Published online Jul 7, 2017. doi: 10.3748/wjg.v23.i25.4579
Peer-review started: January 12, 2017
First decision: February 23, 2017
Revised: March 13, 2017
Accepted: April 12, 2017
Article in press: April 12, 2017
Published online: July 7, 2017
Processing time: 177 Days and 17.4 Hours
To evaluate outcome of acute management and risk of rebleeding in patients with massive hemorrhage due to hepatocellular adenoma (HCA).
This retrospective cohort study included all consecutive patients who presented to our hospital with massive hemorrhage (grade II or III) due to ruptured HCA and were admitted for observation and/or intervention between 1999-2016. The diagnosis of HCA was based on radiological findings from contrast-enhanced magnetic resonance imaging (MRI) or pathological findings from biopsy or resection of the HCA. Hemorrhage was diagnosed based on findings from computed tomography or MRI. Medical records were reviewed for demographic features, clinical presentation, tumor features, initial and subsequent management, short- and long-term complications and patient and lesion follow-up.
All patients were female (n = 23). Treatment in the acute phase consisted of embolization (n = 9, 39.1%), conservative therapy (n = 13, 56.5%), and other intervention (n = 1, 4.3%). Median hemoglobin level decreased significantly more on days 0-3 in the intervention group than in the patients initially treated conservatively (0.9 mmol/L vs 2.4 mmol/L respectively, P = 0.006). In total, 4 patients suffered severe short-term complications, which included hypovolemic shock, acute liver failure and abscess formation. After a median follow-up of 36 mo, tumor regression in non-surgically treated patients occurred with a median reduction of 76 mm down to 25 mm. Four patients underwent secondary (elective) treatment (i.e., tumor resection) to address HCA size of > 5 cm and/or desire for future pregnancy. One case of rebleeding was documented (4.3%). None of the patients experienced long-term complication (mean follow-up time: 36 mo).
With a 4.3% risk of rebleeding, secondary (elective) treatment of HCA after massive hemorrhage may only be considered in patients with persistent HCA > 5 cm.
Core tip: As massive bleeding due to ruptured hepatocellular adenoma (HCA) is rare, we present a unique series of 23 consecutive patients. To our knowledge, this is the first study to assess the outcome and sequelae of the massive bleeding complication, including risk of rebleeding and need for elective tumor resection. In our series, risk of rebleeding was 4.3% and most HCAs regressed spontaneously. No long-term complications were documented. These cases suggest that a wait-and-watch policy may be legitimate and secondary (elective) treatment may only be considered for persistent HCA > 5 cm after follow-up and/or for patients with desire for future pregnancy.
- Citation: Klompenhouwer AJ, de Man RA, Thomeer MG, Ijzermans JN. Management and outcome of hepatocellular adenoma with massive bleeding at presentation. World J Gastroenterol 2017; 23(25): 4579-4586
- URL: https://www.wjgnet.com/1007-9327/full/v23/i25/4579.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i25.4579
Hepatocellular adenoma (HCA) is a rare benign liver tumor that occurs mostly in women in their reproductive phase. An association with the estrogen-containing oral contraceptive (OC) was first described in 1973[1,2]. Currently, the estimated annual incidences are 30-40 per million in long-term (> 2 years) OC users and 1 per million in non-users or women with less than 2 years of OC use[3].
HCAs are most often asymptomatic and discovered during radiologic imaging of the abdomen for unrelated reasons. The best way to diagnose HCA is with contrast-enhanced magnetic resonance imaging (MRI)[4]. In the past decade, much has changed in terms of diagnosis and treatment of HCA, due to the discovery of various subtypes of this tumor[5,6]. The change in treatment strategy is ongoing, due to the apparent differences in risks of complications for the various subtypes.
As HCAs are well vascularized tumors, hemorrhage - as documented on imaging - is a common complication, occurring in approximately 25% of the patients with HCA[7]. OC use, tumor size of > 5 cm, exophytic growth of the tumor and inflammatory subtype (I-HCA) are associated with a higher risk of bleeding[7,8]. Most hemorrhages are intratumoral; however, in cases of massive bleeding, rupture of the HCA can occur, resulting in intraparenchymal hemorrhage and subcapsular hematoma. In some cases, the liver capsule can rupture, causing hemoperitoneum.
Hemorrhagic HCAs cause symptoms such as acute-onset right upper abdominal pain and discomfort. In cases of tumor rupture and excessive bleeding, patients may present with hemodynamically unstable conditions and even may show signs of hypovolemic shock. This complication can be life threatening[3].
Another rare complication of HCAs, not related to risk of bleeding, is malignant degeneration to hepatocellular carcinoma, which occurs mostly in β-catenin-positive HCA[9]. As both hemorrhage and malignant degeneration arise especially in adenomas > 5 cm in size, surgical resection is recommended for HCA which do not regress by 6 mo after cessation of OC and following lifestyle changes, such as weight reduction in overweight patients[10-13].
The management policy for acute bleeding of ruptured HCA has changed over the past years. In the acute phase, a conservative management and hemodynamic stabilization is justified[14]. In case of active bleeding with persistent hemodynamic instability or hemoperitoneum, intervention may be considered[7]. Liver resection in the acute phase is associated with increased morbidity and mortality and, therefore, not advisable[15]. Laparotomy and gauze packing of the liver had been recommended until the introduction of minimally-invasive techniques. The advent of selective arterial embolization (SAE) 10 years ago has now allowed for patients to be treated even less invasively[16,17]. An unanswered question, however, remains in regards to the chance of rebleeding and, correspondingly, the need for elective tumor resection.
In this study, we evaluated the outcome of acute management in patients with massive hemorrhage due to ruptured HCA and its sequelae, including the risk of rebleeding and need for elective tumor resection.
This study was a retrospective cohort study performed in a tertiary referral center for focal liver lesions. With the availability of a large HCA cohort, consisting of 449 patients, we selected all patients who presented with massive hemorrhage as a result of ruptured HCA and who were admitted to a hospital ward for observation and/or intervention between January 1999 (the start of the database) and April 2016, with follow-up time of at least 6 mo.
This study was approved by the accredited local institutional review board (No. MEC-2016-338) and informed consent was waived.
Massive hemorrhage was defined as intrahepatic (grade II) or intraperitoneal (grade III), as reported by Bieze et al[8] in 2014. Patients with intratumoural hemorrhage (grade I) were excluded from this study. The diagnosis of HCA was based on radiological findings from contrast-enhanced magnetic resonance imaging (MRI) or pathological findings from biopsy or resection of the HCA. Hemorrhage was diagnosed based on findings from computed tomography (CT) or MRI. Hepatocellular carcinoma - the main competing differential diagnosis - was excluded by occurrence of tumor regression after hemorrhage, absence of progression in diameter and lack of metastatic disease over time.
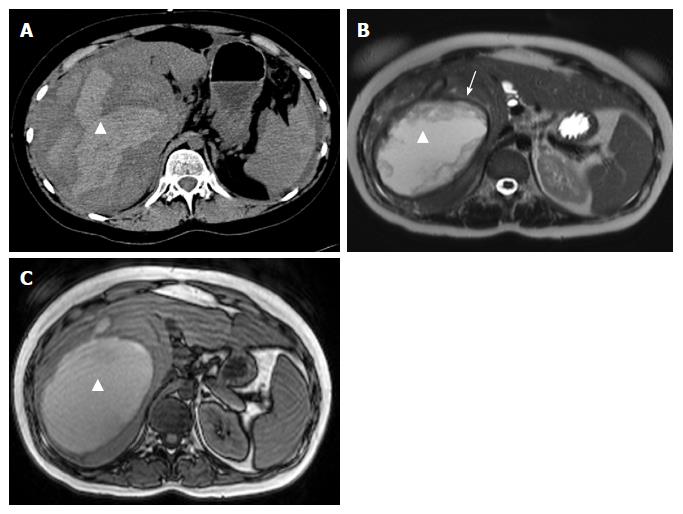
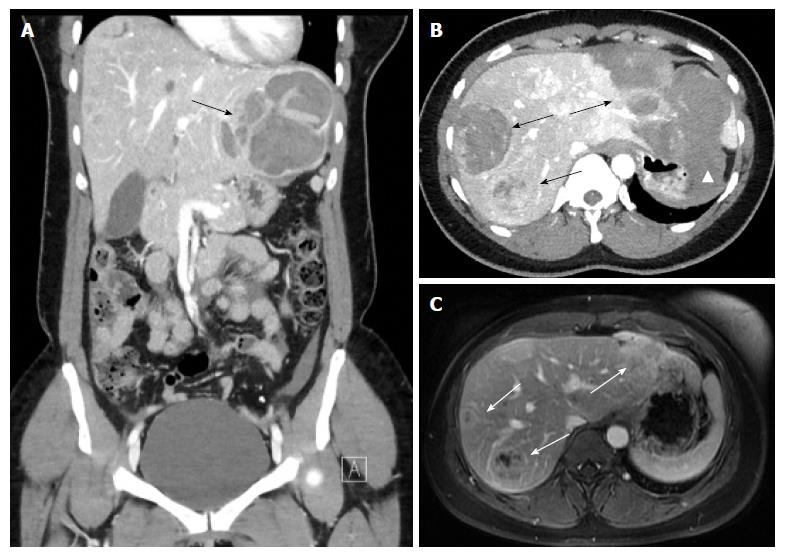
On CT, the density of a hematoma is determined by the time elapsed after the initial event. In the acute phase, a hematoma is hyperdense, becoming more isodense in the chronic phase. On MRI, the intensity of a hematoma also changes over time. On T1-weighting, hematoma is hyperintense in the beginning, becoming more and more isointense in the chronic phase. On T2-weighting, a hematoma starts as hyperintense and resolves in the chronic phase with zones devoid of signal (visualized as black space) due to deposition of hemosiderin, and which occurs mostly in the periphery[18] (Figures 1 and 2).
HCA subtyping - for steatotic, inflammatory, β-catenin-positive and unclassified HCA - was determined based on findings of immunohistochemistry or of the typical MRI features[19-21]. In cases that the HCA subtype was not able to be established by MRI or biopsy, previous available MRI scans were reviewed by an experienced radiologist.
Medical records were reviewed to collect each patient’s demographic features, clinical presentation, tumor features, initial and subsequent management (including surgery or intervention techniques), short- and long term complications and patient and lesion follow-up. If patients had been referred to our center by another hospital, we requested the data from the referring hospital, with consent of the patient.
All statistical analyses were performed using SPSS software, version 21.0 (IBM, Armonk, NY, United States). All non-normal distributed variables were summarized as median and interquartile range (IQR); binary variables were summarized as frequency (n). Differences between groups were investigated using the Mann-Whitney U test for continuous variables. A P value of < 0.05 was considered as the threshold for significance.
We assessed 23 consecutive patients who were admitted to hospital for massive intrahepatic hemorrhage or hemoperitoneum due to ruptured HCA (Table 1). All patients were female, with a median age of 34-years-old (IQR: 30-44 years). Fifteen of the patients presented with grade II hemorrhage, and 8 with grade III hemorrhage. The median lesion size was 76 mm (IQR: 55-92 mm), with 14/23 (60.8%) located in the right or left lateral liver and the remaining 9/23 (39.1%) located in the right medial or central liver (Table 2). Nineteen out of the 23 patients (82.6%) used OCs at the time of presentation. Two patients had an I-HCA, and a third patient had both an I-HCA as well as a H-HCA (steatotic), of which the I-HCA ruptured. The subtype was not defined in all other patients.
| Case no. | Age (yr) | OC use | Type of bleeding, grade | Initial management | ICU | Blood products | Hospital stay (d) | Short-term complications |
| Initially conservatively treated | ||||||||
| 1 | 49 | Yes | II | Cons. | No | No | 19 | - |
| 2 | 43 | Yes | III | Cons. | Yes | Yes | 7 | - |
| 3 | 30 | Yes | II | Cons. | No | No | 7 | - |
| 4 | 36 | No | II | Cons. | No | No | 19 | - |
| Stopped 12 mo prior to bleeding | ||||||||
| 5 | 33 | Yes | II | Cons. | No | No | 8 | - |
| 6 | 23 | Yes | II | Cons. | Yes | Yes | 28 | - |
| 7 | 30 | Yes | II | Cons. | No | No | 12 | - |
| 8 | 39 | Yes | II | Cons. | No | No | 9 | - |
| 9 | 43 | Yes | III | Cons. | No | Yes | 14 | - |
| 10 | 44 | Yes | II | Cons. | No | No | 13 | - |
| 11 | 49 | Yes | III | Cons. | Yes | Yes | 61 | Hypovolemic shock, respiratory insufficiency, kidney failure, abdominal compartment syndrome |
| 12 | 31 | Yes | II | Cons. | No | No | 10 | - |
| 13 | 33 | Yes | III | Cons. | No | No | 13 | - |
| Initially treated with intervention | ||||||||
| 14 | 22 | Unknown | III | SAE + resection | No | Yes | 40 | Postoperative abdominal abscess, drainage pleural effusion |
| 15 | 33 | No | III | SAE | Yes | Yes | Unknown | - |
| Stopped 18 mo prior to bleeding | ||||||||
| 16 | 24 | Yes | III | SAE | Yes | Yes | 18 | - |
| 17 | 36 | Yes | II | SAE | Yes | No | 22 | Acute liver failure after SAE left and right hepatic artery |
| 18 | 48 | Yes | II | SAE | No | No | 10 | - |
| 19 | 48 | Yes | III | SAE | Yes | No | 6 | - |
| 20 | 34 | No | Unknown | SAE | Unknown | Unknown | Unknown | - |
| Stopped 7 mo prior to bleeding | ||||||||
| 21 | 30 | Yes | II | US-guided drainage | No | No | 10 | - |
| 22 | 56 | Yes | II | SAE | Yes | No | Unknown | - |
| 23 | 20 | Yes | III | SAE | No | Yes | 33 | Rebleed after 3 mo, drainage hepatic abscess, drainage pleural effusion |
| Initial conservative, n = 13 | Initial intervention, n = 10 | Total, n = 23 | P value | |
| Median age (yr) | 36 (30.5-43.5) | 33.5 (23.5-48.0) | 34 (30-44) | 0.563 |
| Median HCA diameter at diagnosis (mm) | 76 (55-101.5) | 76.5 (51.3-92.5) | 76 (55-92) | 0.648 |
| Median follow-up time (mo) | 66 (23-87) | 22.5 (12.8-60.3) | 36 (15-79) | 0.257 |
| Type of bleeding | 0.349 | |||
| Grade II | 9 | 5 | 15 | |
| Grade III | 4 | 5 | 8 | |
| Median hospital stay (d) | 13 (8.5-19) | 18 (10-33) | 13 (9.3-21.3) | 0.588 |
| Median Hb level at presentation (mmol/L) | 8.0 (6.0-8.4) | 7.5 (7.0-8.0) | 7.6 (7.0-8.1) | 0.710 |
| Median decrease Hb day 0-3 (mmol/L) | 0.9 (0-1.7) | 2.4 (1.6-3.5) | 1.6 (0.4-2.4) | 0.0061 |
In all 23 patients, hemorrhage was the first presentation, and none of the patients were diagnosed as having HCA before the bleeding. Fifteen out of the 23 patients (65.2%) were hemodynamically stable at presentation, and one patient presented with hypovolemic shock. A total of 13 patients (56.5%) were treated conservatively. Nine patients (39.1%) underwent SAE in the acute phase, and one of them also underwent an acute resection due to persistent bleeding after the SAE. One patient underwent ultrasound-guided drainage of a presumed liver abscess, through which massive bleeding was found. Additional imaging for this patient showed an undetermined liver tumor, which prompted the patient referral to our hospital, where we confirmed the tumor to be an HCA.
Comparison of the patients initially treated conservatively to those treated with intervention showed no statistically significant differences for age, median HCA diameter, median follow-up time, median hospital stay nor median hemoglobin level at presentation. However, the intervention group showed a statistically greater reduction in median hemoglobin level on day 0-3 compared to the conservative treatment group (0.9 mmol/L vs 2.4 mmol/L respectively, P = 0.006) (Table 2).
Of the 23 patients, 4 (17.4%) suffered severe short-term complications (Table 1). In 2 patients, abscesses developed after embolization or resection, necessitating additional percutaneous drainage and resulting in hospital stays of 33 and 40 d respectively. One patient suffered acute liver failure after SAE, affecting both the left and right hepatic artery, with laboratory tests showing increases in aspartate transaminase (350-fold), alanine transaminase (170-fold), bilirubin (5.4-fold), lactate (4.5-fold) and the international normalized ratio (INR; 1.8-fold). Spontaneous recovery occurred within 5 d, and the total duration of hospital stay for this patient was 22 d.
The patient who presented with major hypovolemic shock was treated conservatively and suffered major hemoperitoneum, resulting in respiratory insufficiency, kidney failure and abdominal compartment syndrome. This was a patient who had recent history of oral anticoagulants for treatment of deep vein thrombosis. Initially, her case was classified as venous hemorrhage due to an excessively high INR, and therefore she was treated conservatively. After recovery, she was referred to our hospital and the diagnosis of HCA was only made upon liver imaging after the hematoma had become, more or less, absorbed. The total duration of hospital stay for this patient was 61 d.
Median follow-up time was 36 mo (IQR: 15-79 mo). One patient underwent elective resection, 1 underwent elective SAE and 2 underwent elective radiofrequency ablation (RFA) to address residual HCA (Table 3). These patients either had residual HCA of > 5 cm in size or a smaller lesion but with an expressed desire for future pregnancy. Out of the total 23 patients in the study, 18 (78.3%) were kept under surveillance for > 6 mo, all showing regression of the tumor from a median diameter of 76 mm (IQR: 55-92 mm) to 25 mm (IQR: 17.3-41.5 mm).
| Case no. | Diameter HCA at diagnosis (mm) | Location of HCA | Elective treatment of HCA | Last known HCA diameter (mm) |
| Initially conservatively treated | ||||
| 1 | 200 | Right lateral (sVI/VII) | Surveillance | 6 |
| 2 | 76 | Right lateral (sVI/VII) | Surveillance | 22 |
| 3 | 60 | Right lateral (sVI) | Surveillance | 26 |
| 4 | 80 | Right medial (sVIII) | Surveillance | 53 |
| 5 | 80 | Right lateral (sVI) | Resection | - |
| 6 | 75 | Right lateral (sVI/VII) | RFA | 0 |
| 7 | 75 | Right lateral (sVI/VII) | Surveillance | 8 |
| 8 | 143 | Right medial (sVIII) | Surveillance | 35 |
| 9 | 45 | Right medial (sV/VIII) | Surveillance | 40 |
| 10 | 39 | Right lateral (sVII) | Surveillance | 18 |
| 11 | 50 | Right medial (sV) | Surveillance | 21 |
| 12 | 92 | Right lateral (sVI/VII) | Surveillance | 43 |
| 13 | 111 | Left lateral (sII/III) | Surveillance | 92 |
| Initially treated with intervention | ||||
| 14 | 90 | Central (sIV/VIII) | No adenoma tissue after resection | - |
| 15 | 55 | Right medial (sVIII) | Surveillance | 20 |
| 16 | 40 | Right medial (sV/VIII) | RFA | 0 |
| 17 | 73 | Central (sIV/VIII) | Surveillance | 40 |
| 18 | 80 | Right lateral (sVI/VIII) | Surveillance | 24 |
| 19 | 100 | Left lateral (sII/III) | Surveillance | 45 |
| 20 | 100 | Central (sIV/V/VIII) | SAE | 42 |
| 21 | 55 | Right lateral (sVII) | Surveillance | 38 |
| 22 | 25 | Left lateral (sIII) | Surveillance | 17 |
| 23 | 87 | Right lateral (sVII) | Surveillance | 62 |
Rebleeding was reported in one patient (4.3%), which occurred 3 mo after the initial hemorrhage. This was the only patient in whom OC was continued after the first bleeding because the underlying etiology of the hemorrhage had not yet been established. After the rebleeding, the patient was referred to our tertiary center and the diagnosis of HCA was made. The rebleeding was also managed with a wait-and-watch policy, and cessation of OC lead to regression of the HCA.
None of the patients in this study had long-term complications. Out of the 9 patients who did not have any treatment (initial or elective), 2 became pregnant. In the first patient, the HCA showed growth from 46 mm to 65 mm, but no hemorrhage occurred during pregnancy and after the delivery the HCA regressed. In the second patient, the lesion remained stable in size (30 mm) throughout the pregnancy.
In this retrospective cohort study, we evaluated the outcome of acute management of patients with massive hemorrhage due to ruptured HCA and confirmed that a conservative approach is justified in the acute situation if there is a hemodynamically stable condition. In the case of persistent bleeding, SAE can be a solution. Our cohort showed a significant difference in median decrease in hemoglobin level when the conservatively-treated group was compared to the group of patients who underwent initial intervention, with a greater decrease (median: 2.4 mmol/L) occurring in the intervention group. Four patients in our study suffered the following severe short-term complications: 1 patient who had used oral anticoagulants and then presented with hypovolemic shock developed respiratory insufficiency, kidney failure and abdominal compartment syndrome; 2 patients who developed complications after SAE; and 1 patient who developed postoperative complications after both SAE and resection in the acute phase. All complications resulted in longer hospital stay; however, no long-term complications were documented.
After a median follow-up time of 36 mo, all tumors under surveillance showed spontaneous regression, with a median size decrease from 76 mm at presentation to 25 mm at last follow-up. This finding is comparable to the regression reported for non-hemorrhagic HCA after OC discontinuation[10,11]. Only 1 case of rebleeding was reported (4.3%) in a patient who did not discontinue OC, and this occurred at 3 mo after the initial bleed. Secondary treatment, such as elective tumor resection, SAE or RFA, was only performed in patients with HCA > 5 cm in size after follow-up and/or in patients with an expressed desire for future pregnancy.
This study, however, did not establish whether hemorrhagic HCA should remain in regular follow-up or when, if ever, it will be safe to end surveillance. Most lesions are > 5 cm at the moment of hemorrhage, causing at least a part of the vital adenoma tissue to become necrotic. Therefore, we are unsure if the higher risk of malignant degeneration in HCA > 5 cm is still present in hemorrhagic HCA; indeed, it might be advisable to keep these patients in regular follow-up.
It is important to establish an optimal treatment plan for patients with ruptured HCA, as hemorrhage is a frequent complication of lesions > 5 cm[7]. In 2006, Erdogan et al[14] conducted a study assessing management and outcome in patients treated for ruptured HCA, in which they compared laparotomy and gauze packing with observation. Their results suggested that stable patients could be treated conservatively and that resection of the HCA in the acute situation is accompanied by a higher morbidity. With the advent of SAE a decade ago, Stoot et al[16] conducted a small cohort study including 11 patients, which established the safety and efficacy of SAE for the treatment of ruptured HCA. Recently, the European Association for Study of the Liver issued a European clinical practice guideline for the management of benign liver tumors[13]. In this guideline, patients with massive hemorrhage due to ruptured HCA are recommended to be transferred to a center with an interventional radiology department and the possibility to perform SAE.
To the best of our knowledge, this study presented herein is the first to assess outcome and risk of rebleeding in patients with massive hemorrhage due to ruptured HCA. Unfortunately, for most of the included patients, the HCA subtype remained unknown. This is most likely the result of the hemorrhage itself, which makes it very difficult to distinguish HCA subtypes according to imaging characteristics. As we only knew the subtype for 3 of the patients in our study population (all I-HCA), we cannot make a judgement about the distribution of the various subtypes and the relationship to the different variables. Previous studies have shown that the risk for hemorrhage is the greatest in I-HCA[8], which was confirmed in our population.
It was noticed that in our study 60.8% of the ruptured HCA were located in the right or left lateral liver, and the remaining 39.1% were located medial or central. This does not entirely correspond to the study by Bieze et al[8] that was conducted in 2014, in which those authors identified HCA located in the left lateral liver as a risk factor for hemorrhage. It is thought that the medial or central location and maximum surrounding liver tissue prevent rupture of the HCA by tamponade. Our study likewise suggests that medial or centrally located HCA may cause hemorrhage as well.
The biggest limitation of this retrospective study is the design, which has inherent bias. By requesting data from all of the treatment hospitals, the proportion of missing data was kept to a minimum. In addition, hemorrhage makes the measurement of HCA diameter more difficult and less reliable. Therefore, the reported median diameter of 76 mm for the HCAs at presentation might be an overestimation.
In conclusion, this study confirmed that patients with massive hemorrhage due to ruptured HCA but with cessation of bleeding and in stable condition may be treated conservatively in the acute situation. SAE can be a solution for unstable patients with persistent bleeding and decreasing hemoglobin levels. No long-term complications were documented. As the risk of rebleeding is very low after cessation of OC and most HCAs regress spontaneously, secondary treatments, such as tumor resection, SAE or RFA, may only be considered in patients with HCA > 5 cm after follow-up or in patients with an expressed desire for future pregnancy. However, regular follow-up by imaging in these patients should be considered.
Hepatocellular adenoma (HCA) is a rare benign liver tumor that may present with spontaneous massive hemorrhage.
This study provides unique insights into the outcome of acute management of these patients and the sequelae of massive bleeding, including risk of rebleeding and need for elective tumor resection.
To the best of our knowledge, this is the first study assessing the outcome and the sequelae of massive bleeding, including the risk of rebleeding and need for elective tumor resection, in this patient population. As massive bleeding due to ruptured HCA is rare, we present a unique series of 23 cases.
This study confirms that patients with massive hemorrhage due to ruptured HCA, but with cessation of bleeding and in stable condition, may be treated conservatively in the acute situation. Embolization can be a solution for unstable patients with persistent bleeding and decreasing hemoglobin levels. As the risk of rebleeding is very low after cessation of OC and most HCAs regress spontaneously, secondary treatment may only be considered in patients with HCA > 5 cm after follow-up or in patients with an expressed desire for pregnancy. However, regular follow-up by imaging in these patients should be considered.
HCA is a rare benign liver tumor related to use of estrogen-containing oral contraceptives, and which may present with spontaneous massive hemorrhage.
This is an interesting clinically relevant study. It is well conducted and well written. The outcome is useful in the clinical management of HCA.
| 1. | Baum JK, Bookstein JJ, Holtz F, Klein EW. Possible association between benign hepatomas and oral contraceptives. Lancet. 1973;2:926-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 357] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Baek S, Sloane CE, Futterman SC. Benign liver cell adenoma associated with use of oral contraceptive agents. Ann Surg. 1976;183:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Hill AP, Tyler CW Jr. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA. 1979;242:644-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 54] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | De Kock I, Mortelé KJ, Smet B, Gillardin P, Pauwels W, De Backer AI. Hepatic adenomatosis: MR imaging features. JBR-BTR. 2014;97:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Bioulac-Sage P, Balabaud C, Bedossa P, Scoazec JY, Chiche L, Dhillon AP, Ferrell L, Paradis V, Roskams T, Vilgrain V. Pathological diagnosis of liver cell adenoma and focal nodular hyperplasia: Bordeaux update. J Hepatol. 2007;46:521-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Nault JC, Bioulac-Sage P, Zucman-Rossi J. Hepatocellular benign tumors-from molecular classification to personalized clinical care. Gastroenterology. 2013;144:888-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 7. | van Aalten SM, de Man RA, IJzermans JN, Terkivatan T. Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg. 2012;99:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Bieze M, Phoa SS, Verheij J, van Lienden KP, van Gulik TM. Risk factors for bleeding in hepatocellular adenoma. Br J Surg. 2014;101:847-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Pilati C, Letouzé E, Nault JC, Imbeaud S, Boulai A, Calderaro J, Poussin K, Franconi A, Couchy G, Morcrette G. Genomic profiling of hepatocellular adenomas reveals recurrent FRK-activating mutations and the mechanisms of malignant transformation. Cancer Cell. 2014;25:428-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 10. | Edmondson HA, Reynolds TB, Henderson B, Benton B. Regression of liver cell adenomas associated with oral contraceptives. Ann Intern Med. 1977;86:180-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 145] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Bühler H, Pirovino M, Akobiantz A, Altorfer J, Weitzel M, Maranta E, Schmid M. Regression of liver cell adenoma. A follow-up study of three consecutive patients after discontinuation of oral contraceptive use. Gastroenterology. 1982;82:775-782. [PubMed] |
| 12. | Dokmak S, Belghiti J. Will weight loss become a future treatment of hepatocellular adenoma in obese patients? Liver Int. 2015;35:2228-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines on the management of benign liver tumours. J Hepatol. 2016;65:386-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 355] [Article Influence: 35.5] [Reference Citation Analysis (2)] |
| 14. | Erdogan D, Busch OR, van Delden OM, Ten Kate FJ, Gouma DJ, van Gulik TM. Management of spontaneous haemorrhage and rupture of hepatocellular adenomas. A single centre experience. Liver Int. 2006;26:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Huurman VA, Schaapherder AF. Management of ruptured hepatocellular adenoma. Dig Surg. 2010;27:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Stoot JH, van der Linden E, Terpstra OT, Schaapherder AF. Life-saving therapy for haemorrhaging liver adenomas using selective arterial embolization. Br J Surg. 2007;94:1249-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Terkivatan T, de Wilt JH, de Man RA, van Rijn RR, Tilanus HW, IJzermans JN. Treatment of ruptured hepatocellular adenoma. Br J Surg. 2001;88:207-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Parizel PM, Makkat S, Van Miert E, Van Goethem JW, van den Hauwe L, De Schepper AM. Intracranial hemorrhage: principles of CT and MRI interpretation. Eur Radiol. 2001;11:1770-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 163] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | van Aalten SM, Thomeer MG, Terkivatan T, Dwarkasing RS, Verheij J, de Man RA, Ijzermans JN. Hepatocellular adenomas: correlation of MR imaging findings with pathologic subtype classification. Radiology. 2011;261:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 20. | Ronot M, Bahrami S, Calderaro J, Valla DC, Bedossa P, Belghiti J, Vilgrain V, Paradis V. Hepatocellular adenomas: accuracy of magnetic resonance imaging and liver biopsy in subtype classification. Hepatology. 2011;53:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Laumonier H, Bioulac-Sage P, Laurent C, Zucman-Rossi J, Balabaud C, Trillaud H. Hepatocellular adenomas: magnetic resonance imaging features as a function of molecular pathological classification. Hepatology. 2008;48:808-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: the Netherlands
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Bayraktar Y, El-Salhy M, Lee HC, Perez-Cuadrado-Robles E S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF