Published online Jun 28, 2017. doi: 10.3748/wjg.v23.i24.4369
Peer-review started: December 14, 2016
First decision: February 9, 2017
Revised: February 25, 2017
Accepted: April 12, 2017
Article in press: April 12, 2017
Published online: June 28, 2017
Processing time: 195 Days and 4.2 Hours
To investigate the effects of Ground Cherry (Physalis angulata L.) standardized supercritical CO2 extract in trinitrobenzenesulphonic acid (TNBS) model of rat intestinal inflammation.
The animals were divided into groups that received vehicle or P. angulata extract (PACO2) orally at the doses 25, 50 and 100 mg/kg daily by 5 d before TNBS damage. Protective effects of PACO2 were assessed by macroscopic analysis, biochemical determinations of the levels of myeloperoxidase (MPO), alkaline phosphatase (ALP), glutathione and cytokines (such as INF-γ, IL-1β, IL-6, IL-10 and TNF-α), gene expression evaluation (including Hsp70, heparanase, NF-κB, mitogen-activated protein kinases (Mapk) 1, 3, 6 and 9, and the mucins genes Muc 1, 2, 3 and 4) and histopathological studies using optical, and electronic (transmission and scanning) microscopy.
PACO2 extract promoted a significant reduction in MPO and ALP activities, reducing oxidative stress and neutrophil infiltration. These effects were accompanied by significant reduction of colonic levels of IFN-γ and IL-6 and down-regulation of heparanase, Hsp70, Mapk3, Mapk9, Muc1 and Muc2 genes expression when compared with TNBS-control animals. In addition, protective effects were also evidenced by reduced neutrophil infiltration, recovery of cell architecture and replacement of mucin by histopathological and ultrastructural analysis.
Physalis angulata supercritical CO2 extract is an intestinal anti-inflammatory product that modulates oxidative stress, immune response and expression of inflammatory mediators, with potentially utility for treating inflammatory bowel disease.
Core tip: We report, at the first time, the protective effects of a supercritical CO2 plant extract from aerial parts of Ground Cherry (Physalis angulata L.) in a model of intestinal inflammation induced by trinitrobenzenesulphonic acid in rats. The effects were related to presence of plant steroids, compounds chemically related to glucocorticoids, reference drugs used to treat human inflammatory bowel disease (IBD). The intestinal anti-inflammatory activity of Physalis angulata plant extract was related to its capacity to modulate oxidative stress, immune response and gene expression of inflammatory mediators. This way, the standardized plant extract of Ground Cherry enriched with phytosterols has potential for use to treat IBD.
- Citation: Almeida Junior LD, Quaglio AEV, de Almeida Costa CAR, Di Stasi LC. Intestinal anti-inflammatory activity of Ground Cherry (Physalis angulata L.) standardized CO2 phytopharmaceutical preparation. World J Gastroenterol 2017; 23(24): 4369-4380
- URL: https://www.wjgnet.com/1007-9327/full/v23/i24/4369.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i24.4369
Inflammatory bowel diseases (IBD), comprising ulcerative colitis (UC) and Crohn’s disease (CD), are diseases of modern society associated with a modern and urbanized lifestyle and caused by an increase in stress, bad diet habits and sedentariness[1,2]. CD and UC are chronic inflammatory disorders affecting the gastrointestinal tract and are characterized by periods of exacerbation followed by prolonged intervals of remission of symptoms[3,4]. The etiopathogenesis of these diseases has not been fully elucidated, but is presumed to result from a complex interplay among genetic, environmental, microbial and immune factors[5]. An exaggerated and inappropriate mucosal immune response mediated by mucosal T cells triggers intense synthesis and release of several pro-inflammatory mediators, including reactive species of oxygen and nitrogen, and a multitude of pro- and anti-inflammatory cytokines[6,7].
The available drugs, such as 5-aminosalicylate derivatives, glucocorticoids, immunosuppressive and monoclonal antibodies, have exhibited beneficial effects in the treatment of IBD, but do not represent a definitive cure. Indeed, these drugs have several side effects, and many patients do not respond to these treatments[3,8,9]. Dissatisfaction with the current pharmacological treatments has resulted in an increased interest to use complementary medicine approaches, including medicinal plant extracts or natural active compounds[10]. Based on this, our research group has been interested in studying the pharmacological activities of natural products against intestinal inflammation, focusing on tropical medicinal plants, of which Ground Cherry (Physalis angulata L.) was selected based upon its ethnopharmacological, pharmacological and chemical data.
Ground cherry is a native Brazilian weed from the Solanaceae family, which grows especially in the Brazilian Amazon Forest and other tropical countries of Africa, America and Asia, where it has been used traditionally as an anti-inflammatory herbal product and to treat several health disorders, such as cold, cough, fever, pain, malaria and nervous diseases[11,12]. Fruits from the Ground Cherry are used as food in several countries, particularly as a sophisticated additive to salads. Reputed pharmacological effects, such as antinociceptive, immunosuppressive, anti-protozoal, antineoplastic and anti-inflammatory, have been associated with the constituent presence of sitosterol, stigmasterol and other phytosterols, representing the major components of the plant extract[13-16].
Based on the ethnopharmacological data and pharmacological and chemical studies, a standardized CO2 supercritical preparation from aerial parts of P. angulata containing 10%-18% of phytosterols was patented by our research group, claiming corticoid-like effects evidenced by reduction of TNF-α, IL-6, IL1-β and COX-2 levels in non-stimulated and stimulated human fibroblasts and keratinocytes[17]. In the study presented herein, we evaluated this standardized plant extract from P. angulata in a model of intestinal inflammation induced by trinitrobenzenesulphonic acid (TNBS) in rats.
Physalis angulata L. was cultivated using organic agricultural methods and submitted to taxonomic identification at herbarium Irina Gemtchujinikov (Department of Botany, Institute of Biosciences, Universidade Estadual Paulista - UNESP/SP/BR), where a voucher specimen was deposited. The aerial parts were collected, dehydrated in hothouse with air circulation and renewal, and pulverized in an industrial mill.
A supercritical extraction system (Parker Autoclave Engineers, Erie, PA, United States), under the conditions of 300 bar, 40 °C and CO2 flux of 5 L/min during 150 min, was used to generate a supercritical CO2 extract enriched with phytosterols, including sitosterol, stigmasterol and physalins, which are the main constituents of Physalis plants. The enrichment of supercritical CO2 extract was compared with water:butileneglycol 1:1 extract and evaluated by Liebermann-Burchard reaction and ultraviolet spectrometry at 621 nm (Hewlett-Packard, Palo Alto, CA, United States). P. angulata L. supercritical CO2 extract (PACO2) was standardized in 10%-18% of total phytosterols and provided by Chemyunion Química Ltda (Sorocaba, Brazil).
Male Wistar rats (180-200 g) obtained from ANILAB - Animais de Laboratório, Paulínia, São Paulo (Brazil), were housed in standard environmental conditions (21 °C, 60%-70% humidity) with 12-h light/dark cycle and air filtration. Animals had free access to water and food (Biobase). Experimental protocols met the ‘‘Guidelines of Animal Experimentation’’ approved by the Ethical Committee for Animal Research (Protocol number 042/04-CEEA), Institute of Biosciences, Universidade Estadual Paulista (UNESP).
Colitis was induced using the method originally described by Morris et al[18]. Briefly, animals were fasted overnight and then anaesthetized. Under anesthesia, they were inoculated with 10 mg of TNBS dissolved in 0.25 mL 50% ethanol (v/v), by means of a Teflon cannula inserted 8 cm into the anus. During and after the TNBS administration, the rats remained in a head-down position until they recovered from the anesthesia.
Rats received 25, 50 or 100 mg/kg of the PACO2 extract orally at 96, 72, 48, 24 and 2 h before colitis induction, using an esophageal catheter (volume: 10 mL/kg). Two additional groups were included for reference: a non-colitis group and a colitic group (TNBS-control group) that received vehicle (10 mL/kg methylcellulose) orally. The animal body weights, the occurrence of diarrhea (as detected by perianal fur soiling) and the total food intakes for each group were recorded daily. Animals from all groups (n = 7) were killed at 48 h after colitis induction.
The colonic segments were obtained after laparotomy and the adhesions eventual occurrence between the colon and adjacent organs were noted. These colonic segments were placed on an ice-cold plate, cleaned of fat and mesentery, blotted on filter paper, and then the colon was weighed and its length measured under a constant load (2 g). The colon was opened longitudinally and scored for macroscopically visible damage on a 0-10 scale, according to Bell et al[19]. Subsequently, the colon was longitudinally divided into different pieces to be used for the following determinations: myeloperoxidase (MPO), and alkaline phosphatase (ALP) activity, total glutathione (GSH) content, IL-1β, IL-6, IL-10, TNF-α and INF-γ levels, and gene expression analysis.
MPO activity was measured according to the technique described by Krawisz et al[20] and the results were expressed as MPO units per g of wet tissue. Total GSH content was quantified with the recycling assay[21] and the results were expressed as nmol per g of wet tissue. ALP activity was measured spectrophotometrically, as previously described, and the results were expressed as mU per mg of protein[22,23]. Quantification of cytokines (TNF-α, IL-1β, IL-6, IL-10 and INF-γ) was made on colonic samples previously weighed, homogenized, minced on an ice-cold plate and resuspended in a centrifugation tube containing 10 mmol/L phosphate-buffered saline (pH 7.4; 1:5 w/v). The tubes were placed in a shaker submerged in a 37 °C water bath for 20 min and then centrifuged at 9000 g for 30 s at 4 °C. The supernatants were frozen at -80 °C until assayed. The TNF-α, IL-1β, IL-6, IL-10 and INF-γ levels were quantified by a DuoSet ELISA Kit (R&D Systems, Inc., Minneapolis, MN, United States) to measure the concentration of the natural and recombinant rat enzyme according to the manufacturer’s instructions. The results were expressed as pg per mL.
Colon samples (100 mg) were collected and stored in -80 °C until use for analyses of genes: GAPDH, β-actin, HPRT, HSP70, heparanase, NF-κB, mitogen-activated protein kinase (MAP)K1, MAPK3, MAPK6, MAPK9, MUC1, MUC2, MUC3, and MUC4. For the homogenization, we used 1 mL of Trizol® (Invitrogen-Life Technologies, Carlsbad, CA, United States) and a Polytron homogenizer. The total RNA extraction was made according to the Trizol® manufacturer’s protocol. The purity was determined by A260/A280 ratio using a Nanodrop 2000 (Thermo Scientific, Waltham, MA, United States). After that, 1 μg of the total RNA of colon tissue samples was incubated with DNAse I (1 U/mg RNA; Invitrogen), and then reverse transcribed with SuperScript® III (200 U/mL; Life Technologies) and oligo-d (T) primer. Primers for targets and reference genes were designed based on the rat sequences and using the IDT primer quest software (http://www.idtdna.com/primerquest/Home/Index; Invitrogen). Relative real-time RT-PCR analysis was performed with a StepOne Plus™ (Applied Biosystems Inc., Foster City, CA, United States) using Power SYBR Green PCR Master Mix® (Life Technologies) for all the genes. Amplification efficiencies for target and reference genes were similar. The primer sequences, fragment size, annealing temperature, primer concentration, NCBI reference sequence and sample concentration for each gene are shown in Table 1.
| Target | Sequence | Annealing, °C | Oligo concentration, nmol/L | Fragment size, bp | NCBI reference sequence | cDNA concentration, μL |
| GAPDH | F TGACTCTACCCACGGCAAGTTCAA | 60 | 200 | 141 | NM_017008.3 | 1.000 |
| R ACGACATACTCAGCACCAGCATCA | ||||||
| β-actin | F TTGCTGACAGGATGCAGAAGGAGA | 60 | 100 | 159 | NM_031144.2 | 1.000 |
| R ACTCCTGCTTGCTGATCCACATCT | ||||||
| HPRT | F AGGGAAGTGACAATCTACCTGACG | 60 | 100 | 81 | AA900579.1 | 0.125 |
| R GAAATGTCTGTTGCTGCGTCCCTT | ||||||
| HSP70 | F ACTCCTTCGTTCGGTCTGCAATCA | 60 | 200 | 92 | NM_031971.2 | 0.125 |
| R CTGGGAATGCAAAGCACACGTGAA | ||||||
| Heparanase | F TGTCAAGAGTGAAAGGCCCAGACA | 60 | 200 | 141 | NM_022605.1 | 0.125 |
| R GCAGCTTCAAGTGCTTGGTGACAT | ||||||
| NF-κB | F AAACCAAAGCCCTGAAAGGCCATC | 60 | 200 | 120 | XM_342346.4 | 0.125 |
| R TCGGAAGGCCTCGAATGACATCAA | ||||||
| MAPK1 | F AACAGGTTGTTCCCAAACGCTGAC | 60 | 200 | 187 | NM_053842.1 | 0.500 |
| R AGTCGTCCAGCTCCATGTCAAACT | ||||||
| MAPK3 | F TACCTGGACCAGCTCAACCACATT | 60 | 200 | 173 | NM_017347.2 | 0.500 |
| R AGCAGGTCAAGAGCTTTGGAGTCA | ||||||
| MAPK6 | F AACTGAGCCAGTGGAAGAAGGGAA | 60 | 200 | 164 | NM_031622.2 | 1.000 |
| R TTAACGTGGCCTGGATGGACTTGA | ||||||
| MAPK9 | F TCATGGGAGAGCTGGTGAAAGGTT | 60 | 200 | 106 | NM_017322.2 | 1.000 |
| R ATGAACTCTGCGGATGGTGTTCCT | NM_001270544.1 | |||||
| NM_001270545.1 | ||||||
| MUC1 | F CCGCTACTACCAAGAACTGAAG | 60 | 200 | 102 | NM_012602.1 | 0.500 |
| R GAGCCTGACCTGAACTTGATAG | ||||||
| MUC2 | F GGCTCTGCTCTCTGTGTTATAG | 60 | 200 | 123 | U68172.1 | 1.000 |
| R CAGTTTGGGAAGAAGGTAGGG | ||||||
| MUC3 | F GGGAAATAGACCCTGCAGTTAG | 60 | 200 | 107 | U76551.1 | 0.125 |
| R GATCATCGCTTGCCGTCATA | ||||||
| MUC4 | F ATGTGGAGGTGGGAGAAATG | 60 | 200 | 122 | AF240632.1 | 0.500 |
| R CCCTGGAACTGGAATTAGAGAC |
Reactions were optimized to provide maximum amplification efficiency for each gene. PCR was performed in 25 μL reaction volumes in duplicate, in a MicroAmp® Fast Optical 96-Well Reaction Plate, 0.1 mL (Applied Biosystems Inc.), and the specificity of each PCR product was determined by melting curve analysis. Negative controls (water replacing cDNA) were run in every plate.
The relative expression of each target gene was calculated using the ∆∆Ct method with efficiency correction[24]; the control was a cDNA sample from each cell type analyzed. To select the most stable reference gene for detailed analyses, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), β-actin and HPRT amplification profiles were compared using the RefFinder software (http://www.leonxie.com/referencegene.php?type=reference). All gene expression analysis was performed with β-actin as the reference gene for colon tissue.
A representative colon fragment located 1 cm above the lesion was collected for histological slide preparation and stained with hematoxylin and eosin (HE) for analysis of the microscopic damage. Images were acquired using Zeiss Imager Axio Vision 4.8.2.0 software. Samples of the colon were fixed in 2.5% glutaraldehyde and 4% paraformaldehyde solutions in 0.1 mol/L phosphate buffer (pH 7.3) for transmission electron microscopy (TEM) analysis. Afterwards, the material was post-fixed in a 1% osmium tetroxide solution in 0.1 mol/L phosphate buffer (pH 7.3) at room temperature for 2 h, dehydrated through a graded series of acetone, and embedded in Araldite® resin. Ultra-thin sections (70 ηm) were double-stained with uranyl acetate and lead citrate. All samples were analyzed and images were acquired using a Tecnai Spirit TEM from Fei Company, 80 kV. For scanning electron microscopy (SEM) analysis, small fragments of the colon were fixed in a 2.5% glutaraldehyde solution in 0.1 mol/L phosphate buffer (pH 7.3) and post-fixed in 1% osmium tetroxide in 0.1 mol/L phosphate buffer (pH 7.3) at room temperature for 2 h. Samples were dehydrated through the serial application of increasingly concentrated ethanol, critical point-dried with liquid CO2, and sputter-coated with gold (10 ηm). All samples were analyzed and images were acquired using a QUANTA 200 SEM from Fei Company, 80 kV.
The parametric results are expressed as the mean ± SE of the mean, and the differences between means were tested for statistical significance using one-way analysis of variance followed by Dunnett’s post-test. Nonparametric data (score) are expressed as the median (range) and were analyzed with the Kruskal-Wallis test, followed by Dunn post-test. Differences between proportions were analyzed by Fisher’s exact test. Statistical significance was set at P ≤ 0.05.
TNBS instillation resulted in colonic inflammation, which was evidenced after 48 h by severe necrosis of the mucosa (extending for 2 to 3 cm along the colon), hyperemia, significant increase in the colonic weight/length ratio, incidence of diarrhea and adherence to adjacent organs (Table 2). These effects were related to significant reduction in both body weight and food intake (data not shown).
| Group | Damage score, 0-101 | Extension of lesion, cm2 | Colonic weight, mg/cm2 | Diarrhea3 | Adherence3 |
| Non-colitic | 0a | 0a | 82.7 ± 2.10a | 0%a | 0%a |
| TNBS-control | 7 (2-8) | 2.55 ± 0.28 | 136.0 ± 5.87 | 87.50% | 81.25% |
| PACO2 - 25 mg/kg | 7 (5-8) | 2.44 ± 0.18 | 135.0 ± 6.86 | 100.00% | 71.42% |
| PACO2 - 50 mg/kg | 7 (2-9) | 2.11 ± 0.34 | 124.0 ± 7.72 | 85.71% | 71.42% |
| PACO2 - 100 mg/kg | 7 (4-9) | 2.93 ± 0.20 | 162.0 ± 5.04 | 100.00% | 57.14% |
Biochemically, the colonic damage induced by TNBS was characterized by increased activities of MPO (13-fold) and ALP (4-fold) and high levels of colonic TNF-α, IL-1β, IL-6, IFN-γ and IL-10 compared with healthy animals. Furthermore, significant colonic GSH depletion took place in the inflamed colon.
Administration of P. angulata extract (PACO2) at the doses of 50 and 100 mg/kg decreased ALP activity relative to TNBS-control group. Reduction of INF-γ and IL-6 colonic levels, and MPO activity were observed after treatment with 50 and 100 mg/kg, respectively. The 25 mg/kg dose of PACO2 did not alter any cytokine analyzed (Tables 3 and 4).
| Group | IFN-γ | TNF-α | IL1-β | IL-6 | IL-10 |
| Non-colitic | 78.2 ± 7.9c | 121.2 ± 7.0a | 1395.0 ± 136.9a | 421.1 ± 45.3a | 467.1 ± 31.1a |
| TNBS control | 125 ± 20.4 | 240.3 ± 34.3 | 2205.0 ± 147.4 | 893.8 ± 105.5 | 675.5 ± 38.6 |
| PACO2, 25 mg/kg | 92.1 ± 8.6 | 246.6 ± 34.3 | 1867.0 ± 221.4 | 891.9 ± 87.09 | 674.5 ± 40.4 |
| PACO2, 50 mg/kg | 67 ± 11.7c | 167.9 ± 23.6 | 2106.0 ± 128.9 | 563.4 ± 55.5c | 626.9 ± 66.5 |
| PACO2, 100 mg/kg | 76.4 ± 12.7c | 205.5 ± 34.5 | 2323.0 ± 154.1 | 649.5 ± 86.9 | 537.4 ± 41.1 |
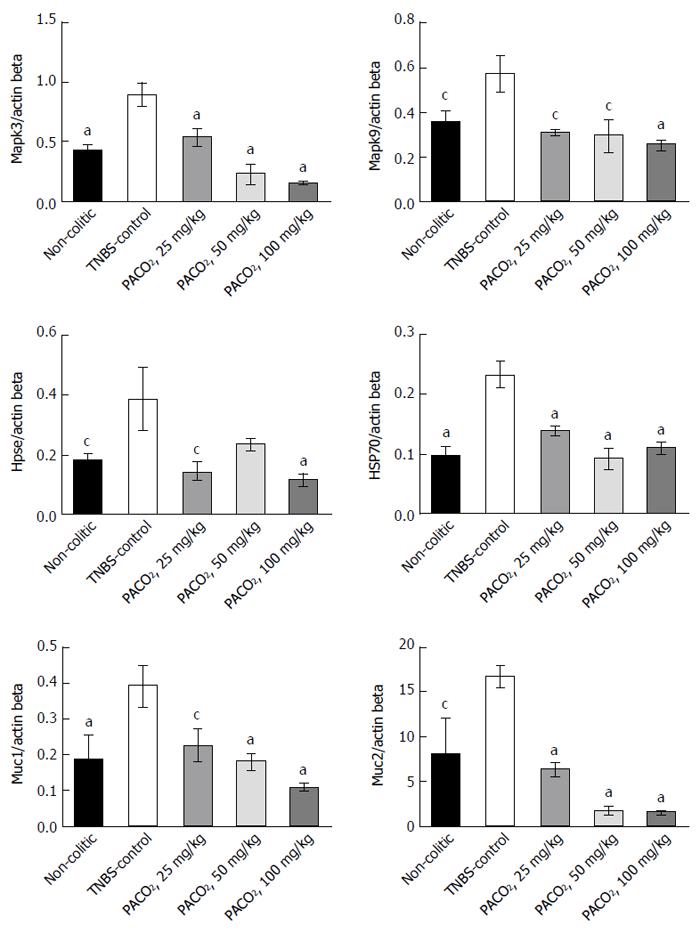
Expression of genes involved in the inflammatory process (Hpse, Hsp70, Mapk1, Mapk3, Mapk6, Mapk9, and NF-κB) and genes involved in the protective functions (Muc1, Muc2, Muc3, and Muc4) were differentially affected by the plant extract treatment. PACO2 was able to diminish the expression of Hsp70, Mapk3, Mapk9, Muc1, and Muc2 at doses of 25, 50 and 100 mg/kg and Hpse at doses of 25 and 100 mg/kg, as compared with the TNBS-control group (Figure 1).
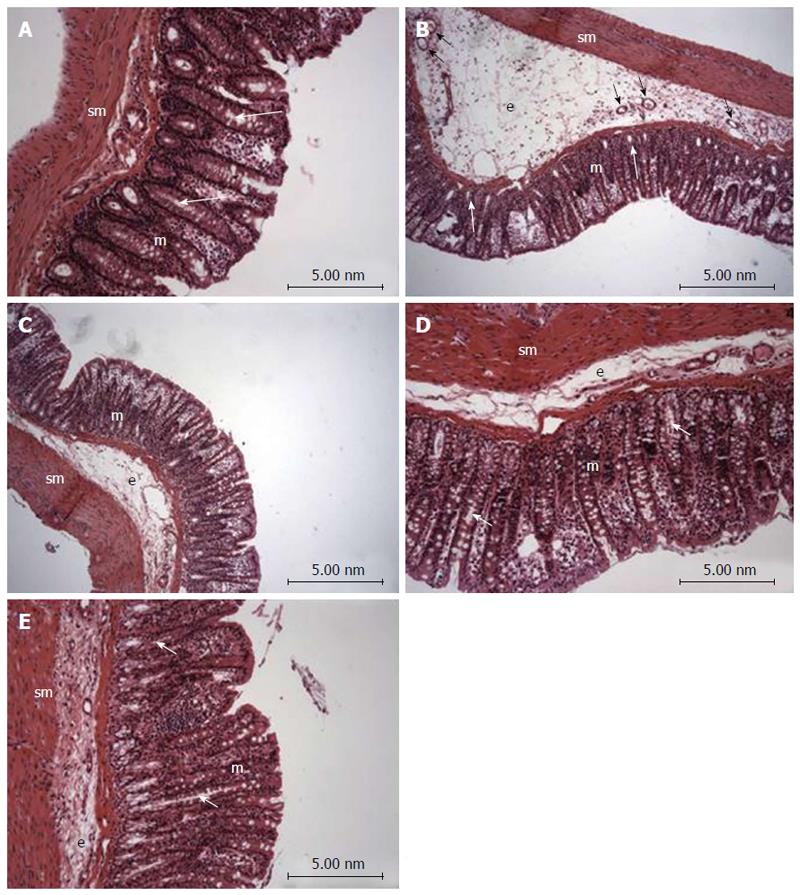
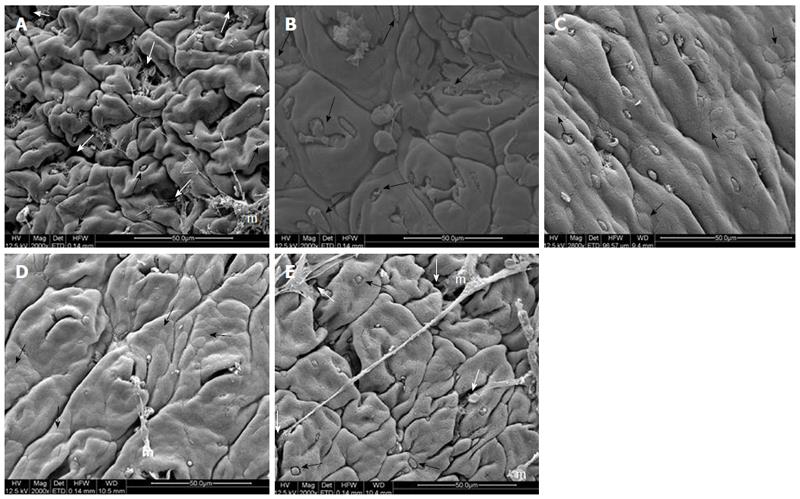
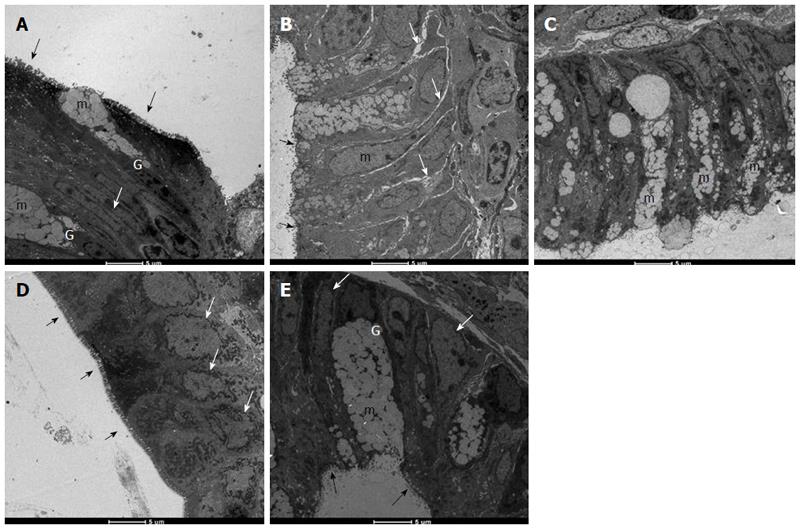
Histopathological evaluation demonstrated that treatments with PACO2 led to improved colon cytoarchitecture, including the restoration of tubular glands containing goblet cells, especially at the doses of 50 mg/kg and 100 mg/kg; moreover, edema was significantly reduced, as compared to the TNBS-control group (Figure 2). In SEM, PACO2 at a dose of 25 mg/kg showed a slight recovery of the polygonal shape of absorptive cells; however, the number of goblet cells remained elevated. At a dose of 50 mg/kg, a greater recovery of polygonal appearance and increase of the main was observed compared to the TNBS-control group. PACO2 at a dose of 100 mg/kg exhibited a good recovery of mucosal architecture, with goblet cells similar to healthy animals, including crypts and mucin (Figure 3). In transmission ultrastructure analysis, reduced mucin granules were observed in colonic goblet cells at a dose of 25 mg/kg, but the intercellular edema was lower. Already at dose of 50 mg/kg, the colonocytes are juxtaposed with nucleus in basal position and preserved microvilli. The 100 mg/kg-treated group showed goblet cells with mucus granules similar to healthy animals, the nucleus in basal position, mild intercellular edema and atypical microvilli (Figure 4).
The combination of products that improve the anti-inflammatory activity of drugs would be an important approach for IBD treatment, since the current pharmacological treatments for IBD, including aminosalicylates, corticosteroids, biological agents and immunosuppressant, result in serious side effects and most patients do not respond to these therapies[3,8,9]. The results of this study showed that a phytosterol standardized extract obtained from aerial parts of P. angulata improved the response of animals throughout the duration of intestinal damage induced by TNBS, modulating oxidative stress, immune response and inflammatory genes expression.
The biochemical changes that occur after administration of TNBS were similar to what occurs in humans, mainly excessive production of reactive oxygen and nitrogen metabolites[25]. Neutrophils are responsible for tissue injury in inflammatory diseases, producing free radicals and hypochlorous acid that increase the local oxidative stress and cause damage[26]. In this process, MPO plays a central role, generating reactive intermediates (primarily hypochlorous acid) and leading to oxidative damage of lipids and proteins, thereby acting as an enzyme strongly linked to both inflammation and oxidative stress[27]. Indeed, MPO acts as an inflammatory marker, so that activity reduction of this enzyme is also related to lower cellular infiltration into the colon.
ALP is also a sensitive inflammatory process biochemical marker, which is increased as a consequence of tissue-non-specific isoform and associated with an influx of inflammatory cells[28,29]. However, studies suggest that ALP release occurs as a consequence of lipid peroxidation of membranes[30]. Although oxidative stress can also be measured by GSH levels (the first line of defense against the toxic products of oxygen and other hydroperoxides[31,32]), PACO2 at the tested dose was ineffective for avoiding the GSH depletion induced by inflammatory process. Nevertheless, the inhibitory effects on the MPO and ALP activity can be interpreted either by antioxidant or anti-inflammatory properties of the PACO2.
The focus on the adaptive immune response in IBD led to a consensus that the mucosa is dominated by CD4+ (Th1) lymphocyte phenotypes, characterized by the production of INF-γ[33]. Immune response in the colon after TNBS administration is also characterized by increased production of IFN-γ[34]. The increased synthesis of IFN-γ has been associated with the aggravation of disease in patients with IBD[35]. IL-6 plays a central role in diverse inflammatory responses via recruiting CD4 (Th17) lymphocyte phenotypes, so it is evident that neutralization of IL-6 has a beneficial effect on intestinal inflammation, acting in tissue remodeling and neutrophil infiltration[35-37]. Our results showed that PACO2 anti-inflammatory properties were also related to down-regulation of colonic IFN-γ and IL-6 levels; but, at the tested doses, no effects were observed on other cytokines, such as TNF-α, IL-1β and IL-10.
The genetic profiling identified a number of genes or genetic loci that have been associated with IBD conditions, and many of these genes are directly related to signaling pathways that regulate the innate and adaptive immune systems, triggering complex intracellular cascades[38,39]. One of these ways of controlling signaling are MAPKs, an evolutionarily conserved family of serine/threonine kinases involved in a wide range of biological processes, such as cell growth, proliferation, differentiation, migration and apoptosis[40,41]. Studies show that Mapk1 and Mapk3 were significantly expressed in animals from the TNBS-control group, indicating that these genes play a significant role in the inflammation caused by TNBS, and are expressed in the active phases of IBD[38,42]. Indeed, evidence has shown that Mapk3 activation plays an essential role in the signaling mechanisms that lead to increased neutrophil adhesion[43]. Our data showed that PACO2 decreased expression of Mapk3 genes, probably contributing to the reduced neutrophil infiltration evidenced by histopathological analysis. In fact, reduced neutrophil infiltration promoted by PACO2 can be closely associated to inhibition of MPO activity and reduction of IL-6 colonic levels.
Another kinase that has the ability to phosphorylate other protein targets is Mapk9, also known as JNK2[38]. In our research, we found increased Mapk9 gene expression after TNBS administration, indicating a participation of this MAPK in the development of inflammation. Mapk9 has been implicated as an important regulator of cytokine release and in the response of the neutrophils to release inflammatory stimuli[44]. Indeed, a specific inhibitor of Mapk9 in the murine IBD model reduced production of inflammatory cytokines, including IFN-γ and IL-6[45]. This way, it is possible to suggest that modulatory effects of PACO2 on cytokine production was dependent of down-regulation of Mapk9 gene expression.
A complex network involving heparanase and other inflammatory pathways, such as hsp70 and NF-κB, has been proposed to explain the immunomodulatory response in inflammatory conditions[46,47]. Heparanase has an important role in body physiology and inflammatory responses, where its expression is an important mechanism underlying chronic colonic inflammation[48]. In fact, it has been hypothesized that heparanase activates macrophages, increasing NF-κB signaling and releasing several cytokines and reactive oxygen species, as well as heparanase, via TNF-α-dependent mechanisms, promoting disruption of the cell membrane[46-49].
Recently, the relationship among heparanase, hsp70, NF-κB and cytokine profile was identified by our research group in the TNBS-model of intestinal inflammation, demonstrating the pharmacological importance of the heparanase modulation as a target of new drugs[50]. Indeed, in the complex inflammatory response, heat shock proteins have been implicated in the pathogenesis of IBD, mainly Hsp70 that confers resistance to various tissues and are synthesized rapidly after exposure to the offending agent, being useful in maintaining homeostasis, facilitating the repair of damaged areas and providing protection against injuries[51,52]. According to results, the anti-inflammatory properties of PACO2 were closely related to inhibitory gene expression of both heparanase and Hsp70, leading to a lower colonic damage as observed by histopathological analysis.
Another important factor related to gut homeostasis is mucus production, responsible for formation of gel layers covering the gastrointestinal tract and providing a semi-permeable barrier between the lumen and the epithelium[53]. The mucus gel barrier, formed by building blocks of mucins, which determine the thickness and properties of mucus, contributes to maintaining the integrity of the epithelium in the colon and plays a key role in IBD[53,54]. In small and large intestine, Muc2 is the major secretory mucin synthesized and secreted by goblet cells, whereas goblet and absorptive cells express the membrane-bound mucins Muc1, Muc3, Muc4 and Muc13 in the apical membrane[53-55]. Although the hypothesis that mucins and mucus barrier are protective factors in IBD is difficult to prove in humans, animal models, including the TNBS model, permit detailed analysis of the role of mucins in intestinal inflammation. In fact, our results showed that TNBS increased colonic Muc1 and Muc2 expression, probably as a response against inflammatory process and to maintain the mucosal barrier integrity[56]. Although PACO2 reversed these effects, an increased production of mucus was observed by histopathological analysis and was clearly evidenced in the TEM and SEM studies.
The main structural damage induced by TNBS was detected by histopathological analysis, and included membrane disruption, reduction in mucus production and increase of inflammatory cells. These effects were clearly related to reduction in MPO activity and modulation of IL-6 and INF-γ, whereas membrane integrity was affected by heparanase and Hsp70 up-regulation. These damaged effects were completely reversed by treatment with different doses of PACO2, which acted as an anti-inflammatory agent modulating oxidative stress, immune response and inflammatory gene expression.
All protective effects produced by PACO2 can be partially attributed to presence of phytosterols in the standardized plant extract, natural compounds with several pharmacological activities able to regulate the balance of Th1 and Th2 response, and inhibition of nitric oxide production, TNF-α and IFN-γ[9,57]. The main group of sterols in this plant are the seco-sterols (named physalins) and molecules derived from ergostane[9,58], which were identified as active compounds from P. angulata, reducing vascular permeability, neutrophil infiltration[59], inflammatory cytokine production such as of IFN-γ and IL-6, and modulating the expression of Mapks[60], as observed in our experiments. Indeed, administration of seco-sterols of P. angulata has been useful to treat autoimmune diseases, allergies or in cases of transplant rejection with lower toxicity when compared to glucocorticoids[59]. These studies showed that phytosterols of P. angulata are promising compounds for treatment of inflammatory diseases, suggesting that the search for new natural compounds similar to steroids such as glucocorticoids is a promising field of research to obtain new drugs with efficacy and safety.
In conclusion, the standardized extract of P. angulata L.-PACO2-exerts intestinal anti-inflammatory effect by modulating a series of pathways and mediators of the inflammatory response, mainly related to oxidative stress and immune response. These effects were related to the presence of phytosterols, natural compounds structurally related to steroids, which are promising substances for the treatment of inflammatory diseases. In this way, PACO2 from Physalis angulata is an innovative active ingredient and a phytopharmaceutical preparation with potential clinical applications in the control of intestinal inflammation.
The authors acknowledge technical support provided by Aline W Fantinati, Alexandre S Chagas, Alexandre Tanimoto, Adriana Del Ben, Tainan FS Curimbaba, Juliana Severi e Juliana R Ribeiro. Chemyunion Chemistry provided us with the standard extract of Physalis angulata (PACO2).
Inflammatory bowel disease (IBD) is a chronic inflammatory process of the gut, including two different disorders: ulcerative colitis and Crohn’s disease. Nowadays, the search for new drugs to treat IBD is based in immunomodulators, modulators of intestinal microbiota and antioxidative natural and synthetic compounds. This manuscript describes a study of a potential product for use as complementary therapy based on a phytopharmaceutical preparation with Ground Cherry (Physalis angulata), a rich phytosterols medicinal plant.
The study demonstrated that a CO2 supercritical herbal preparation containing steroidal natural compounds produces a potent intestinal anti-inflammatory activity. These data open a new perspective for use of supercritical extraction method as well as for the study of phytosterols as active components with intestinal anti-inflammatory effects.
Several medicinal plants have been studied as immunomodulators in several chronic diseases, but this research is the first to investigate Ground Cherry using a supercritical carbon dioxide extraction and fractionation for its effects on chemically-induced intestinal inflammation.
The main application of these data is their potential application as a phytopharmaceutical preparation complementary to current drugs to treat IBD.
This is very well done study, very supported, results are excellent, and observations do support the conclusion. The article is good work, perfectly analyzed, presented the results and discussed them perfectly.
| 1. | Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 949] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 2. | Crohn BB, Ginzburg L, Oppenheimer GD. Regional ileitis: a pathologic and clinical entity. 1932. Mt Sinai J Med. 2000;67:263-268. [PubMed] |
| 3. | Pithadia AB, Jain S. Treatment of inflammatory bowel disease (IBD). Pharmacol Rep. 2011;63:629-642. [PubMed] |
| 4. | Gitnick G. Inflammatory bowel disease: a new assessment. Scand J Gastroenterol Suppl. 1996;220:83-86. [PubMed] |
| 5. | Witaicenis A, Luchini AC, Hiruma-Lima CA, Felisbino SL, Garrido-Mesa N, Utrilla P, Gálvez J, Di Stasi LC. Suppression of TNBS-induced colitis in rats by 4-methylesculetin, a natural coumarin: comparison with prednisolone and sulphasalazine. Chem Biol Interact. 2012;195:76-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Tang C, Chen S, Qian H, Huang W. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology. 2012;135:112-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 238] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 7. | Műzes G, Molnár B, Tulassay Z, Sipos F. Changes of the cytokine profile in inflammatory bowel diseases. World J Gastroenterol. 2012;18:5848-5861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 194] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 8. | Yamamoto T, Nakahigashi M, Saniabadi AR. Review article: diet and inflammatory bowel disease--epidemiology and treatment. Aliment Pharmacol Ther. 2009;30:99-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Morrison G, Headon B, Gibson P. Update in inflammatory bowel disease. Aust Fam Physician. 2009;38:956-961. [PubMed] |
| 10. | Kong SC, Hurlstone DP, Pocock CY, Walkington LA, Farquharson NR, Bramble MG, McAlindon ME, Sanders DS. The Incidence of self-prescribed oral complementary and alternative medicine use by patients with gastrointestinal diseases. J Clin Gastroenterol. 2005;39:138-141. [PubMed] |
| 11. | Di Stasi LC, Hiruma CA, Guimarães EM, Santos CM. Medicinal Plants popularly used in brazilian amazon. Fitoterapia. 1994;65:529-540. |
| 12. | Richter RK, Carlson TJ. Reporting biological assay results on tropical medicinal plants to host country collaborators. J Ethnopharmacol. 1998;62:85-88. [PubMed] |
| 13. | Jin Z, Mashuta MS, Stolowich NJ, Vaisberg AJ, Stivers NS, Bates PJ, Lewis WH, Hammond GB. Physangulidines A, B, and C: three new antiproliferative withanolides from Physalis angulata L. Org Lett. 2012;14:1230-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Lima Mda S, Evangelista AF, Santos GG, Ribeiro IM, Tomassini TC, Pereira Soares MB, Villarreal CF. Antinociceptive properties of physalins from Physalis angulata. J Nat Prod. 2014;77:2397-2403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Wu SY, Leu YL, Chang YL, Wu TS, Kuo PC, Liao YR, Teng CM, Pan SL. Physalin F induces cell apoptosis in human renal carcinoma cells by targeting NF-kappaB and generating reactive oxygen species. PLoS One. 2012;7:e40727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Yu Y, Sun L, Ma L, Li J, Hu L, Liu J. Investigation of the immunosuppressive activity of Physalin H on T lymphocytes. Int Immunopharmacol. 2010;10:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Velasquez Pereda MC, Nogueira C, de Campos Diemant G, Berlin S, Mussi L, De Tarso Vieira e Rosa P, Polezel MA. inventors. 2009;. |
| 18. | Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795-803. [PubMed] |
| 19. | Bell CJ, Gall DG, Wallace JL. Disruption of colonic electrolyte transport in experimental colitis. Am J Physiol. 1995;268:G622-G630. [PubMed] |
| 20. | Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344-1350. [PubMed] |
| 21. | Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548-555. [PubMed] |
| 22. | Bessey OA, Lowry OH, Brock MJ. A method for the rapid determination of alkaline phosphates with five cubic millimeters of serum. J Biol Chem. 1946;164:321-329. [PubMed] |
| 23. | Smith GP, Harris H, Peters TJ. Studies of the biochemical and immunological properties of human neutrophil alkaline phosphatase with comparison to the established alkaline phosphatase isoenzymes. Clin Chim Acta. 1984;142:221-230. [PubMed] |
| 24. | Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [PubMed] |
| 25. | Kruidenier L, Verspaget HW. Antioxidants and mucosa protectives: realistic therapeutic options in inflammatory bowel disease? Mediators Inflamm. 1998;7:157-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Dhiman M, Estrada-Franco JG, Pando JM, Ramirez-Aguilar FJ, Spratt H, Vazquez-Corzo S, Perez-Molina G, Gallegos-Sandoval R, Moreno R, Garg NJ. Increased myeloperoxidase activity and protein nitration are indicators of inflammation in patients with Chagas’ disease. Clin Vaccine Immunol. 2009;16:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Karakas M, Koenig W. Myeloperoxidase production by macrophage and risk of atherosclerosis. Curr Atheroscler Rep. 2012;14:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Sánchez de Medina F, Vera B, Gálvez J, Zarzuelo A. Effect of quercitrin on the early stages of hapten induced colonic inflammation in the rat. Life Sci. 2002;70:3097-3108. [PubMed] |
| 29. | Tuin A, Poelstra K, de Jager-Krikken A, Bok L, Raaben W, Velders MP, Dijkstra G. Role of alkaline phosphatase in colitis in man and rats. Gut. 2009;58:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 30. | Dalaklioglu S, Genc GE, Aksoy NH, Akcit F, Gumuslu S. Resveratrol ameliorates methotrexate-induced hepatotoxicity in rats via inhibition of lipid peroxidation. Hum Exp Toxicol. 2013;32:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | DeLeve LD, Kaplowitz N. Glutathione metabolism and its role in hepatotoxicity. Pharmacol Ther. 1991;52:287-305. [PubMed] |
| 32. | Masella R, Di Benedetto R, Varì R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005;16:577-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 685] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 33. | Corridoni D, Arseneau KO, Cominelli F. Inflammatory bowel disease. Immunol Lett. 2014;161:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1028] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 35. | Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280-4288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 440] [Cited by in RCA: 531] [Article Influence: 29.5] [Reference Citation Analysis (11)] |
| 36. | Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1248] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 37. | Shen W, Durum SK. Synergy of IL-23 and Th17 cytokines: new light on inflammatory bowel disease. Neurochem Res. 2010;35:940-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Broom OJ, Widjaya B, Troelsen J, Olsen J, Nielsen OH. Mitogen activated protein kinases: a role in inflammatory bowel disease? Clin Exp Immunol. 2009;158:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 39. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3412] [Article Influence: 179.6] [Reference Citation Analysis (12)] |
| 40. | Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279-3290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1960] [Cited by in RCA: 2281] [Article Influence: 120.1] [Reference Citation Analysis (0)] |
| 41. | Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3924] [Cited by in RCA: 4033] [Article Influence: 161.3] [Reference Citation Analysis (33)] |
| 42. | Quaglio AE, Castilho AC, Di Stasi LC. Experimental evidence of MAP kinase gene expression on the response of intestinal anti-inflammatory drugs. Life Sci. 2015;136:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | József L, Khreiss T, Fournier A, Chan JS, Filep JG. Extracellular signal-regulated kinase plays an essential role in endothelin-1-induced homotypic adhesion of human neutrophil granulocytes. Br J Pharmacol. 2002;135:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Mitsuyama K, Suzuki A, Tomiyasu N, Tsuruta O, Kitazaki S, Takeda T, Satoh Y, Bennett BL, Toyonaga A, Sata M. Pro-inflammatory signaling by Jun-N-terminal kinase in inflammatory bowel disease. Int J Mol Med. 2006;17:449-455. [PubMed] |
| 45. | Assi K, Pillai R, Gómez-Muñoz A, Owen D, Salh B. The specific JNK inhibitor SP600125 targets tumour necrosis factor-alpha production and epithelial cell apoptosis in acute murine colitis. Immunology. 2006;118:112-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 46. | Hermano E, Lerner I, Elkin M. Heparanase enzyme in chronic inflammatory bowel disease and colon cancer. Cell Mol Life Sci. 2012;69:2501-2513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 47. | Goldberg R, Meirovitz A, Hirshoren N, Bulvik R, Binder A, Rubinstein AM, Elkin M. Versatile role of heparanase in inflammation. Matrix Biol. 2013;32:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 48. | Vlodavsky I, Beckhove P, Lerner I, Pisano C, Meirovitz A, Ilan N, Elkin M. Significance of Heparanase in Cancer and Inflammation. Cancer Microenvironment. 2011;5:115-132. [RCA] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 49. | Edovitsky E, Lerner I, Zcharia E, Peretz T, Vlodavsky I, Elkin M. Role of endothelial heparanase in delayed-type hypersensitivity. Blood. 2006;107:3609-3616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 50. | Quaglio AE, Castilho AC, Di Stasi LC. Experimental evidence of heparanase, Hsp70 and NF-κB gene expression on the response of anti-inflammatory drugs in TNBS-induced colonic inflammation. Life Sci. 2015;141:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Kaufmann SH. Heat shock proteins and the immune response. Immunol Today. 1990;11:129-136. [PubMed] |
| 52. | Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 860] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 53. | Einerhand AW, Renes IB, Makkink MK, van der Sluis M, Büller HA, Dekker J. Role of mucins in inflammatory bowel disease: important lessons from experimental models. Eur J Gastroenterol Hepatol. 2002;14:757-765. [PubMed] |
| 54. | Buisine MP, Desreumaux P, Debailleul V, Gambiez L, Geboes K, Ectors N, Delescaut MP, Degand P, Aubert JP, Colombel JF. Abnormalities in mucin gene expression in Crohn’s disease. Inflamm Bowel Dis. 1999;5:24-32. [PubMed] |
| 55. | Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 821] [Cited by in RCA: 1009] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 56. | Hoebler C, Gaudier E, De Coppet P, Rival M, Cherbut C. MUC genes are differently expressed during onset and maintenance of inflammation in dextran sodium sulfate-treated mice. Dig Dis Sci. 2006;51:381-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Sun L, Liu J, Liu P, Yu Y, Ma L, Hu L. Immunosuppression effect of Withangulatin A from Physalis angulata via heme oxygenase 1-dependent pathways. Process Biochemistry. 2011;46:482-488. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Bastos GN, Santos AR, Ferreira VM, Costa AM, Bispo CI, Silveira AJ, Do Nascimento JL. Antinociceptive effect of the aqueous extract obtained from roots of Physalis angulata L. on mice. J Ethnopharmacol. 2006;103:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 59. | Vieira AT, Pinho V, Lepsch LB, Scavone C, Ribeiro IM, Tomassini T, Ribeiro-dos-Santos R, Soares MB, Teixeira MM, Souza DG. Mechanisms of the anti-inflammatory effects of the natural secosteroids physalins in a model of intestinal ischaemia and reperfusion injury. Br J Pharmacol. 2005;146:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Sun L, Liu J, Cui D, Li J, Yu Y, Ma L, Hu L. Anti-inflammatory function of Withangulatin A by targeted inhibiting COX-2 expression via MAPK and NF-kappaB pathways. J Cell Biochem. 2010;109:532-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Al-Rejaie SS, Prasad S S- Editor: Yu J L- Editor: Filipodia E- Editor: Zhang FF