Published online Jul 14, 2017. doi: 10.3748/wjg.v23.i26.4701
Peer-review started: November 18, 2016
First decision: April 17, 2017
Revised: June 5, 2017
Accepted: June 18, 2017
Article in press: June 19, 2017
Published online: July 14, 2017
Processing time: 237 Days and 4.2 Hours
To determine the mechanistic role of fibrinogen, a key regulator of inflammation and fibrosis, in early and delayed radiation enteropathy.
Fibrinogen wild-type (Fib+/+), fibrinogen heterozygous (Fib+/-), and fibrinogen knockout (Fib-/-) mice were exposed to localized intestinal irradiation and assessed for early and delayed structural changes in the intestinal tissue. A 5-cm segment of ileum of mice was exteriorized and exposed to 18.5 Gy of x-irradiation. Intestinal tissue injury was assessed by quantitative histology, morphometry, and immunohistochemistry at 2 wk and 26 wk after radiation. Plasma fibrinogen level was measured by enzyme-linked immunosorbent assay.
There was no difference between sham-irradiated Fib+/+ and Fib+/- mice in terms of fibrinogen concentration in plasma and intestinal tissue, intestinal histology, morphometry, intestinal smooth muscle cell proliferation, and neutrophil infiltration. Therefore, Fib+/- mice were used as littermate controls. Unlike sham-irradiated Fib+/+ and Fib+/- mice, no fibrinogen was detected in the plasma and intestinal tissue of sham-irradiated Fib-/- mice. Moreover, fibrinogen level was not elevated after irradiation in the intestinal tissue of Fib-/- mice, while significant increase in intestinal fibrinogen level was noticed in irradiated Fib+/+ and Fib+/- mice. Importantly, irradiated Fib-/- mice exhibited substantially less overall intestinal structural injury (RIS, P = 0.000002), intestinal wall thickness (P = 0.003), intestinal serosal thickness (P = 0.009), collagen deposition (P = 0.01), TGF-β immunoreactivity (P = 0.03), intestinal smooth muscle proliferation (P = 0.046), neutrophil infiltration (P = 0.01), and intestinal mucosal injury (P = 0.0003), compared to irradiated Fib+/+ and Fib+/- mice at both 2 wk and 26 wk.
These data demonstrate that fibrinogen deficiency directly attenuates development of early and delayed radiation enteropathy. Fibrinogen could be a novel target in treating intestinal damage.
Core tip: Fibrinogen, a plasma protein, and fibrin (breakdown product of fibrinogen) induce inflammation, and fibrosis. Suppression of coagulation, inflammation, and fibrosis attenuate intestinal radiation injury. While fibrinogen has been presumed to be involved in intestinal fibrosis development, a direct role has only been supported by indirect evidence. We investigated the direct role of fibrinogen deficiency in early and delayed intestinal radiation injury. Radiation caused less intestinal injury in mice deficient in the fibrinogen gene, than in mice bearing two or one wild type fibrinogen alleles. We conclude that fibrinogen is essential for full-blown intestinal radiation fibrosis to occur.
- Citation: Wang J, Pathak R, Garg S, Hauer-Jensen M. Fibrinogen deficiency suppresses the development of early and delayed radiation enteropathy. World J Gastroenterol 2017; 23(26): 4701-4711
- URL: https://www.wjgnet.com/1007-9327/full/v23/i26/4701.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i26.4701
The intestine is an important dose-limiting organ during radiation therapy of abdominal and pelvic tumors. Despite advances in radiation delivery techniques having substantially reduced intestinal exposure and clinical bowel toxicity, early intestinal radiation toxicity (early radiation enteropathy) remains a significant clinical problem resulting in treatment delays, increased patient hospitalization rates and short term morbidity. Also, delayed radiation enteropathy continues to adversely affect the quality of life of a large number of long-term cancer survivors[1,2].
Radiation enteropathy is classified as early or delayed, depending on when it presents relative to radiation therapy. Early radiation enteropathy occurs during or within weeks of completion of radiation therapy. The cause is clonogenic crypt cell death, disruption of the epithelial barrier, and mucosal inflammation. Delayed radiation enteropathy is a chronic condition, characterized mainly by vascular sclerosis and progressive intestine wall fibrosis[1,2]. Clinical and experimental data from our laboratory show that intestinal radiation toxicity is associated with marked endothelial dysfunction, interstitial accumulation of enzymatically active thrombin, and interstitial fibrin deposition[3-5].
Fibrinogen, a plasma protein, and fibrin (breakdown product of fibrinogen), in addition to its known role in blood clotting, is considered a key modulator of tissue injury and inflammation[6-8], as well as in fibrosis development[9-13]. However, the putative significance of fibrinogen in radiation-induced inflammation and fibrosis is mostly based on correlative observations, and the results with pharmacological modulators of coagulation have been inconclusive. The availability of fibrinogen-deficient mice provides the means to directly evaluate the contribution of fibrinogen to radiation-induced intestinal injury. We used fibrinogen knockout (Fib-/-), heterozygous (Fib+/-), and homozygous (Fib+/+) mice to determine the role of fibrinogen in radiation-induced early and delayed intestinal injuries. The results showed that the lack of fibrinogen was associated with a significant protection from radiation-induced early and delayed intestinal injuries, thus confirming the role of fibrinogen.
A α-chain fibrinogen-deficient (Fib-/-) male and fibrinogen-heterozygous (Fib+/-) female breeder mice were a gift from Dr. Jay L Degen (Cincinnati Children’s Hospital, Cincinnati, OH, United States). Breeding was performed at Department of Laboratory Animal Medicine, University of Arkansas for Medical Sciences. The animals were housed in conventional cages with free access to tap drinking water and standard mouse chow (TD8640, Harlan Teklad, Madison, WI, United States). A pathogen-free environment with controlled humidity, temperature, and 12-12 h light-dark cycle was maintained. All experimental protocols were approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee.
Fib-/- mice lack all components of fibrinogen in the circulation[14]. Because A α-chain of fibrinogen is not the rate-limiting step for fibrinogen biosynthesis in normal mice, heterozygous (Fib+/-) mice maintain plasma fibrinogen concentrations that are 75%-100% of that in wild-type (Fib+/+) mice[15]. Both male and female mice were included in the study. Genotype analysis of mice was performed by mouse tail biopsy and PCR as described elsewhere[14]. Briefly, approximately 2-3 mm mouse tail was incubated with direct PCR Lysis Reagent (Viagen Biotech, Los Angeles, CA, United States) including freshly prepared proteinase K at 55 °C overnight and 2 μL of lysate was used per PCR reaction. The sequences of three primers were used in PCR reaction (kindly provided by Dr. Jay L Degen, Cincinnati Children’s Hospital, Cincinnati, OH, United States). 1). 5’-TAT TAC CAG TGA ATC TTT GTC AGC AG-3’, 2) 5’- TGC TGG ATC AAT CCC CAG CAA CCG TGA GAG-3’, and 3) 5’- GCT TCA GCT CCA GTT CTC CTC ATG AGC CAT-3’.
Mice were between 10 and 15 wk of age at the initiation of experiments. Mice were anesthetized with 60 mg/kg sodium pentobarbital administered intraperitoneally (Abbott Laboratories, Chicago, IL, United States). A 5 cm segment of ileum, located 10 cm from the ileocecal junction, was exteriorized through a midline abdominal incision and marked for future identification with a tantalum clip on the mesentery. The mice were placed on a heating pad (maintained at 38 °C). The exteriorized ileum was covered with saline-moistened gauze and exposed to 18.5 Gy localized single dose x-irradiation using a Seifert Isovovolt 320 X-ray machine (Seifert X-Ray Corp., Fairview Village, PA) operated at 250 kVp and 15 mA with 3 mm aluminum added filtration. The half-value layer was 0.85 mm Cu, and the dose rate was 4.49 Gy/min. Our previous experience with localized intestinal radiation model in mice suggests that 18.5 Gy radiation dose causes consistent structural, cellular, and molecular changes. After irradiation, the ileum was replaced into the abdomen and the incision was closed with 5-0 polypropylene. Antimicrobial prophylaxis was not used. Each strain of mice (Fib+/+, Fib+/-, Fib-/-) was randomly assigned into 2 groups (2 wk and 26 wk), which represent, respectively, early (acute) and delayed (chronic) radiation enteropathy in our model system[16]. This model allows for accurate delineation and dosimetry to the exposed intestinal segment, whereas, the rest of the intestine is not exposed to radiation.
Groups of mice were euthanized 2 wk and 26 wk after irradiation. Specimens of irradiated and un-irradiated intestine were procured and fixed in methanol-Carnoy’s solution for histological and immunohistochemical studies and snap-frozen for fibrinogen enzyme-linked immunosorbent assay (ELISA) analysis.
Radiation injury score: The overall severity of structural radiation injury was assessed using the radiation injury score (RIS) system. The RIS is a composite histopathological scoring system that provides a global measure of the severity of structural radiation injury. It has been extensively used and validated in our laboratory[16,17]. Briefly, we assessed and graded (from 0-3) seven histopathologic parameters of radiation injury (mucosal ulcerations, epithelial atypia, subserosal thickening, vascular sclerosis, intestinal wall fibrosis, ileitis cystica profunda, and lymph congestion). The sum of the scores for the individual alterations constitutes the RIS. All specimens were evaluated in a blinded fashion by two separate researchers.
Mucosal surface area: Mucosal surface area was measured in vertical sections using a stereologic projection/cycloid method as described by Baddeley et al[18] and adapted by us for use in our model system[19]. The advantage of this technique is that it does not require assumptions about the shape or orientation distribution of the specimens and thus circumvents problems associated with most other procedures for surface area measurement.
Thickness of the intestinal wall and subserosa: Intestinal wall thickening is a measure of both reactive intestinal wall fibrosis and intestinal smooth muscle cell hyperplasia. In contrast, subserosal thickening reflects mainly reactive fibrosis. Intestinal wall thickness (encompassing submucosa, muscularis externa, and subserosa) and subserosal thickness were measured with computer-assisted image analysis (Image-Pro Plus, Media Cybernetics, Silver Spring, MD). All measurements were done with a 10 × objective lens. A total of 5 areas, 500 μm apart, were chosen for the measurement, with 3 measurements taken per area. The average of all 5 areas was used as a single value for statistical calculations[20].
Immunohistochemical staining was performed with standard technique using avidin-biotin complex, diaminobenzidine chromogen, and hematoxylin counterstaining. Appropriate positive and negative controls were included. The primary antibodies, incubation times, dilutions, and sources were as follows: polyclonal anti-myeloperoxidase antibody (MPO), 2 h, 1:100 dilution, Dako (Carpinteria, CA); monoclonal antibody against proliferating cell nuclear antigen (PCNA), 2 h, 1:100 dilution, Calbiochem (Cambridge, MA), and polyclonal antibody against transforming growth factor-β (TGF-β), 2 h, 1:300 dilution, R&D (Minneapolis, MN).
Computer-assisted immunohistochemistry and histochemistry image analysis (Image-Pro Plus, Media Cybernetics, Silver Spring, MD) were used to assess the following established indicators of intestinal radiation injury: (1) neutrophil infiltration; (2) proliferation of intestinal smooth muscle cells; (3) deposition of collagen in the intestinal wall; and (4) expression of extracellular matrix-associated TGF-β as described in detail and validated previously[20].
Neutrophil infiltration: The number of myeloperoxidase-positive cells was determined by color thresholding and counting in 20 fields at 40 × magnification, selected according to a predetermined grid pattern. Smooth muscle cell proliferation: Intestinal smooth muscle cell proliferation was assessed in the smooth muscle layer of intestine (muscularis propria). The numbers of total smooth muscle cells and PCNA-positive smooth muscle cells were determined in 20 fields at 40 × magnification using color thresholding and normalizing PCNA-positive smooth muscle cells per thousand smooth muscle cells. Collagen deposition: Masson’s trichrome 2000 stain kit (American Master Tech, Lodi, CA) was used for collagen staining to evaluate collagen deposition. The percentages of areas (relative to the total intestinal wall area) positive for collagen were determined in 20 fields (under 40 × magnification), according to the procedure established by Raviv et al[21] and adapted to our model system[22].
TGF-β immunoreactivity: Areas relatively positive for TGF-β were determined in 20 fields (under 40 × magnification) according to the method described by Raviv et al[21] and adapted to our model system[22].
Quantitative determination of fibrinogen in mouse plasma and small intestinal samples was done by double antibody sandwich ELISA method following manufacturer’s instructions (Kamiya Biomedical Company, Seattle, WA). For plasma preparation, whole blood was collected and centrifuged at 2000 × g for 15 min at 4 °C. Following the centrifugation, the supernatant (plasma) was collected and stored at -20 °C until further use. For intestinal tissue sample preparation, frozen tissues were homogenized in ice-cold PBS containing protease inhibitors and sonicated using cell disrupter. The samples were centrifuged at 2000 x g for 15 min at 4 °C and the supernatants were taken for ELISA procedure. The total protein concentration was first quantified using Bicinchoninic Acid protein assay kit following the manufacture’s protocol (Pierce Biotechnology, Rockford, IL, United States).
Samples and standards were added to appropriate polystyrene microtiter wells pre-coated with anti-fibrinogen antibodies. After incubation and following extensive washing steps to remove any unbound proteins, Horseradish Peroxidase conjugated anti-fibrinogen antibody solution was added to the wells and incubated. After another extensive washing step, the enzyme bound to the immunosorbent was assayed by the addition of chromogenic substrate 3,3′,5,5′-Tetramethylbenzidine and was measured at a wavelength of 450 nm. The quantity of bound fibrinogen was proportional to the magnitude of the absorbance at 450 nm and thus, was a measure of concentration of fibrinogen in the test samples.
The localization of fibrinogen in the intestinal tissue was performed by immunohistochemical staining. Rabbit anti-mouse fibrinogen antibody (kindly gifted by Dr. Jay L Degen) was incubated for 2 h at 1:100 dilution.
Sample size calculation was performed with PASS 2000 for Windows (NCSS, Kaysville, UT). Differences between experimental groups and variability for the early and delayed endpoints was derived from similar experiments conducted in our laboratory and used for calculations, making sure statistical power was at least 0.8. Statistical analyses were performed with the software package NCSS2007 for Windows (NCSS, Kaysville, UT). Differences in endpoints between two groups were assessed with Equal-Variance T-Test of two samples t-Test. Differences in endpoints among the groups Fixed factor analysis of variance (different times and different mice such as Fib+/+, Fib+/-, or Fib-/- mice) was performed with ANOVA General Linear Models analysis. A P value less than 0.05 was considered statistically significant.
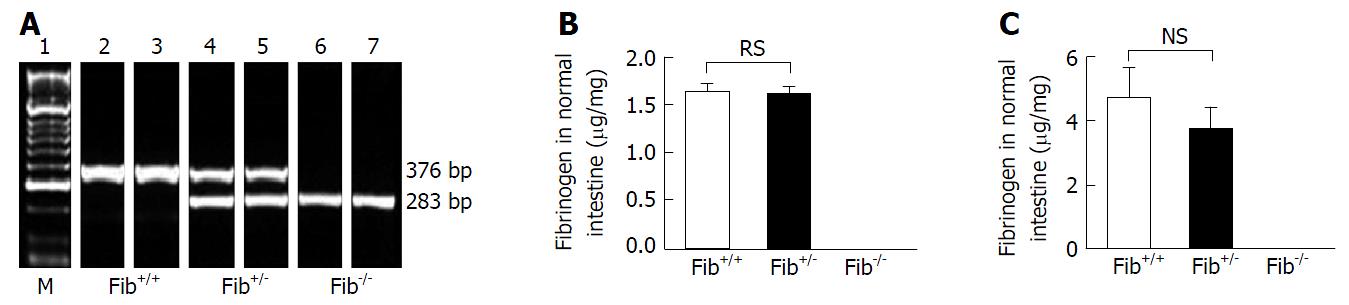
Genotype of the offspring were determined by identifying the number and position of DNA bands in agarose gel. Single band of about 283 bp region indicates that the alleles are truncated, which means homozygous null (Fib-/-) genotype, single band at about 376 bp region signifies endogenous allele that means wild-type (Fib+/+) genotype, and double bands, one at 283 bp region and the other at 376 bp region indicate heterozygous (Fib+/-) genotype (Figure 1A).
Quantitative determination of fibrinogen levels was performed by ELISA. In plasma, fibrinogen was not detected in Fib-/- mice, while fibrinogen was easily detected in the plasma of Fib+/+ and Fib+/- mice. There was no significant difference in plasma fibrinogen levels between Fib+/+ and Fib+/- mice (Figure 1B). Similarly, in intestinal tissue, fibrinogen was not detected in Fib-/- mice, whereas, fibrinogen was detected in Fib+/+ and Fib+/- mice. Again, there was no significant difference between in Fib+/+ and Fib+/- mice in terms of intestinal tissue fibrinogen levels (Figure 1C).
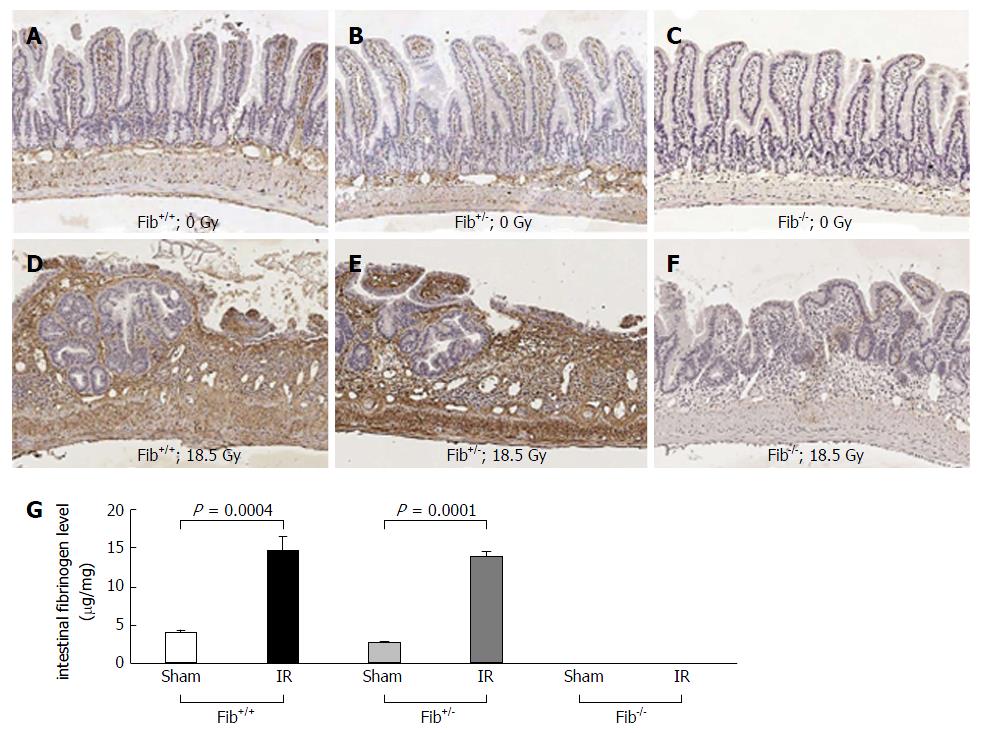
Intestinal fibrinogen level was measured by immunohistochemical staining. Fibrinogen immunoreactivity recognizes both fibrinogen and fibrin mainly located in the intravascular space, endothelial surface, connective tissues of the lamina propria, submucosa, muscularis, and serosa in the intestinal sections. Fibrinogen was detected in the intestinal tissue of sham-irradiated Fib+/+ and Fib+/- mice, however, no significant difference in fibrinogen immunoreactivity was observed. In un-irradiated intestine of Fib-/- mice, fibrinogen immunoreactivity was undetectable (Figure 2A-C).
Like previously published studies, we used Fib+/- mice as littermate controls for Fib-/- mice[11,23,24]. Irradiation significantly increased intestinal fibrinogen level in both Fib+/+ (P = 0.0004) and Fib+/- mice (P < 0.0001) but no detectable increase in fibrinogen level was observed in Fib-/- mice after radiation exposure, compared to the respective sham-irradiated groups. (Figure 2D-G). However, the increase in intestinal fibrinogen level between irradiated Fib+/+ and Fib+/- mice was not significantly different (Figure 2G).
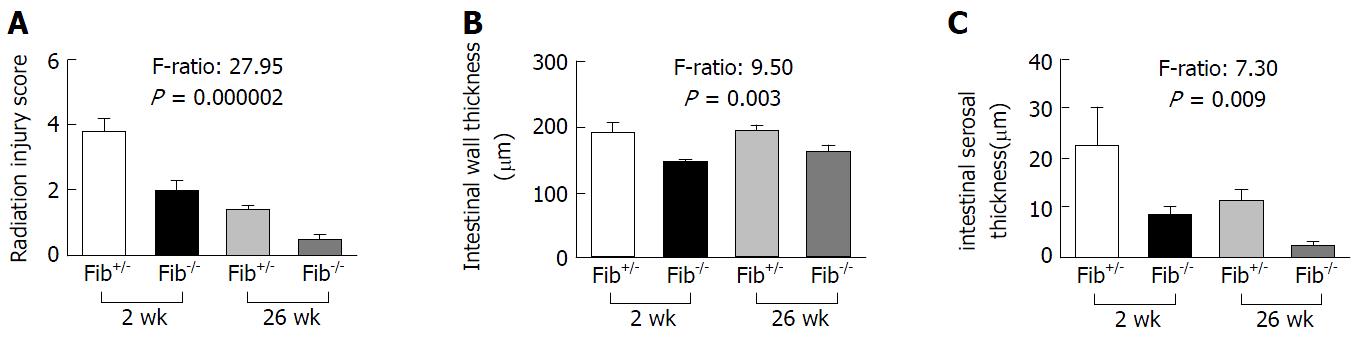
Similar to previous studies performed in our laboratory[19], irradiated intestine exhibited epithelial atypia, reactive thickening of the subserosa and mucosal ulceration at 2 wk, and chronic ulceration, vascular and intestinal wall fibrosis at 26 wk. Compared with Fib+/- mice, Fib-/- mice showed much less intestinal radiation structural injury (RIS) (F-Ratio: 27.95, P = 0.000002), less intestinal wall thickening (F-Ratio: 9.50, P = 0.003) and serosal thickening (F-Ratio: 7.30, P = 0.009) at 2 wk and 26 wk after radiation (Figure 3A-C).
Enhanced myeloperoxidase activity is a well-documented inflammation marker. Myeloperoxidase enzymatic activity in leukocytes correlates directly with neutrophil number (r = 0.99) and myeloperoxidase activity in tissue extract correlates directly with cellular infiltration (r = 0.94)[25]. Un-irradiated intestine from Fib+/- and Fib-/- mice showed few MPO immunoreactive cells and there was no difference between Fib+/- and Fib-/- mice (P > 0.05) (supplemental Figure 1D). After irradiation, the number of MPO positive cells in the intestine of Fib+/- and Fib-/- mice increased significantly (P < 0.01) at 2 wk and 26 wk (data not shown). However, compared with Fib+/-, Fib-/- mice showed significant decrease in the number of positive MPO cells at 2 wk and 26 wk (F-Ratio: 6.37, P = 0.01) as shown in Figure 4A-C.
A decrease in intestinal mucosal surface area is a sensitive parameter of small bowel radiation injury[19]. Compared to un-irradiated mice, irradiated mice showed significant decreased (P < 0.01) mucosal surface area in both Fib+/- and Fib-/- mice at 2 wk and 26 wk (data not shown). However, Fib-/- mice showed significant improvement (F-Ratio: 6.37, P = 0.01) in mucosal surface area at both 2 wk and 26 wk compared to Fib+/- mice (Figure 4D-F).
Effects of fibrinogen deficiency on early and delayed radiation-induced intestinal smooth muscle cell proliferation, collagen deposition, and TGF-β immunoreactivity
In the intestine, collagen is mainly produced by intestinal smooth muscle cells, rather than by fibroblasts. Intestinal smooth muscle cell proliferation rate is very low at baseline, but increases steeply after irradiation[26]. In the current study, PCNA was used as intestinal smooth muscle cell proliferation marker. Un-irradiated intestine of Fib+/- and Fib-/- mice showed low proliferation rate. After irradiation, the proliferation rate of intestinal smooth muscle cells increased significantly (P < 0.01) at 2 wk and 26 wk (data not shown). However, compared to Fib+/-, Fib-/- mice showed a borderline significant decrease (F-Ratio: 4.19, P = 0.046) in the proliferation of intestinal smooth muscle cells at both 2 wk and 26 wk (Figure 5A-C).
Collagen is the major component of fibrous tissue. We previously showed that collagen accumulation is increased in the intestine after irradiation[27]. The current study is consistent with this observation. Radiation significantly increased (P < 0.01) collagen accumulation at both 2 wk and 26 wk in Fig+/- mice, as well as in Fib-/- mice compared to sham-irradiated groups (data not shown). However, in comparison to Fib+/-, Fib-/- mice showed significant decrease (F-Ratio: 6.86, P = 0.01) in collagen accumulation at both 2 wk and 26 wk after radiation (Figure 5D-F).
TGF-β is overexpressed in many fibrotic conditions, including radiation fibrosis[28], and is mechanistically involved in radiation enteropathy[22]. Extracellular matrix-associated TGF-β staining was similar in the intestines of Fib+/- and Fib-/- mice before irradiation (data not shown). TGF-β immunoreactivity increased significantly (P < 0.01) in the intestine after radiation in both Fib+/- and Fib-/- mice compared to sham-irradiated mice at both 2 wk and 26 wk (data not shown). However, compared to Fib+/-, Fib-/- mice showed a significant decrease (F-Ratio: 4.67, P = 0.03) in TGF-β immunoreactivity at both 2 wk and 26 wk after radiation (Figure 5G-I).
The current study demonstrated that there is no statistically significant difference with regard to intestinal wall thickness, mucosal surface area, smooth muscle cell proliferation, and neutrophil infiltration among three mouse strains (Fib+/+, Fib+/- and Fib+/-) before irradiation, both at 2 and 26 wk time points. Moreover, irradiated Fib+/+ and Fib+/- mice show no significant difference in intestinal radiation injury score (F-Ratio: 0.19, P = 0.66), mucosal surface area (F-Ratio: 0.04, P = 0.84), and smooth muscle cell proliferation (F-Ratio: 0.00, P = 0.99), at both time intervals. In addition, ELISA results demonstrated that un-irradiated Fib+/+ mice have similar plasma and intestinal fibrinogen level as un-irradiated Fib+/- mice. No significant difference in intestinal fibrinogen immunoreactivity was observed between Fib+/+ and Fib+/- mice before and after irradiation, thus justifying the use of Fib+/- mice as littermate controls in the current study as in previous studies. Importantly, we demonstrated that fibrinogen deficiency profoundly attenuated early and delayed radiation-induced intestinal inflammation, mucosal injury and fibrosis, intestinal wall and serosal thickness, collagen deposition, intestinal smooth muscle proliferation, TGF-β immunoreactivity, and loss of intestinal mucosal surface area. This study thus confirms a significant role for fibrinogen in radiation enteropathy and suggests that fibrinogen is a potential target in suppressing radiation-induced gut injury.
Fibrinogen is a soluble glycoprotein synthesized by the liver with a molecular weight of 340 kDa. It circulates in the blood, where it is converted by thrombin into fibrin during coagulation and inflammation[29]. In addition, fibrinogen and their degradation products have been implicated in regulating fibrotic process[6-13], including radiation-induced enteropathy and nephropathy[5,30]. However, direct evidence supporting this notion has been lacking.
In pathological conditions, such as vascular wall injury, infection or inflammation, the blood concentration of fibrinogen increases significantly[31]. Even before extravasation into the perivascular space, increased fibrinogen and its derivative peptides in the blood induce blood mononuclear cells to synthesize pro-inflammatory cytokines, such as TNF-α, IL-6 and IL-1β[32,33], activate neutrophils[34], mediate leukocyte adhesion to the vascular endothelium[35,36], and increase leukocyte migration[36], notably leukocyte transendothelial migration[37]. Fibrinogen can bind to both endothelial cells and platelets increasing vascular permeability and causing their local aggregation, as well as inducing fibrin and thrombin production[6,38,39] permitting fibrinogen, fibrin, thrombin, and other plasma constituents to escape the vasculature[40]. Increased interstitial deposition of fibrinogen and/or fibrin has been observed in inflammatory conditions and is considered an early and persistent hallmark of inflammatory responses[8]. Our previous and current studies shows that radiation significantly increase the deposition of both intravascular and extravascular fibrinogen, as well as thrombin deposition in the intestinal tissue at both early and delayed stage[5].
Extravasated fibrinogen promotes leukocyte adhesion and stimulates macrophage production of a select set of chemokines such as macrophage inflammatory protein-1alpha (MIP-1α), MIP-1β, MIP-2, and monocyte chemoattractant protein-1, resulting in the attraction of inflammatory cells such as T cells, neutrophils and additional macrophages to the site of the insult[41]. Direct evidence of the proinflammatory role of fibrinogen comes from animal studies using genetic and pharmacologic approaches. Fibrinogen deficiency delays endotoxin induced inflammatory responses[42], decreases inflammation and delays the onset of demyelination in a tumor necrosis factor transgenic mouse model[43]. Fibrinogen deficiency also diminishes macrophage infiltration and activation in a model of Duchenne muscular dystrophy[13] and in a model of crescentic glomerulonephritis[23]. Ancrod, a defibrinogenating agent, reduces macrophage infiltration and activation, and production of multiple cytokines in a model of Duchenne muscular dystrophy[13]. Reduction of fibrin formation by hirudin is accompanied by a diminution of inflammation and disease severity in experimental arthritis[6] and radiation-induced intestinal injury[5]. Current results are consistent with these and other previous studies, supporting that fibrinogen is a key regulator in inflammation and tissue injury.
Progressive vascular and intestinal wall fibrosis are characteristics of delayed radiation enteropathy, which involves excessive accumulation of collagen and other extracellular matrix (ECM) components. During inflammation and tissue injury, the extravasated fibrinogen is cleaved by the thrombin to form insoluble fibrin, which appears as an eosinophilic meshwork of threads and provides an initial scaffold along which the fibroblasts adhere and migrate. Fibrinogen and its degradation products act as a mitogen to stimulate fibroblast proliferation, resulting in collagen deposition and leading to tissue fibrosis[9-11]. Accordingly, genetic deletion of fibrinogen diminishes crescentic glomerulonephritis[23] and induced wound-healing defects[24]. Fibrin, once extravasated into the tissue, resists degradation[10]. The persistent fibrin is associated with enhanced collagen accumulation that may result in the development of fibrotic disorders[9-13]. Fibrinogen and/or fibrin deposition has been involved in the pathogenesis of arterial intimal thickening and atherosclerosis[44,45]. Studies show that fibrinogen and its degradation products stimulate vascular smooth muscle cells to produce inflammatory cytokines[46] and induce smooth muscle cell proliferations[47,48], resulting in collagen and other ECM production[47]. Fibrinogen and fibrin are also chemoattractant for smooth muscle cell[49,50]. This could be involved in the pathogenesis of vascular sclerosis, as well as intestinal wall fibrosis in radiation enteropathy, since collagen is produced largely by smooth muscle cells in the intestine. Moreover, because the intestinal fibrosis becomes more prominent with increasing radiation dose, the protective effect of fibrinogen deficiency would likely increase with increasing radiation doses.
Fibrinogen and fibrin can bind multiple growth factors, including TGF-β[51]. TGF-β has been implicated in many fibrotic conditions, including radiation injury in skin, liver, heart, kidney, lung and intestine and plays important role in fibrotic condition. Fibrinogen in the blood stream serves as a carrier for latent TGF-β[52]. The fibrinogen-bound latent TGF-β leaks into the interstitial tissue after vascular injury and is activated, leading to the formation of active TGF-β and activation of downstream signaling pathways. Fibrin also triggers endogenous TGFβ1 expression[10,13]. Genetic or pharmacologic depletion of fibrinogen reduces active TGF-β and inhibits the fibrinogen-induced effects on glial scar formation[52]. The current study showed that fibrinogen deficiency significantly reduced TGF-β expression. These results thus suggest that TGF-β could be a molecular link between fibrinogen and intestinal fibrosis formation.
In conclusion, the current study shows that fibrinogen deficiency attenuated early and delayed radiation-induced intestinal inflammation, mucosal injury and fibrosis, suggesting that fibrinogen play a key role in early and delayed radiation enteropathy. Pharmacological inhibition of fibrinogen could be a novel strategy to reduce the risk of radiation enteropathy.
The authors express their gratitude to Prof. Jay L. Degen, Developmental Biology and the Department of Pediatrics, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, for providing the fibrinogen-deficient mice breeders.
One third of total cancer patients suffer from either abdominal or pelvic malignancies. About 14%-68% of patients with abdominal or pelvic malignancies undergo radiotherapy. The major limitation of abdominal or pelvic radiotherapy is the risk of development of radiation enteropathy, an adverse side effect.
Radiation enteropathy is a well-known complication after abdominal or pelvic radiotherapy and causes substantial morbidity in patients. The underlying mechanisms involved in the development of intestinal injury from therapeutic radiation are largely unknown. A role for fibrinogen in intestinal fibrosis has been suggested, but not unequivocally confirmed.
To the best of our knowledge, this study for the first time show that deficiency of fibrinogen suppresses radiation-induced early and delayed intestinal injury using a fibrinogen knock-out mouse model.
Fibrinogen may represent a novel target to reduce intestinal damage in patients undergoing abdominopelvic radiotherapy or in case of accidental or intentional exposure to ionizing radiation.
Radiation enteropathy, or intestinal damage caused by therapeutic radiation is a global problem and its successful mitigation has not been achieved. The mechanisms underlying the development of radiation enteropathy are highly complex and controversial. The present study demonstrated that deficiency of fibrinogen, a plasma protein that induces coagulation, inflammation, and fibrosis, substantially reduces radiation-induced intestinal damage.
Fibrinogen deficiency directly attenuates development of early and delayed radiation enteropathy. Fibrinogen could be a novel target in treating intestinal damage. The manuscript is an interesting piece of good work.
| 1. | Hauer-Jensen M, Wang J, Boerma M, Fu Q, Denham JW. Radiation damage to the gastrointestinal tract: mechanisms, diagnosis, and management. Curr Opin Support Palliat Care. 2007;1:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Hauer-Jensen M, Denham JW, Andreyev HJ. Radiation enteropathy--pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol. 2014;11:470-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 343] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 3. | Wang J, Zheng H, Ou X, Fink LM, Hauer-Jensen M. Deficiency of microvascular thrombomodulin and up-regulation of protease-activated receptor-1 in irradiated rat intestine: possible link between endothelial dysfunction and chronic radiation fibrosis. Am J Pathol. 2002;160:2063-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 127] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Wang J, Boerma M, Fu Q, Hauer-Jensen M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol. 2007;13:3047-3055. [PubMed] |
| 5. | Wang J, Zheng H, Ou X, Albertson CM, Fink LM, Herbert JM, Hauer-Jensen M. Hirudin ameliorates intestinal radiation toxicity in the rat: support for thrombin inhibition as strategy to minimize side-effects after radiation therapy and as countermeasure against radiation exposure. J Thromb Haemost. 2004;2:2027-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34:43-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 695] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 7. | Rowland FN, Donovan MJ, Gillies C, O’Rourke J, Kreutzer DL. Fibrin: mediator of in vivo and in vitro injury and inflammation. Curr Eye Res. 1985;4:537-553. [PubMed] |
| 8. | Andreotti F, Burzotta F, Maseri A. Fibrinogen as a marker of inflammation: a clinical view. Blood Coagul Fibrinolysis. 1999;10 Suppl 1:S3-S4. [PubMed] |
| 9. | Brown LF, Dvorak AM, Dvorak HF. Leaky vessels, fibrin deposition, and fibrosis: a sequence of events common to solid tumors and to many other types of disease. Am Rev Respir Dis. 1989;140:1104-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Davila HH, Ferrini MG, Rajfer J, Gonzalez-Cadavid NF. Fibrin as an inducer of fibrosis in the tunica albuginea of the rat: a new animal model of Peyronie’s disease. BJU Int. 2003;91:830-838. [PubMed] |
| 11. | Sörensen I, Susnik N, Inhester T, Degen JL, Melk A, Haller H, Schmitt R. Fibrinogen, acting as a mitogen for tubulointerstitial fibroblasts, promotes renal fibrosis. Kidney Int. 2011;80:1035-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Tawfik S, El-Sawy MA, Zeina AA, Azzam ZA. Fibrinogen, fibrin degradation products and fibrinolysis in bilharzial (schistosomal) hepatic fibrosis. Trans R Soc Trop Med Hyg. 1977;71:359-360. [PubMed] |
| 13. | Vidal B, Serrano AL, Tjwa M, Suelves M, Ardite E, De Mori R, Baeza-Raja B, Martínez de Lagrán M, Lafuste P, Ruiz-Bonilla V. Fibrinogen drives dystrophic muscle fibrosis via a TGFbeta/alternative macrophage activation pathway. Genes Dev. 2008;22:1747-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Suh TT, Holmbäck K, Jensen NJ, Daugherty CC, Small K, Simon DI, Potter S, Degen JL. Resolution of spontaneous bleeding events but failure of pregnancy in fibrinogen-deficient mice. Genes Dev. 1995;9:2020-2033. [PubMed] |
| 15. | Palumbo JS, Potter JM, Kaplan LS, Talmage K, Jackson DG, Degen JL. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res. 2002;62:6966-6972. [PubMed] |
| 16. | Langberg CW, Sauer T, Reitan JB, Hauer-Jensen M. Tolerance of rat small intestine to localized single dose and fractionated irradiation. Acta Oncol. 1992;31:781-787. [PubMed] |
| 17. | Jensen MH, Sauer T, Devik F, Nygaard K. Late changes following single dose roentgen irradiation of rat small intestine. Acta Radiol Oncol. 1983;22:299-303. [PubMed] |
| 18. | Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142:259-276. [PubMed] |
| 19. | Langberg CW, Sauer T, Reitan JB, Hauer-Jensen M. Relationship between intestinal fibrosis and histopathologic and morphometric changes in consequential and late radiation enteropathy. Acta Oncol. 1996;35:81-87. [PubMed] |
| 20. | Cenac N, Coelho AM, Nguyen C, Compton S, Andrade-Gordon P, MacNaughton WK, Wallace JL, Hollenberg MD, Bunnett NW, Garcia-Villar R. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am J Pathol. 2002;161:1903-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 295] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 21. | Raviv G, Kiss R, Vanegas JP, Petein M, Danguy A, Schulman C, Wespes E. Objective measurement of the different collagen types in the corpus cavernosum of potent and impotent men: an immunohistochemical staining with computerized-image analysis. World J Urol. 1997;15:50-55. [PubMed] |
| 22. | Zheng H, Wang J, Koteliansky VE, Gotwals PJ, Hauer-Jensen M. Recombinant soluble transforming growth factor beta type II receptor ameliorates radiation enteropathy in mice. Gastroenterology. 2000;119:1286-1296. [PubMed] |
| 23. | Drew AF, Tucker HL, Liu H, Witte DP, Degen JL, Tipping PG. Crescentic glomerulonephritis is diminished in fibrinogen-deficient mice. Am J Physiol Renal Physiol. 2001;281:F1157-F1163. [PubMed] |
| 24. | Drew AF, Liu H, Davidson JM, Daugherty CC, Degen JL. Wound-healing defects in mice lacking fibrinogen. Blood. 2001;97:3691-3698. [PubMed] |
| 25. | Boughton-Smith NK, Wallace JL, Whittle BJ. Relationship between arachidonic acid metabolism, myeloperoxidase activity and leukocyte infiltration in a rat model of inflammatory bowel disease. Agents Actions. 1988;25:115-123. [PubMed] |
| 26. | Zheng H, Wang J, Hauer-Jensen M. Role of mast cells in early and delayed radiation injury in rat intestine. Radiat Res. 2000;153:533-539. [PubMed] |
| 27. | Wang J, Zheng J, Kulkarni A, Wang W, Garg S, Prather PL, Hauer-Jensen M. Palmitoylethanolamide regulates development of intestinal radiation injury in a mast cell-dependent manner. Dig Dis Sci. 2014;59:2693-2703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Richter KK, Langberg CW, Sung CC, Hauer-Jensen M. Association of transforming growth factor beta (TGF-beta) immunoreactivity with specific histopathologic lesions in subacute and chronic experimental radiation enteropathy. Radiother Oncol. 1996;39:243-251. [PubMed] |
| 29. | Weisel JW. Fibrinogen and fibrin. Adv Protein Chem. 2005;70:247-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 641] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 30. | Cohen EP, Bonsib SA, Whitehouse E, Hopewell JW, Robbins ME. Mediators and mechanisms of radiation nephropathy. Proc Soc Exp Biol Med. 2000;223:218-225. [PubMed] |
| 31. | Adams RA, Passino M, Sachs BD, Nuriel T, Akassoglou K. Fibrin mechanisms and functions in nervous system pathology. Mol Interv. 2004;4:163-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 32. | Jensen T, Kierulf P, Sandset PM, Klingenberg O, Joø GB, Godal HC, Skjønsberg OH. Fibrinogen and fibrin induce synthesis of proinflammatory cytokines from isolated peripheral blood mononuclear cells. Thromb Haemost. 2007;97:822-829. [PubMed] |
| 33. | Perez RL, Roman J. Fibrin enhances the expression of IL-1 beta by human peripheral blood mononuclear cells. Implications in pulmonary inflammation. J Immunol. 1995;154:1879-1887. [PubMed] |
| 34. | de Almeida VV, Calado A, Rosário HS, Saldanha C. Differential effect of soluble fibrinogen as a neutrophil activator. Microvasc Res. 2012;83:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Languino LR, Plescia J, Duperray A, Brian AA, Plow EF, Geltosky JE, Altieri DC. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1-dependent pathway. Cell. 1993;73:1423-1434. [PubMed] |
| 36. | Pearson MJ, Lipowsky HH. Effect of fibrinogen on leukocyte margination and adhesion in postcapillary venules. Microcirculation. 2004;11:295-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Languino LR, Duperray A, Joganic KJ, Fornaro M, Thornton GB, Altieri DC. Regulation of leukocyte-endothelium interaction and leukocyte transendothelial migration by intercellular adhesion molecule 1-fibrinogen recognition. Proc Natl Acad Sci USA. 1995;92:1505-1509. [PubMed] |
| 38. | Muradashvili N, Qipshidze N, Munjal C, Givvimani S, Benton RL, Roberts AM, Tyagi SC, Lominadze D. Fibrinogen-induced increased pial venular permeability in mice. J Cereb Blood Flow Metab. 2012;32:150-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Tyagi N, Roberts AM, Dean WL, Tyagi SC, Lominadze D. Fibrinogen induces endothelial cell permeability. Mol Cell Biochem. 2008;307:13-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Dvorak HF, Senger DR, Dvorak AM, Harvey VS, McDonagh J. Regulation of extravascular coagulation by microvascular permeability. Science. 1985;227:1059-1061. [PubMed] |
| 41. | Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887-2894. [PubMed] |
| 42. | Cruz-Topete D, Iwaki T, Ploplis VA, Castellino FJ. Delayed inflammatory responses to endotoxin in fibrinogen-deficient mice. J Pathol. 2006;210:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Akassoglou K, Adams RA, Bauer J, Mercado P, Tseveleki V, Lassmann H, Probert L, Strickland S. Fibrin depletion decreases inflammation and delays the onset of demyelination in a tumor necrosis factor transgenic mouse model for multiple sclerosis. Proc Natl Acad Sci USA. 2004;101:6698-6703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Smith EB. Fibrinogen, fibrin and fibrin degradation products in relation to atherosclerosis. Clin Haematol. 1986;15:355-370. [PubMed] |
| 45. | Naito M, Hayashi T, Kuzuya M, Funaki C, Asai K, Kuzuya F. Effects of fibrinogen and fibrin on the migration of vascular smooth muscle cells in vitro. Atherosclerosis. 1990;83:9-14. [PubMed] |
| 46. | Lu PP, Liu JT, Liu N, Guo F, Ji YY, Pang X. Pro-inflammatory effect of fibrinogen and FDP on vascular smooth muscle cells by IL-6, TNF-α and iNOS. Life Sci. 2011;88:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Ahmann KA, Weinbaum JS, Johnson SL, Tranquillo RT. Fibrin degradation enhances vascular smooth muscle cell proliferation and matrix deposition in fibrin-based tissue constructs fabricated in vitro. Tissue Eng Part A. 2010;16:3261-3270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 48. | Sturge J, Carey N, Davies AH, Powell JT. Fibrin monomer and fibrinopeptide B act additively to increase DNA synthesis in smooth muscle cells cultured from human saphenous vein. J Vasc Surg. 2001;33:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Paulhe F, Bogyo A, Chap H, Perret B, Racaud-Sultan C. Vascular smooth muscle cell spreading onto fibrinogen is regulated by calpains and phospholipase C. Biochem Biophys Res Commun. 2001;288:875-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Naito M, Hayashi T, Kuzuya M, Funaki C, Asai K, Kuzuya F. Fibrinogen is chemotactic for vascular smooth muscle cells. FEBS Lett. 1989;247:358-360. [PubMed] |
| 51. | Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci USA. 2013;110:4563-4568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 359] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 52. | Schachtrup C, Ryu JK, Helmrick MJ, Vagena E, Galanakis DK, Degen JL, Margolis RU, Akassoglou K. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J Neurosci. 2010;30:5843-5854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 308] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Mayol J, Sergi CM S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF