Published online Jul 21, 2017. doi: 10.3748/wjg.v23.i27.5034
Peer-review started: March 29, 2017
First decision: May 12, 2017
Revised: June 9, 2017
Accepted: June 18, 2017
Article in press: June 19, 2017
Published online: July 21, 2017
Processing time: 117 Days and 18.5 Hours
We are reporting a rare case of acute liver injury that developed after an internal hemorrhoid treatment with the aluminum potassium sulfate and tannic acid (ALTA) regimen. A 41-year-old man developed a fever and liver injury after undergoing internal hemorrhoid treatment with a submucosal injection of ALTA with lidocaine. The acute liver injury was classified clinically as hepatocellular and pathologically as cholestastic. We could not classify the mechanism of injury. High eosinophil and immunoglobulin E levels characterized the injury, and a drug lymphocyte stimulation test was negative on postoperative day 25. Fluid replacement for two weeks after hospitalization improved the liver injury. ALTA therapy involves injecting chemicals into the submucosa, from the rectum to the anus, and this is the first description of a case that developed a severe liver disorder after this treatment; hence, an analysis of future cases as they accumulate is desirable.
Core tip: The definition of drug-induced liver injury has diversified in recent years. This report describes the characteristics of a case of acute liver injury that developed after internal hemorrhoid treatment using the aluminum potassium sulfate and tannic acid regimen, and it is the first report of a case of drug-induced liver injury caused by a rectal submucosal injection.
- Citation: Yoshikawa K, Kawashima R, Hirose Y, Shibata K, Akasu T, Hagiwara N, Yokota T, Imai N, Iwaku A, Kobayashi G, Kobayashi H, Kinoshita A, Fushiya N, Kijima H, Koike K, Saruta M. Liver injury after aluminum potassium sulfate and tannic acid treatment of hemorrhoids. World J Gastroenterol 2017; 23(27): 5034-5040
- URL: https://www.wjgnet.com/1007-9327/full/v23/i27/5034.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i27.5034
Drug-induced liver injury (DILI) is a liver disorder caused by the administration of drugs, but in recent years the definition of this term has diversified to include supplements, health foods, and traditional Chinese medicines[1-3]. Drugs that cause hepatopathy are usually administered orally or intravenously, but there are few case reports that describe DILI cause by drugs administered by other routes, for example, subcutaneously or intravesically[4-6].
In this report, we describe a case of DILI that developed after internal hemorrhoid treatment with an aluminum potassium sulfate and tannic acid (ALTA) regimen with lidocaine. This is the first case report that describes the development and management of a severe liver disorder after ALTA therapy.
A 41-year-old Japanese man was admitted to our hospital with liver damage, itchy skin, and pyrexia. His medical history was unremarkable, but one year previously his gamma-glutamyl transpeptidase levels had risen to 271 IU/L (normal: < 30 IU/L). Prior to this, the patient had consumed 540 mL of sake three times a week, and he had been reducing the amount of alcohol he consumed for about one year. He did not smoke or take any recreational drugs. He was single, was not homosexual, and he had not had sexual intercourse for over one year.
The patient had undergone treatment of his internal hemorrhoids as an outpatient at a nearby hospital that comprised an aluminum potassium sulfate and tannic acid (ALTA) injection. He developed a fever of 38 °C, dark colored urine, and itchy skin on postoperative day (POD) 1, and he took loxoprofen sodium, as required. He consulted another doctor on POD 7, because the symptoms had not improved, and his blood test results led to a diagnosis of liver dysfunction. His liver dysfunction had not improved by POD 14, and he was admitted to our hospital on POD 15.
The patient was 167 cm tall and weighed 52.5 kg. He presented with a blood pressure of 103/59 mmHg, a heart rate of 82 beats per minute, a temperature of 36.6 °C, and 99% oxygen saturation in room air. No specific physical findings were evident during the clinical examination on the day of hospitalization, but the patient had a rash, edema, hepatosplenomegaly, lymphadenopathy, and jaundice. The subjective symptoms, comprising the fever, dark colored urine, and itchy skin, had disappeared by POD 10.
The laboratory test results showed that the patient’s complete blood count was almost normal with a white blood cell count of 6900 cells/μL, a hemoglobin concentration of 14.7 g/dL, a hematocrit level of 43.1%, and a platelet count of 3.98 × 105 cells/μL, but a higher percentage of eosinophils was present (6.8%) (normal: 1.0%-5.0%), which peaked at 13.9% on POD 20. The patient’s serum liver enzyme and bilirubin levels were elevated at the time of admission. The aspartate aminotransferase (AST) level was 432 IU/L (normal: 10-40 IU/L), the alanine aminotransferase (ALT) level was 911 IU/L (normal: 10-40 IU/L), the alkaline phosphatase (ALP) level was 473 IU/L (normal: 115-359 IU/L), and the total bilirubin level was 1.3 mg/dL (normal: 0.2-1.2 mg/dL). Thus, compared with the ALP level, the AST and ALT levels showed greater magnitudes of elevation. The patient’s other blood test results were within the normal ranges, as follows: serum albumin: 4.0 g/dL (normal: 3.8-5.2 g/dL); serum creatinine: 0.86 mg/dL (normal: 0.65-1.09 mg/dL); C-reactive protein: 0.1 mg/dL (normal: 0-0.3 mg/dL); prothrombin time: 100% (normal: 80%-120 %); thyroid-stimulating hormone: 0.5 μIU/mL (normal: 0.34-4.04 μIU/mL); triiodothyronine: 2.98 pg/dL (normal: 2.36-5.00 pg/dL); and thyroxin: 1.18 ng/dL (normal: 0.88-1.67 ng/dL). The serological tests for autoantibodies generated weakly positive results (1:40) for anti-nuclear antibodies, and negative results for anti-smooth muscle and anti-mitochondrial antibodies. Negative results were obtained from the tests for the hepatitis B virus surface antigen, the hepatitis C virus antibody, rapid plasma reagin, the anti-Epstein-Barr virus immunoglobulin M (IgM) antibody, and the anti-cytomegalovirus IgM antibody, and from the Treponema pallidum hemagglutination test. The IgG level was within the normal range at 1415 mg/dL (normal: 800-1600 mg/dL) and the IgM level was within the normal range at 100 mg/dL (normal: 60-250 mg/dL). The IgE level was elevated at 1333 mg/dL (normal: < 250 mg/dL). Chest radiography did not reveal any abnormalities. No morphological changes were evident following abdominal ultrasonography, magnetic resonance cholangiopancreatography, and abdominal computed tomography (Figure 1).
When the patient was hospitalized, we considered viral, alcohol-induced, autoimmune, drug-induced, biliary tract, and thyroid function-based diseases as frequent causes of acute liver dysfunction for the differential diagnosis, and, based on the patient’s medical history, and the serological and imaging results, we considered that alcohol and DILI were highly likely causes of the patient’s acute liver dysfunction. However, the patient had stopped drinking during the past year, so we thought that there was a high likelihood of DILI. Therefore, we began conservative treatment with a small amount of extracellular fluid replacement.
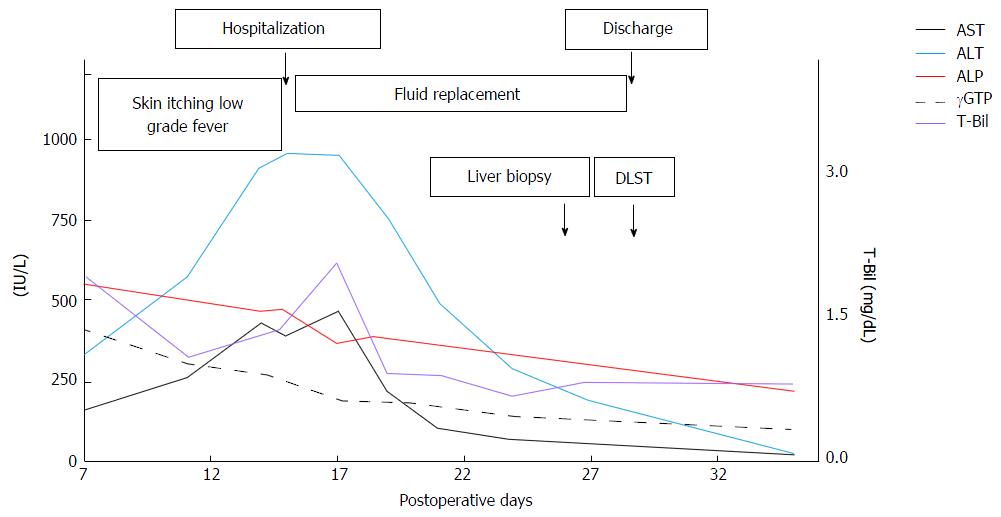
Figure 2 presents the patient’s symptoms, laboratory test results, and treatment over the entire course. The liver transaminase and bilirubin levels decreased rapidly after admission, and the maximum levels were as follows: ALT: 950 IU/L on POD 15; AST: 470 IU/L on POD 17; and total bilirubin: 2.1 mg/dL on POD 17. The ALP and gamma-glutamyl transpeptidase levels showed consistent downward trends from POD 7. The patient's symptoms disappeared on POD 10, and no new symptoms appeared after hospitalization. We performed a liver biopsy on POD 25, and a drug-induced lymphocyte stimulation test (DLST) of the components of the ALTA injection and lidocaine on POD 28. The patient was discharged on POD 29, and he was managed as an outpatient without any prescriptions. At the time of the patient’s first outpatient appointment on POD 35, his liver transaminase and bilirubin levels had declined and were almost within the normal ranges.
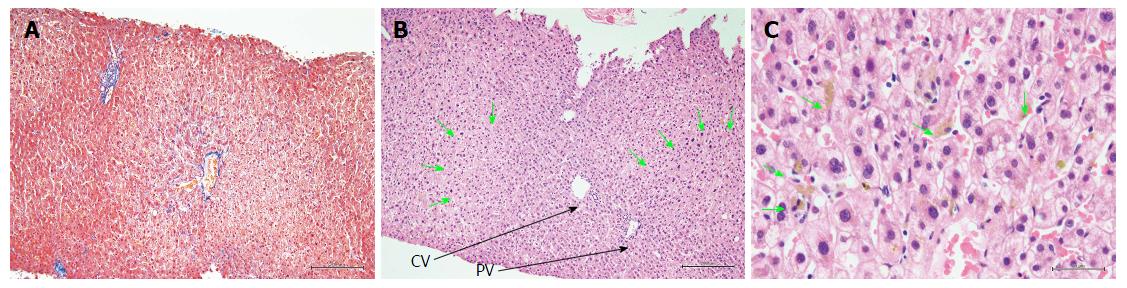
Figure 3 presents hematoxylin and eosin (H and E)-stained sections of the liver biopsy performed on POD 25. The H and E staining showed that the basic structure of the liver had been maintained without any hepatocyte dropout or disruption, and that the bile duct had not been disrupted. At a higher magnification, the H and E staining showed that the parenchymal cells were partially dilated and that a mild inflammatory cell infiltration was present in the area of the central vein. Neither eosinophil nor plasma cell infiltrations were detected within the liver tissue. Bile plugs were found at multiple sites within the parenchyma and sinusoids. Masson staining did not show the presence of liver tissue fibrosis.
The lymphocyte proliferation activity levels, which were determined from the DLST performed on POD 28, were 676 counts per minute (cpm) for the control and 871 cpm for the ALTA with lidocaine. The stimulation index, which was calculated as the ALTA with lidocaine cpm divided by the control cpm, was 1.29 (normal in Japanese people: < 1.8).
This is the first case report that describes suspected severe acute liver injury caused by ALTA therapy for the treatment of internal hemorrhoids. Our discussion focusses on the cause of the acute liver injury, the components of the ALTA regimen used to treat the hemorrhoids in this case, the positions of the biopsy and the DLST in the diagnosis, and the properties of the administration route.
We primarily suspected DILI caused by the ALTA therapy in this case. The laboratory test results ruled out viral, autoimmune, and biliary tract diseases, and a thyroid gland malfunction as causes of the acute hepatic injury. Given that the IgG levels were normal and the autoantibody test results were negative or only weakly positive in this case, a comprehensive judgement based on a histopathological evaluation was necessary[7]. Accounting for the consumption of alcohol during the pathological assessments was necessary based on the patient’s history. Interface hepatitis with lymphocytic/lymphoplasmacytic inflammatory activity, rosettes, and emperipolesis, which are characteristic of autoimmune hepatitis[8,9], and lobular neutrophilic infiltration, ballooning degeneration with apoptosis, and Mallory bodies, which are characteristic of alcoholic liver injury[10], were not observed in the pathological specimens from this case. Therefore, we diagnosed the patient as having DILI, which was a diagnosis of exclusion. This patient did not regularly use supplements or health foods, so the suspect drugs were loxoprofen and the ALTA with lidocaine injection. Loxoprofen is a non-steroidal anti-inflammatory agent, is one of the most frequently used drugs by patients with DILI[11], and it was used once by this patient. Given that it was used to treat the symptoms after their onset, we decided that loxoprofen did not cause this patient’s liver injury. Therefore, we concluded that the ALTA with lidocaine injection caused the acute liver injury in this case.
ALTA is an improved formulation of Xiaozhiling®[12], and ALTA injections were approved by the Japanese Government in 2005 for the treatment of hemorrhoids. Traditionally, 5% phenol almond oil was injected into the submucosal layer to treat internal hemorrhoids, which reduced the blood flow to the thick hemorrhoidal vein by causing an inflammatory reaction in the surrounding area and promoting fibrosis, and the short-term one-year results were good[12]. However, the long-term outcomes from phenol almond oil injections were not positive in relation to curing hemorrhoids or regarding the treatment of internal Goligher grade III and IV hemorrhoids. On the other hand, ALTA sclerotherapy is now widely used to treat internal hemorrhoids in Japan, because it performs well over one-to-five years, with cumulative successive rates at one, three, and five years of 95.9%, 89.3%, and 89.3%, respectively, for grade II hemorrhoids, and 93.1%, 83.7%, and 78.2%, respectively, for grade III hemorrhoids[13]. The treatment comprises a four-step process, and it is injected locally into the submucosa of the rectum and the anus via the lamina propria, and not into the vascular lumen[14]. Aluminum potassium sulfate causes inflammation inside the hemorrhoids followed by sclerosis, and tannic acid is an anti-inflammatory substance that prevents excessive inflammation. The complications associated with this treatment include bleeding (1.4%), pyrexia (1.4%), and rectal ulcers (1.4%)[15]; however, no case reports that describe acute liver injury following ALTA injections have been published.
The formulation used to treat the current case comprised aluminum potassium sulfate, tannic acid, and lidocaine. This patient had undergone local anesthesia involving the use of lidocaine in the past; hence, we considered that the likelihood of lidocaine involvement in the acute liver injury was low. Aluminum is less essential in living organisms, and it is generally considered to be less toxic. Clinically, aluminum encephalopathy can become a problem in dialysis patients, and the possibility of its accumulation in the central nervous system has been noted when renal excretion becomes insufficient[16]. Aluminum as aluminum chloride is used in an external preparation to manage hyperhidrosis, and sucralfate is a sucrose sulfate-aluminum complex that is used to treat peptic ulcers. Although a case report has been published that describes liver injury following sucralfate treatment[17], it is very rare and the underlying mechanism is unknown. There are no data that describe the transferability of aluminum into the blood following its external application or oral administration. In a rat model, intraperitoneal administrations of aluminum increased the aluminum concentrations in the blood and caused morphological changes in the liver, but the mechanism that underlies these changes remains unclear[18,19]. Tannic acid is used to treat gastric bleeding, diarrhea, and ulcerative colitis[20,21]. Although a liver disorder has been described that involved an advanced degree of destruction in the region of the central vein following the administration of a barium enema containing tannic acid, the causative component was not identified. Lidocaine is used as an anesthetic or as an antiarrhythmic agent, and only two cases of hepatopathy caused by tocainide, which is a lidocaine analog that is used as an oral preparation for ventricular arrhythmias, have been published[22,23].
Some scoring systems have been used to diagnose DILI[24], and the Digestive Disease Week Japan 2004 scale and the Roussel-Uclaf Causality Assessment Method are considered particularly useful[25-27]. The current case scored six points using these scoring systems, which suggested that DILI was probable in this patient. DILI is classified in several ways based on its clinical presentation, histological findings, and the hepatotoxicity mechanism[28]. When the current case was admitted, the R value, defined as the ALT level/the upper limit of the normal (ULN) ALT level divided by the ALP level/the ULN ALP level, was 17.3, and given that it was ≥ 5, we diagnosed DILI with a hepatocellular injury pattern[27]. DILI mechanisms can be separated into the “intrinsic” type, which is dose-dependent and can be predicted, and the “idiosyncratic” type, which is dose-independent and unpredictable, and the idiosyncratic type can be further classified into the immune-mediated and metabolic types[29]. Immune-mediated DILI develops one-to-eight weeks after the administration of the drug, and T-cell-dependent liver injury is accompanied by a positive DLST, because the drug or its metabolite binds to protein to acquire antigenicity. Metabolic DILI develops against a background of the genetic variations in the enzymes that metabolize drugs, it develops after the long-term oral administration of drugs, and the DLST is often negative, because the mechanism is not mediated by T cells. A mechanism has not been described for any hepatic disorder caused by the drugs used to treat this case. The DLST was negative at one-to-two weeks after the administration of the drugs, and the elevated eosinophil and IgE levels suggested that the DILI was immune mediated. The mechanism underlying this patient’s DILI cannot be specified, because there was a problem relating to the timing of the DLST that is described below; however, given that the DILI developed in association with specific agents, we think the mechanism was idiosyncratic hepatotoxicity in this case.
The histopathological patterns in DILI can be classified as the hepatocellular type, the cholestatic type, and the mixed type. A characteristic pathological feature of the hepatocellular type is cyclic necrosis in the central vein zone (zone 3), and biliary plugs in the bile capillaries are a characteristic pathological feature of the cholestatic type. The histological pattern is basically classified as cholestatic in the idiosyncratic clinical disease type[30], and the current case seemed to be consistent with the idiosyncratic type. A report that compares the clinical disease and the pathological types[31] explains that a pathological image can exhibit the cholestatic type, even if the clinical disease type is hepatocellular, which corresponds to the current case. Although the scoring systems can diagnose DILI with high levels of sensitivity and specificity, they may produce high scores for autoimmune hepatitis[31]. Drugs may cause autoimmune hepatitis, therefore, careful evaluations must be undertaken[32]. We confirmed the liver biopsy findings while taking this fact into account.
A DLST determines the proliferative response by lymphocytes to the addition of drugs. Based on the observed responses of sensitized T cells, positive DLST results tend to be generated by immune-mediated DILI[33]. Currently, DLST is positioned as one item that contributes to the scoring systems used to diagnose DILI, because it is only positive in association with some types of DILI[34], and because of the diversification of substances that have been determined to cause DILI in recent years. The number of reports describing DILI caused by supplements and health foods has increased in recent years[1-3], and the causal agents include garlic, egg yolk, turmeric, glucosamine, and noni juice[35]. Moreover, traditional Chinese medicine is thought to generate false-positive results from DLSTs[36], and their interpretation is often difficult. The DLST positive response rate for DILI overall is 45.7%[1].
On the other hand, Popple et al[37] and Pichler and Tilch[38] suggested that DLSTs should be undertaken following remission, that is, at about four-to-eight weeks, as the tests may generate false-negative results, because of the strong activation of regulatory T cells or the uneven distribution of memory T cells caused by ongoing hypersensitivity reactions[37,38]. Because the patient consent is not obtained, a second DLST cannot be conducted on samples from that patient. However, if the test appears to be positive, it may be possible to determine whether or not the mechanism is immune mediated.
DILI usually occurs following the oral or intravenous administration of drugs or when they are administered as suppositories. In relation to intravesical instillations, 18 cases (0.7%) of pneumonia or hepatitis occurred following Bacillus Calmette-Guérin therapy for bladder cancer[4]. Regarding the subcutaneous administration of drugs, insulin and interferon injections can cause hepatic injury[5,6]. This is the first report that describes a case of hepatic injury caused by the administration of drugs to the rectal submucosa. The median rectal vein and the inferior rectal vein are present in the middle-to-lower regions of the rectum, and it is possible that the administered drugs entered the systemic circulation from the submucosal blood vessels and caused DILI in this patient.
We have described the first case of DILI following an ALTA injection to treat internal hemorrhoids. The accumulation and analysis of further cases are necessary to clarify the mechanism, frequency, and the clinical variations of this disease type.
My deepest appreciation goes to Professor Masayuki Saruta whose comments and suggestions were of inestimable value to my report.
A Japanese male patient presented with fever, dark-colored urine, and itchy skin.
The authors diagnosed acute liver disorder after aluminum potassium sulfate and tannic acid (ALTA) therapy.
The diseases to be considered are liver injury induced by autoimmune hepatitis, alcohol, and other drugs, which can be estimated by liver biopsy.
The patient had elevated liver enzymes, with an aspartate aminotransferase level of 432 IU/L, an alanine aminotransferase level of 911 IU/L, an alkaline phosphatase level of 473 IU/L, and a total bilirubin level of 1.3 mg/dL.
Computed tomography scan showed no morphological changes.
Histological examination showed liver injury of the cholestatic type characterized by bile plugs within the parenchyma and sinusoids, with mild inflammation of hepatocytes around the area of the central vein.
The patient received conservative treatment, with a small amount of extracellular fluid replacement.
There is no other case report of acute liver injury associated with ALTA therapy. There are reports of a case of liver injury accompanying barium enema containing tannic acid and of two cases of liver injury accompanying lidocaine, but neither pathophysiology nor pathological features have been elucidated.
ALTA sclerotherapy is now widely used to treat internal hemorrhoids in Japan and consists of aluminum potassium sulfate, tannic acid and lidocaine.
This is the first case of drug-induced liver injury following an ALTA injection to treat internal hemorrhoids and discusses the clinical and pathological features. The accumulation and analysis of further cases are necessary to clarify the mechanism, frequency and clinical variations of this disease type.
This case report is very informative and well written. It should be worth sharing to people in the field as well as to the general public.
| 1. | Takikawa H, Murata Y, Horiike N, Fukui H, Onji M. Drug-induced liver injury in Japan: An analysis of 1676 cases between 1997 and 2006. Hepatol Res. 2009;39:427-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 2. | Stickel F, Patsenker E, Schuppan D. Herbal hepatotoxicity. J Hepatol. 2005;43:901-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Navarro VJ, Seeff LB. Liver injury induced by herbal complementary and alternative medicine. Clin Liver Dis. 2013;17:715-735, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Lamm DL, van der Meijden PM, Morales A, Brosman SA, Catalona WJ, Herr HW, Soloway MS, Steg A, Debruyne FM. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol. 1992;147:596-600. [PubMed] |
| 5. | Quesada JR, Talpaz M, Rios A, Kurzrock R, Gutterman JU. Clinical toxicity of interferons in cancer patients: a review. J Clin Oncol. 1986;4:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 413] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 6. | Tawata M, Ikeda M, Kodama Y, Aida K, Onaya T. A type 2 diabetic patient with liver dysfunction due to human insulin. Diabetes Res Clin Pract. 2000;49:17-21. [PubMed] |
| 7. | Balitzer D, Shafizadeh N, Peters MG, Ferrell LD, Alshak N, Kakar S. Autoimmune hepatitis: review of histologic features included in the simplified criteria proposed by the international autoimmune hepatitis group and proposal for new histologic criteria. Mod Pathol. 2017;30:773-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Papamichalis PA, Zachou K, Koukoulis GK, Veloni A, Karacosta EG, Kypri L, Mamaloudis I, Gabeta S, Rigopoulou EI, Lohse AW. The revised international autoimmune hepatitis score in chronic liver diseases including autoimmune hepatitis/overlap syndromes and autoimmune hepatitis with concurrent other liver disorders. J Autoimmune Dis. 2007;4:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Qiu D, Wang Q, Wang H, Xie Q, Zang G, Jiang H, Tu C, Guo J, Zhang S, Wang J. Validation of the simplified criteria for diagnosis of autoimmune hepatitis in Chinese patients. J Hepatol. 2011;54:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 10. | Singal AK, Kodali S, Vucovich LA, Darley-Usmar V, Schiano TD. Diagnosis and Treatment of Alcoholic Hepatitis: A Systematic Review. Alcohol Clin Exp Res. 2016;40:1390-1402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Björnsson ES. Hepatotoxicity by Drugs: The Most Common Implicated Agents. Int J Mol Sci. 2016;17:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 12. | Lim SW. Aluminum potassium sulfate and tannic Acid injection for hemorrhoids. J Korean Soc Coloproctol. 2012;28:73-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Miyamoto H, Hada T, Ishiyama G, Ono Y, Watanabe H. Aluminum potassium sulfate and tannic acid sclerotherapy for Goligher Grades II and III hemorrhoids: Results from a multicenter study. World J Hepatol. 2016;8:844-849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Takano M, Iwadare J, Ohba H, Takamura H, Masuda Y, Matsuo K, Kanai T, Ieda H, Hattori Y, Kurata S. Sclerosing therapy of internal hemorrhoids with a novel sclerosing agent. Comparison with ligation and excision. Int J Colorectal Dis. 2006;21:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Abe T, Hachiro Y, Ebisawa Y, Hishiyama H, Kunimoto M. Distal hemorrhoidectomy with ALTA injection: a new method for hemorrhoid surgery. Int Surg. 2014;99:295-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Alfrey AC, LeGendre GR, Kaehny WD. The dialysis encephalopathy syndrome. Possible aluminum intoxication. N Engl J Med. 1976;294:184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1246] [Cited by in RCA: 1003] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 17. | Odeh M, Oliven A. Hepatotoxicity related to sucralfate. Hepatol Res. 2001;20:255-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Kutlubay R, Oguz EO, Abban G, Turgut S. Amelioration of aluminium-induced liver damage by vitamin E. Saudi Med J. 2007;28:197-200. [PubMed] |
| 19. | Abubakar MG, Taylor A, Ferns GA. Aluminium administration is associated with enhanced hepatic oxidant stress that may be offset by dietary vitamin E in the rat. Int J Exp Pathol. 2003;84:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Lucke HH, Hodge KE, Patt NL. Fatal Liver Damage After Barium Enemas Containing Tannic Acid. Can Med Assoc J. 1963;89:1111-1114. [PubMed] |
| 21. | Hupkens P, Boxma H, Dokter J. Tannic acid as a topical agent in burns: historical considerations and implications for new developments. Burns. 1995;21:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Higa K, Hirata K, Dan K. Mexiletine-induced severe skin eruption, fever, eosinophilia, atypical lymphocytosis, and liver dysfunction. Pain. 1997;73:97-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Geisler C, Keiding S. [Cholestatic hepatitis during treatment with mexiletine/lidocaine]. Ugeskr Laeger. 1984;146:1212-1213. [PubMed] |
| 24. | Lucena MI, Camargo R, Andrade RJ, Perez-Sanchez CJ, Sanchez De La Cuesta F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001;33:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 167] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Tajiri K, Shimizu Y. Practical guidelines for diagnosis and early management of drug-induced liver injury. World J Gastroenterol. 2008;14:6774-6785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 127] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Hanatani T, Sai K, Tohkin M, Segawa K, Kimura M, Hori K, Kawakami J, Saito Y. A detection algorithm for drug-induced liver injury in medical information databases using the Japanese diagnostic scale and its comparison with the Council for International Organizations of Medical Sciences/the Roussel Uclaf Causality Assessment Method scale. Pharmacoepidemiol Drug Saf. 2014;23:984-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ; Practice Parameters Committee of the American College of Gastroenterology. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950-966; quiz 967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 522] [Article Influence: 43.5] [Reference Citation Analysis (1)] |
| 28. | Chang CY, Schiano TD. Review article: drug hepatotoxicity. Aliment Pharmacol Ther. 2007;25:1135-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Suk KT, Kim DJ. Drug-induced liver injury: present and future. Clin Mol Hepatol. 2012;18:249-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 30. | Maddur H, Chalasani N. Idiosyncratic drug-induced liver injury: a clinical update. Curr Gastroenterol Rep. 2011;13:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Tsutsui A, Nakanuma Y, Takaguchi K, Nakamura S, Shibata H, Baba N, Senoh T, Nagano T, Ikeda H. Comparison of Liver Biopsy Findings with the Digestive Disease Week Japan 2004 Scale for Diagnosis of Drug-Induced Liver Injury. Mediators Inflamm. 2015;2015:913793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Teschke R, Frenzel C. Drug induced liver injury: do we still need a routine liver biopsy for diagnosis today? Ann Hepatol. 2013;13:121-126. [PubMed] |
| 33. | Shibuya A, Watanabe M, Fujita Y, Saigenji K, Kuwao S, Takahashi H, Takeuchi H. An autopsy case of troglitazone-induced fulminant hepatitis. Diabetes Care. 1998;21:2140-2143. [PubMed] |
| 34. | Maria VA, Pinto L, Victorino RM. Lymphocyte reactivity to ex-vivo drug antigens in drug-induced hepatitis. J Hepatol. 1994;21:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Mrzljak A, Kosuta I, Skrtic A, Kanizaj TF, Vrhovac R. Drug-Induced Liver Injury Associated with Noni (Morinda citrifolia) Juice and Phenobarbital. Case Rep Gastroenterol. 2013;7:19-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Mantani N, Kogure T, Sakai S, Goto H, Shibahara N, Kita T, Shimada Y, Terasawa K. Incidence and clinical features of liver injury related to Kampo (Japanese herbal) medicine in 2,496 cases between 1979 and 1999: problems of the lymphocyte transformation test as a diagnostic method. Phytomedicine. 2002;9:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Popple A, Williams J, Maxwell G, Gellatly N, Dearman RJ, Kimber I. The lymphocyte transformation test in allergic contact dermatitis: New opportunities. J Immunotoxicol. 2016;13:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59:809-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 453] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Leardkamolkarn V S- Editor: Ma YJ L- Editor: A E- Editor: Li D