Published online Jul 28, 2017. doi: 10.3748/wjg.v23.i28.5068
Peer-review started: February 16, 2017
First decision: April 5, 2017
Revised: April 13, 2017
Accepted: July 12, 2017
Article in press: July 12, 2017
Published online: July 28, 2017
Processing time: 164 Days and 4.4 Hours
Inflammatory bowel disease (IBD) is a chronic recurrent condition whose etiology is unknown, and it includes ulcerative colitis, Crohn’s disease, and microscopic colitis. These three diseases differ in clinical manifestations, courses, and prognoses. IBD reduces the patients’ quality of life and is an economic burden to both the patients and society. Interactions between the gastrointestinal (GI) neuroendocrine peptides/amines (NEPA) and the immune system are believed to play an important role in the pathophysiology of IBD. Moreover, the interaction between GI NEPA and intestinal microbiota appears to play also a pivotal role in the pathophysiology of IBD. This review summarizes the available data on GI NEPA in IBD, and speculates on their possible role in the pathophysiology and the potential use of this information when developing treatments. GI NEPA serotonin, the neuropeptide Y family, and substance P are proinflammatory, while the chromogranin/secretogranin family, vasoactive intestinal peptide, somatostatin, and ghrelin are anti-inflammatory. Several innate and adaptive immune cells express these NEPA and/or have receptors to them. The GI NEPA are affected in patients with IBD and in animal models of human IBD. The GI NEPA are potentially useful for the diagnosis and follow-up of the activity of IBD, and are candidate targets for treatments of this disease.
Core tip: Approximately 80% of the body immune cells (IC) are localized in the gastrointestinal (GI) tract close to the GI neuroendocrine regulatory system (NES). Many IC express GI neuroendocrine peptides/amines (NEPA) and possess receptors to several NEPA. Several GI NEPA are abnormal during active inflammatory bowel disease (IBD) in both patients and animal models of IBD. The changes in the GI NEPA are correlated with those of the IC during the inflammatory process. Studying the interactions between the GI NES and the immune system in IBD may improve our understanding of the pathophysiology of IBD and provide us with new tools for treatment.
- Citation: El-Salhy M, Solomon T, Hausken T, Gilja OH, Hatlebakk JG. Gastrointestinal neuroendocrine peptides/amines in inflammatory bowel disease. World J Gastroenterol 2017; 23(28): 5068-5085
- URL: https://www.wjgnet.com/1007-9327/full/v23/i28/5068.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i28.5068
Inflammatory bowel disease (IBD) is a lifelong recurrent disorder that comprises three diseases: ulcerative colitis (UC), Crohn’s disease (CD), and microscopic colitis (MC). These three diseases have different clinical manifestations, courses, and prognoses[1-3]. Whereas the onset of UC and CD occurs mostly at a young age, MC onset occurs in old age[4,5]. In UC and CD, the activity of the disease varies considerably between patients, from frequent relapses, persistent active disease, to several years of complete remission[4], whereas all MC patients exhibit chronic active disease[6-8]. The inflammation in CD is transmural, in UC it is superficial, and in MC it is in the form of the mucosal and submucosal infiltration of immune cells (IC). CD can arise at any part of the gastrointestinal (GI) tract, while UC and MC affect the recto-colonic mucosa[8,9]. In contrast to UC and CD, spontaneous symptomatic remission occurs in 59%-93% of MC patients[10,11].
IBD diminishes the quality of life considerably and represents an economic problem to both the patients themselves and society[4,9]. The prevalence of IBD amounts to 1.4 million patients in North America and 2.2 million patients in Europe, with 3-20 new cases occurring per 100000 persons annually[12-16]. The prevalence of IBD does not differ among Hispanics, blacks, and Caucasians[17,18]. The incidence of IBD is lower in Asia than in North America and Europe[19-21], but it has been increasing worldwide in recent years[19,21].
The etiology of IBD is not completely understood[9], and the available treatments are not ideal[1-4,22-31]. Typically 70%-80% of the body IC are present in the GI tract in close proximity to the GI neuroendocrine regulatory system (NES)[32,33]. Interactions between the GI neuroendocrine peptides/amines (NEPA) and the immune system have recently been discussed, and it is believed that these interactions play an important part in the pathophysiology of IBD[33-45]. Understanding the role of the GI NEPA in IBD would increase our understanding of the mechanisms underlying the pathophysiology of IBD, and may yield tools for treating these conditions using agonists or antagonists to the GI NEPA[43].
The aim of the present review was to summarize the available data on GI NEA in IBD and to speculate on their possible role in the underlying pathophysiology, and the potential utilization of these peptides/amines in treatments.
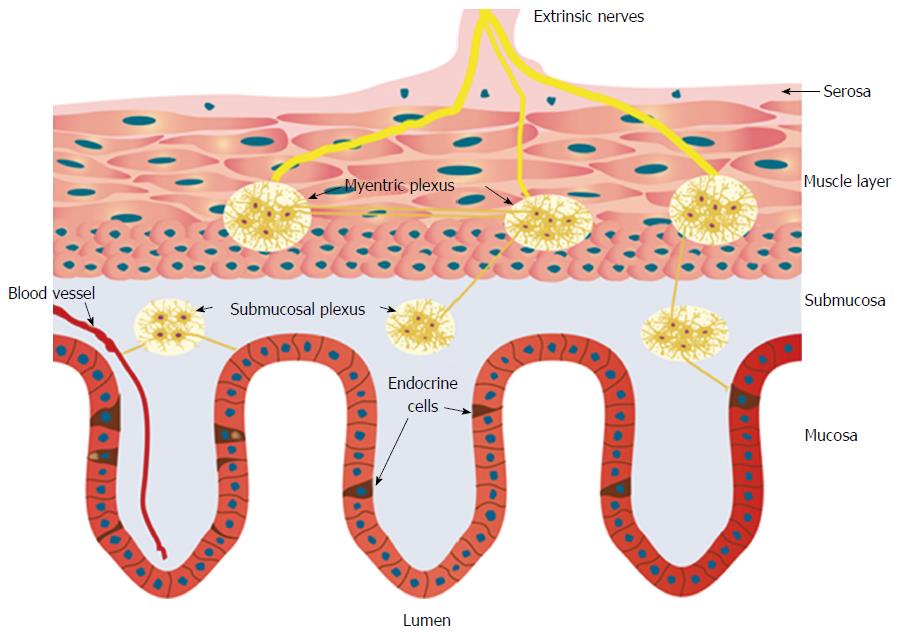
The NES comprises two parts: the GI endocrine cells in the mucosa and the enteric nervous system (ENS) (Figure 1). The GI endocrine cells occur in all segments of the GI tract except for the esophagus[46,47]. These cells lie between the mucosal epithelial cells facing the GI lumen, and they comprise about 1% of all epithelial cells and produce a large number of hormonal peptides/amines[48-56]. The GI endocrine cells are divided into at least 15 different types depending on the hormone they produce[48,49]. Two hormones can be colocalized in the same type of endocrine cell, such as glucagon-like peptide-1 and glucose-stimulated insulinotropic peptide in the small intestine, and peptide YY (PYY) and oxyntomodulin (enteroglucagon) in the distal small and large intestines[57-60]. It has been shown recently that mature GI endocrine cells can express up to seven different hormones[51,52,61-64].
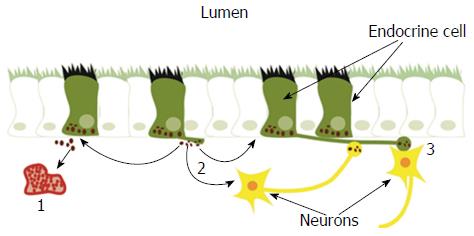
The GI endocrine cells have specialized sensory microvilli that project into the lumen, and they respond to luminal stimuli (mostly nutrients and/or bacteria byproducts) by releasing their hormones into the lamina propria[32,42,65-85]. The cells also possess a basal cytoplasmic process about 70 μm long that is believed to be involved in their paracrine mode of action[86-90]. It has been shown recently that this process exhibits neuronal axon-like characteristics, and it has been named a neuropod[88,91-93]. The GI endocrine cells also exhibit synaptic vesicles and synthetize presynaptic proteins: synapsin 1, piccolo, bassoon, MUNC13B, RIMS2, latrophilin, and transsynaptic neurexin[88,91-93]. These cells also synthetize transsynaptic neuroligins 2 and 3, homer 3, and postsynaptic density 95[93]. Thus, the GI endocrine cells possess the elements necessary for both afferent and efferent synaptic transmission[93]. These data suggest that the GI hormones released in the lamina propria could act locally on close by cells or neurons (paracrine mode), through the circulating blood (endocrine mode), or by afferent and efferent synaptic transmission[94-97] (Figure 2).
Recent observations of GI endocrine cells exhibiting both endocrine and neuron-like characteristics support a long-standing hypothesis about the evolution of the GI NES[98]. The absence of mammalian GI hormonal peptides in the gut of invertebrates, and he occurrence of these peptides in the central nervous system (CNS)[99-101] resulted in the hypothesis that the GI endocrine cells of vertebrates initiated in the nervous system of a common ancestor of invertebrates and vertebrates and then moved during a later stage of evolution into the gut as endocrine cells[98].
The ENS is an independent nervous system within the GI tract that consists of two plexi: one located in the submucosa (the submucosa plexus) and one situated between the longitudinal and circular muscle layers (the myenteric plexus)[102-104]. The neurons of the ENS (about 100 million) are modulated by afferent and efferent nerve fibers from the CNS and the autonomic nervous system[102-104]. The GI endocrine cells integrate and interact with each other and the ENS[105].
The NES regulates GI motility, secretion, absorption, visceral sensitivity, local immune defense, cell proliferation, and appetite[105].
It has long been believed that IBD is caused bacterial infection, and this belief lead to the introduction of salazopyrine (5-aminosalicylic acid-sulfapyridine) for the treat of IBD[106,107]. However, A specific microbe(s) could not be identified as the cause of IBD[106]. Recent studies have shown, however, that intestinal microbiota plays an important role in the pathophysiology of IBD[106]. Thus, low intestinal microbiome diversity and dysbiosis appear to be important factors in the pathophysiology of IBD[106]. The short-chain fatty acids produced upon fermentation of dietary fibers in the large intestine affect both the immune system and the NES. Butyrate is one of these short-chain fatty acids[108,109]. Butyrate suppresses large intestinal inflammation by inducing T-cell apoptosis, and by suppressing IFN-γ-mediated inflammation[110-112]. The short-chain fatty acids affect several GI peptides, such as PYY and glucagon-like peptide-1[80,113-115]. Furthermore butyrate has been found to affect neurons of the ENS[113,116].
Several NEPA of the GI NES have been shown to interact with the immune system, including members of the chromogranin/secretogranin family, serotonin, vasoactive intestinal peptide (VIP), members of the neuropeptide Y (NPY) family, substance P, somatostatin, and ghrelin.
All of the GI endocrine cell types produce members of the granins family (including chromogranins A and B) that are co-stored and co-released from the GI endocrine cells[34,117-120]. Chromogranin A (CgA) occurs in all GI tract endocrine cell types[121-124]. CgA-derived peptides decrease interleukin (IL)-16 and IL-5 release, and hence decrease the density of lymphocytes at inflammatory sites and thus the proinflammatory action of lymphocytes and monocytes[125-127]. Members of the chromogranin/secretogranin family are believed to exert anti-inflammatory effects.
About 95% of the body serotonin occurs in the GI, of which only 10% occurs in the neurons of the ENS and the rest in the enterochromaffin cells[34,128]. Serotonin is believed to play a pivotal role in intestinal inflammation[34,38,40,125,129,130]. Mast cells, macrophages/monocytes, and T cells are capable of producing serotonin[131]. Serotonin receptors occur in numerous innate IC such as neutrophils, eosinophils, monocytes, macrophages, dendritic cell, mast cells, and natural killer (NK) cells, and in cells of the adaptive immune system such as lymphocytes[130-132]. Serotonin promotes the activation of lymphocytes, whose proliferation protects NK cells and T-helper cells, hinders the apoptosis of IC, and endorses the recruitment of T cells[133-137]. The number of intestinal serotonin cells is decreased in knockout mice lacking T-lymphocyte receptors[125]. Serotonin cells express IL-13 receptors[138]. Against this background, serotonin is considered to be a proinflammatory amine during the inflammatory process.
VIP is a 28-amino-acid peptide exhibiting structural similarities with secretin[139]. VIP is secreted by neurons, endocrine cells, and IC, and it occurs in almost all body organs[140]. In GI tract, VIP occurs in endocrine cells and neurons of the ENS[141,142]. VIP is believed to be a major immune-regulating neuropeptide that plays an important role in inflammatory disorders, and is considered to be a natural anti-inflammatory agent[142,143].
Both CD4 and CD8T cells produce VIP, especially following antigen stimulation[144,145]. The VIP receptor VPAC1 occurs in lymphocytes, macrophages, monocytes, dendritic cells, microglia, and mast cells[146,147]. VIP inhibits the production of proinflammatory cytokines such as tumor necrosis factor α (TNFα), IL-6, IL-12, iNOS, and promotes the production anti-inflammatory cytokines such as IL-10[148-153]. VIP also inhibits the transcription factors AP-1, nuclear factor-κB (NFκB), CREB, and IRF-1[142,147,153,154], and impairs the acquisition of the macrophage proinflammatory polarization profile[155].
The NPY family includes three neuroendocrine peptides that act as hormones and/or neurotransmitters/neuromodulators: NPY, PYY, and pancreatic polypeptide (PP)[156-160]. These peptides consists of 36-amino-acid residues and are structurally related[161]. Whereas NPY is expressed in neurons of the CNS and NES[158,159,162], PYY and PP are expressed by endocrine cells of the ileum, colon, and rectum[163-165]. PP occurs also in endocrine cells in pancreatic islets of Langerhans[160]. NPY and PYY exert similar biological effects[105,161,165], and they act through binding to receptors Y1 and Y2[166-169]. T lymphocytes, macrophages, and dendritic cells produce NPY during inflammation[170]. NPY Y1/Y2 receptors are localized on IC[171,172], and the binding of NPY to these receptors induces the release of proinflammatory cytokines and nitric oxide from macrophages, neutrophils, and lymphocytes[171,173]. NPY therefore exerts proinflammatory effects in the presence of an inflammatory process. The role of PYY and PP in inflammation is not yet known.
Substance P is a member of the tachykinin family and substance P nerve fibers are widely distributed in the GI wall. Substance P is localized in enteric efferent neurons[174-176] and is expressed by several IC including T cells, macrophages, dendritic cells, and eosinophil cells[177-182]. It also plays an important role in the migration of innate IC such as neutrophils and macrophages, and of adaptive IC such as T lymphocytes[183-190]. Furthermore, substance P regulates the proliferation of lymphocytes and modulates the activities of innate and adaptive IC[179,183,191]. Substance P is therefore considered to be one of the main proinflammatory mediators in the GI tract.
The GI tract and the pancreas contain most of the body somatostatin[192,193]. About 90% of GI somatostatin is localized in GI endocrine cells, and the remaining 10% is in neurons of the ENS[194]. Somatostatin binds to five membrane G-protein-coupled receptor subtypes (SSTR 1-5)[195]. Several innate and adaptive IC such as monocytes/macrophages, B lymphocytes, T lymphocytes, and dendritic cells expressed these receptors[195-204]. Somatostatin stimulates B-lymphoblast proliferation with the enhancement of immunoglobulin formation[205], inhibits T lymphocytes and granulocyte proliferation, and reduces proinflammatory cytokines such as IFN-g[194,196,206-213]. Somatostatin is considered to be an anti-inflammatory peptide[37,214,215].
Ghrelin is a peptide composed of 28-amino-acid that occurs mostly in X/A endocrine cells in the oxyntic mucosa of the stomach[42,50,216-221]. Ghrelin performs several functions, including controlling food intake, energy homeostasis, and GI motility[217,218,221-224]. It also mediates the immune response and inflammation[146,225-227]. The anti-inflammatory prosperities of ghrelin are due to it modulating the secretion of pro-and anti-inflammatory cytokines from LPS-stimulated macrophages[225].
Changes in the ENS in IBD such as an increase in the number of enteric neurons, and altered neurotransmitter synthesis and release have been described[228-237]. Similarly, the density of the GI endocrine cells, the proportions of different endocrine cell types, and the release of GI NEPA are affected in both IBD patients and animal models of human IBD.
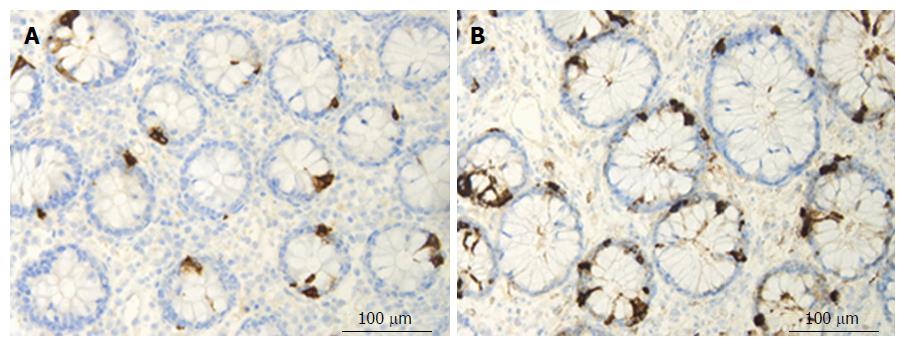
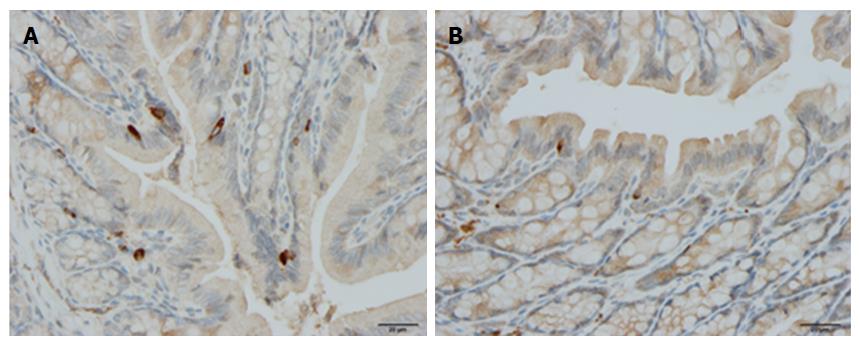
Chromogranin/secretogranin family: The circulating level of CgA is elevated in IBD patients and is reduced following treatment with certain biological agents[56,238-241]. Patients with IBD exhibit elevated concentrations of fecal CgA and secretogranins[242,243]. The CgA cell density is increased in patients with IBD, and in animal models of human UC and CD, with the exception of trinitrobenzene sulfonic acid (TNBS)-induced colitis[9,117,244-248] (Figure 3). The administration of the proinflammatory cytokines INFγ and TNFα and the induction of colitis by dextran sodium sulfate (DSS) in mice were found to increase the number of CgA cells[249].
Serotonin: The density of colonic serotonin cells is elevated in patients with UC, CD, and lymphocytic colitis[117,250] (Figure 4). The serotonin cell density was also increased in an animal model of human UC (TNBS-induced colitis in rats) and in an animal model of human CD (DSS-induced colitis in rats), as well as in other animal models of human UC and CD, and in IL-2-knockout mice[230,244,245,251,252].
VIP: Studies of VIP in patients with IBD have produced conflicting results. The immunohistochemical examination and quantification of tissue extracts from rectal biopsy samples obtained from patients with UC and CD showed an increased number of VIP-positive nerve fibers and an increased VIP concentration in CD but not in UC[253]. Other studies found that the number of VIP-positive nerve fibers was either decreased or unchanged in patients with UC and CD[245,254,255]. These contradictory results for VIP in patients with IBD could be explained by VIP occurring mostly in neurons of the ENS and that analyzing VIP in small mucosal biopsy specimens obtained during an endoscopic examination does not produce reliable results. However, changes in VIP have been found in animal models of human IBD, especially knockout mice[251]. In IL-2 gene-knockout mice, the relative volume density of VIP-positive nerve fibers and the level of VIP in tissue extracts were both decreased[251].
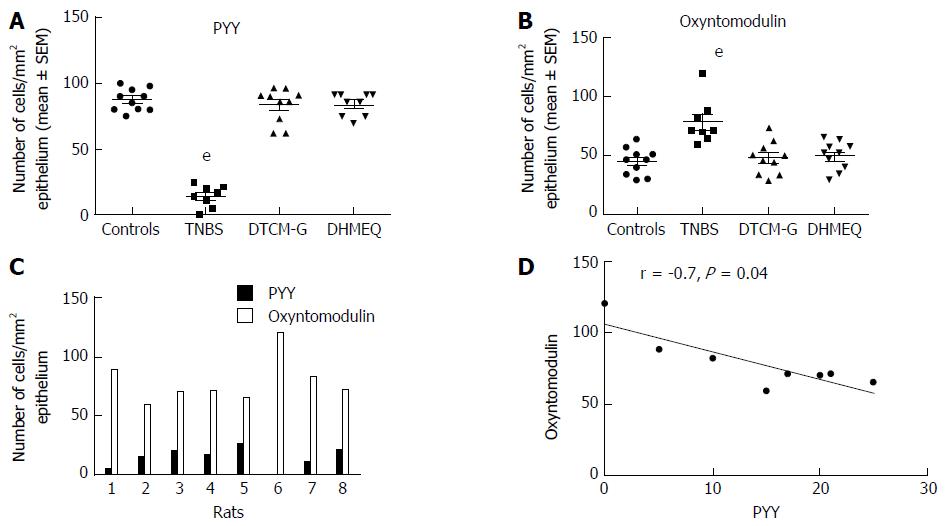
NPY family: The density of NPY enteric neurons increased as well as hyperplasia of NPY nerve fibers have been observed in mice with colitis induced either by DSS or streptomycin-pretreated Salmonella typhimurium[256,257]. The PYY cell density is increased in patients with UC and lymphocytic colitis as well as in colitis induced by DSS in rats and in IL-2 gene-knockout mice[117,245,250,251]. The PYY cell density is decreased in CD, with this change being correlated positively with the increased disease severity[117]. Similarly, the density of PYY cells was reduced in an animal model of human CD, namely TNBS-induced colitis in rats[244]. The robust positive correlation between the PYY cells and IC found in colitis induced either by DSS, or TNBS in rats is suggestive of an interaction between PYY cells and the IC[244,245]. It is noteworthy that PYY and oxyntomodulin (enteroglucagon) are produced from the same endocrine cell (L cells)[57]. Whereas the density of oxyntomodulin-containing cells is increased in patients with CD and in both DSS- and TNBS-induced colitis, and in IL-2 gene-knockout mice, it is unchanged in patients with UC[118,244,251,258]. The PP cell density is decreased in patients with CD and in colitis induced by either DSS, or TNBS in rats[117,245,248].
Substance P: The levels of substance P are increased in tissue extracts from the colon and in the rectum of patients with UC and CD, and were correlated with disease activity[253,259-261]. The density of nerve fibers immunoreactive to substance P is decreased in the colon of UC patients[262]. The density of substance-P-immunoreactive fibers has been reported to be both increased[253,262] and unchanged[262] in the colon of CD patients. The concentration of substance P in the colon of IL-2-knockout mice is decreased, while substance-P-immunoreactive cells were unchanged[251].
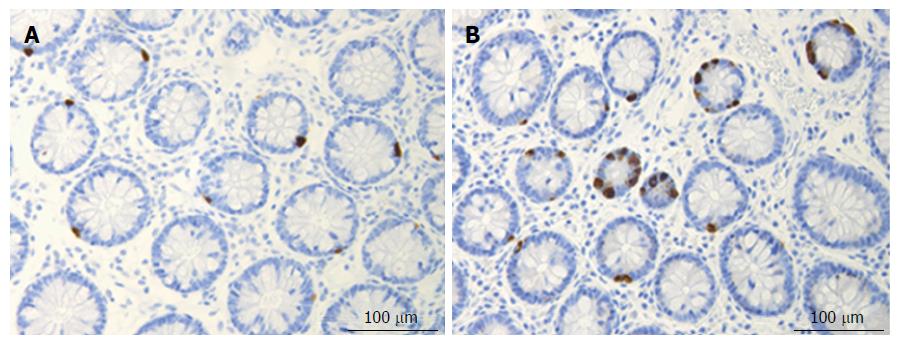
Somatostatin: The number of somatostatin cells is decreased in the colon of patients with IBD, and in animal models of human IBD, except for TNBS-induced colitis where it is increased[245,263-265] (Figure 5).
Ghrelin: The circulating levels of ghrelin are elevated in patients with IBD with active inflammation[266,267]. Moreover, circulating ghrelin levels in UC and CD patients are correlated with TNFα, C-reactive protein, the erythrocyte sedimentation rate, and fibrinogen, and negatively correlated with nutritional status parameters[42,228,268,269].
The mechanisms underlying the changes in ENS during inflammation in IBD remain unclear. However, recent studies have shed some light on the possible mechanisms of the inflammation-induced changes in the GI endocrine cells in IBD[258,270].
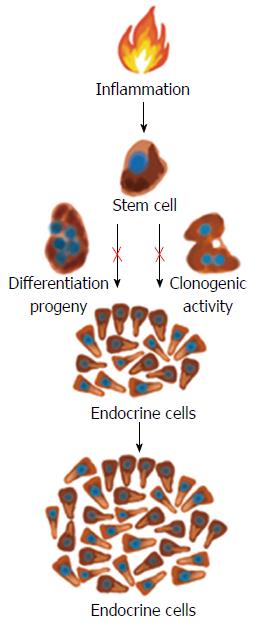
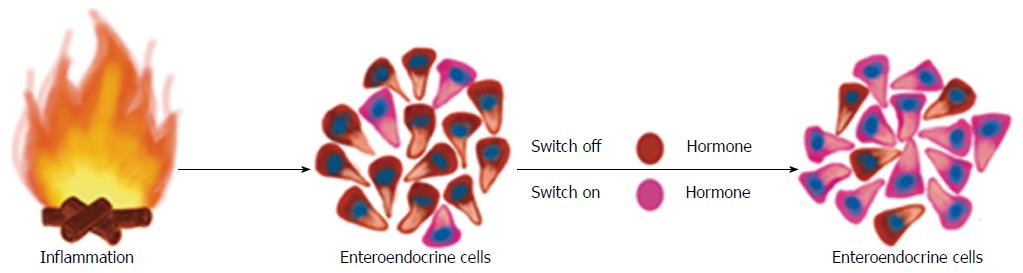
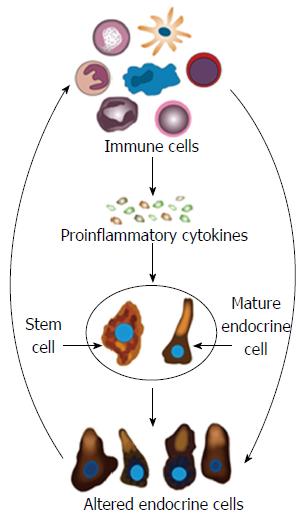
Whereas changes in GI endocrine cells do occur in UC, CD, lymphocytic colitis, and animal models of human IBD, the nature of these changes differ between the different IBDs and animal models of human IBD[9,117,244-248,250,259]. The changes in GI endocrine cells can be explained by two different mechanisms: abnormal stem cell clonogenic and differentiation progeny toward endocrine cells activities (Figure 6), and switching on and off of the expression of certain GI NEPA (Figure 7).
Abnormal stem cell clonogenic and differentiation activities: Each intestinal crypt contains four to six stem cells that either divide into identical new stem cells (clonogenic) or differentiate into all types of epithelial cells through a series of progenitors[270-281]. This differentiation into epithelial cells includes the secretory and absorptive lineages. The secretory lineage gives rise to endocrine, goblet, and Paneth cells. The absorptive lineage results to absorptive enterocytes[270-281]. In rats with TNBS-induced colitis, which is an animal model of human CD, the colonic density of Musashi-1 (Musi-1) immunoreactive cells was found to be reduced[258]. In contrast, the colonic density of Musi-1 cells was unaffected in rats with DSS-induced colitis, which is an animal model of human UC[268]. Musi-1 is located in both intestinal stem cells and early progenitors[282-284]. These observations indicate that the clonogenic activity of stem cells is affected in an animal model of CD but not in one of UC. This is probably due to the inflammation associated with CD being deep while that associated with UC being superficial.
In rats with both TNBS- and DSS-induced colitis, the colonic Math-1 cell density was found to be unaffected. Math-1 occurs early progenitor in the secretory lineage, and mutant (Math-1-⁄-) mice have no secretory cells[285].
The colonic neurogenin 3 (Neurog3) cell density is reduced in rats with TNBS-induced colitis, while it is increased in rats with DSS-induced colitis[259,270]. Neurog3 is localized in an early progenitor belonging to the secretory lineage, which contributes to the differentiation into endocrine cells[286]. Transgenic mice (Neurog3-⁄-) do not have enteroendocrine cells, but normal densities of goblet and Paneth cells[286-288]. Similar to Neurog3, the colonic NeuroD1 cell density is decreased in rats with TNBS-induced colitis while it is increased in rats with DSS-induced colitis[269,282]. NeuroD1 is located in progenitors originated from Neurog3 progenitors[289,290]. Mice deficient in NeuroD1 lacks certain types of enteroendocrine cells[53,291]. These findings show that the differentiation progeny toward endocrine cells is affected in animal models of human IBD.
Switching the expression of NEPA on and off: As mentioned above, mature GI endocrine cells can express up to seven different hormones[51,52,61-64]. It seems that the changes in the proportion of GI endocrine cells during inflammation occur via switching off the synthesis of a neuroendocrine peptide/amine and switching on the synthesis of another[270]. Such a phenomenon has been reported in rats with TNBS-induced colitis (Figure 8).
Hypothesis: It may be speculated that during the inflammation that occurs in active IBD, the IC produce proinflammatory cytokines and other substances that affect the GI stem cells and mature endocrine cells. This will induce abnormal clonogenic and differentiation activities of stem cells. Moreover, the mature endocrine cells switch off the expression of a certain hormone in favor of switching on the synthesis of another hormone. This would result in changes in the total density of endocrine cells and in the proportion of different endocrine cell types. NPA produced by the altered endocrine cells would in return affect the IC via their NPA receptors (Figure 9).
The changes in the intestinal NES associated with inflammation in IBD patients are believed to be useful tools for the diagnosis and follow-up of disease activity. Furthermore, the GI NEA could be candidate targets of IBD treatments. Thus, agonists to anti-inflammatory NEPA and antagonists to proinflammatory NEPA can be used not only for their pharmacological effects but also to correct a pre-existing imbalance in GI NEPA caused by inflammation.
The colonic CgA cell density has been shown to be a good biomarker for diagnosing lymphocytic colitis, with a high sensitivity and specificity[9]. The blood and fecal levels of CgA and secretogranins have been proposed for the diagnosis and follow-up of the disease activity in IBD[56,238-243].
Treatment with CgA-derived peptides of mice with DSS-induced colitis decreases the disease activity index, macroscopic and histology scores, and the colonic levels of IL-1β, IL-6, and TNFα[34].
Antagonists of serotonin receptors 5-HTR3 and 5-HTR7 such as tropisetron, granisetron, ondansetron, ramosetron, and SB-269970 have shown anti inflammatory effects in animal models of human IBD[292-300]. These serotonin receptor antagonists act via reducing the ynthesis of proinflammatory cytokines IL-1, IL-6, and TNFα. The usefulness of selective inhibition of mucosal serotonin by these receptor antagonists in the clinical treatment of IBD remains to be determined[301].
VIP is believed to be a potential agent for treating IBD since it targets both the innate and adaptive immune responses and inhibits the secretion of numerous proinflammatory cytokines via its actions on AP-1 and NFκB[142]. Administering VIP reduced inflammation in TNBS-induced colitis in mice[142], and it has been used successfully in the clinic as an inhalator for treating pulmonary hypertension and sarcoidosis[142]. However, delivering VIP is problematic since it is degraded rapidly in the blood circulation (with a half-life of only 1-2 min) and systemic administration causes both cardiovascular and intestinal side effects[140,302,303].
NPY occupies a key position during the inflammatory process in IBD, and NPY antagonists could be potentially useful in treatments for the inflammation in IBD[43]. This suggestion is supported by observations made in animal models of UC, namely DSS-induced colitis in rats[303,304]. Treatment with NPY antisense oligodeoxy-nucleotides in colitis induced by DSS in rats reduced the inflammation as well the concentration of NPY, TNFα, p-Akt, and asp-NFκB[304]. The NPY Y1 receptor is involved in several biological functions[302-305], and so using an NPY Y2 receptor antagonist is preferable in future clinical implications in order to minimize side effects.
Blocking substance P receptors with either substance P antibodies or with CP 96345 (NK-1R antagonist) diminished jejunal inflammation[306,307].
The effects of ghrelin treatment were tested in an animal model of human UC, namely TNBS-induced colitis in mice[147,307]. Ghrelin decreased both the clinical and histopathological severity of the colitis and increased the survival rate[147,307]. These effects seem to be attributable to the decrease of both inflammatory and Th1-driven autoimmune responses via affecting several inflammatory mediators, and by the involvement of IL-10/transforming growth factor-ß1-secreting regulatory T cells[147,307].
IBD is a chronic disease with unknown etiology that affects a large number of individuals worldwide. About 80% of the body IC are in the GI tract close to the NES of the gut. Several innate and adaptive IC express and release a considerable number of GI NEPA. Furthermore, the IC possess receptors for several GI NEPA. The enteroendocrine cells and the neurons of the ENS are abnormal during the inflammation that occurs in IBD. The changes in these two compartments of the GI NES are strongly correlated with the changes in IC in active IBD. These observations indicate the presence of interactions between GI NEPA and the immune system in active IBD. These interactions seem to play a significant role in the pathophysiology of IBD. The changes in the GI NEPA during active IBD occur in proinflammatory GI NEPA such as serotonin, members of the NPY family, and substance P, and in anti-inflammatory GI NEPA such as members of the chromogranin/secretogranin family, VIP, somatostatin, and ghrelin. Antagonists to the proinflammatory GI NEPA and agonists to the anti-inflammatory GI NEPA could therefore be useful tools for treating IBD.
| 1. | Prantera C, Marconi S. Glucocorticosteroids in the treatment of inflammatory bowel disease and approaches to minimizing systemic activity. Therap Adv Gastroenterol. 2013;6:137-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2770] [Article Influence: 115.4] [Reference Citation Analysis (3)] |
| 3. | Podolsky DK. The current future understanding of inflammatory bowel disease. Best Pract Res Clin Gastroenterol. 2002;16:933-943. [PubMed] |
| 4. | Carter MJ, Lobo AJ, Travis SP; IBD Section, British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53 Suppl 5:V1-V16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 777] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 5. | Rasmussen MA, Munck LK. Systematic review: are lymphocytic colitis and collagenous colitis two subtypes of the same disease - microscopic colitis? Aliment Pharmacol Ther. 2012;36:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Danese S, Fiocchi C. Etiopathogenesis of inflammatory bowel diseases. World J Gastroenterol. 2006;12:4807-4812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 196] [Cited by in RCA: 229] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Nunes T, Fiorino G, Danese S, Sans M. Familial aggregation in inflammatory bowel disease: is it genes or environment? World J Gastroenterol. 2011;17:2715-2722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Clinical presentation, diagnosis, pathogenesis and treatment options for lymphocytic colitis (Review). Int J Mol Med. 2013;32:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Chromogranin A cell density as a diagnostic marker for lymphocytic colitis. Dig Dis Sci. 2012;57:3154-3159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Baert F, Wouters K, D’Haens G, Hoang P, Naegels S, D’Heygere F, Holvoet J, Louis E, Devos M, Geboes K. Lymphocytic colitis: a distinct clinical entity? A clinicopathological confrontation of lymphocytic and collagenous colitis. Gut. 1999;45:375-381. [PubMed] |
| 11. | Mullhaupt B, Güller U, Anabitarte M, Güller R, Fried M. Lymphocytic colitis: clinical presentation and long term course. Gut. 1998;43:629-633. [PubMed] |
| 12. | Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517. [PubMed] |
| 13. | Loftus EV Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:1-20. [PubMed] |
| 14. | Saleh M, Elson CO. Experimental inflammatory bowel disease: insights into the host-microbiota dialog. Immunity. 2011;34:293-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Betteridge JD, Armbruster SP, Maydonovitch C, Veerappan GR. Inflammatory bowel disease prevalence by age, gender, race, and geographic location in the U.S. military health care population. Inflamm Bowel Dis. 2013;19:1421-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 16. | Andres PG, Friedman LS. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:255-281, vii. [PubMed] |
| 17. | Mendoza Ladd A, Jia Y, Yu C, Elhanafi S, Dwivedi A, Liu J, Song G, Hall M, Zuckerman MJ. Demographic and Clinical Characteristics of a Predominantly Hispanic Population with Inflammatory Bowel Disease on the US-Mexico Border. South Med J. 2016;109:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of Inflammatory Bowel Disease Among Adults Aged ≥18 Years - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1166-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 493] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 19. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1600] [Article Influence: 106.7] [Reference Citation Analysis (3)] |
| 20. | Yang SK, Loftus EV Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease in Asia. Inflamm Bowel Dis. 2001;7:260-270. [PubMed] |
| 21. | Goh K, Xiao SD. Inflammatory bowel disease: a survey of the epidemiology in Asia. J Dig Dis. 2009;10:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Sands BE. New therapies for the treatment of inflammatory bowel disease. Surg Clin North Am. 2006;86:1045-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Sands BE. The risks and benefits of early immunosuppression and biological therapy. Dig Dis. 2012;30 Suppl 3:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Prantera C, Pallone F, Brunetti G, Cottone M, Miglioli M. Oral 5-aminosalicylic acid (Asacol) in the maintenance treatment of Crohn’s disease. The Italian IBD Study Group. Gastroenterology. 1992;103:363-368. [PubMed] |
| 25. | Lopez A, Billioud V, Peyrin-Biroulet C, Peyrin-Biroulet L. Adherence to anti-TNF therapy in inflammatory bowel diseases: a systematic review. Inflamm Bowel Dis. 2013;19:1528-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 26. | Danese S. Anti TNF-alpha treatment for Crohn’ disease: “ménage a trois”. Curr Drug Targets. 2010;11:136-137. [PubMed] |
| 27. | Danese S, Angelucci E. New and emerging biologics in the treatment of inflammatory bowel disease: quo vadis? Gastroenterol Clin Biol. 2009;33 Suppl 3:S217-S227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Danese S, Angelucci E, Malesci A, Caprilli R. Biological agents for ulcerative colitis: hypes and hopes. Med Res Rev. 2008;28:201-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Danese S, Colombel JF, Peyrin-Biroulet L, Rutgeerts P, Reinisch W. Review article: the role of anti-TNF in the management of ulcerative colitis -- past, present and future. Aliment Pharmacol Ther. 2013;37:855-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Danese S, Colombel JF, Reinisch W, Rutgeerts PJ. Review article: infliximab for Crohn’s disease treatment--shifting therapeutic strategies after 10 years of clinical experience. Aliment Pharmacol Ther. 2011;33:857-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Danese S, Semeraro S, Armuzzi A, Papa A, Gasbarrini A. Biological therapies for inflammatory bowel disease: research drives clinics. Mini Rev Med Chem. 2006;6:771-784. [PubMed] |
| 32. | Furness JB, Kunze WA, Clerc N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol. 1999;277:G922-G928. [PubMed] |
| 33. | Shajib MS, Wang H, Kim JJ, Sunjic I, Ghia JE, Denou E, Collins M, Denburg JA, Khan WI. Interleukin 13 and serotonin: linking the immune and endocrine systems in murine models of intestinal inflammation. PLoS One. 2013;8:e72774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Khan WI, Ghia JE. Gut hormones: emerging role in immune activation and inflammation. Clin Exp Immunol. 2010;161:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Margolis KG, Gershon MD. Neuropeptides and inflammatory bowel disease. Curr Opin Gastroenterol. 2009;25:503-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Bampton PA, Dinning PG. High resolution colonic manometry--what have we learnt?--A review of the literature 2012. Curr Gastroenterol Rep. 2013;15:328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Ameri P, Ferone D. Diffuse endocrine system, neuroendocrine tumors and immunity: what’s new? Neuroendocrinology. 2012;95:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Margolis KG, Gershon MD. Enteric Neuronal Regulation of Intestinal Inflammation. Trends Neurosci. 2016;39:614-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 39. | Moran GW, Leslie FC, Levison SE, Worthington J, McLaughlin JT. Enteroendocrine cells: neglected players in gastrointestinal disorders? Therap Adv Gastroenterol. 2008;1:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Worthington JJ. The intestinal immunoendocrine axis: novel cross-talk between enteroendocrine cells and the immune system during infection and inflammatory disease. Biochem Soc Trans. 2015;43:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 41. | Shajib MS, Khan WI. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol (Oxf). 2015;213:561-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 274] [Article Influence: 24.9] [Reference Citation Analysis (1)] |
| 42. | El-Salhy M. Ghrelin in gastrointestinal diseases and disorders: a possible role in the pathophysiology and clinical implications (review). Int J Mol Med. 2009;24:727-732. [PubMed] |
| 43. | El-Salhy M, Hausken T. The role of the neuropeptide Y (NPY) family in the pathophysiology of inflammatory bowel disease (IBD). Neuropeptides. 2016;55:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | El-Salhy M, Mazzawi T, Gundersen D, Hatlebakk JG, Hausken T. The role of peptide YY in gastrointestinal diseases and disorders (review). Int J Mol Med. 2013;31:275-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | El-Salhy M, Suhr O, Danielsson A. Peptide YY in gastrointestinal disorders. Peptides. 2002;23:397-402. [PubMed] |
| 46. | El-Salhy M, Mazzawi T, Hausken T, Hatlebakk JG. Interaction between diet and gastrointestinal endocrine cells. Biomed Rep. 2016;4:651-656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | El-Salhy M, Hausken T, Gilja OH, Hatlebakk JG. The possible role of gastrointestinal endocrine cells in the pathophysiology of irritable bowel syndrome. Expert Rev Gastroenterol Hepatol. 2017;11:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | May CL, Kaestner KH. Gut endocrine cell development. Mol Cell Endocrinol. 2010;323:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 49. | Gunawardene AR, Corfe BM, Staton CA. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int J Exp Pathol. 2011;92:219-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 50. | Massironi S, Zilli A, Cavalcoli F, Conte D, Peracchi M. Chromogranin A and other enteroendocrine markers in inflammatory bowel disease. Neuropeptides. 2016;58:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Egerod KL, Engelstoft MS, Grunddal KV, Nøhr MK, Secher A, Sakata I, Pedersen J, Windeløv JA, Füchtbauer EM, Olsen J. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153:5782-5795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 256] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 52. | Engelstoft MS, Egerod KL, Lund ML, Schwartz TW. Enteroendocrine cell types revisited. Curr Opin Pharmacol. 2013;13:912-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Schonhoff SE, Giel-Moloney M, Leiter AB. Minireview: Development and differentiation of gut endocrine cells. Endocrinology. 2004;145:2639-2644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 237] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 54. | Shulkes A. Gastrointestinal hormones: from basic science to a clinical perspective. Aust N Z J Surg. 1990;60:575-578. [PubMed] |
| 55. | Solcia E, Fiocca R, Rindi G, Villani L, Cornaggia M, Capella C. The pathology of the gastrointestinal endocrine system. Endocrinol Metab Clin North Am. 1993;22:795-821. [PubMed] |
| 56. | Zissimopoulos A, Vradelis S, Konialis M, Chadolias D, Bampali A, Constantinidis T, Efremidou E, Kouklakis G. Chromogranin A as a biomarker of disease activity and biologic therapy in inflammatory bowel disease: a prospective observational study. Scand J Gastroenterol. 2014;49:942-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Spångéus A, Forsgren S, el-Salhy M. Does diabetic state affect co-localization of peptide YY and enteroglucagon in colonic endocrine cells? Histol Histopathol. 2000;15:37-41. [PubMed] |
| 58. | Pyarokhil AH, Ishihara M, Sasaki M, Kitamura N. The developmental plasticity of colocalization pattern of peptide YY and glucagon-like peptide-1 in the endocrine cells of bovine rectum. Biomed Res. 2012;33:35-38. [PubMed] |
| 59. | Mortensen K, Christensen LL, Holst JJ, Orskov C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept. 2003;114:189-196. [PubMed] |
| 60. | El-Salhy M, Wilander E, Grimelius L. Immunocytochemical localization of gastric inhibitory peptide (GIP) in the human foetal pancreas. Ups J Med Sci. 1982;87:81-85. [PubMed] |
| 61. | Ghia JE, Li N, Wang H, Collins M, Deng Y, El-Sharkawy RT, Côté F, Mallet J, Khan WI. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 322] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 62. | Dryden S, Wang Q, Frankish HM, Pickavance L, Williams G. The serotonin (5-HT) antagonist methysergide increases neuropeptide Y (NPY) synthesis and secretion in the hypothalamus of the rat. Brain Res. 1995;699:12-18. [PubMed] |
| 63. | Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CA, Parker HE, Morley TC, Yeo GS, Reimann F, Gribble FM. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. 2012;153:3054-3065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 310] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 64. | Habib AM, Richards P, Rogers GJ, Reimann F, Gribble FM. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia. 2013;56:1413-1416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 65. | Sandström O, El-Salhy M. Ageing and endocrine cells of human duodenum. Mech Ageing Dev. 1999;108:39-48. [PubMed] |
| 66. | Tolhurst G, Reimann F, Gribble FM. Intestinal sensing of nutrients. Handb Exp Pharmacol. 2012;309-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 67. | Lee J, Cummings BP, Martin E, Sharp JW, Graham JL, Stanhope KL, Havel PJ, Raybould HE. Glucose sensing by gut endocrine cells and activation of the vagal afferent pathway is impaired in a rodent model of type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol. 2012;302:R657-R666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | Parker HE, Reimann F, Gribble FM. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev Mol Med. 2010;12:e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 69. | Raybould HE. Nutrient sensing in the gastrointestinal tract: possible role for nutrient transporters. J Physiol Biochem. 2008;64:349-356. [PubMed] |
| 70. | San Gabriel A, Nakamura E, Uneyama H, Torii K. Taste, visceral information and exocrine reflexes with glutamate through umami receptors. J Med Invest. 2009;56 Suppl:209-217. [PubMed] |
| 71. | Rudholm T, Wallin B, Theodorsson E, Näslund E, Hellström PM. Release of regulatory gut peptides somatostatin, neurotensin and vasoactive intestinal peptide by acid and hyperosmolal solutions in the intestine in conscious rats. Regul Pept. 2009;152:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of ‘taste’ in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes. 2008;15:73-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 283] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 73. | Sternini C. Taste receptors in the gastrointestinal tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing. Am J Physiol Gastrointest Liver Physiol. 2007;292:G457-G461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 74. | Buchan AM. Nutrient Tasting and Signaling Mechanisms in the Gut III. Endocrine cell recognition of luminal nutrients. Am J Physiol. 1999;277:G1103-G1107. [PubMed] |
| 75. | Montero-Hadjadje M, Elias S, Chevalier L, Benard M, Tanguy Y, Turquier V, Galas L, Yon L, Malagon MM, Driouich A. Chromogranin A promotes peptide hormone sorting to mobile granules in constitutively and regulated secreting cells: role of conserved N- and C-terminal peptides. J Biol Chem. 2009;284:12420-12431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 76. | Shooshtarizadeh P, Zhang D, Chich JF, Gasnier C, Schneider F, Haïkel Y, Aunis D, Metz-Boutigue MH. The antimicrobial peptides derived from chromogranin/secretogranin family, new actors of innate immunity. Regul Pept. 2010;165:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 77. | Reber SO, Obermeier F, Straub RH, Falk W, Neumann ID. Chronic intermittent psychosocial stress (social defeat/overcrowding) in mice increases the severity of an acute DSS-induced colitis and impairs regeneration. Endocrinology. 2006;147:4968-4976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 78. | Milde AM, Murison R. A study of the effects of restraint stress on colitis induced by dextran sulphate sodium in singly housed rats. Integr Physiol Behav Sci. 2002;37:140-150. [PubMed] |
| 79. | Hassani H, Lucas G, Rozell B, Ernfors P. Attenuation of acute experimental colitis by preventing NPY Y1 receptor signaling. Am J Physiol Gastrointest Liver Physiol. 2005;288:G550-G556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 80. | Cani PD, Everard A, Duparc T. Gut microbiota, enteroendocrine functions and metabolism. Curr Opin Pharmacol. 2013;13:935-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 272] [Article Influence: 20.9] [Reference Citation Analysis (1)] |
| 81. | Cani PD, Hoste S, Guiot Y, Delzenne NM. Dietary non-digestible carbohydrates promote L-cell differentiation in the proximal colon of rats. Br J Nutr. 2007;98:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 82. | Petitto JM, Huang Z, McCarthy DB. Molecular cloning of NPY-Y1 receptor cDNA from rat splenic lymphocytes: evidence of low levels of mRNA expression and [125I]NPY binding sites. J Neuroimmunol. 1994;54:81-86. [PubMed] |
| 83. | De la Fuente M, Bernaez I, Del Rio M, Hernanz A. Stimulation of murine peritoneal macrophage functions by neuropeptide Y and peptide YY. Involvement of protein kinase C. Immunology. 1993;80:259-265. [PubMed] |
| 84. | Shibata M, Hisajima T, Nakano M, Goris RC, Funakoshi K. Morphological relationships between peptidergic nerve fibers and immunoglobulin A-producing lymphocytes in the mouse intestine. Brain Behav Immun. 2008;22:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 85. | Painsipp E, Herzog H, Sperk G, Holzer P. Sex-dependent control of murine emotional-affective behaviour in health and colitis by peptide YY and neuropeptide Y. Br J Pharmacol. 2011;163:1302-1314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 86. | El-Salhy M, Grimelius L, Wilander E, Abu-Sinna G, Lundqvist G. Histological and immunohistochemical studies of the endocrine cells of the gastrointestinal mucosa of the toad (Bufo regularis). Histochemistry. 1981;71:53-65. [PubMed] |
| 87. | Sandstrom O. Age-related changes in the neuroendocrine system of the gut. Umea. Univ Med Diss. 1999;617:1-46. |
| 88. | Bohórquez DV, Chandra R, Samsa LA, Vigna SR, Liddle RA. Characterization of basal pseudopod-like processes in ileal and colonic PYY cells. J Mol Histol. 2011;42:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 89. | Gustafsson BI, Bakke I, Hauso Ø, Kidd M, Modlin IM, Fossmark R, Brenna E, Waldum HL. Parietal cell activation by arborization of ECL cell cytoplasmic projections is likely the mechanism for histamine induced secretion of hydrochloric acid. Scand J Gastroenterol. 2011;46:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 90. | Gustafsson BI, Bakke I, Tømmerås K, Waldum HL. A new method for visualization of gut mucosal cells, describing the enterochromaffin cell in the rat gastrointestinal tract. Scand J Gastroenterol. 2006;41:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Pang XH, Li TK, Xie Q, He FQ, Cui DJ, Chen YQ, Huang XL, Gan HT. Amelioration of dextran sulfate sodium-induced colitis by neuropeptide Y antisense oligodeoxynucleotide. Int J Colorectal Dis. 2010;25:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 92. | Bohórquez DV, Samsa LA, Roholt A, Medicetty S, Chandra R, Liddle RA. An enteroendocrine cell-enteric glia connection revealed by 3D electron microscopy. PLoS One. 2014;9:e89881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 93. | Bohórquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, Wang F, Liddle RA. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest. 2015;125:782-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 374] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 94. | Rindi G, Inzani F, Solcia E. Pathology of gastrointestinal disorders. Endocrinol Metab Clin North Am. 2010;39:713-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 95. | Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 811] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 96. | Bertrand PP. The cornucopia of intestinal chemosensory transduction. Front Neurosci. 2009;3:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 97. | Bertrand PP, Bertrand RL. Serotonin release and uptake in the gastrointestinal tract. Auton Neurosci. 2010;153:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 210] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 98. | El-Salhy M. On the phylogeny of the gastro-entero-pancreatic (GEP) neuroendocrine system. Acta Uni Uppsal. 1981;385:1-39. |
| 99. | El-Salhy M, Abou-el-Ela R, Falkmer S, Grimelius L, Wilander E. Immunohistochemical evidence of gastro-entero-pancreatic neurohormonal peptides of vertebrate type in the nervous system of the larva of a dipteran insect, the hoverfly, Eristalis aeneus. Regul Pept. 1980;1:187-204. [PubMed] |
| 100. | El-Salhy M, Falkmer S, Kramer KJ, Speirs RD. Immunohistochemical investigations of neuropeptides in the brain, corpora cardiaca, and corpora allata of an adult lepidopteran insect, Manduca sexta (L). Cell Tissue Res. 1983;232:295-317. [PubMed] |
| 101. | El-Salhy M, Falkmer S, Kramer KJ, Speirs RD. Immunocytochemical evidence for the occurrence of insulin in the frontal ganglion of a Lepidopteran insect, the tobacco hornworm moth, Manduca sexta L. Gen Comp Endocrinol. 1984;54:85-88. [PubMed] |
| 102. | Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G775-G785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 291] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 103. | Camilleri M. Physiological underpinnings of irritable bowel syndrome: neurohormonal mechanisms. J Physiol. 2014;592:2967-2980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 104. | Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2012;367:1626-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 234] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 105. | El-Salhy M, Seim I, Chopin L, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Front Biosci (Elite Ed). 2012;4:2783-2800. [PubMed] |
| 106. | Kaunitz J, Nayyar P. Bugs, genes, fatty acids, and serotonin: Unraveling inflammatory bowel disease? F1000Res. 2015;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 107. | Kirsner JB. Historical aspects of inflammatory bowel disease. J Clin Gastroenterol. 1988;10:286-297. [PubMed] |
| 108. | Eswaran S, Muir J, Chey WD. Fiber and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:718-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 303] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 109. | Chutkan R, Fahey G, Wright WL, McRorie J. Viscous versus nonviscous soluble fiber supplements: mechanisms and evidence for fiber-specific health benefits. J Am Acad Nurse Pract. 2012;24:476-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 110. | Zimmerman MA, Singh N, Martin PM, Thangaraju M, Ganapathy V, Waller JL, Shi H, Robertson KD, Munn DH, Liu K. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1405-G1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 111. | Klampfer L, Huang J, Sasazuki T, Shirasawa S, Augenlicht L. Inhibition of interferon gamma signaling by the short chain fatty acid butyrate. Mol Cancer Res. 2003;1:855-862. [PubMed] |
| 112. | Stempelj M, Kedinger M, Augenlicht L, Klampfer L. Essential role of the JAK/STAT1 signaling pathway in the expression of inducible nitric-oxide synthase in intestinal epithelial cells and its regulation by butyrate. J Biol Chem. 2007;282:9797-9804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 113. | Holzer P, Farzi A. Neuropeptides and the microbiota-gut-brain axis. Adv Exp Med Biol. 2014;817:195-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 311] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 114. | Everard A, Cani PD. Gut microbiota and GLP-1. Rev Endocr Metab Disord. 2014;15:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 115. | Arora T, Loo RL, Anastasovska J, Gibson GR, Tuohy KM, Sharma RK, Swann JR, Deaville ER, Sleeth ML, Thomas EL. Differential effects of two fermentable carbohydrates on central appetite regulation and body composition. PLoS One. 2012;7:e43263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 116. | Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, Neunlist M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138:1772-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 390] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 117. | El-Salhy M, Danielsson A, Stenling R, Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413-419. [PubMed] |
| 118. | Buffa R, Marè P, Gini A, Salvadore M. Chromogranins A and B and secretogranin II in hormonally identified endocrine cells of the gut and the pancreas. Basic Appl Histochem. 1988;32:471-484. [PubMed] |
| 119. | D'amico MA, Ghinassi B, Izzicupo P, Manzoli L, Di Baldassarre A. Biological function and clinical relevance of chromogranin A and derived peptides. Endocr Connect. 2014;3:R45-R54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 120. | Helle KB. Regulatory peptides from chromogranin A and secretogranin II: putative modulators of cells and tissues involved in inflammatory conditions. Regul Pept. 2010;165:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 121. | El-Salhy M, Lomholt-Beck B, Hausken T. Chromogranin A as a possible tool in the diagnosis of irritable bowel syndrome. Scand J Gastroenterol. 2010;45:1435-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 122. | Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 635] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 123. | Wiedenmann B, Huttner WB. Synaptophysin and chromogranins/secretogranins--widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;58:95-121. [PubMed] |
| 124. | Deftos LJ. Chromogranin A: its role in endocrine function and as an endocrine and neuroendocrine tumor marker. Endocr Rev. 1991;12:181-187. [PubMed] |
| 125. | Spiller R. Serotonin and GI clinical disorders. Neuropharmacology. 2008;55:1072-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 178] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 126. | Egger M, Beer AG, Theurl M, Schgoer W, Hotter B, Tatarczyk T, Vasiljevic D, Frauscher S, Marksteiner J, Patsch JR. Monocyte migration: a novel effect and signaling pathways of catestatin. Eur J Pharmacol. 2008;598:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 127. | Feistritzer C, Mosheimer BA, Colleselli D, Wiedermann CJ, Kähler CM. Effects of the neuropeptide secretoneurin on natural killer cell migration and cytokine release. Regul Pept. 2005;126:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 128. | Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 988] [Cited by in RCA: 1162] [Article Influence: 61.2] [Reference Citation Analysis (2)] |
| 129. | Shahbazkhani B, Forootan M, Merat S, Akbari MR, Nasserimoghadam S, Vahedi H, Malekzadeh R. Coeliac disease presenting with symptoms of irritable bowel syndrome. Aliment Pharmacol Ther. 2003;18:231-235. [PubMed] |
| 130. | Baganz NL, Blakely RD. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem Neurosci. 2013;4:48-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 131. | Ahern GP. 5-HT and the immune system. Curr Opin Pharmacol. 2011;11:29-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 132. | Cloëz-Tayarani I, Changeux JP. Nicotine and serotonin in immune regulation and inflammatory processes: a perspective. J Leukoc Biol. 2007;81:599-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 133. | Stefulj J, Cicin-Sain L, Schauenstein K, Jernej B. Serotonin and immune response: effect of the amine on in vitro proliferation of rat lymphocytes. Neuroimmunomodulation. 2001;9:103-108. [PubMed] |
| 134. | Betten A, Dahlgren C, Hermodsson S, Hellstrand K. Serotonin protects NK cells against oxidatively induced functional inhibition and apoptosis. J Leukoc Biol. 2001;70:65-72. [PubMed] |
| 135. | Laberge S, Cruikshank WW, Beer DJ, Center DM. Secretion of IL-16 (lymphocyte chemoattractant factor) from serotonin-stimulated CD8+ T cells in vitro. J Immunol. 1996;156:310-315. [PubMed] |
| 136. | Soga F, Katoh N, Inoue T, Kishimoto S. Serotonin activates human monocytes and prevents apoptosis. J Invest Dermatol. 2007;127:1947-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 137. | León-Ponte M, Ahern GP, O’Connell PJ. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood. 2007;109:3139-3146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 258] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 138. | Wang H, Steeds J, Motomura Y, Deng Y, Verma-Gandhu M, El-Sharkawy RT, McLaughlin JT, Grencis RK, Khan WI. CD4+ T cell-mediated immunological control of enterochromaffin cell hyperplasia and 5-hydroxytryptamine production in enteric infection. Gut. 2007;56:949-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 139. | El-Salhy M. Gastrointestinal transit in nonobese diabetic mouse: an animal model of human diabetes type 1. J Diabetes Complications. 2001;15:277-284. [PubMed] |
| 140. | Henning RJ, Sawmiller DR. Vasoactive intestinal peptide: cardiovascular effects. Cardiovasc Res. 2001;49:27-37. [PubMed] |
| 141. | Polak JM, Bloom SR. Regulatory peptides of the gastrointestinal and respiratory tracts. Arch Int Pharmacodyn Ther. 1986;280:16-49. [PubMed] |
| 142. | Ganea D, Hooper KM, Kong W. The neuropeptide vasoactive intestinal peptide: direct effects on immune cells and involvement in inflammatory and autoimmune diseases. Acta Physiol (Oxf). 2015;213:442-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 143. | Chedid P, Boussetta T, Dang PM, Belambri SA, Marzaioli V, Fasseau M, Walker F, Couvineau A, El-Benna J, Marie JC. Vasoactive intestinal peptide dampens formyl-peptide-induced ROS production and inflammation by targeting a MAPK-p47<sup>phox</sup> phosphorylation pathway in monocytes. Mucosal Immunol. 2017;10:332-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 144. | Delgado M, Ganea D. Cutting edge: is vasoactive intestinal peptide a type 2 cytokine? J Immunol. 2001;166:2907-2912. [PubMed] |
| 145. | Leceta J, Martinez MC, Delgado M, Garrido E, Gomariz RP. Lymphoid cell subpopulations containing vasoactive intestinal peptide in the rat. Peptides. 1994;15:791-797. [PubMed] |
| 146. | Gonzalez-Rey E, Delgado M. Anti-inflammatory neuropeptide receptors: new therapeutic targets for immune disorders? Trends Pharmacol Sci. 2007;28:482-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 147. | Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56:249-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 310] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 148. | Chorny A, Delgado M. Neuropeptides rescue mice from lethal sepsis by down-regulating secretion of the late-acting inflammatory mediator high mobility group box 1. Am J Pathol. 2008;172:1297-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 149. | Delgado M, Ganea D. Neuroprotective effect of vasoactive intestinal peptide (VIP) in a mouse model of Parkinson’s disease by blocking microglial activation. FASEB J. 2003;17:944-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 150. | Delgado M, Ganea D. Vasoactive intestinal peptide inhibits IL-8 production in human monocytes. Biochem Biophys Res Commun. 2003;301:825-832. [PubMed] |
| 151. | Higyno PM, Mendes PF, Miranda MB, Pereira DE, Mota AP, Nogueira Kde O, Caldas IS, Moura SA, Menezes CA. Vasoactive intestinal peptide reduces the inflammatory profile in mice infected with Trypanosoma cruzi. Exp Parasitol. 2015;159:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 152. | Ran WZ, Dong L, Tang CY, Zhou Y, Sun GY, Liu T, Liu YP, Guan CX. Vasoactive intestinal peptide suppresses macrophage-mediated inflammation by downregulating interleukin-17A expression via PKA- and PKC-dependent pathways. Int J Exp Pathol. 2015;96:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 153. | Delgado M. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit the MEKK1/MEK4/JNK signaling pathway in endotoxin-activated microglia. Biochem Biophys Res Commun. 2002;293:771-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 154. | Delgado M, Ganea D. Inhibition of endotoxin-induced macrophage chemokine production by VIP and PACAP in vitro and in vivo. Arch Physiol Biochem. 2001;109:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 155. | Carrión M, Pérez-García S, Martínez C, Juarranz Y, Estrada-Capetillo L, Puig-Kröger A, Gomariz RP, Gutiérrez-Cañas I. VIP impairs acquisition of the macrophage proinflammatory polarization profile. J Leukoc Biol. 2016;100:1385-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 156. | Tatemoto K, Mutt V. Isolation of two novel candidate hormones using a chemical method for finding naturally occurring polypeptides. Nature. 1980;285:417-418. [PubMed] |
| 157. | Tatemoto K. Isolation and characterization of peptide YY (PYY), a candidate gut hormone that inhibits pancreatic exocrine secretion. Proc Natl Acad Sci USA. 1982;79:2514-2518. [PubMed] |
| 158. | Tatemoto K, Siimesmaa S, Jörnvall H, Allen JM, Polak JM, Bloom SR, Mutt V. Isolation and characterization of neuropeptide Y from porcine intestine. FEBS Lett. 1985;179:181-184. [PubMed] |
| 159. | Tatemoto K. Neuropeptide Y: complete amino acid sequence of the brain peptide. Proc Natl Acad Sci USA. 1982;79:5485-5489. [PubMed] |
| 160. | Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070-1077. [PubMed] |
| 161. | Vona-Davis LC, McFadden DW. NPY family of hormones: clinical relevance and potential use in gastrointestinal disease. Curr Top Med Chem. 2007;7:1710-1720. [PubMed] |
| 162. | El-Salhy M, Grimelius L, Wilander E, Ryberg B, Terenius L, Lundberg JM, Tatemoto K. Immunocytochemical identification of polypeptide YY (PYY) cells in the human gastrointestinal tract. Histochemistry. 1983;77:15-23. [PubMed] |
| 163. | El-Salhy M, Wilander E, Grimelius L, Terenius L, Lundberg JM, Tatemoto K. The distribution of polypeptide YY (PYY) - and pancreatic polypeptide (PP) - immunoreactive cells in the domestic fowl. Histochemistry. 1982;75:25-30. [PubMed] |
| 164. | El-Salhy M, Wilander E, Juntti-Berggren L, Grimelius L. The distribution and ontogeny of polypeptide YY (PYY)- and pancreatic polypeptide (PP)-immunoreactive cells in the gastrointestinal tract of rat. Histochemistry. 1983;78:53-60. [PubMed] |
| 165. | El-Salhy M, Gundersen D, Gilja OH, Hatlebakk JG, Hausken T. Is irritable bowel syndrome an organic disorder? World J Gastroenterol. 2014;20:384-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 166. | Cox HM, Tough IR. Neuropeptide Y, Y1, Y2 and Y4 receptors mediate Y agonist responses in isolated human colon mucosa. Br J Pharmacol. 2002;135:1505-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 167. | Cox HM, Pollock EL, Tough IR, Herzog H. Multiple Y receptors mediate pancreatic polypeptide responses in mouse colon mucosa. Peptides. 2001;22:445-452. [PubMed] |
| 168. | Hyland NP, Cox HM. The regulation of veratridine-stimulated electrogenic ion transport in mouse colon by neuropeptide Y (NPY), Y1 and Y2 receptors. Br J Pharmacol. 2005;146:712-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 169. | Hyland NP, Sjöberg F, Tough IR, Herzog H, Cox HM. Functional consequences of neuropeptide Y Y 2 receptor knockout and Y2 antagonism in mouse and human colonic tissues. Br J Pharmacol. 2003;139:863-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 170. | Wheway J, Mackay CR, Newton RA, Sainsbury A, Boey D, Herzog H, Mackay F. A fundamental bimodal role for neuropeptide Y1 receptor in the immune system. J Exp Med. 2005;202:1527-1538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 171. | Chandrasekharan B, Nezami BG, Srinivasan S. Emerging neuropeptide targets in inflammation: NPY and VIP. Am J Physiol Gastrointest Liver Physiol. 2013;304:G949-G957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 172. | Dimitrijević M, Stanojević S, Vujić V, Beck-Sickinger A, von Hörsten S. Neuropeptide Y and its receptor subtypes specifically modulate rat peritoneal macrophage functions in vitro: counter regulation through Y1 and Y2/5 receptors. Regul Pept. 2005;124:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 173. | Dimitrijević M, Stanojević S, Mitić K, Kustrimović N, Vujić V, Miletić T, Kovacević-Jovanović V. Modulation of granulocyte functions by peptide YY in the rat: age-related differences in Y receptors expression and plasma dipeptidyl peptidase 4 activity. Regul Pept. 2010;159:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 174. | Severini C, Improta G, Falconieri-Erspamer G, Salvadori S, Erspamer V. The tachykinin peptide family. Pharmacol Rev. 2002;54:285-322. [PubMed] |
| 175. | Ekblad E, Winther C, Ekman R, Håkanson R, Sundler F. Projections of peptide-containing neurons in rat small intestine. Neuroscience. 1987;20:169-188. [PubMed] |
| 176. | Brodin E, Sjölund K, Håkanson R, Sundler F. Substance P-containing nerve fibers are numerous in human but not in feline intestinal mucosa. Gastroenterology. 1983;85:557-564. [PubMed] |
| 177. | Lai JP, Douglas SD, Ho WZ. Human lymphocytes express substance P and its receptor. J Neuroimmunol. 1998;86:80-86. [PubMed] |
| 178. | Lai JP, Douglas SD, Zhao M, Ho WZ. Quantification of substance P mRNA in human mononuclear phagocytes and lymphocytes using a mimic-based RT-PCR. J Immunol Methods. 1999;230:149-157. [PubMed] |
| 179. | Marriott I, Bost KL. IL-4 and IFN-gamma up-regulate substance P receptor expression in murine peritoneal macrophages. J Immunol. 2000;165:182-191. [PubMed] |
| 180. | Lambrecht BN, Germonpré PR, Everaert EG, Carro-Muino I, De Veerman M, de Felipe C, Hunt SP, Thielemans K, Joos GF, Pauwels RA. Endogenously produced substance P contributes to lymphocyte proliferation induced by dendritic cells and direct TCR ligation. Eur J Immunol. 1999;29:3815-3825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 181. | Metwali A, Blum AM, Ferraris L, Klein JS, Fiocchi C, Weinstock JV. Eosinophils within the healthy or inflamed human intestine produce substance P and vasoactive intestinal peptide. J Neuroimmunol. 1994;52:69-78. [PubMed] |
| 182. | Weinstock JV, Blum A, Walder J, Walder R. Eosinophils from granulomas in murine schistosomiasis mansoni produce substance P. J Immunol. 1988;141:961-966. [PubMed] |
| 183. | Mashaghi A, Marmalidou A, Tehrani M, Grace PM, Pothoulakis C, Dana R. Neuropeptide substance P and the immune response. Cell Mol Life Sci. 2016;73:4249-4264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 347] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 184. | Guo CJ, Lai JP, Luo HM, Douglas SD, Ho WZ. Substance P up-regulates macrophage inflammatory protein-1beta expression in human T lymphocytes. J Neuroimmunol. 2002;131:160-167. [PubMed] |
| 185. | Sun J, Ramnath RD, Zhi L, Tamizhselvi R, Bhatia M. Substance P enhances NF-kappaB transactivation and chemokine response in murine macrophages via ERK1/2 and p38 MAPK signaling pathways. Am J Physiol Cell Physiol. 2008;294:C1586-C1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 186. | Lembeck F, Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979;310:175-183. [PubMed] |
| 187. | Ahluwalia A, De Felipe C, O’Brien J, Hunt SP, Perretti M. Impaired IL-1beta-induced neutrophil accumulation in tachykinin NK1 receptor knockout mice. Br J Pharmacol. 1998;124:1013-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 188. | Castellani ML, Vecchiet J, Salini V, Conti P, Theoharides TC, Caraffa A, Antinolfi P, Teté S, Ciampoli C, Cuccurullo C. Stimulation of CCL2 (MCP-1) and CCL2 mRNA by substance P in LAD2 human mast cells. Transl Res. 2009;154:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 189. | O'Connor TM, O’Connell J, O’Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 579] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 190. | Gross KJ, Pothoulakis C. Role of neuropeptides in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:918-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 191. | Simeonidis S, Castagliuolo I, Pan A, Liu J, Wang CC, Mykoniatis A, Pasha A, Valenick L, Sougioultzis S, Zhao D. Regulation of the NK-1 receptor gene expression in human macrophage cells via an NF-kappa B site on its promoter. Proc Natl Acad Sci USA. 2003;100:2957-2962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 192. | Patel YC, Wheatley T, Ning C. Multiple forms of immunoreactive somatostatin: comparison of distribution in neural and nonneural tissues and portal plasma of the rat. Endocrinology. 1981;109:1943-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 135] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 193. | Shulkes A. Somatostatin: physiology and clinical applications. Baillieres Clin Endocrinol Metab. 1994;8:215-236. [PubMed] |
| 194. | Penman E, Wass JA, Butler MG, Penny ES, Price J, Wu P, Rees LH. Distribution and characterisation of immunoreactive somatostatin in human gastrointestinal tract. Regul Pept. 1983;7:53-65. [PubMed] |
| 195. | Ferone D, Resmini E, Boschetti M, Arvigo M, Albanese V, Ceresola E, Pivonello R, Albertelli M, Bianchi F, Giusti M. Potential indications for somatostatin analogues: immune system and limphoproliferative disorders. J Endocrinol Invest. 2005;28:111-117. [PubMed] |
| 196. | ten Bokum AM, Hofland LJ, van Hagen PM. Somatostatin and somatostatin receptors in the immune system: a review. Eur Cytokine Netw. 2000;11:161-176. [PubMed] |
| 197. | Ferone D, Pivonello R, Kwekkeboom DJ, Gatto F, Ameri P, Colao A, de Krijger RR, Minuto F, Lamberts SW, van Hagen PM. Immunohistochemical localization and quantitative expression of somatostatin receptors in normal human spleen and thymus: Implications for the in vivo visualization during somatostatin receptor scintigraphy. J Endocrinol Invest. 2012;35:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 198. | Ferone D, Pivonello R, Van Hagen PM, Dalm VA, Lichtenauer-Kaligis EG, Waaijers M, Van Koetsveld PM, Mooy DM, Colao A, Minuto F. Quantitative and functional expression of somatostatin receptor subtypes in human thymocytes. Am J Physiol Endocrinol Metab. 2002;283:E1056-E1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 199. | Dalm VA, van Hagen PM, van Koetsveld PM, Achilefu S, Houtsmuller AB, Pols DH, van der Lely AJ, Lamberts SW, Hofland LJ. Expression of somatostatin, cortistatin, and somatostatin receptors in human monocytes, macrophages, and dendritic cells. Am J Physiol Endocrinol Metab. 2003;285:E344-E353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 138] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 200. | Lichtenauer-Kaligis EG, Dalm VA, Oomen SP, Mooij DM, van Hagen PM, Lamberts SW, Hofland LJ. Differential expression of somatostatin receptor subtypes in human peripheral blood mononuclear cell subsets. Eur J Endocrinol. 2004;150:565-577. [PubMed] |
| 201. | Armani C, Catalani E, Balbarini A, Bagnoli P, Cervia D. Expression, pharmacology, and functional role of somatostatin receptor subtypes 1 and 2 in human macrophages. J Leukoc Biol. 2007;81:845-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 202. | Taniyama Y, Suzuki T, Mikami Y, Moriya T, Satomi S, Sasano H. Systemic distribution of somatostatin receptor subtypes in human: an immunohistochemical study. Endocr J. 2005;52:605-611. [PubMed] |
| 203. | Hagströmer L, Emtestam L, Stridsberg M, Talme T. Expression pattern of somatostatin receptor subtypes 1-5 in human skin: an immunohistochemical study of healthy subjects and patients with psoriasis or atopic dermatitis. Exp Dermatol. 2006;15:950-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 204. | Talme T, Ivanoff J, Hägglund M, Van Neerven RJ, Ivanoff A, Sundqvist KG. Somatostatin receptor (SSTR) expression and function in normal and leukaemic T-cells. Evidence for selective effects on adhesion to extracellular matrix components via SSTR2 and/or 3. Clin Exp Immunol. 2001;125:71-79. [PubMed] |
| 205. | Rosskopf D, Schürks M, Manthey I, Joisten M, Busch S, Siffert W. Signal transduction of somatostatin in human B lymphoblasts. Am J Physiol Cell Physiol. 2003;284:C179-C190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 206. | Casnici C, Lattuada D, Perego C, Franco P, Marelli O. Inhibitory effect of somatostatin on human T lymphocytes proliferation. Int J Immunopharmacol. 1997;19:721-727. [PubMed] |
| 207. | Radosević-Stasić B, Trobonjaca Z, Lucin P, Cuk M, Polić B, Rukavina D. Immunosuppressive and antiproliferative effects of somatostatin analog SMS 201-995. Int J Neurosci. 1995;81:283-297. [PubMed] |
| 208. | Sirianni MC, Annibale B, Fais S, Delle Fave G. Inhibitory effect of somatostatin-14 and some analogues on human natural killer cell activity. Peptides. 1994;15:1033-1036. [PubMed] |
| 209. | Eglezos A, Andrews PV, Helme RD. In vivo inhibition of the rat primary antibody response to antigenic stimulation by somatostatin. Immunol Cell Biol. 1993;71:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 210. | Goetzl EJ, Payan DG. Inhibition by somatostatin of the release of mediators from human basophils and rat leukemic basophils. J Immunol. 1984;133:3255-3259. [PubMed] |
| 211. | Payan DG, Hess CA, Goetzl EJ. Inhibition by somatostatin of the proliferation of T-lymphocytes and Molt-4 lymphoblasts. Cell Immunol. 1984;84:433-438. [PubMed] |
| 212. | Malec P, Zeman K, Markiewicz K, Tchórzewski H, Nowak Z, Baj Z. Short-term somatostatin infusion affects T lymphocyte responsiveness in humans. Immunopharmacology. 1989;17:45-49. [PubMed] |
| 213. | Pawlikowski M, Stepien H, Kunert-Radek J, Schally AV. Effect of somatostatin on the proliferation of mouse spleen lymphocytes in vitro. Biochem Biophys Res Commun. 1985;129:52-55. [PubMed] |
| 214. | Helyes Z, Elekes K, Németh J, Pozsgai G, Sándor K, Kereskai L, Börzsei R, Pintér E, Szabó A, Szolcsányi J. Role of transient receptor potential vanilloid 1 receptors in endotoxin-induced airway inflammation in the mouse. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1173-L1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 215. | Helyes Z, Pintér E, Sándor K, Elekes K, Bánvölgyi A, Keszthelyi D, Szoke E, Tóth DM, Sándor Z, Kereskai L. Impaired defense mechanism against inflammation, hyperalgesia, and airway hyperreactivity in somatostatin 4 receptor gene-deleted mice. Proc Natl Acad Sci USA. 2009;106:13088-13093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 216. | Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255-4261. [PubMed] |
| 217. | Hosoda H, Kojima M, Kangawa K. Ghrelin and the regulation of food intake and energy balance. Mol Interv. 2002;2:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 218. | Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5961] [Cited by in RCA: 5959] [Article Influence: 220.7] [Reference Citation Analysis (0)] |
| 219. | Kojima M, Hosoda H, Kangawa K. Purification and distribution of ghrelin: the natural endogenous ligand for the growth hormone secretagogue receptor. Horm Res. 2001;56 Suppl 1:93-97. [PubMed] |
| 220. | Kojima M, Hosoda H, Matsuo H, Kangawa K. Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol Metab. 2001;12:118-122. [PubMed] |
| 221. | Hataya Y, Akamizu T, Takaya K, Kanamoto N, Ariyasu H, Saijo M, Moriyama K, Shimatsu A, Kojima M, Kangawa K. A low dose of ghrelin stimulates growth hormone (GH) release synergistically with GH-releasing hormone in humans. J Clin Endocrinol Metab. 2001;86:4552. [PubMed] |
| 222. | Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. [PubMed] |
| 223. | Cheung CK, Wu JC. Role of ghrelin in the pathophysiology of gastrointestinal disease. Gut Liver. 2013;7:505-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 224. | Korbonits M, Goldstone AP, Gueorguiev M, Grossman AB. Ghrelin--a hormone with multiple functions. Front Neuroendocrinol. 2004;25:27-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 397] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 225. | Waseem T, Duxbury M, Ito H, Ashley SW, Robinson MK. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery. 2008;143:334-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 193] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 226. | Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW Jr, Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 275] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 227. | Peracchi M, Bardella MT, Caprioli F, Massironi S, Conte D, Valenti L, Ronchi C, Beck-Peccoz P, Arosio M, Piodi L. Circulating ghrelin levels in patients with inflammatory bowel disease. Gut. 2006;55:432-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 228. | Mawe GM. Colitis-induced neuroplasticity disrupts motility in the inflamed and post-inflamed colon. J Clin Invest. 2015;125:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 229. | Furness JB, Jones C, Nurgali K, Clerc N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog Neurobiol. 2004;72:143-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 266] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 230. | Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207-G216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 218] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 231. | Linden DR, Foley KF, McQuoid C, Simpson J, Sharkey KA, Mawe GM. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol Motil. 2005;17:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 232. | Linden DR, Sharkey KA, Mawe GM. Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon. J Physiol. 2003;547:589-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 160] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 233. | Lomax AE, Mawe GM, Sharkey KA. Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig. J Physiol. 2005;564:863-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 234. | Roberts JA, Lukewich MK, Sharkey KA, Furness JB, Mawe GM, Lomax AE. The roles of purinergic signaling during gastrointestinal inflammation. Curr Opin Pharmacol. 2012;12:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 235. | Strong DS, Cornbrooks CF, Roberts JA, Hoffman JM, Sharkey KA, Mawe GM. Purinergic neuromuscular transmission is selectively attenuated in ulcerated regions of inflamed guinea pig distal colon. J Physiol. 2010;588:847-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 236. | Roberts JA, Durnin L, Sharkey KA, Mutafova-Yambolieva VN, Mawe GM. Oxidative stress disrupts purinergic neuromuscular transmission in the inflamed colon. J Physiol. 2013;591:3725-3737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 237. | Roberts SA, Hendry JH, Potten CS. Intestinal crypt clonogens: a new interpretation of radiation survival curve shape and clonogenic cell number. Cell Prolif. 2003;36:215-231. [PubMed] |
| 238. | Conlon JM. Granin-derived peptides as diagnostic and prognostic markers for endocrine tumors. Regul Pept. 2010;165:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 239. | Sciola V, Massironi S, Conte D, Caprioli F, Ferrero S, Ciafardini C, Peracchi M, Bardella MT, Piodi L. Plasma chromogranin a in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:867-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 240. | Sidhu R, Drew K, McAlindon ME, Lobo AJ, Sanders DS. Elevated serum chromogranin A in irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD): a shared model for pathogenesis? Inflamm Bowel Dis. 2010;16:361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 241. | Spadaro A, Ajello A, Morace C, Zirilli A, D’arrigo G, Luigiano C, Martino F, Bene A, Migliorato D, Turiano S. Serum chromogranin-A in hepatocellular carcinoma: diagnostic utility and limits. World J Gastroenterol. 2005;11:1987-1990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 242. | Strid H, Simrén M, Lasson A, Isaksson S, Stridsberg M, Öhman L. Fecal chromogranins and secretogranins are increased in patients with ulcerative colitis but are not associated with disease activity. J Crohns Colitis. 2013;7:e615-e622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 243. | Wagner M, Stridsberg M, Peterson CG, Sangfelt P, Lampinen M, Carlson M. Increased fecal levels of chromogranin A, chromogranin B, and secretoneurin in collagenous colitis. Inflammation. 2013;36:855-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 244. | El-Salhy M, Hatlebakk JG. Changes in enteroendocrine and immune cells following colitis induction by TNBS in rats. Mol Med Rep. 2016;14:4967-4974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 245. | El-Salhy M, Hatlebakk JG, Gilja OH. Abnormalities in endocrine and immune cells are correlated in dextransulfatesodiuminduced colitis in rats. Mol Med Rep. 2017;15:12-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 246. | El-Salhy M, Lomholt-Beck B, Gundersen TD. High chromogranin A cell density in the colon of patients with lymphocytic colitis. Mol Med Rep. 2011;4:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 247. | El-Salhy M, Umezawa K. Treatment with novel AP-1 and NF-κB inhibitors restores the colonic endocrine cells to normal levels in rats with DSS-induced colitis. Int J Mol Med. 2016;37:556-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 248. | El-Salhy M, Umezawa K. Effects of AP1 and NFκB inhibitors on colonic endocrine cells in rats with TNBSinduced colitis. Mol Med Rep. 2016;14:1515-1522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 249. | Hernández-Trejo JA, Suárez-Pérez D, Gutiérrez-Martínez IZ, Fernandez-Vargas OE, Serrano C, Candelario-Martínez AA, Meraz-Ríos MA, Citalán-Madrid AF, Hernández-Ruíz M, Reyes-Maldonado E. The pro-inflammatory cytokines IFNγ/TNFα increase chromogranin A-positive neuroendocrine cells in the colonic epithelium. Biochem J. 2016;473:3805-3818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 250. | El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. High densities of serotonin and peptide YY cells in the colon of patients with lymphocytic colitis. World J Gastroenterol. 2012;18:6070-6075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 251. | Qian BF, El-Salhy M, Melgar S, Hammarström ML, Danielsson A. Neuroendocrine changes in colon of mice with a disrupted IL-2 gene. Clin Exp Immunol. 2000;120:424-433. [PubMed] |
| 252. | O'Morain C, Bishop AE, McGregor GP, Levi AJ, Bloom SR, Polak JM, Peters TJ. Vasoactive intestinal peptide concentrations and immunocytochemical studies in rectal biopsies from patients with inflammatory bowel disease. Gut. 1984;25:57-61. [PubMed] |
| 253. | Mazumdar S, Das KM. Immunocytochemical localization of vasoactive intestinal peptide and substance P in the colon from normal subjects and patients with inflammatory bowel disease. Am J Gastroenterol. 1992;87:176-181. [PubMed] |
| 254. | Kimura M, Masuda T, Hiwatashi N, Toyota T, Nagura H. Changes in neuropeptide-containing nerves in human colonic mucosa with inflammatory bowel disease. Pathol Int. 1994;44:624-634. [PubMed] |
| 255. | Padua D, Vu JP, Germano PM, Pisegna JR. The Role of Neuropeptides in Mouse Models of Colitis. J Mol Neurosci. 2016;59:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 256. | Chandrasekharan B, Bala V, Kolachala VL, Vijay-Kumar M, Jones D, Gewirtz AT, Sitaraman SV, Srinivasan S. Targeted deletion of neuropeptide Y (NPY) modulates experimental colitis. PLoS One. 2008;3:e3304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 257. | Björck S, Jennische E, Dahlström A, Ahlman H. Influence of topical rectal application of drugs on dextran sulfate-induced colitis in rats. Dig Dis Sci. 1997;42:824-832. [PubMed] |
| 258. | El-Salhy M, Mazzawi T, Umezawa K, Gilja OH. Enteroendocrine cells, stem cells and differentiation progenitors in rats with TNBS-induced colitis. Int J Mol Med. 2016;38:1743-1751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 259. | Koch TR, Carney JA, Go VL. Distribution and quantitation of gut neuropeptides in normal intestine and inflammatory bowel diseases. Dig Dis Sci. 1987;32:369-376. [PubMed] |
| 260. | Bernstein CN, Robert ME, Eysselein VE. Rectal substance P concentrations are increased in ulcerative colitis but not in Crohn’s disease. Am J Gastroenterol. 1993;88:908-913. [PubMed] |
| 261. | Sjölund K, Schaffalitzky OB, Muckadell DE, Fahrenkrug J, Håkanson R, Peterson BG, Sundler F. Peptide-containing nerve fibres in the gut wall in Crohn’s disease. Gut. 1983;24:724-733. [PubMed] |
| 262. | Kimura K, Chen D, Lindström E, Yamada H, Zhao CM, Håkanson R. Functional impairment of the individual rat stomach ECL cell in response to sustained hypergastrinemia. Regul Pept. 1997;72:69-77. [PubMed] |
| 263. | Watanabe T, Kubota Y, Sawada T, Muto T. Distribution and quantification of somatostatin in inflammatory disease. Dis Colon Rectum. 1992;35:488-494. [PubMed] |
| 264. | Koch TR, Carney JA, Morris VA, Go VL. Somatostatin in the idiopathic inflammatory bowel diseases. Dis Colon Rectum. 1988;31:198-203. [PubMed] |
| 265. | Ahonen A, Kyösola K, Penttilä O. Enterochromaffin cells in macrophages in ulcerative colitis and irritable colon. Ann Clin Res. 1976;8:1-7. [PubMed] |
| 266. | Eissa N, Ghia JE. Immunomodulatory effect of ghrelin in the intestinal mucosa. Neurogastroenterol Motil. 2015;27:1519-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 267. | Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Voudouri T, Kouroumalis EA. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 237] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 268. | Ates Y, Degertekin B, Erdil A, Yaman H, Dagalp K. Serum ghrelin levels in inflammatory bowel disease with relation to disease activity and nutritional status. Dig Dis Sci. 2008;53:2215-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 269. | Hosomi S, Oshitani N, Kamata N, Sogawa M, Yamagami H, Watanabe K, Tominaga K, Watanabe T, Fujiwara Y, Maeda K. Phenotypical and functional study of ghrelin and its receptor in the pathogenesis of Crohn’s disease. Inflamm Bowel Dis. 2008;14:1205-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 270. | El-Salhy M, Umezawa K, Hatlebakk JG, Gilja OH. Abnormal differentiation of stem cells into enteroendocrine cells in rats with DSS-induced colitis. Mol Med Rep. 2017;15:2106-2112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 271. | Darlington GJ. Molecular mechanisms of liver development and differentiation. Curr Opin Cell Biol. 1999;11:678-682. [PubMed] |
| 272. | Cardoso WV, Lü J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 427] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 273. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1225] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 274. | Rawlins EL, Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol. 2008;295:L231-L234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 229] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 275. | Barker N, Clevers H. Tracking down the stem cells of the intestine: strategies to identify adult stem cells. Gastroenterology. 2007;133:1755-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 276. | Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 487] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 277. | Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4485] [Article Influence: 236.1] [Reference Citation Analysis (0)] |
| 278. | Barker N, van Oudenaarden A, Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell. 2012;11:452-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 250] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 279. | Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1207] [Cited by in RCA: 1218] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 280. | Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1050] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 281. | Rawdon BB, Andrew A. Origin and differentiation of gut endocrine cells. Histol Histopathol. 1993;8:567-580. [PubMed] |
| 282. | Hoffman J, Kuhnert F, Davis CR, Kuo CJ. Wnts as essential growth factors for the adult small intestine and colon. Cell Cycle. 2004;3:554-557. [PubMed] |
| 283. | Montgomery RK, Breault DT. Small intestinal stem cell markers. J Anat. 2008;213:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 284. | Potten CS, Booth C, Tudor GL, Booth D, Brady G, Hurley P, Ashton G, Clarke R, Sakakibara S, Okano H. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation. 2003;71:28-41. [PubMed] |
| 285. | Kayahara T, Sawada M, Takaishi S, Fukui H, Seno H, Fukuzawa H, Suzuki K, Hiai H, Kageyama R, Okano H. Candidate markers for stem and early progenitor cells, Musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett. 2003;535:131-135. [PubMed] |
| 286. | He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 371] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 287. | Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 728] [Article Influence: 29.1] [Reference Citation Analysis (7)] |
| 288. | Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002;21:6338-6347. [PubMed] |
| 289. | Wang J, Cortina G, Wu SV, Tran R, Cho JH, Tsai MJ, Bailey TJ, Jamrich M, Ament ME, Treem WR. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med. 2006;355:270-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 229] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 290. | Lee CS, Perreault N, Brestelli JE, Kaestner KH. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 2002;16:1488-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 240] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 291. | Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 1997;11:2323-2334. [PubMed] |
| 292. | Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development. 1996;122:1409-1416. [PubMed] |
| 293. | Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol. 2004;270:443-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 243] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 294. | Fakhfouri G, Rahimian R, Daneshmand A, Bahremand A, Rasouli MR, Dehpour AR, Mehr SE, Mousavizadeh K. Granisetron ameliorates acetic acid-induced colitis in rats. Hum Exp Toxicol. 2010;29:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 295. | Mousavizadeh K, Rahimian R, Fakhfouri G, Aslani FS, Ghafourifar P. Anti-inflammatory effects of 5-HT receptor antagonist, tropisetron on experimental colitis in rats. Eur J Clin Invest. 2009;39:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 296. | Vega Lde L, Muñoz E, Calzado MA, Lieb K, Candelario-Jalil E, Gschaidmeir H, Färber L, Mueller W, Stratz T, Fiebich BL. The 5-HT3 receptor antagonist tropisetron inhibits T cell activation by targeting the calcineurin pathway. Biochem Pharmacol. 2005;70:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 297. | Motavallian-Naeini A, Minaiyan M, Rabbani M, Mahzuni P. Anti-inflammatory effect of ondansetron through 5-HT3 receptors on TNBS-induced colitis in rat. EXCLI J. 2012;11:30-44. [PubMed] |
| 298. | Kato S. Role of serotonin 5-HT3 receptors in intestinal inflammation. Biol Pharm Bull. 2013;36:1406-1409. [PubMed] |
| 299. | Fakhfouri G, Rahimian R, Ghia JE, Khan WI, Dehpour AR. Impact of 5-HT3 receptor antagonists on peripheral and central diseases. Drug Discov Today. 2012;17:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 300. | Kim JJ, Bridle BW, Ghia JE, Wang H, Syed SN, Manocha MM, Rengasamy P, Shajib MS, Wan Y, Hedlund PB. Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation. J Immunol. 2013;190:4795-4804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 301. | Levin AD, van den Brink GR. Selective inhibition of mucosal serotonin as treatment for IBD? Gut. 2014;63:866-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 302. | Chu TG, Orlowski M. Soluble metalloendopeptidase from rat brain: action on enkephalin-containing peptides and other bioactive peptides. Endocrinology. 1985;116:1418-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 122] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 303. | Bloom SR, Polak JM, Pearse AG. Vasoactive intestinal peptide and watery-diarrhoea syndrome. Lancet. 1973;2:14-16. [PubMed] |
| 304. | Balasubramaniam AA. Neuropeptide Y family of hormones: receptor subtypes and antagonists. Peptides. 1997;18:445-457. [PubMed] |
| 305. | Agro A, Stanisz AM. Inhibition of murine intestinal inflammation by anti-substance P antibody. Reg Immunol. 1993;5:120-126. [PubMed] |
| 306. | Kataeva G, Agro A, Stanisz AM. Substance-P-mediated intestinal inflammation: inhibitory effects of CP 96,345 and SMS 201-995. Neuroimmunomodulation. 1994;1:350-356. [PubMed] |
| 307. | Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology. 2006;130:1707-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 207] [Article Influence: 10.4] [Reference Citation Analysis (5)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Norway
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Gassler N S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF