
Revised: March 30, 2017
Accepted: April 21, 2017
Article in press: April 21, 2017
Published online: August 21, 2017
Processing time: 184 Days and 7.4 Hours
To elucidate the impact of Schistosoma (S.) japonicum infection on inflammatory bowel disease by studying the effects of exposure to S. japonicum cercariae on dextran sodium sulfate (DSS)-induced colitis.
Infection was percutaneously established with 20 ± 2 cercariae of S. japonicum, and colitis was induced by administration of 3% DSS at 4 wk post infection. Weight change, colon length, histological score (HS) and disease activity index (DAI) were evaluated. Inflammatory cytokines, such as IL-2, IL-10 and IFN-γ, were tested by a cytometric bead array and real-time quantitative polymerase chain reaction (RT-PCR). Protein and mRNA levels of IRE1α, IRE1β, GRP78, CHOP, P65, P-P65, P-IκBα and IκBα in colon tissues were examined by Western blot and RT-PCR, respectively. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling positive cells, cleaved-caspase 3 expression and Bcl2/Bax were investigated to assess the apoptosis in colon tissues.
Mice infected with S. japonicum cercariae were less susceptible to DSS. Mice infected with S. japonicum cercariae and treated with DSS showed decreased weight loss, longer colon, and lower HS and DAI compared with mice treated with DSS alone. A substantial decrease in Th1/Th2/Th17 response was observed after infection with S. japonicum. Endoplasmic reticulum (ER) stress and the nuclear factor-kappa B (NF-κB) pathway were reduced in mice infected with S. japonicum cercariae and treated with DSS, along with ameliorated celluar apoptosis, in contrast to mice treated with DSS alone.
Exposure to S. japonicum attenuated inflammatory response in a DSS-induced colitis model. In addition to the Th1/Th2/Th17 pathway and NF-κB pathway, ER stress was shown to be involved in mitigating inflammation and decreasing apoptosis. Thus, ER stress is a new aspect in elucidating the relationship between helminth infection and inflammatory bowel disease (IBD), which may offer new therapeutic methods for IBD.
Core tip:Schistosoma (S.) japonicum has been demonstrated to participate in the development of colitis in animal experiments as well as clinical trials. However, the effects of Schistosoma infection on colitis and the underlying mechanism are still elusive. Here, we studied the effects of exposure to S. japonicum cercariae on dextran sodium sulfate (DSS)-induced colitis. We found that S. japonicum attenuated DSS-induced colitis in mice by reducing inflammatory response and apoptosis in colon tissues. Besides Th1/Th2/Th17 pathway and nuclear factor-kappa B pathway, endoplasmic reticulum stress played an important role in the preventive effects of parasite infection on DSS-induced colitis.
- Citation: Liu Y, Ye Q, Liu YL, Kang J, Chen Y, Dong WG. Schistosoma japonicum attenuates dextran sodium sulfate-induced colitis in mice via reduction of endoplasmic reticulum stress. World J Gastroenterol 2017; 23(31): 5700-5712
- URL: https://www.wjgnet.com/1007-9327/full/v23/i31/5700.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i31.5700
Inflammatory bowel disease (IBD), which involves chronic and complicated inflammatory lesions of the intestinal tract, includes ulcerative colitis (UC) and Crohn’s disease (CD)[1,2]. Epidemiological investigations have shown that the incidence and morbidity of IBD are increasing worldwide[3,4]. However, the mechanism of IBD is still unclear, and the environment, individual genetics, infection and host immunity are involved[1,5]. Elucidating the exact mechanism of IBD and developing effective treatments will be valuable for ameliorating patients’ quality of life.
Exposures to infectious agents (such as parasites) are suggested to have fundamental effects on the development and behavior of the immune system, which may decrease the incidence of both allergic and autoimmune diseases[6]. Thus, several studies were performed to investigate the effects of helminth infections on IBD. Patients with UC or CD showed significant disease remission after administration of viable and embryonated eggs of T. suis (the porcine whipworm), suggesting the therapeutic effects of parasites on IBD[7,8]. Seven of nine Crohn’s patients infected with larvae of Necator, the human hookworm, showed an improved disease score, and their inflammatory cytokine (IFN-γ and IL-17) responses in duodenal biopsies were reduced compared to those of placebo-treated patients[9,10]. In addition, a mouse model of experimental colitis induced with trinitrobenzene sulfonic acid (TNBS) or dextran sodium sulfate (DSS) also showed decreased susceptibility to IBD or attenuated symptoms after treatment with eggs or larvae of Schistosoma (S.)[11-13]. However, infection with S. mansoni soluble egg antigen had no effect on mice with DSS–induced colitis[14]. Moreover, Smith et al [15] demonstrated that mice with colitis induced with DSS displayed worsening results after injection of S. mansoni eggs. These dissimilar outcomes of the impact of Schistosoma on IBD have yet to be investigated since the underlying mechanism is still unclear.
Here, we infected mice with S. japonicum cercariae via contact with the abdomen skin, which is a classical and natural method to infect hosts, such as mice[16]. Then, we investigated the effects of S. japonicum infection on the relatively simple and replicable DSS-induced colitis model due to the limitations of human clinical trials. DSS-induced colitis has been widely used as an animal model of experimental IBD, especially UC, due to its similarities to IBD characteristics, such as diarrhea, mucosal ulceration, rectal bleeding and body weight loss[17-19]. In the present study, we aimed to explore the effects of exposure to S. japonicum cercariae on DSS-induced colitis and identify the underlying pathogenesis of IBD.
All animal experiments during this research were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of Wuhan University.
Oncomelania hupensis snails infected with S. japonicum were obtained from the Institute of Parasitic Disease Control and Prevention, Jiangsu Province, China. Male Kunming mice, weighing 14-16 g, were purchased from the Hubei Provincial Center for Disease Control.
DSS for inducing acute colitis was purchased from MP Biochemicals (Solon, OH, United States). Antibodies for immunofluorescence, Western blot and cytokine tests were purchased from Abcam (Cambridge, MA, United States), Bioworld Technology (Minneapolis, MN, United States), Millipore (Billerica, MA, United States), Santa Cruz Biotechnology (Santa Cruz, CA, United States) and Cell Signaling Technology (Danvers, MA, United States).
Forty mice were randomly divided into four groups with ten mice in each group. The mice only treated with DSS were termed the DSS group, and those only infected with cercariae were called the CER group. The mice in the CER + DSS group were infected with cercariae and subsequently treated with DSS. In the control group, the mice were treated with saline instead.
The infected snails were illuminated to induce cercarial shedding. The mice from the CER group and CER + DSS group were percutaneously infected with 20 ± 2 cercariae of S. japonicum, while those in the control group and DSS group were treated with saline by dermal contact.
After 4 wk of the infection, the mice in the CER + DSS group and DSS group were fed 3% (wt/vol) DSS freely to induce acute colitis. The status of each mouse, including weight, morbidity, stool properties, and bleeding, was recorded daily. Disease activity index (DAI) was assessed as described in Table 1[20].
| Score | Body weight loss | Stool consistency | Rectal bleeding |
| 0 | 0%-1% | Normal | None |
| 1 | 1%-5% | Soft and shaped | Between |
| 2 | 5%-10% | Loose | Slight |
| 3 | 10%-15% | Between | Between |
| 4 | > 15% | Diarrhea | Gross bleeding |
All mice were sacrificed on day 7 after DSS feeding. Blood samples were collected and centrifuged to isolate serum, which was subsequently stored at -80 °C. The entire colon was cut away and gently washed with saline precooled to 4 °C, most of which was dried and frozen in liquid nitrogen immediately for subsequent Western blot or RT-PCR analysis. The distal segment of the colon (approximately 0.5 cm) was fixed in 10% buffered formalin for histological observation.
To assess colon inflammation, the formalin-treated colon was embedded into paraffin. Serial sections with 5 mm thickness were cut and stained with hematoxylin and eosin (H and E, Richard Allen Scientific, Kalamazoo, MI, United States). Histological score (HS) was calculated in a blinded manner three times as shown in Table 2[21]. CD3 and Ly6G monoclonal antibodies were used to assess the infiltration of inflammatory cells. Cleaved caspase 3 (C-C3) was tested to identify the cellular apoptosis in colon tissues. All digital images were taken using a fluorescence microscope (Olympus DX51, Olympus, Tokyo, Japan) and processed with a digital image analysis system (Image-Pro Plus version 6.0, Media Cybernetics, Bethesda, MD, United States). Then the original pictures were merged and the positive cells were quantified. Data were obtained from 3 or more mice per group.
| Score | Damaged area | Mucodepletion of glands | Tissue damage | Inflammatory cell infiltration |
| 0 | N/A | None | No mucosal damage | Occasional inflammatory cells in the lamina propria |
| 1 | ≤ 25% | Mild | Discrete epithelial lesions | Increased numbers of inflammatory cells in the lamina propria |
| 2 | ≤ 50% | Moderate | Surface mucosal erosion or focal ulceration | Confluence inflammatory cells, extending into the submucosa |
| 3 | ≤ 75% | Moderate | Extensive mucosal damage and extension into deeper structures of the bowel wall | Transmural extension of the infiltrate |
| 4 | ≤ 100% | Severe |
Cytometric bead array (CBA) was used to detect the expression of several inflammatory cytokines (IL-2, IL-4, IL-6, IL-10, IL-17A, TNFα and IFN-γ) in the serum of different groups. Serum samples were assayed with a mouse Th1/Th2/Th17 Cytokine Kit (560484, BD), which included seven specific capture beads. After reacting with the bead mixture for 3 h in a dark environment at room temperature, blood samples were analyzed by flow cytometry (Aira III, BD) to identify the numbers of different positive beads. FCAP Array v3 version 3.0.1 from BD Biosciences was employed to translate the images into data.
The proteins extracted from colon tissues of the mice in different groups were boiled with loading buffer and subjected to SDS-PAGE. Then, they were blotted onto polyvinylidene fluoride membranes (IPVH00010, Millipore), which were then washed and blocked with Tris-buffered saline containing 5% milk and 0.1% Tween-20 for 2 h. The treated membranes were incubated with particular antibodies overnight at 4 °C. An enhanced chemiluminescence reagent (LiDE110, Canon) was used to detect the expression of proteins according to the protocol. All images were analyzed with AlphaEaseFC software, and GAPDH was used as the reference.
Total RNA was obtained from 1 mg of colon tissue using TRIzol reagent (15596-026, Invitrogen) and converted into cDNA using a PrimeScriptTM RT reagent Kit with gDNA eraser (RR047A, TaKaRa). The parameters for RT-PCR amplification were as follows: 95 °C for 1 min, followed by 40 cycles of 95 °C for 15 s, 58 °C for 20 s and 72 °C for 45 s. Primers for RT-PCR are listed in Table 3, and GAPDH was used as the housekeeping gene.
| Primer name | Primer sequence | Product length (bp) | |
| M-GAPDH | Forward | 5’-TGAAGGGTGGAGCCAAAAG-3’ | 227 |
| Reverse | 5’-AGTCTTCTGGGTGGCAGTGAT-3’ | ||
| M-IL-6 | Forward | 5’-CTTCTTGGGACTGATGCTGGT-3’ | 171 |
| Reverse | 5’-CACAACTCTTTTCTCATTTCCACG-3’ | ||
| M-IL-10 | Forward | 5’-TACAGCCGGGAAGACAATAACT-3’ | 142 |
| Reverse | 5’-AGGAGTCGGTTAGCAGTATGTTG-3’ | ||
| M-TNF-α | Forward | 5`-TCCCCAAAGGGATGAGAAGTT-3’ | 298 |
| Reverse | 5’-GAGGAGGTTGACTTTCTCCTGG-3’ | ||
| M-TGF-β | Forward | 5’-AGAGCCCTGGATACCAACTATTG-3’ | 286 |
| Reverse | 5’-TGCGACCCACGTAGTAGACG-3’ | ||
| M-IFN-γ | Forward | 5’-CTCAAGTGGCATAGATGTGGAAG-’ | 250 |
| Reverse | 5’-GACCTCAAACTTGGCAATACTCA-3’ | ||
| M-IL-1b | Forward | 5’-GGGCCTCAAAGGAAAGAATCT-3’ | 195 |
| Reverse | 5’-GAGGTGCTGATGTACCAGTTGG-3’ | ||
| M-IL-17a | Forward | 5’-GTCTTTAACTCCCTTGGCGC-3’ | 136 |
| Reverse | 5’-GGCACTGAGCTTCCCAGATC-3’ | ||
| M-IRE1a | Forward | 5’-ACACACCGACCACCGTATCTC-3’ | 157 |
| Reverse | 5’-GGGTAAGTGATGATGAACGCC-3’ | ||
| M-IRE1b | Forward | 5’-TGGACGGTCCCACAACAGAT-3’ | 140 |
| Reverse | 5’-GGGAGGTTCGTGGTATCCAA-3’ | ||
| M-GRP78 | Forward | 5’-GATGAAATTGTTCTGGTTGGTGG-3’ | 203 |
| Reverse | 5’-AGTGTAAGGGGACAAACATCAAG-3’ | ||
| M-CHOP | Forward | 5’-GGAGCTGGAAGCCTGGTATG-3’ | 285 |
| Reverse | 5’-GGGCACTGACCACTCTGTTTC-3’ | ||
Transferase-mediated dUTP nick-end labeling (TUNEL) (ApopTag Plus In Situ Apoptosis Fluorescein Detection Kit, Millipore) assay was performed to assess cellular apoptosis. Paraffin-embedded tissue sections were treated with protease K solution for 15-30 min at 37 °C after dewaxing and rehydrating. Sections were washed and incubated with TUNEL reaction mixture for 60 min at 37 °C in a moist chamber. Addition of converter-POD for 30 min at 37 °C and substrate solution for 30 min at room temperature was performed sequentially. Fluorescence microscopy was used to analyze the sections, and Image-Pro Plus version 6.0 was adopted to process the digital images. Then the original pictures were merged and the positive cells were quantified. Data obtained from 3 or more mice per group were calculated in a blinded manner.
One-way analysis of variance or Kruskal-Wallis H test was performed to analyze all the data (SPSS 20.0 software). P < 0.05 was considered statistically significant.
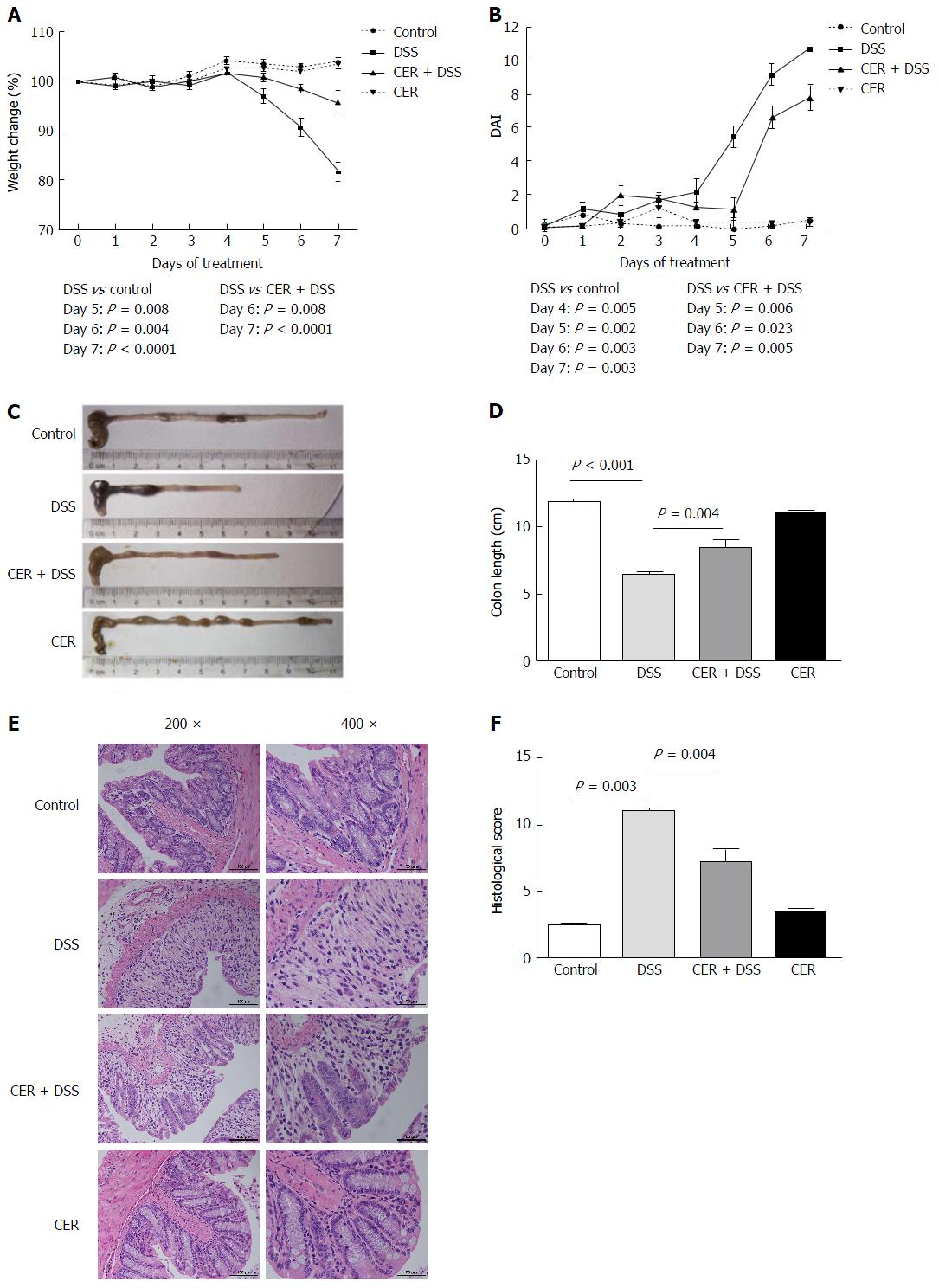
To evaluate the effect of exposure to S. japonicum cercariae on the development of colitis, a chemical model of mucosal inflammation induced by oral administration of 3% DSS was established in mice infected with cercariae for 4 wk. Various indexes, including body weight, DAI, colon length, histological observation and HS, were used to assess the severity of acute colitis in mice.
On the 7th day of DSS treatment, three mice from the DSS group had died due to substantial weight loss (15.4%-19.3%). One of ten mice in the CER + DSS group died on the 5th day due to collision with the cage, and another died for unclear reasons before DSS treatment. From the 5th day, the body weights of mice in both the CER + DSS and DSS groups began to decline, while those in the control and CER groups remained stable. On the 7th day, the mice in the DSS group presented a more significant weight loss than those in the CER + DSS group (Figure 1A; P < 0.0001). In addition, the DAI scores of the mice in the CER + DSS group were lower than those in the DSS group (Figure 1B, P = 0.005). Among the mice in the four groups, the colon length was shortest in DSS mice and shorter in CER + DSS mice (Figure 1C and D; P < 0.001 DSS vs control, P = 0.004 DSS vs CER + DSS). There was no significant difference between control mice and CER mice with regard to colon length. Although evident colon hyperemia and edema were observed in both colon tissues of DSS mice and CER + DSS mice, reduced architecture loss, goblet damage and inflammatory cell infiltration were observed in colon tissues of CER + DSS mice compared to those of DSS mice under a microscope (Figure 1E). No pathological lesions were found in the control and CER groups. According to blind assessments from three independent experimenters, the HS of DSS mice was much larger than that of CER + DSS mice (Figure 1F; P = 0.004).
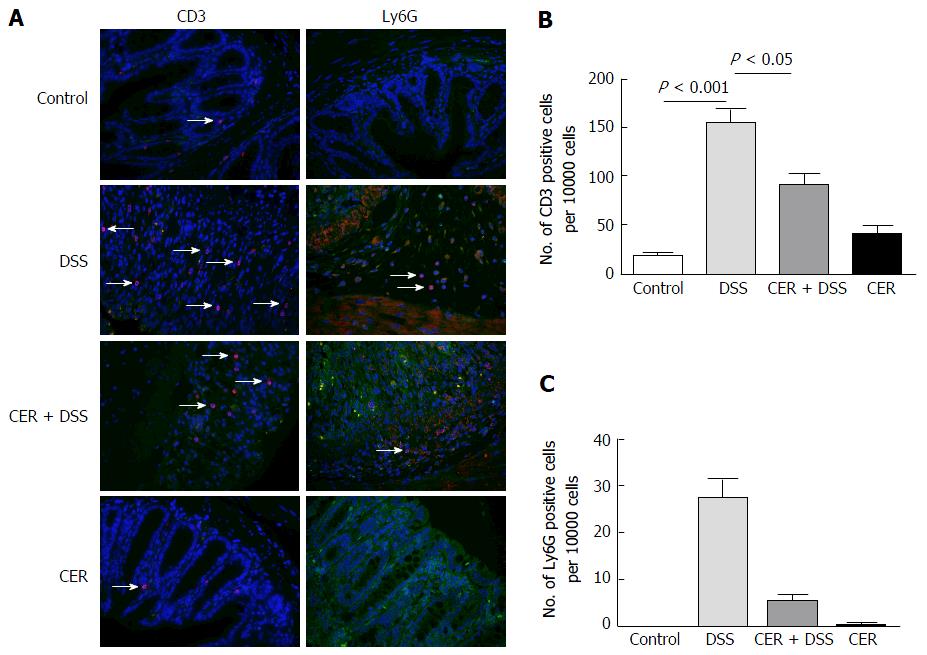
CD3 (indicates lymphocytes) and Ly6G (indicates neutrophils) were detected to assess the infiltration of inflammatory cells in colon tissues. CD3 and Ly6G were both labeled red, and nuclei were stained with 4’, 6-diamidino-2-phenylindole (DAPI). CD3 positive cells were more abundant in DSS mice than those in control mice after merging CD3 with nuclei using Image-Pro Plus (Figure 2A and B; P < 0.001). Mice in the CER + DSS group presented fewer CD3 positive cells compared to those of mice in the DSS group (Figure 2A and B; P < 0.05). As for Ly6G, there were differences among the four groups (Figure 2C; P = 0.019). The number of Ly6G positive cells was larger in the DSS group than that in mice of the CER+DSS group (Figure 2C). Nevertheless, mice in the control group and CER group showed no difference in the abundance of CD3 positive cells and Ly6G positive cells.
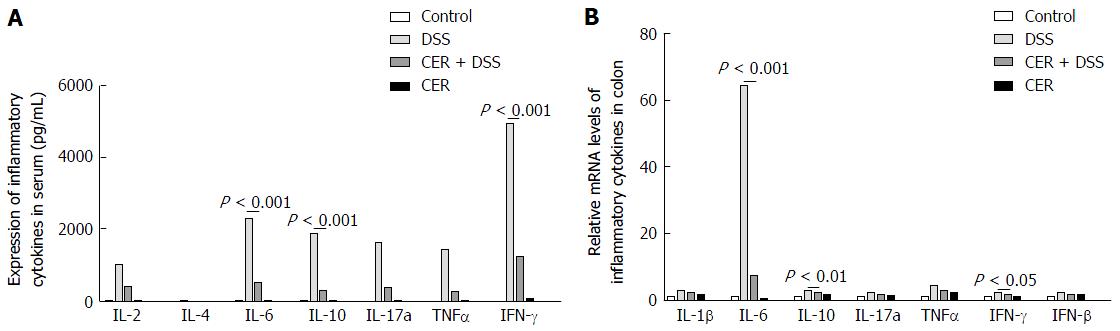
The expression levels of IL-2, IFN-γ and TNFα were detected to assess the Th1 response in mice of each group, and IL-4, IL-6 and IL-10 were examined to evaluate the Th2 response. In addition, IL-17A was tested to assess the Th17 response. CBA tests indicated that the mice in the CER + DSS and DSS groups showed higher levels of inflammatory cytokines compared with control and CER mice (Figure 3). The expression levels of IL-2, IFN-γ, TNFα, IL-6, IL-10 and IL-17a in CER + DSS mice were much lower than those in DSS mice, especially for IL-6, IL-10 and IFN-γ (Figure 3A, P < 0.001 for IL-6, IL-10 and IFN-γ). However, there were no significant differences between control and CER mice. IL-4 was hardly detected in any samples. Higher mRNA levels of IL-1β, IL-6, IL-10, IL-17a, IFN-γ, TNFα and TGFβ were observed in colon tissues of CER + DSS and DSS mice compared to control mice (Figure 3B). Among the four groups, mice in the DSS group displayed the highest expression of IL-6, while mice in the CER + DSS group presented much lower expression of IL-6 compared with that in mice of the DSS group (Figure 3B; P < 0.001). Additionally, IL-10 and IFN-γ were lower in the CER+DSS group than in the DSS group (Figure 3B; P < 0.001 for IL-10; P < 0.05 for IFN-γ). No significant difference was observed between mice of the control group and CER group.
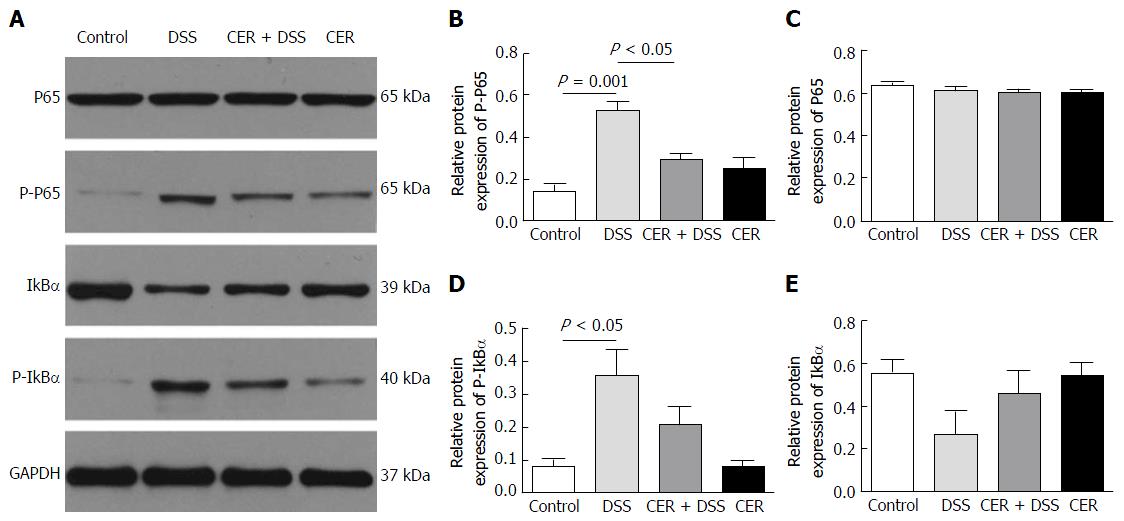
The expression level of phosphorylated-p65 (P-p65) in colon tissues of DSS mice was the highest among the four groups (Figure 4A and B). Mice in the CER + DSS group presented lower P-p65 expression than mice in the DSS group (Figure 4A and B; P < 0.05). Consistent with the P-p65 results, mice in the DSS group showed much higher expression of phosphorylated-IκBα (P-IκBα) compared with the control group (Figure 4A and D; P < 0.05). The expression level of P-IκBα in CER + DSS mice was lower than that in mice of the DSS group (Figure 4A and D). There were no differences in expression levels of P-p65, P65, P-IκBα and IκBα between mice of the control group and CER group.
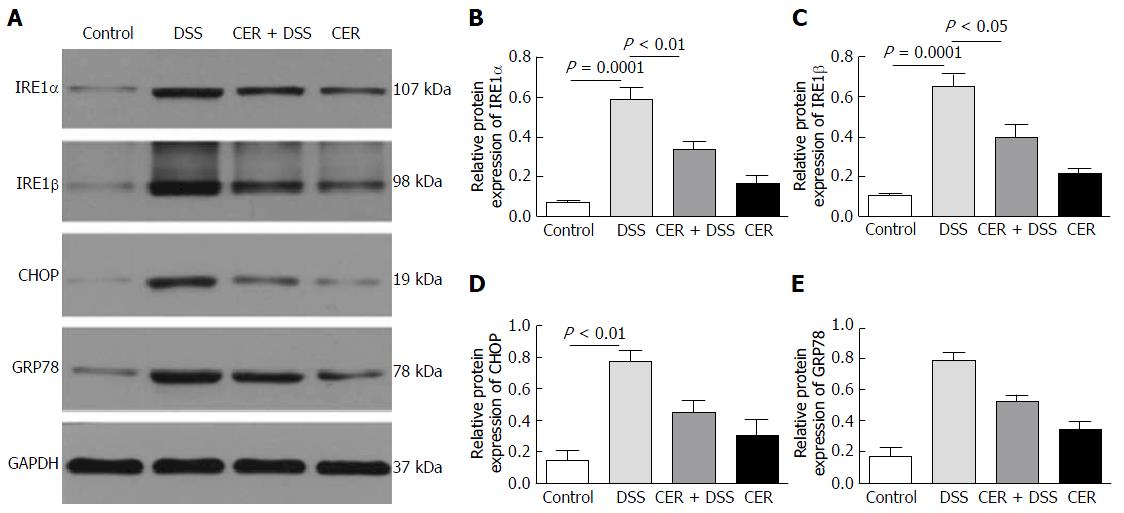
For endoplasmic reticulum (ER) stress markers, the expression levels of IRE1α, IRE1β and CHOP were significantly higher in DSS mice than in control mice as revealed by both Western blot (Figure 5A-D, P < 0.0001 for IRE1α and IRE1β, P < 0.01 for CHOP) and RT-PCR (Supplementary Figure 1). Compared with mice in the DSS group, mice in the CER + DSS group presented much lower expression levels of IRE1α and IRE1β (Figure 5A and B; P < 0.01; Figure 5A and C; P < 0.05). Furthermore, there were clear differences in the expression levels of GRP78 among the four groups (Figure 5A and E; P = 0.0006 for 4 groups). The expression levels of GRP78 and CHOP in mice of the CER + DSS group were lower than those in mice of the DSS group (Figure 5A, D and E). Mice in the control group and CER group showed no difference in ER stress markers.
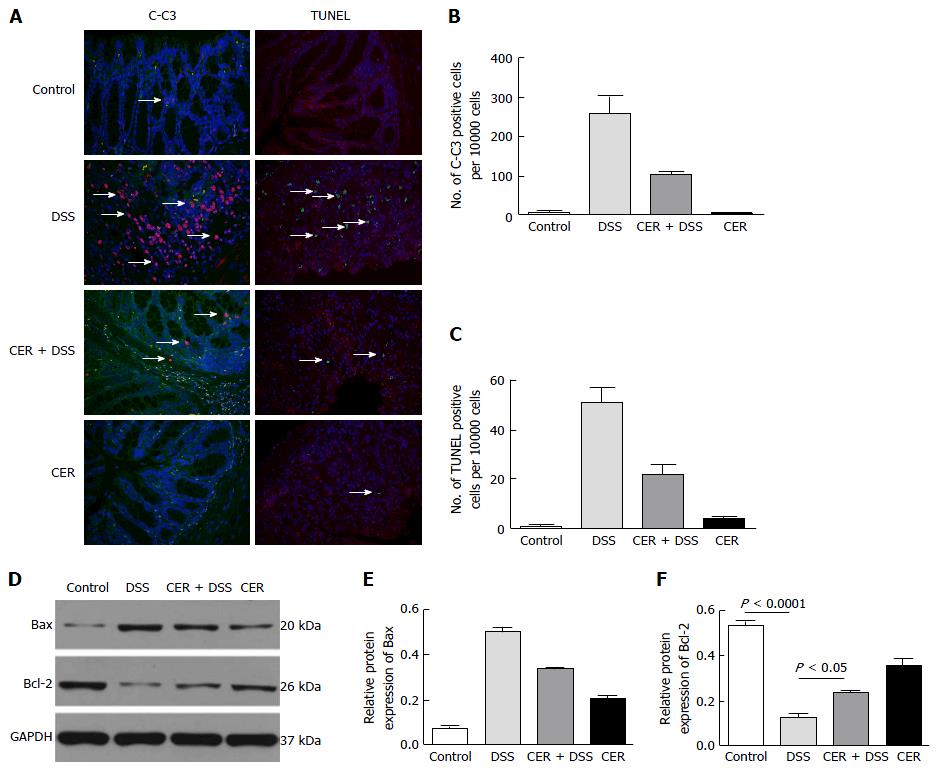
To estimate celluar apoptosis in colon tissues, TUNEL and C-C3 positive cells were investigated. C-C3 and TUNEL positive cells were labeled red and green, respectively. Nuclei were stained with DAPI. There were significant difference in the numbers of C-C3 positive cells and TUNEL positive cells among the mice of the four groups (Figure 6A-C, P = 0.015 for the four groups). Mice of the DSS group presented a dramatically larger number of C-C3 positive cells than mice of the control group as well as the CER group (Figure 6A and B). Large numbers of TUNEL positive cells were observed in colon tissues of DSS mice (Figure 6A and C). There were fewer C-C3 positive cells and TUNEL positive cells in CER+DSS mice compared with DSS mice (Figure 6A-C). Hardly any difference in the abundance of C-C3 positive cells and TUNEL positive cells was observed between mice in the control group and CER group.
In addition, mice in the DSS group showed distinctly lower Bcl-2 expression than mice in the control group (Figure 6D and F; P < 0.0001). The expression level of Bcl-2 was much higher in mice of the CER + DSS group than in mice of the DSS group (Figure 6D and F; P < 0.05). Additionally, there was a difference in Bax levels among the four groups (Figure 6D and E; P = 0.015 for 4 groups). Mice of the CER + DSS group had much lower Bax expression than mice of the DSS group (Figure 6D and E). Mice in the control group and CER group displayed no difference in the expression levels of Bcl-2 and Bax.
According to the “hygiene hypothesis”, which was first proposed by Strachan[22] in 1958, early childhood infections were associated with decreased atopy in children. Although the concept began with allergic disorders, it has been extended and is currently related to autoimmunity, neuroinflammatory disorders, atherosclerosis, and some cancers[23]. In the 1990s, the “inflammatory bowel disease hygiene hypothesis” was first proposed, which stated that children in extremely hygienic environments that might impede the proper maturation of their immune system were predisposed to IBD later in life[11]. According to epidemiological investigations, helminth infection was suggested to decrease the susceptibility to or prevent the development of IBD[11]. Here, we investigated the effects of S. japonicum infection on a DSS-induced colitis model, and we found that S. japonicum infection reduced the susceptibility to DSS and decreased the infiltration of inflammatory cells, ER stress and the NF-κB pathway.
There were no significant differences in colon length, weight loss, DAI and HS between the mice in the control and CER groups. The colon length of mice in the DSS group was not only shorter than that of control mice but also shorter than that of mice in the CER + DSS group. The weights of the mice in the DSS group decreased significantly from the 5th day of DSS treatment, while the mice in the CER + DSS group showed a slower decrease from the 6th day of DSS treatment. Both DAI and HS were highest in the DSS group mice and were significantly higher than those of mice infected with S. japonicum cercariae prior to DSS treatment (CER + DSS mice). In addition, less lymphocyte and neutrophil infiltration was observed in colon tissues of CER + DSS mice compared with DSS mice. All results indicated that exposure to S. japonicum contributed to preventing the development of colitis induced with DSS.
Although the mechanism underlying IBD is complicated, the influence of Schistosoma or its molecules on animal colitis was predominantly suggested to be related to the balance between Th1 response and Th2 response[12,14,24,25]. Research by Elliott et al[12] illustrated that infection with eggs of S. mansoni attenuated TNBS-induced colitis by reducing Th1 response and increasing Th2 response. However, DSS-induced colitis mice presented diminished expression levels of TNFα, IL-2 and IL-4 mRNA after exposure to S. mansoni larvae[14]. Recombinant cystatin from S. japonicum showed a therapeutic effect on mice with TNBS-induced colitis by suppressing IFN-γ and IL-17a and enhancing IL-4 and IL-13[24]. In addition, Ruyssers et al[25] reported augmented IL-10 mRNA expression and diminished IFN-γ mRNA and IL-17 mRNA expression. In our study, compared with DSS mice, significant decreases in IL-6, IL-2, IL-10, IL-17, IFN-γ and TNFα were observed in CER + DSS mice as revealed by the CBA technique, which are consistent with the diminished levels of inflammatory cytokines and transcription factors detected by RT-PCR, especially for IL-10, IL-6 and IFN-γ. In other words, we observed that S. japonicum infection ameliorated DSS-induced colitis with decreased Th1, Th2 and Th17 responses. Thus, we hypothesize that the Th1/Th2/Th17 pathway may not be the dominant mediator during colitis following exposure to S. japonicum. Additionally, these results might be caused by the different time points we chose to induce colitis, diverse infection methods, disparate animal models and other factors compared to those of other studies. For example, Elliott et al[12] and Xia et al[26] adopted TNBS-induced colitis and Bodammer et al[14] used S. mansoni larvae to infect mice.
Oh et al[27] identified an important effect of NF-κB in regulating differentiation and maturation of Th cells. Furthermore, connections between the NF-κB pathway and IBD have been reported in previous studies[28,29]. In our previous study, CARD3 deficiency alleviated the infiltration of inflammatory cells and cellular apoptosis during DSS-induced colitis followed by decreased NF-κB activation[21]. Hence, we examined the protein levels of P65, P-P65, IκBα and P-IκBα in colon tissues of mice. The results showed high expression of P-P65 and P-IκBα in DSS mice. Consistent with the cytokine results of serum samples and colon tissues, CER + DSS mice displayed lower P-P65 and P-IκBα levels compared to DSS mice. It was reported that NF-κB was essential for the production of IL-2[27], and the absence of P65 could impair IFN-γ production in Th1 cells[30]. Additionally, NF-κB activation played an important role in regulating the expression of the GATA3 and RORγt genes, which are transcription factors of Th2 cells and Th17 cells, respectively[31,32]. Thus, the attenuation of the DSS-induced colitis in mice via exposure to S. japonicum as well as the change in cytokines related to Th1, Th2 and Th17 responses was probably due NF-κB activation.
ER stress participates in many inflammatory diseases, such as CD and T2DM, and has shown great potential in regulating inflammatory responses through the unfolded protein response (UPR) pathway[33,34]. Increased expression of ER stress markers was observed in ileal and/or colonic epithelial tissues of active IBD patients[34,35]. Adolph et al[36] also demonstrated spontaneous transmural ileitis similar to CD accompanied by activated IRE1α in intestinal epithelial cells of mice with Xbp1 and Atg1611 deletions. However, addition of ER stress inhibitors, such as tauroursodeoxycholate and 4-phenylbutyrate, mitigated intestinal inflammation in IL-10-/- and TnfΔARE mice induced with DSS[37]. In addition, taurine supplement attenuated hepatic granuloma and fibrosis in S. japonicum-infected mice, with a significant decrease in ER stress[38]. In our study, the expression levels of IRE1α, IRE1β, GRP78 and CHOP and their coding genes were notably highest in DSS mice among the four groups. Although the expression levels of these molecules were still higher in CER + DSS mice compared with those in control mice, they were much lower than those in DSS mice. Coupling of the UPR in cells triggered inflammation and played an important role in the pathogenesis of inflammatory diseases[39]. Additionally, IRE1α activation by binding with TNFα receptor-associated factor 2 (TRAF2) may affect TRAF2 and subsequently activate the NF-κB pathway[40,41]. Inflammation induced by Brucella abortus accompanied by high expression levels of IL-6 and ER stress in an NOD1/NOD2-dependent manner was suppressed by IRE1α kinase inhibitor[42]. Hence, we hypothesized that the decrease in NF-κB activation detected in the CER + DSS group might be influenced by ER stress. Two hypotheses were proposed here: first, the damage in enterocytes caused by DSS treatment triggered inflammation and ER stress/UPR, and UPR probably aggravated inflammation via the NF-κB pathway simultaneously; second, S. japonicum infection regulated the activation of ER stress or UPR directly.
Moreover, colon inflammation was reported to lead to dysfunction in epithelial barrier and apoptosis of intestinal epithelial cells[43]. In this study, we tested the expression of C-C3, TUNEL, Bcl-2 and Bax to assess the apoptosis in colon tissues of the treated mice. DSS mice showed more C-C3 positive cells and TUNEL positive cells compared with control mice. However, fewer C-C3 positive cells and TUNEL positive cells were observed in CER + DSS mice than in DSS mice. DSS mice displayed the highest expression level of Bax and the lowest expression level of Bcl-2 among the mice in the four groups. CER + DSS mice exhibited significantly lower Bax levels and higher Bcl-2 levels than DSS mice. On one hand, this remissive apoptosis in colon tissues might be caused by the attenuation of colon inflammation in DSS-induced colitis mice infected with S. japonicum. On the other hand, Chen et al[44] showed that activation of the NF-κB signaling pathway enhanced the apoptosis of dental epithelial cells in vitro; thus, the high apoptosis level in DSS-treated intestinal cells might be affected by the strong response of NF-κB, which is consistent with our previous research showing that diminished NF-κB activation accompanied by decreased apoptosis in colon tissues, especially enterocytes in CARD3-/- mice induced with DSS.
Additionally, ER stress also exhibited a connection with apoptosis through multiple mechanisms. Wang et al[45] found that overexpression of IRE1 could promote apoptosis of HEK193 human embryonic kidney cells. Under ER stress, IRE1α could not only prevent cell apoptosis caused by sustained ER stress via activation of Xbp1[36] but also promote mitochondrion-dependent cell death by binding to Bax on the outer membrane of mitochondria[46,47]. When IRE1 associates with TRAF2 and Aask1 to form the IRE1-TRAF2-ASK1 complex, it can play a pro-apoptotic role by enhancing the JNK pathway. Additionally, enhanced expression of CHOP might lead to apoptosis by reducing expression of Bcl-2, decreasing endocellular glutathione and increasing generation of reactive oxygen intermediates[48]. By forming a dimer with cAMP response element binding protein, CHOP could also promote apoptosis by inhibiting the expression of Bcl-2 and enhancing the mitochondria sensitivity to pro-apoptotic factors[49]. Thus, we propose that the less severe apoptosis in CER + DSS mice was associated with a lower level of ER stress in contrast to DSS mice.
In conclusion, exposure to S. japonicum cercariae can prevent inflammation progression and apoptosis in colon tissues of DSS-induced experimental colitis. In addition to the Th1/Th2/Th17 pathway and NF-κB pathway, ER stress was also shown to be involved in attenuating the inflammatory response by parasite infection in DSS-induced colitis. Further investigations are needed to determine the exact mechanism of its action for further development as a therapeutic strategy for IBD treatment.
The authors thank Professor Hui-Fen Dong at Department of Medical Parasitology and Research Laboratory of Schistosomiasis, Wuhan University School of Medicine for experimental guidance.
Schistosoma (S.) and its protein extracts have shown therapeutic effects in ameliorating colitis. It was supposed that the Th1/Th2/Th17 pathway might be involved in the induction of remission while the outcomes were dissimilar and the underlying mechanism is still unclear. To further elucidate the role and mechanism of S. japonicum infection on inflammatory bowel disease (IBD), the authors studied the effects of exposure to S. japonicum cercariae on dextran sodium sulfate (DSS)-induced colitis.
Nuclear factor-kappa B (NF-κB) has been reported to play an important role in regulating differentiation and maturation of Th cells. In previous studies, NF-κB also showed a pro-inflammation and pro-apoptosis effect on DSS-induced colitis. In addition, endoplasmic reticulum (ER) stress, which has a close relationship with inflammation and apoptosis, takes a significant part in the development of IBD.
This is the first time to elucidate the effect of S. japonicum infection on IBD from the aspect of considering NF-κB pathway, ER stress pathway and apoptosis together.
S. japonicum attenuates DSS-induced colitis in mice and decreases the cellular apoptosis in colon tissues accompanied by reduced expression of NF-κB and ER stress proteins, especially ER stress proteins. Therefore, ER stress-related proteins might be potential therapeutic targets for IBD.
ER stress is a cellular stress response related to the endoplasmic reticulum, which has been found to be conserved between all mammalian species, as well as yeast and worm organisms. Unfolded protein response is activated by the accumulation of unfolded or misfolded proteins in the lumen of the ER.
Authors demonstrated down-regulated expression of cytokines and transcriptional factors such as IL-6, IL-10 and IFN-γ as well as phosphorylated-P65 and ER stress-related proteins after exposure to S. japonicum in DSS-induced colitis mice. Besides, ER stress probably contributed to the cellular apoptosis in colon tissues during S. japonicum infection in colitis mice.
| 1. | Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1587] [Cited by in RCA: 1571] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 2. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3412] [Article Influence: 179.6] [Reference Citation Analysis (12)] |
| 3. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3598] [Article Influence: 257.0] [Reference Citation Analysis (6)] |
| 4. | Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol. 2012;27:1266-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 270] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 5. | Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology. 2011;140:1704-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 311] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 6. | Briggs N, Weatherhead J, Sastry KJ, Hotez PJ. The Hygiene Hypothesis and Its Inconvenient Truths about Helminth Infections. PLoS Negl Trop Dis. 2016;10:e0004944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 7. | Summers RW, Elliott DE, Qadir K, Urban JF, Thompson R, Weinstock JV. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2003;98:2034-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 310] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 8. | Summers RW, Elliott DE, Urban JF, Thompson R, Weinstock JV. Trichuris suis therapy in Crohn’s disease. Gut. 2005;54:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 532] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 9. | Croese J, O’neil J, Masson J, Cooke S, Melrose W, Pritchard D, Speare R. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut. 2006;55:136-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 196] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | McSorley HJ, Gaze S, Daveson J, Jones D, Anderson RP, Clouston A, Ruyssers NE, Speare R, McCarthy JS, Engwerda CR. Suppression of inflammatory immune responses in celiac disease by experimental hookworm infection. PLoS One. 2011;6:e24092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Elliott DE, Urban JF JR, Argo CK, Weinstock JV. Does the failure to acquire helminthic parasites predispose to Crohn’s disease? FASEB J. 2000;14:1848-1855. [PubMed] |
| 12. | Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban JF, Weinstock JV. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G385-G391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 186] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | Zhao Y, Zhang S, Jiang L, Jiang J, Liu H. Preventive effects of Schistosoma japonicum ova on trinitrobenzenesulfonic acid-induced colitis and bacterial translocation in mice. J Gastroenterol Hepatol. 2009;24:1775-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Bodammer P, Waitz G, Loebermann M, Holtfreter MC, Maletzki C, Krueger MR, Nizze H, Emmrich J, Reisinger EC. Schistosoma mansoni infection but not egg antigen promotes recovery from colitis in outbred NMRI mice. Dig Dis Sci. 2011;56:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Smith P, Mangan NE, Walsh CM, Fallon RE, McKenzie AN, van Rooijen N, Fallon PG. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J Immunol. 2007;178:4557-4566. [PubMed] |
| 16. | Wu J, Xu W, Ming Z, Dong H, Tang H, Wang Y. Metabolic changes reveal the development of schistosomiasis in mice. PLoS Negl Trop Dis. 2010;4:pii: e807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Arseneau KO, Pizarro TT, Cominelli F. Discovering the cause of inflammatory bowel disease: lessons from animal models. Curr Opin Gastroenterol. 2000;16:310-317. [PubMed] |
| 18. | Shang J, Li L, Wang X, Pan H, Liu S, He R, Li J, Zhao Q. Disruption of Tumor Necrosis Factor Receptor-Associated Factor 5 Exacerbates Murine Experimental Colitis via Regulating T Helper Cell-Mediated Inflammation. Mediators Inflamm. 2016;2016:9453745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Yu SJ, Liu Y, Deng Y, Zhu XY, Zhan N, Dong WG. CARD3 deficiency protects against colitis through reduced epithelial cell apoptosis. Inflamm Bowel Dis. 2015;21:862-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 616] [Cited by in RCA: 634] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 21. | Ohya S, Fukuyo Y, Kito H, Shibaoka R, Matsui M, Niguma H, Maeda Y, Yamamura H, Fujii M, Kimura K. Upregulation of KCa3.1 K(+) channel in mesenteric lymph node CD4(+) T lymphocytes from a mouse model of dextran sodium sulfate-induced inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2014;306:G873-G885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Ben-Ami Shor D, Harel M, Eliakim R, Shoenfeld Y. The hygiene theory harnessing helminths and their ova to treat autoimmunity. Clin Rev Allergy Immunol. 2013;45:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Wang S, Xie Y, Yang X, Wang X, Yan K, Zhong Z, Wang X, Xu Y, Zhang Y, Liu F. Therapeutic potential of recombinant cystatin from Schistosoma japonicum in TNBS-induced experimental colitis of mice. Parasit Vectors. 2016;9:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Ruyssers NE, De Winter BY, De Man JG, Loukas A, Pearson MS, Weinstock JV, Van den Bossche RM, Martinet W, Pelckmans PA, Moreels TG. Therapeutic potential of helminth soluble proteins in TNBS-induced colitis in mice. Inflamm Bowel Dis. 2009;15:491-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Xia CM, Zhao Y, Jiang L, Jiang J, Zhang SC. Schistosoma japonicum ova maintains epithelial barrier function during experimental colitis. World J Gastroenterol. 2011;17:4810-4816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Oh H, Ghosh S. NF-κB: roles and regulation in different CD4(+) T-cell subsets. Immunol Rev. 2013;252:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 337] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 28. | Hollenbach E, Vieth M, Roessner A, Neumann M, Malfertheiner P, Naumann M. Inhibition of RICK/nuclear factor-kappaB and p38 signaling attenuates the inflammatory response in a murine model of Crohn disease. J Biol Chem. 2005;280:14981-14988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008;263:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 666] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 30. | Balasubramani A, Shibata Y, Crawford GE, Baldwin AS, Hatton RD, Weaver CT. Modular utilization of distal cis-regulatory elements controls Ifng gene expression in T cells activated by distinct stimuli. Immunity. 2010;33:35-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Das J, Chen CH, Yang L, Cohn L, Ray P, Ray A. A critical role for NF-kappa B in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat Immunol. 2001;2:45-50. [PubMed] |
| 32. | Ruan Q, Kameswaran V, Zhang Y, Zheng S, Sun J, Wang J, DeVirgiliis J, Liou HC, Beg AA, Chen YH. The Th17 immune response is controlled by the Rel-RORγ-RORγ T transcriptional axis. J Exp Med. 2011;208:2321-2333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 33. | Montane J, Cadavez L, Novials A. Stress and the inflammatory process: a major cause of pancreatic cell death in type 2 diabetes. Diabetes Metab Syndr Obes. 2014;7:25-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1177] [Cited by in RCA: 1169] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 35. | Hu S, Ciancio MJ, Lahav M, Fujiya M, Lichtenstein L, Anant S, Musch MW, Chang EB. Translational inhibition of colonic epithelial heat shock proteins by IFN-gamma and TNF-alpha in intestinal inflammation. Gastroenterology. 2007;133:1893-1904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Adolph TE, Tomczak MF, Niederreiter L, Ko HJ, Böck J, Martinez-Naves E, Glickman JN, Tschurtschenthaler M, Hartwig J, Hosomi S. Paneth cells as a site of origin for intestinal inflammation. Nature. 2013;503:272-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 584] [Cited by in RCA: 593] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 37. | Cao SS, Zimmermann EM, Chuang BM, Song B, Nwokoye A, Wilkinson JE, Eaton KA, Kaufman RJ. The unfolded protein response and chemical chaperones reduce protein misfolding and colitis in mice. Gastroenterology. 2013;144:989-1000.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 199] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 38. | Yu YR, Ni XQ, Huang J, Zhu YH, Qi YF. Taurine drinking ameliorates hepatic granuloma and fibrosis in mice infected with Schistosoma japonicum. Int J Parasitol Drugs Drug Resist. 2016;6:35-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1641] [Cited by in RCA: 1616] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 40. | Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664-666. [PubMed] |
| 41. | Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3842] [Cited by in RCA: 4722] [Article Influence: 337.3] [Reference Citation Analysis (1)] |
| 42. | Keestra-Gounder AM, Byndloss MX, Seyffert N, Young BM, Chávez-Arroyo A, Tsai AY, Cevallos SA, Winter MG, Pham OH, Tiffany CR. NOD1 and NOD2 signalling links ER stress with inflammation. Nature. 2016;532:394-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 437] [Cited by in RCA: 423] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 43. | Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, Taylor CT. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 349] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 44. | Chen G, Sun W, Liang Y, Chen T, Guo W, Tian W. Maternal diabetes modulates offspring cell proliferation and apoptosis during odontogenesis via the TLR4/NF-κB signalling pathway. Cell Prolif. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998;17:5708-5717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 636] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 46. | Iwakoshi NN, Pypaert M, Glimcher LH. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J Exp Med. 2007;204:2267-2275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 251] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 47. | Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2167] [Cited by in RCA: 2158] [Article Influence: 143.9] [Reference Citation Analysis (0)] |
| 48. | McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 1547] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Hashimoto N, Tomizawa M S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Li D