Published online Jul 7, 2017. doi: 10.3748/wjg.v23.i25.4604
Peer-review started: December 19, 2016
First decision: February 10, 2017
Revised: April 7, 2017
Accepted: June 1, 2017
Article in press: June 1, 2017
Published online: July 7, 2017
Processing time: 203 Days and 8.6 Hours
To investigate the fatty acid-based functional lipidomics of patients on long-term home parenteral nutrition receiving different intravenous lipid emulsions.
A cross-sectional comparative study was carried out on 3 groups of adults on home parenteral nutrition (HPN), receiving an HPN admixture containing an olive-soybean oil-based intravenous lipid emulsion (IVLE) (OO-IVLE; n = 15), a soybean- medium-chain triacylglycerol-olive-fish oil-based IVLE (SMOF-IVLE; n = 8) or HPN without IVLE (No-IVLE; n = 8) and 42 healthy controls (HCs). The inclusion criteria were: duration of HPN ≥ 3 mo, current HPN admixtures ≥ 2 mo and HPN infusions ≥ 2/wk. Blood samples were drawn 4-6 h after the discontinuation of the overnight HPN infusion. The functional lipidomics panel included: the red blood cell (RBC) fatty acid (FA) profile, molecular biomarkers [membrane fluidity: saturated/monounsaturated FA ratio = saturated fatty acid (SFA)/monounsaturated fatty acid (MUFA) index; inflammatory risk: n-6/n-3 polyunsaturated fatty acid (PUFA) ratio = n-6/n-3 index; cardiovascular risk: sum of n-3 eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) = n-3 index; free radical stress: sum of FA trans isomers = %trans index] and FA pathway enzyme activity estimate (delta-9-desaturase = D9D; delta-6-desaturase = D6D; delta-5-desaturase = D5D; elongase = ELO). Statistics were carried out using nonparametric tests. The amount of each FA was calculated as a percentage of the total FA content (relative%).
In the OO-IVLE group, the percentage of oleic acid in the RBCs was positively correlated with the weekly load of OO-IVLE (r = 0.540, P = 0.043). In the SMOF-IVLE cohort, the RBC membrane EPA and DHA were positively correlated with the daily amount of SMOF-IVLE (r = 0.751, P = 0.044) and the number of HPN infusions per week (r = 0.753; P = 0.046), respectively. The SMOF-IVLE group showed the highest EPA and DHA and the lowest arachidonic acid percentages (P < 0.001). The RBC membrane linoleic acid content was lower, and oleic and vaccenic acids were higher in all the HPN groups in comparison to the HCs. Vaccenic acid was positively correlated with the weekly HPN load of glucose in both the OO-IVLE (r = 0.716; P = 0.007) and the SMOF-IVLE (r = 0.732; P = 0.053) groups. The estimated activity of D9D was higher in all the HPN groups than in the HCs (P < 0.001). The estimated activity of D5D was lower in the SMOF-IVLE group than in the HCs (P = 0.013). The SFA/MUFA ratio was lower in all the HPN groups than in the HCs (P < 0.001). The n-6/n-3 index was lower and the n-3 index was higher in the SMOF-IVLE group in comparison to the HCs and to the other HPN groups (P < 0.001). The %trans index did not differ among the four groups.
The FA profile of IVLEs significantly influenced the cell membrane functional lipidomics. The amount of glucose in the HPN may play a relevant role, mediated by the insulin regulation of the FA pathway enzyme activities.
Core tip: Fatty acid-based “functional lipidomics” investigates the structural and functional roles played by lipids and their in vivo changes, and provides the rationalisation of these changes in connection with their biological significance. In this study, the effects of two intravenous lipid emulsions with different fatty acids profiles on the red blood cell membrane lipidome in patients on long-term home parenteral nutrition for chronic intestinal failure were investigated. The results were analysed in terms of functional lipidomics. The membrane lipidome was significantly modified by the fatty acid profile of the intravenous lipid emulsions. Functional lipidomics indicated that both the lipid emulsion fatty acid profile and the glucose amount of the parenteral nutrition admixture play a role in regulating the activity of the enzymes of the fatty acid metabolism pathways.
- Citation: Pironi L, Guidetti M, Verrastro O, Iacona C, Agostini F, Pazzeschi C, Sasdelli AS, Melchiorre M, Ferreri C. Functional lipidomics in patients on home parenteral nutrition: Effect of lipid emulsions. World J Gastroenterol 2017; 23(25): 4604-4614
- URL: https://www.wjgnet.com/1007-9327/full/v23/i25/4604.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i25.4604
Chronic intestinal failure (CIF) is the persistent “reduction of gut function below the minimum necessary for the absorption of macronutrients and/or water and electrolytes, such that intravenous supplementation is required to maintain health and/or growth”[1]. Intravenous supplementation is required for a long period or for the rest of the patient’s life. These patients are metabolically stable, and they and/or their relatives are trained to become independent in managing intravenous (IV) feeding at home (home parenteral nutrition, HPN)[2]. In HPN programs, intravenous supplementation consists of parenteral nutrition (PN)-admixtures containing water, macronutrients (amino acids, glucose, lipids), electrolytes, vitamins and trace elements[3].
Intravenous lipid emulsions (IVLEs) are oil-in-water emulsions consisting of one or more triacylglycerol-containing oils, a phospholipid emulsifier and glycerol. In PN-admixtures, IVLEs are primarily used as a source of non-glucose energy and of essential fatty acids (EFAs). For clinical purposes, the fatty acid (FA) profile is the most relevant characteristic of the IVLE. The first IVLE developed was a soybean oil-based IVLE with a high content of n-6 polyunsaturated fatty acid (PUFA) and linoleic acid (18:2; n-6) which may result in a pro-inflammatory response. In order to decrease the n-6 PUFA content, the second generation of IVLEs consisted of a 50:50 (by weight) physical mixture of soybean oil and medium-chain triacylglycerols (MCTs). The third-generation of IVLE consisted of 80% olive oil and 20% soybean oil by weight, and the fourth included fish oil, either in combination with one or more of the oils used in previous IVLEs, or alone. Fish oil is rich in n-3 PUFAs, which may exert anti-inflammatory properties[4-7].
The cell membrane FA profile (membrane lipidome) depends on the interaction among genetic, metabolic and nutritional factors, and represents a comprehensive biomarker of the homeostatic (or allostatic) condition of the subject[8]. Fatty acid-based “functional lipidomics” investigates the structural and functional roles played by lipids and their in vivo changes due to metabolic or degradation pathways, and provides the rationalisation of these changes in connection with their biological significance. Membrane lipidomics of red blood cells (RBCs) is considered a snapshot of the individual situation due to the close relationship between the structure and the function of the RBC membrane and to the RBC distribution in all body districts[8].
The aim of this study was to investigate the effects of two IVLEs with different FA profiles on the RBC membrane lipidome in patients on HPN for CIF and to analyse the results in terms of functional lipidomics.
This was a cross-sectional study carried out on a cohort of adult patients on HPN for benign CIF at the Chronic Intestinal Failure Centre of S. Orsola-Malpighi University Hospital of Bologna, Italy. The study was approved by the Local Ethics Committee (n 1468/2015). Voluntary informed written consent was obtained from all patients.
The patient inclusion criteria were: age ≥ 18 years; duration of HPN for ≥ 3 mo; days of HPN infusion ≥ 2 per week; HPN schedule, oral feeding and drug therapy unchanged during the 2 mo before inclusion in the study; PN-admixture containing Clinoleic 20%®(Baxter SAS, Maurepas-Cedex 78311, France), an olive-soybean oil-based IVLE (20% soybean oil, 80% olive oil; OO-IVLE) or Smoflipid® 20% (Fresenius Kabi, Bad Homburg, Germany), a soybean-MCT-olive-fish oil-based IVLE (30% soybean oil, 30% MCT, 25% olive oil, 15% fish oil; SMOF-IVLE) or PN without IVLE (No-IVLE).
The patient exclusion criteria were: the current use of experimental drugs, pregnancy or the presence of malignant disease.
The healthy control (HC) group consisted of healthy subjects from the Lipinutragen s.r.l. (Bologna, Italy) database, having age, gender and body mass index (BMI) matched with the HPN patients.
Demographic, anthropometric and CIF characteristics: The parameters recorded were: patient age, gender, BMI (kg/m2), primary disease and the pathophysiological mechanism of CIF.
HPN schedule: The duration of HPN, the duration of the current PN-admixture, the weekly frequency of PN infusion and the composition of the PN-admixture were recorded. The amount of lipids and glucose infused was calculated as the amount per day of infusion, amount per patient body weight per day of infusion and total amount per week. Energy (kcal) was calculated as the amount per day of infusion and as the percentage of basal energy expenditure (BEE) on a weekly basis [(kcal/infusion × number of infusions per week/7/BEE) × 100]. The patient BEE was calculated using the Harris-Benedict equation[9].
Oral food intake and intestinal fat absorption: Oral food intake was evaluated using a 5-d self-reported weighed food intake record during which patients were invited to maintain their usual diet. All food records were reviewed by a dietitian. Energy intake was calculated using a food database and expressed in kcal/day. The assessment of the intestinal fat absorption was carried out over a 3-d period. The coefficient of the net digestive absorption was expressed as the percentage of fat representing the proportion of oral food not recovered in fecal output (% fat absorption). Fecal lipid output was measured on homogenized aliquots of 3-d pooled samples using the Van de Kamer method[10].
The food intake evaluation was computed ± 7 d from the blood sampling for RBC lipidome analysis and the assessment of fat absorption ± 1 mo from blood sampling.
The lipidomics analysis was carried out by Lipinutragen s.r.l., a spin-off of the National Research Council (Consiglio Nazionale delle Ricerche, CNR).
The venous blood samples were drawn in the morning, in the fasting state, 4-6 h after discontinuation of the overnight HPN infusion. Blood (approximately 2 mL) was collected in vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA). Samples were stored at 4 °C until analysis which was carried out 10-15 d after collection.
Lipid extraction and lipid transesterification to fatty acid methyl esters (FAMEs) was performed using an automated protocol which included a selection of RBCs which had been aged for three months[11]. The erythrocytes were separated from the plasma by centrifugation (4000 rpm for 5 min at 4 °C), they were then suspended in pure water, vortexed and subsequently centrifuged (14000 × g for 15 min at 4 °C) to isolate the membrane pellets. Phospholipids, extracted from pellets using the Bligh and Dyer method[10], were transesterified to FAMEs by treatment with a potassium hydroxide (KOH)/methyl alcohol (MeOH) solution (0.5 mol/L) for 10 min at room temperature and were subsequently extracted using n-hexane (2 mL). The FAMEs were analysed using capillary column gas chromatography (GC). The GC analysis was run on the Agilent 6850 Network GC System, equipped with a fused silica capillary column Agilent DB23 (60 m × 0.25 mm × 0.25 μm) having a flame ionisation detector.
The GC analysis of the fatty acids released showed the separation of all fatty acids and their isomers. Identification was made by comparing them to commercially available standards and to a library of isomers trans monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) available at Lipinutragen S.r.l.[12]. The amount of each FA was calculated as a percentage of the total FA content (relative%).
Functional lipidomics is based on the analysis of the RBC membrane FA profile. The panel of functional lipidomics carried out by Lipinutragen s.r.l. included some molecular biomarkers of the physical properties of the membrane and of specific risks. Furthermore, the activity of elongase and desaturase, the two classes of enzymes of the MUFA and PUFA biosynthetic pathways was estimated by calculating the precursor/product ratio of the individual FAs.
Membrane FA profile (lipidome): The FA panel included saturated fatty acids (SFAs: palmitic acid: C16:0; stearic acid: C18:0); MUFAs (palmitoleic acid: C16:1;11c; oleic acid: C18:1;9c; vaccenic acid: C18:1;11c); n-6 PUFAs [linoleic acid: C18:2; dihomo-γ-linoleic acid (DGLA): 20:3; arachidonic acid (ARA): C20:4] and n-3 PUFAs [eicosapentaenoic acid (EPA): C20:5; Docosahexaenoic acid (DHA): C22:6] and the trans isomers of oleic acid (trans-C18:1, 9c) and of arachidonic acid (mono trans-C20:4; n-6).
Molecular biomarkers: The membrane fluidity index (SFA/MUFA ratio): The ratio of saturated and unsaturated fatty acids is one of the factors regulating the fluidity of the cell membrane; the higher the value of the SFA/MUFA ratio, the lower the fluidity of the membrane (reference range 1.7-2.0)[8].
The inflammatory risk index (n-6/n-3 ratio): The ratio of n6 and n3 PUFAs was involved in regulating the inflammatory responses. An elevated value of the n-6/n-3 ratio can drive the lipid metabolic pathways to the production of pro-inflammatory mediators (reference range 3.5-5.5)[8].
The n-3 cardiovascular risk index (n-3 index): The n-3 index is the sum of the relative percentage of two prominent n-3 PUFAs in the RBC membrane: EPA and DHA. The n-3 index risk is categorised as high (< 4%), intermediate (4%-8%) and low ( > 8%)[13].
The free radical stress index (%Transindex): This index is the sum of the relative percentage of the trans isomer of oleic acid and 5- and 8-monotrans isomers of arachidonic acid (reference range 0%-0.4%). Trans isomers of unsaturated FAs are present in industrially produced and partially hydrogenated vegetable oils. It has been demonstrated that they are also formed in vivo by means of the double bond isomerisation process, catalysed by radical species. The perturbation produced by the unnatural trans geometry to membrane properties negatively affects eukaryotic cell survival[14-16].
Enzyme activity estimate: The desaturase and elongase activities were estimated by calculating the precursor/product or product/precursor ratio of individual FAs.
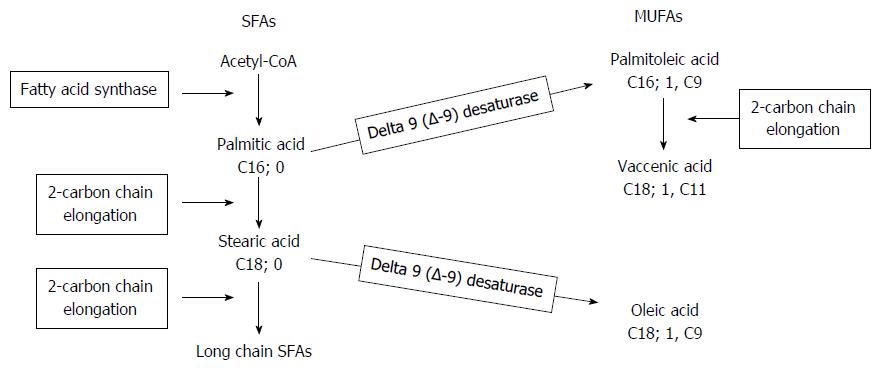
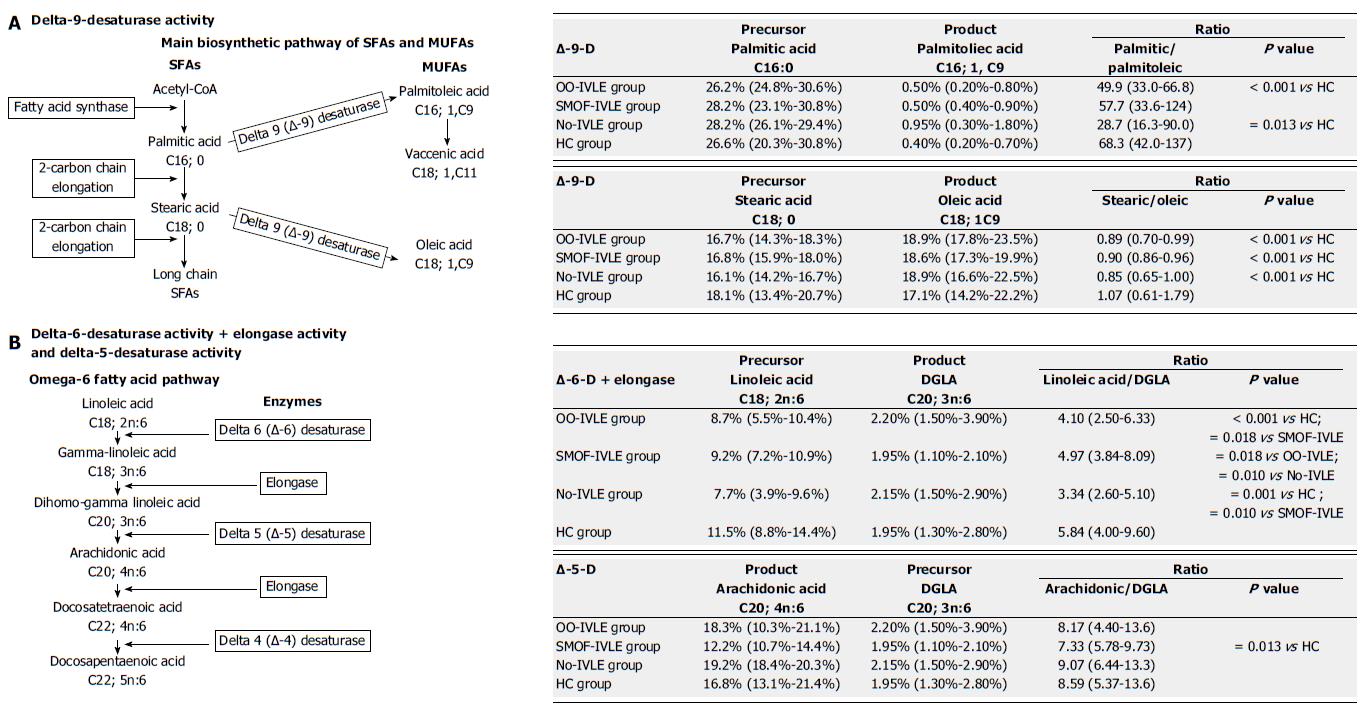
Metabolic pathway of SFAs and MUFAs: Delta-9-desaturase (D9D) (Figure 1): This enzyme catalyses the double bond formation in the biosynthetic pathways of saturated and monounsaturated FAs. It converts palmitic acid (C16:0) and stearic acid (C18:0) into palmitoleic acid (C16:1; 9c) and oleic acid (18:1; 9c), respectively. Its activity was estimated using the palmitic acid/palmitoleic acid ratio and by the stearic acid/oleic acid ratio. The lower the ratio, the higher the D9D activity.
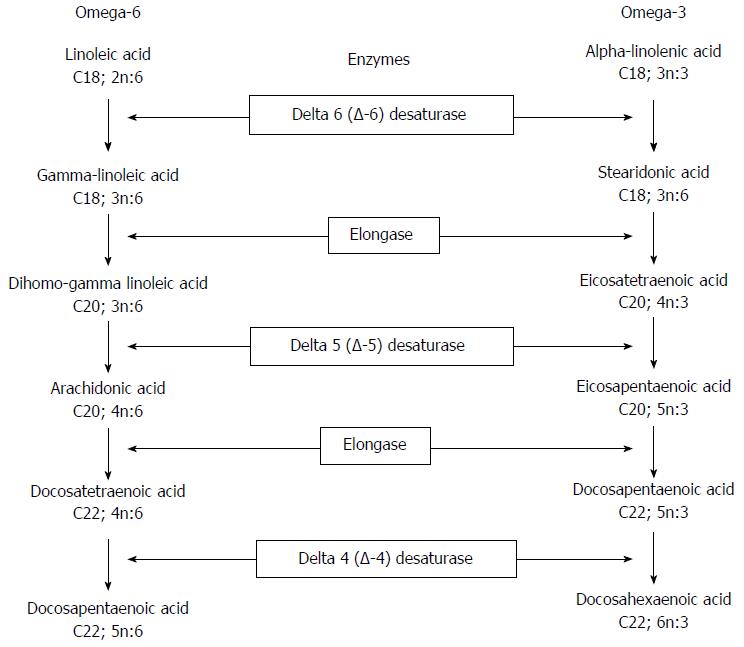
Metabolic pathway of n-6 PUFAs: Delta-6-desaturase + Elongase (D6D+ELO) (Figure 2): Delta-6-desaturase converts linoleic acid (C18:2; n-6) into γ-linoleic acid (GLA; C18:3; n-6); ELO then catalyses the conversion of GLA into DGLA (C20:3; n-6). The ratio between the relative percentages of linoleic acid (1C8:2; n-6) and DGLA (C20:3; n-6) estimates the activity of these two enzymes. The lower the ratio, the higher the D6D and ELO activity.
Delta-5-desaturase (D5D) (Figure 2): This enzyme catalyses the next step of the n-6 PUFA metabolic pathway, converting DGLA (C20:3; n-6) into arachidonic acid (20:4; n-6). Due to the higher relative percentage of arachidonic acid, the estimated activity of this enzyme was calculated as product/precursor ratio (arachidonic acid/DGLA. The higher the ratio, the lower the D5D activity.
The FA profiles of OO-IVLE and SMOF-IVLE were also analysed by testing three different batches of each IVLE.
Variables are reported as medians (ranges) and percentages. The Mann-Whitney U-test and the Kruskall-Wallis test were used for group comparison, and the Spearman’s rank correlation was used to investigate the correlations between the continuous variables. Frequencies were compared using the χ2 test. The statistical analysis was carried out by running the Statgraphics Centurion Professional statistical package (Version XVI, Statpoint Technologies.inc, Warrenton, VA, United States) on a personal computer. P values less than 0.05 were considered to be significant.
Thirty-one patients were included in the study: 15 in the OO-IVLE group, 8 in the SMOF-IVLE group and 8 in the No-IVLE group. The HC group consisted of 42 healthy subjects, age, gender and BMI matched with the patients. Table 1 shows the characteristics of the four groups of subjects. The groups were similar with respect to gender, age and BMI. The duration of the HPN and the weekly frequency of PN infusions did not differ among the HPN groups. The amount of IVLE infused was similar between the OO-IVLE and SMOF-IVLE groups.
| OO-IVLE | SMOF-IVLE | No-IVLE | HC | P value (4 groups) | P value (OO vs SMOF) | |
| Demographic, anthropometric and disease characteristics | ||||||
| N of subjects (M/F) | 15 (8/7) | 8 (3/5) | 8 (2/6) | 42 (19/23) | 0.4051 | |
| Age (yr) median (range) | 56 (19-78) | 44 (19-73) | 56 (29-64) | 46 (16-78) | 0.4051 | |
| BMI (kg/m2) median (range) | 22 (12-25) | 20 (17-23) | 21 (16-29) | 22 (17-26) | 0.1407 | |
| Cause of CIF n (%) | 0.0791 | |||||
| SBS | 9 (60) | 5 (62) | 8 (100) | |||
| Fistulas | - | 2 (25) | - | |||
| Dysmotility | 5 (33) | 1 (12) | - | |||
| Mucosal disease | 1 (7) | - | - | |||
| Primary disease n (%) | 0.0037 | |||||
| Mesenteric ischemia | 6 (40) | 1 (12) | 1 (12) | 0.0627 | ||
| CIPO | 5 (32) | 1 (12) | 4 (50) | |||
| Crohn’s disease | 1 (7) | 3 (36) | 3 (38) | |||
| Others | 3 (21) | 3 (36) | ||||
| Home parenteral nutrition schedule and oral feeding characteristics | ||||||
| HPN schedule: median (range) | ||||||
| Duration (mo) | 65 (2-261) | 29 (5-53) | 17 (6-278) | 0.2765 | - | |
| Current duration (mo) | 26 (2-96) | 7 (5-14) | 7 (2-19) | 0.0620 | 0.5682 | |
| Infusions (n/wk) | 7 (3.5-7) | 7 (2-7) | 7 (2-7) | 0.8028 | ||
| Lipids (n inf/wk) | 7 (3.5-7) | 7 (2-7) | - | - | 0.6946 | |
| (g/inf) | 48 (26-60) | 45 (28-56) | - | - | 0.8601 | |
| (g/kg of BW/inf) | 0.79 (0.38-1.10) | 0.86 (0.43-1.20) | - | - | 0.6260 | |
| (g/wk) | 210 (100-406) | 203 (180-350) | - | - | 0.8210 | |
| Glucose (g/inf) | 169 (84-350) | 198 (115-375) | 150 (36-265) | 0.3370 | - | |
| (g/kg of BW/inf) | 3.3 (1.4-5.5) | 3.8 (2.4-5.8) | 2.3 (0.9-5.0) | 0.5959 | - | |
| (g/wk) | 1014 (300-2450) | 1382 (473-2625) | 1050 (300-1855) | 0.4265 | - | |
| Amino acids (g/inf) | 50 (24-106) | 61 (50-100) | 29 (0-72) | 0.0553 | 0.2070 | |
| Energy (Kcal/inf) | 1400 (739-2104) | 1544 (837-2180) | 700 (144-1345) | 0.0117 | 0.4578 | |
| (Kcal/BEE × 100)1 | 99% (26%-149%) | 122% (59%-147%) | 54% (7%-116%) | 0.0568 | 0.4114 | |
| Oral feeding: median (range) | ||||||
| Intake (Kcal/d) | 1510 (0-2657) | 1071 (0-2827) | 1834 (930-2965) | 0.1114 | - | |
| Fat Absorption (% ingested) | 38 (0-88) | 27 (0-68) | 71 (21-95) | 0.1816 | - | |
The FA profile of the IVLEs was in agreement with that stated by the manufacturers. The OO-IVLE had a higher percentage of MUFAs and an n-6/n-3 ratio of 10:1. The SMOF-IVLE showed higher linoleic and alpha-linolenic acid percentages, approximately 3% of EPA (20:5; n 3) and DHA (22:6; n 3), and an n-6/n-3 ratio of 3:1. In both IVLEs, neither the trans isomer of oleic acid nor the 5-and 8-monotrans isomers of arachidonic acid were detected (Table 2).
| Fatty acids, % total FAs | OO-IVLE | SMOF-IVLE | OO-IVLE group | SMOF-IVLE group | No-IVLE group | HC group | P value |
| Palmitic (16:0) | 12.8 | 14.1 | 26.2 (23.1-30.6) | 28.1 (24.8-30.6) | 28.1 (26.1-29.4) | 26.6 (20.3-29.6) | 0.040 |
| Stearic (18:0) | 3.7 | 4.4 | 16.7 (14.3-18.3) | 16.8 (15.9-18.3) | 16.1 (14.2-16.7) | 18.1 (13.4-20.7) | < 0.001 |
| Palmitoleic (16:1) | 0 | 0 | 0.5 (0.4-0.9) | 0.5 (0.2-0.8) | 1.0 (0.3-1.8) | 0.4 (0.2-0.7) | < 0.001 |
| Oleic (18:1; 9c) | 61.8 | 43.3 | 18.9 (17.8-23.5) | 18.6 (17.3-19.9) | 18.9 (16.6-22.5) | 17.1 (14.2-22.2) | < 0.001 |
| Vaccenic (18:1; 11c) | 2.3 | 2.5 | 1.9 (1.6-2.7) | 1.7 (1.5-2.3) | 2.2 (1.7-3.3) | 1.4 (0.9-2.6) | < 0.001 |
| Linoleic (18:2; n-6) | 17.7 | 26.9 | 8.7 (5.5-10.4) | 9.2 (7.2-10.9) | 7.7 (3.9-9.6) | 11.6 (8.8-14.4) | < 0.001 |
| DGLA (20:3; n-6) | 0 | 0 | 2.2 (1.5-3.9) | 1.9 (1.1-2.1) | 2.2 (1.5-2.9) | 2.0 (1.5-2.8) | 0.049 |
| Arachidonic (20:4; n-6) | 0 | 0 | 18.3 (10.0-21.1) | 12.2 (10.7-14.4) | 19.2 (18.4-20.3) | 16.8 (13.1-21.4) | < 0.001 |
| Alfa-linoleic (18:3; n-3) | 1.7 | 2.4 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | |
| EPA (20:5; n-3) | 0 | 3.4 | 0.6 (0.4-4.5) | 3.2 (2.1-5.0) | 0.5 (0.4-1.0) | 0.7 (0.2-6.0) | < 0.001 |
| DHA (22:6; n-3) | 0 | 3 | 4.8 (3.4-8.4) | 7.6 (6.2-10.4) | 3.9 (3.0-4.7) | 5.2 (2.3-8.4) | < 0.001 |
| trans-Oleic | 0 | 0 | 0.1 (0.1-0.2) | 0.1 (0.0-0.2) | 0.2 (0.0-0.2) | 0.1 (0.0-0.2) | 0.275 |
| 5-8-trans-Arachidonic | 0 | 0 | 0.1 (0.1-0.2) | 0.1 (0.0-0.2) | 0.2 (0.0-0.1) | 0.1 (0.0-0.3) | 0.109 |
| Total SFAs | 16.5 | 18.5 | 43.5 (41.1-46.4) | 44.9 (40.7-47.4) | 44.0 (40.8-45.6) | 44.3 (37.7-50.1) | 0.786 |
| Total MUFAs | 65.1 | 45.8 | 21.5 (20.7-25.6) | 20.9 (19.7-21.9) | 21.9 (20.7-27.1) | 19.5 (15.8-24.8) | 0.001 |
| Total PUFAs | 19.4 | 35.7 | 34.4 (30.6-37.1) | 33.7 (31.0-39.2) | 33.8 (28.9-35.7) | 35.7 (24.4-39.2) | 0.937 |
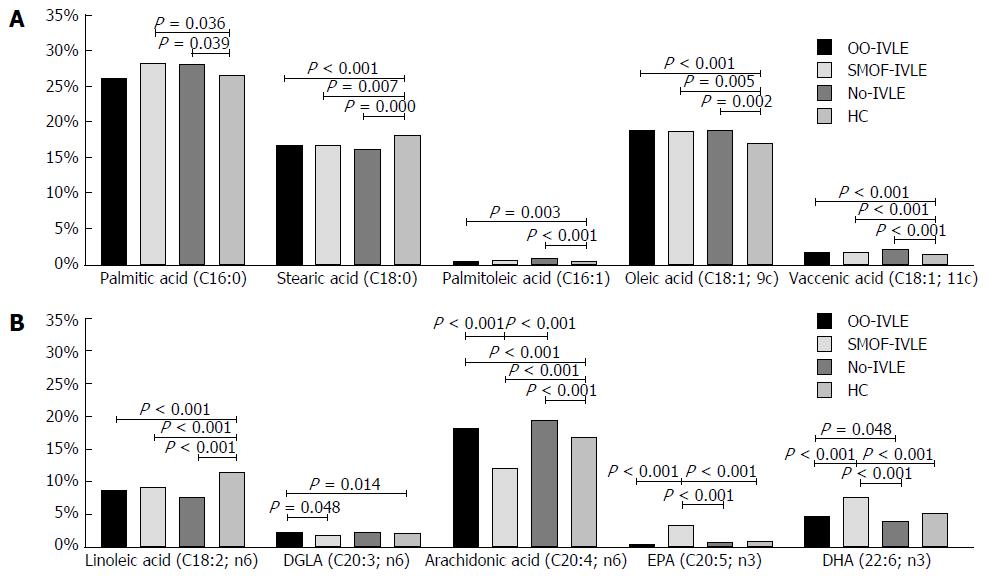
Total SFAs did not differ among the groups. In comparison with the HCs, the palmitic acid (16:0) concentration was significantly higher in the SMOF and No-IVLE groups whereas stearic acid (18:0) was lower in all the HPN groups (Table 2 and Figure 3).
Total MUFAs, as well as oleic acid (18:1, 9C) and vaccenic acid (18:1, 11C), were higher in the HPN groups than in the HCs. Among the HPN groups, total MUFAs showed higher values in the OO-IVLE and No-IVLE groups than in the SMOF-IVLE group. Palmitoleic acid (16:1) was higher in the OO-IVLE and No-IVLE groups than in the HCs.
Total PUFA content did not differ among the groups. The amount of linoleic acid (18:2, n-6) was lower in the HPN groups than in the HCs. The DGLA (20:3, n-6) was higher in the OO-IVLE group than in the SMOF-IVLE group and in the HCs. Arachidonic acid (20:4, n-6) was lower, and EPA (20:5, n-3) and DHA (22-6, n-3) were higher in the SMOF-IVLE group than in the other study groups. The highest value of arachidonic acid, and the lowest value of both EPA and DHA, were found in the No-IVLE group .
The percentages of trans-oleic acid (C18:1, 9C) and 5-8 mono-trans-arachidonic acid (C20:4) did not differ among the groups.
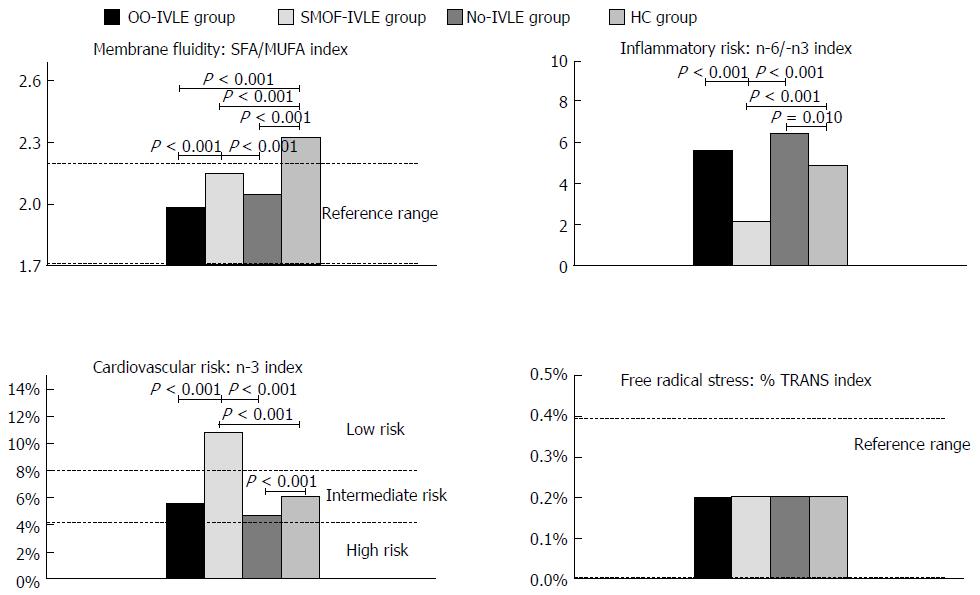
Membrane fluidity index: The SFA/MUFA ratio was lower in the HPN groups than in the HCs. The OO-IVLE group showed the lowest value (1.99; range: 1.66-2.12) (Figure 4).
Inflammatory risk index: The lowest n-6/n-3 ratio was found in the SMOF-IVLE group (2.05, range 1.56-3.01) and the highest in the No-IVLE group (6.5, range 4.78-9.17). No statistical difference was present between the OO-IVLE group and the HCs [5.65 (2.44-12.8) and 4.90 (1.57-7.05), respectively].
n-3 cardiovascular risk index: The n-3 index value ranged from 4 to 8% (intermediate risk) in the OO-IVLE and No-IVLE groups, and the HCs [5.5 (3.8-12.9), 4,7 (3.4-5.3), 6.1 (2.6-10.6), respectively] whereas it was > 8% (low risk) in the SMOF-IVLE group [(10.8 (8.3-14.0)].
Free radical stress index: The median value of the %Trans index was within the reference ranges (0%-0.4%) in all the groups (range 0.2%-0.3% in OO-IVLE and No-IVLE, 0.1%-0.2% in SMOF-IVLE and 0.2%-0.2% in HCs).
Metabolic pathway of the SFAs (D9D): The palmitic/palmitoleic acid ratio was lower in the OO-IVLE and the No-IVLE groups than in the HCs, suggesting increased D9D activity in converting palmitic acid (16:0) into palmitoleic acid (16:1) in both groups. No significant difference was found between the SMOF-IVLE group and the HCs (Figure 5).
Metabolic pathway of the MUFAs (D9D): The stearic/oleic acid ratio was lower in all the HPN groups than in the HCs, suggesting increased activity of D9D in converting stearic (18:0) into oleic acid (18:1; 9C) in patients on HPN (Figure 5).
Metabolic pathway of the n-6 PUFAs: The linoleic/DGLA ratio was lower in the OO-IVLE and No-IVLE groups than in both the SMOF-IVLE group and the HCs, suggesting higher activity of D6D and ELO in converting linoleic acid (18:2; n6) into DGLA (20:3; n6) in both the OO-IVLE and No-IVLE groups. No difference was found between the SMOF-IVLE group and the HCs.
The ARA/GLA ratio did not statistically differ among the HPN groups, suggesting similar D5D activity in converting DGLA (20:3; n6) into ARA (20:4; n6). The ARA/GLA ratio was lower in the SMOF-IVLE group than in the HCs, suggesting decreased D5D activity in the SMOF-IVLE group.
In the OO-IVLE group, a positive significant correlation was found between the weekly amount of lipid infused with PN (g/W) and the RBC oleic acid (18:1:9c) (n = 15; r = 0.540, P = 0.043).
In the SMOF-IVLE group, the RBC EPA (20:5, n = 3) was positively associated with the weekly amount of lipid infused (g/inf) (n = 8; r = 0.751, P = 0.044).
Red blood cell vaccenic acid (18:1; 11c) was positively correlated with the weekly amount of glucose infused with PN (g/W) in both the OO-IVLE (n = 15; r = 0.716; P = 0.007) and SMOF-IVLE (n = 8; r = 0.732; P = 0.053) groups.
In this study, the functional lipidomics analysis was used to interpret the effects of two IVLEs on the RBC membrane FA profile (membrane lipidome) of patients on HPN for CIF. The results of the membrane lipidome analysis are in agreement with previous findings[7]. The functional lipidomics analysis allowed these findings to be translated into their potential biological effects, previously unreported data. The limitations of the present study were similar to those of the previous studies[7]. They were mainly represented by the small size of the patient cohorts and by the lack of detailed information regarding oral fat intake. However, in the present study, the low intestinal fat absorption observed in both the OO-IVLE and SMOF-IVLE groups would suggest that oral lipids had a limited impact on the RBC membrane lipidome. In fact, in patients receiving the OO-IVLE, the percentage of oleic acid in the RBC membrane showed the highest numeric value and was positively correlated with the weekly load of OO-IVLE infused with PN. The group receiving the SMOF-IVLE showed the statistically significant highest percentage of EPA and DHA in the membrane lipidome. The former was positively correlated with the weekly amount of infused SMOF-IVLE.
In comparison with the HCs, the total MUFA percentage, as well as the oleic acid and vaccenic acid percentages in the RBC membrane, were increased in all the HPN groups. The functional lipidomics indicated that the activity of the D9D, which converts palmitic acid into palmitoleic acid and stearic acid into oleic acid was increased in all the HPN cohorts. A positive correlation was found between the weekly load of glucose infused with PN and the percentage of vaccenic acid in the RBC membrane. As it is known that insulin activates all the enzymes of the PUFA pathways[8,17,18], it could be suggested that the increase in MUFAs in the RBC membrane may be due to the insulin stimulation of D9D by the iv glucose load with HPN.
The RBC membrane linoleic acid percentage was lower in all the HPN groups than in the HCs, although it was the second most abundant FA in the IVLEs. These data have also been described in previous studies investigating patients receiving a soybean-based IVLE, the IVLE with the highest linoleic acid content[7,19-22]. The low linoleic acid percentage in the RBC membrane did not seem to be justified by an inadequate supply with PN as both the OO-IVLE and SMOF-IVLE groups received an amount of linoleic acid (8% and 6% of total PN calories, respectively) greater that the 1%-4% needed to prevent EFA deficiency[23]. In both the OO-IVLE and the No-IVLE groups, the low linoleic acid percentages were associated with increased arachidonic acid percentages. Also in this case, the functional lipidomics analysis suggested that the low linoleic acid percentages could have been due to the insulin stimulation of the activity of enzymes of the n-6 pathway, that catalysed the desaturation and elongation of Linoleic acid to DGLA (D6D and ELO) and the desaturation of DGLA to Arachidonic acid (D5D). In the SMOF-IVLE group, the low linoleic acid percentage was associated with an arachidonic acid percentage significantly lower than in the OO-IVLE and No-IVLE groups, suggesting a decrease in D5D. This could also have been due to the higher n-3 PUFA content of SMOF-IVLE because the affinity of both D6D and D5D for the n-3 PUFA is higher than that for the n-6 PUFAs, provided that the dietary n-3 to n-6 PUFA ratio is in the range of 1:1 to 4:1[24].
The molecular biomarkers of FA-related functions indicated some differences among the HPN groups. The membrane fluidity index, represented by the SFA/MUFA ratio, was normal in all the HPN groups, with the OO-IVLE group showing the greatest membrane fluidity. The maintenance of physiological cell membrane fluidity is a prerequisite for proper membrane function because it has a pivotal role in modulating the activity of the membrane enzymes, receptors, channels and transporters[25]. The inflammatory index, (expressed by the n-6/n-3 PUFA ratio), and the cardiovascular index (represented by the sum of the n-3 PUFAs) showed better values in the SMOF-IVLE group, reflecting the replacement of n-6 PUFAs with n-3 PUFAs in the RBC membrane, in agreement with the higher n-3 and the lower n-6 contents of the SMOF-IVLE. A decrease in cell membrane n-6-PUFA content, mainly arachidonic acid, associated with an increase in n-3 PUFAs, EPA and DHA, modified the balance of eicosanoid and cytokine production from a generally pro-inflammatory profile to a less inflammatory and even inflammation-resolving profile[26,27]. The n-6/n-3 ratio of patients receiving the OO-IVLE was similar to that of the HCs. In the No-IVLE patients, the n-6/n-3 index showed an increased inflammatory index. This could have been due to the lack of PN supply of FAs, and/or to insufficient oral intake and/or absorption of n-3 PUFAs associated with the above-mentioned insulin secretion due to the long-term PN infusion of glucose[17,18]. Finally, in all the PN-groups, the %trans index was within the normal range. This would indicate that, in our patients, there was no increased radical stress. Furthermore, trans isomers of oleic and arachidonic acids were not found in either of the IVLEs tested, indicating that the oil refining process utilised for their production appeared to be safe and free from trans isomer formation.
In conclusion, this study confirmed that the FA profile of IVLEs significantly influenced the cell membrane lipidome, indicated its functional relevance and suggested that other factors of the PN-admixture, such as the glucose content, may play a relevant role.
Fatty acid-based “functional lipidomics” investigates the structural and functional roles played by cell membrane lipids and their in vivo changes, and provides the rationalisation of these changes in connection with their biological significance.
The data of this study indicated that the fatty acid profile of intravenous lipid emulsions significantly influenced the cell membrane lipidome and highlighted its functional relevance. New studies should be carried out with the aim of detecting the optimal fatty acid composition of intravenous lipid emulsions in order to achieve the best functional asset of cell membrane.
The functional lipidomics analysis was used for the first time to interpret the effects of two intravenous lipid emulsions.
The fatty acid profiles of lipid emulsions can be a criterion for modifying the membrane lipidome of patients on home parenteral nutrition.
Functional lipidomics, based on the analysis of the red blood cell membrane fatty acid profile, investigates the structural and functional roles played by lipids and provides the rationalisation of these changes in connection with their biological significance.
The novelty of this manuscript is good, and the result can help to explain the research purpose.
| 1. | Pironi L, Arends J, Baxter J, Bozzetti F, Peláez RB, Cuerda C, Forbes A, Gabe S, Gillanders L, Holst M. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr. 2015;34:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 446] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 2. | Pironi L, Arends J, Bozzetti F, Cuerda C, Gillanders L, Jeppesen PB, Joly F, Kelly D, Lal S, Staun M. ESPEN guidelines on chronic intestinal failure in adults. Clin Nutr. 2016;35:247-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 516] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 3. | Staun M, Pironi L, Bozzetti F, Baxter J, Forbes A, Joly F, Jeppesen P, Moreno J, Hébuterne X, Pertkiewicz M. ESPEN Guidelines on Parenteral Nutrition: home parenteral nutrition (HPN) in adult patients. Clin Nutr. 2009;28:467-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 4. | Vanek VW, Borum P, Buchman A, Fessler TA, Howard L, Jeejeebhoy K, Kochevar M, Shenkin A, Valentine CJ. A.S.P.E.N. position paper: recommendations for changes in commercially available parenteral multivitamin and multi-trace element products. Nutr Clin Pract. 2012;27:440-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Wanten GJ, Calder PC. Immune modulation by parenteral lipid emulsions. Am J Clin Nutr. 2007;85:1171-1184. [PubMed] |
| 6. | Mirtallo JM, Dasta JF, Kleinschmidt KC, Varon J. State of the art review: Intravenous fat emulsions: Current applications, safety profile, and clinical implications. Ann Pharmacother. 2010;44:688-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Pironi L, Agostini F, Guidetti M. Intravenous lipids in home parenteral nutrition. World Rev Nutr Diet. 2015;112:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Ferreri C, Chatgilialoglu C. Role of fatty acid-based functional lipidomics in the development of molecular diagnostic tools. Expert Rev Mol Diagn. 2012;12:767-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Harris JA, Benedict FG. A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci USA. 1918;4:370-373. [PubMed] |
| 10. | Van de kamer JH, Ten bokkel huinink H, Weyers HA. Rapid method for the determination of fat in feces. J Biol Chem. 1949;177:347-355. [PubMed] |
| 11. | Ghezzo A, Visconti P, Abruzzo PM, Bolotta A, Ferreri C, Gobbi G, Malisardi G, Manfredini S, Marini M, Nanetti L. Oxidative Stress and Erythrocyte Membrane Alterations in Children with Autism: Correlation with Clinical Features. PLoS One. 2013;8:e66418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (11)] |
| 12. | Ferreri C, Faraone Mennella MR, Formisano C, Landi L, Chatgilialoglu C. Arachidonate geometrical isomers generated by thiyl radicals: the relationship with trans lipids detected in biological samples. Free Radic Biol Med. 2002;33:1516-1526. [PubMed] |
| 13. | Harris WS. RBC omega-3 predicts risk for death. Atherosclerosis. 2016;252:192-193. [PubMed] |
| 14. | Ferreri C, Chatgilialoglu C. Membrane Lipidomics and the Geometry of Unsaturated Fatty Acids: from Biomimetic Models to Biological Consequences. In: Lipidomics, Vol. 1: Methods and Protocols, Armstrong, D., Ed, Humana Press: New York, 2009: 391-412. . |
| 15. | Chatgilialoglu C, Ferreri C, Melchiorre M, Sansone A, Torreggiani A. Lipid geometrical isomerism: from chemistry to biology and diagnostics. Chem Rev. 2014;114:255-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 16. | Kummerow FA, Zhou Q, Mahfouz MM. Effect of trans fatty acids on calcium influx into human arterial endothelial cells. Am J Clin Nutr. 1999;70:832-838. [PubMed] |
| 17. | Vessby B, Gustafsson IB, Tengblad S, Boberg M, Andersson A. Desaturation and elongation of Fatty acids and insulin action. Ann N Y Acad Sci. 2002;967:183-195. [PubMed] |
| 18. | Brenner RR. Hormonal modulation of delta6 and delta5 desaturases: case of diabetes. Prostaglandins Leukot Essent Fatty Acids. 2003;68:151-162. [PubMed] |
| 19. | Pironi L, Belluzzi A, Miglioli M. Low levels of essential fatty acids in the red blood cell membrane phospholipid fraction of long-term home parenteral nutrition patients. JPEN J Parenter Enteral Nutr. 1996;20:377-378. [PubMed] |
| 20. | Jeppesen PB, Høy CE, Mortensen PB. Essential fatty acid deficiency in patients receiving home parenteral nutrition. Am J Clin Nutr. 1998;68:126-133. [PubMed] |
| 21. | Ling PR, Ollero M, Khaodhiar L, McCowen K, Keane-Ellison M, Thibault A, Tawa N, Bistrian BR. Disturbances in essential fatty acid metabolism in patients receiving long-term home parenteral nutrition. Dig Dis Sci. 2002;47:1679-1685. [PubMed] |
| 22. | Chambrier C, Garcia I, Bannier E, Gerard-Boncompain M, Bouletreau P. Specific changes in n -6 fatty acid metabolism in patients with chronic intestinal failure. Clin Nutr. 2002;21:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Bistrian BR. Clinical aspects of essential fatty acid metabolism: Jonathan Rhoads Lecture. JPEN J Parenter Enteral Nutr. 2003;27:168-175. [PubMed] |
| 24. | Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J Nutr Metab. 2012;2012:539426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 530] [Cited by in RCA: 568] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 25. | Maulucci G, Cohen O, Daniel B, Sansone A, Petropoulou PI, Filou S, Spyridonidis A, Pani G, De Spirito M, Chatgilialoglu C. Fatty acid-related modulations of membrane fluidity in cells: detection and implications. Free Radic Res. 2016;50:S40-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 26. | Walker CG, West AL, Browning LM, Madden J, Gambell JM, Jebb SA, Calder PC. The Pattern of Fatty Acids Displaced by EPA and DHA Following 12 Months Supplementation Varies between Blood Cell and Plasma Fractions. Nutrients. 2015;7:6281-6293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495-505. [PubMed] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Hu S S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF