Published online Jun 28, 2017. doi: 10.3748/wjg.v23.i24.4324
Peer-review started: February 8, 2017
First decision: March 7, 2017
Revised: March 19, 2017
Accepted: May 9, 2017
Article in press: May 9, 2017
Published online: June 28, 2017
Processing time: 148 Days and 18.2 Hours
Despite significant therapeutic progress in recent years, inflammatory bowel disease (IBD), which includes Crohn’s disease and ulcerative colitis, remains a challenge regarding its pathogenesis and long-term complications. New concepts have emerged in the management of this disease, such as the “treat-to-target” concept, in which mucosal healing plays a key role in the evolution of IBD, the risk of recurrence and the need for surgery. Endoscopy is essential for the assessment of mucosal inflammation and plays a pivotal role in the analysis of mucosal healing in patients with IBD. Endoscopy is also essential in the detection of dysplasia and in the identification of the risk of colon cancer. The current surveillance strategy for dysplasia in IBD patients indicates white-light endoscopy with non-targeted biopsies. The new chromoendoscopy techniques provide substantial benefits for both clinicians and patients. Narrow-band imaging (NBI) has similar rates of dysplastic lesion detection as white-light endoscopy, and it seems that NBI identifies more adenoma-like lesions. Because it is used instinctively by many endoscopists, the combination of these two techniques might improve the rate of dysplasia detection. Flexible spectral imaging color enhancement can help differentiate dysplastic and non-dysplastic lesions and can also predict the risk of recurrence, which allows us to modulate the treatment to gain better control of the disease. The combination of non-invasive serum and stool biomarkers with endoscopy will improve the monitoring and limit the evolution of IBD because it enables the use of a personalized approach to each patient based on that patient’s history and risk factors.
Core tip: New concepts have emerged in the management of inflammatory bowel disease, such as the “treat-to-target” concept in which mucosal healing plays a key role in the evolution, risk of recurrence and need for surgery. Endoscopy is essential for the assessment of mucosal inflammation and plays a pivotal role in the analysis of mucosal healing in patients with inflammatory bowel disease (IBD) and in the detection of dysplasia and assessment of the risk of colon cancer. The current surveillance strategy for dysplasia in IBD patients indicates white-light endoscopy with non-targeted biopsies. Despite the screening program, the high rate of colorectal cancer among IBD patients illustrates the need for better and more efficient techniques for dysplasia recognition. Classical chromoendoscopy and new digital techniques have provided promising results. In addition to the endoscopy techniques, stool and blood biomarkers are beneficial for the assessment of disease progress and disease monitoring. When used wisely and combined with the endoscopic methods, these techniques are promising in terms of the selection of patients for the early detection of dysplastic lesions and the prevention of inflammatory relapse.
- Citation: Goran L, Negreanu L, Negreanu AM. Role of new endoscopic techniques in inflammatory bowel disease management: Has the change come? World J Gastroenterol 2017; 23(24): 4324-4329
- URL: https://www.wjgnet.com/1007-9327/full/v23/i24/4324.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i24.4324
Although there has been significant therapeutic progress, inflammatory bowel disease (IBD), which includes Crohn’s disease and ulcerative colitis (UC), remains a challenge regarding its pathogenesis and long-term complications.
New concepts have emerged in the management of this disease, such as the “treat-to-target” concept, in which mucosal healing plays a key role in the assessments of the evolution, risk of recurrence and need for surgery.
Endoscopy is essential for the assessment of mucosal inflammation and plays a pivotal role in the analysis of mucosal healing in patients with IBD and in the detection of dysplasia and assessment of the risk of colon cancer. The current surveillance strategy for dysplasia in IBD patients indicates white-light endoscopy with non-targeted biopsies. The high rate of colorectal cancer (CRC) in IBD patients despite the screening program indicates the need for better and more efficient techniques for dysplasia recognition. Classical chromoendoscopy and new digital techniques have exhibited promising results.
Long-standing colitis represents an increased risk for the development of CRC in patients with IBD[1]. UC patients exhibit a 2.4-fold increased risk for the development of a colorectal cancer compared with the general population[2], and this risk increases with time by 2% after 10 years of disease, 8% at 20 years and 18% at 30 years[3]. The recognized risk factors for CRC are extensive colitis[4], early-age onset of the disease[5] (which supports the fact that longer duration inflammation is associated with a greater[6]), a family history of CRC[7] and concomitant primary sclerosing cholangitis[8]. Based on these factors, a personalized approach to each patient can be proposed and would involve individualized surveillance intervals depending on the risk factors of each patient[9].
Prevention represents the first step of treatment, and the regular follow-up of IBD patients through colonoscopy screenings for dysplasia reduces the mortality and morbidity of CRC by enabling action during earlier stages of tumors[10].
The detection of dysplasia represents the primary goal of colonoscopy surveillance in IBD patients, although it does not reduce the incidence of CRC to zero.
Although optimal surveillance strategies for CRC in IBD patients are not yet established, most gastroenterologists use white-light endoscopy with targeted and random biopsies. The recommendation is that colonoscopy surveillance should start 8-10 years after the diagnosis and encourages annual white-light examinations of the colonic mucosa, 4-quadrant non-targeted biopsies every 10 cm of the colon[9] that obtain at least 32 biopsies from the entire colon, and biopsy or resection of any suspicious lesion when possible. There is no significant difference in dysplasia detection between targeted and random biopsies, but the cost of targeted biopsies is lower[11].
However, this technique has its own disadvantages because flat and subtle dysplastic lesions are not readily identifiable, and active colonic inflammation can make the lesions difficult to interpret. Non-targeted biopsies will not always identify dysplasia because only a very small portion of the colonic mucosa is sampled. Moreover, this method is time-consuming and expensive.
An alternative to white-light colonoscopy (WLC) emerged with the introduction of chromoendoscopy (CME) to clinical practice. CME is an imaging technique that uses contrast agents to identify abnormalities of the colonic mucosa. Dysplastic lesions are better highlighted by the addition of two topical dyes, i.e., the contrast agent indigo carmine and the absorptive dye methylene blue (which is differentially taken up by normal colonic cells and neoplastic cells) and thus enable the endoscopist to differentiate between normal colonic mucosa and dysplastic mucosa. Polypoid lesions are easier to detect, and the dyes help the endoscopist delineate the lesion border[12].
In 2003, Kiesslich’s clinical trial that included 165 UC patients revealed that more flat dysplasias[13] and intraepithelial neoplasias were identified by CME than by WLC. In another study of 102 IBD patients, Marion compared WLC with 4-quadrant random biopsies every 10 cm with targeted biopsies alone and CME with targeted biopsies and concluded that CME-targeted biopsies resulted in a notably higher rate of dysplasia identification than WLC with non-targeted biopsies[14].
Chromoendoscopy is able to detect dysplasia almost 7% more often than WLC, and the detection of flat dysplastic lesions increases by 27% with CME[15]. In 4 studies in which patients were examined by both WLE and CME, CME identified nearly twice as many dysplastic lesions.
All of these data have resulted in the acceptance of CME as an alternative to non-targeted biopsies; thus, in 2010, the AGA guidelines stated that CME can be used as an alternative to the actual dysplasia surveillance technique[16,17], but well-established protocols are not yet available.
However, why is CME not the optimal technique for dysplasia surveillance in IBD patients? Why it is not routinely and widely used?
The main reasons that temper the enthusiasm for the adoption of CME as the optimal surveillance method are the bias effect of expert centers (it is less likely that every average endoscopist will obtain the same dysplasia detection rates as an expert center) and the lack of studies that demonstrate sustained benefits in terms of CRC morbidity and mortality. Other impediments include the increased time required for colonoscopy (CME increases the colonoscopy time by 9-12 min), the need to train endoscopists in this method and the lack of established quality measures.
With the recent advent of the new generation scopes, several image-enhancing techniques were introduced to routine practice to increase the sensitivity of the detection of dysplasia.
Narrow-band imaging (NBI) highlights the vascular and mucosal architectures with specialized light filters[18], but up-to-date studies have demonstrated no improvements in the recognition of dysplasia compared to WLC[19,20].
NBI with targeted biopsies seem to result in a greater rate of dysplasia detection compared to WLE, but the difference is not statistically significant. Moreover, the miss rates of the two methods are comparable. The advantages of NBI could be that fewer biopsies are needed to diagnose neoplastic lesions, which might reduce costs, and a lower withdrawal time[21]. Although NBI and CME identified similar numbers of neoplastic lesions, the miss rate was higher for NBI[22].
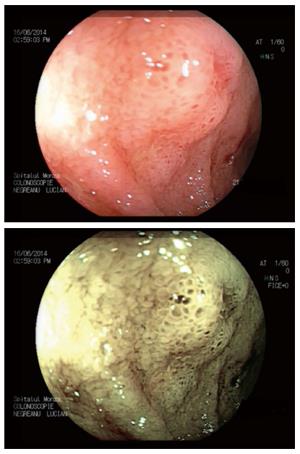
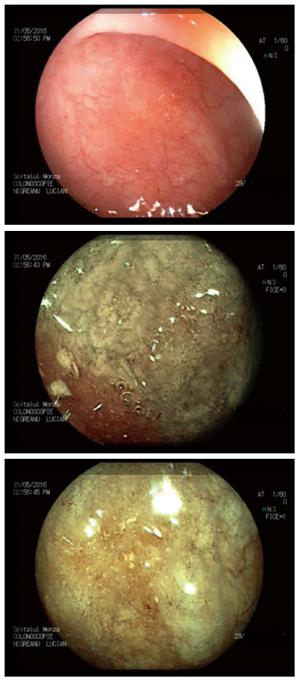
Two other contrast-based technologies are available, i.e., flexible spectral imaging color enhancement (FICE-Fujifilm) and i-scan (Pentax). Both use a computer algorithm that modifies the white-light image[23]. However, there are no clinical trials that support their efficacies in detecting dysplasia in IBD patients, but it has been shown that these methods result in a 3-fold increase in polyp identification (Figures 1 and 2)[24].
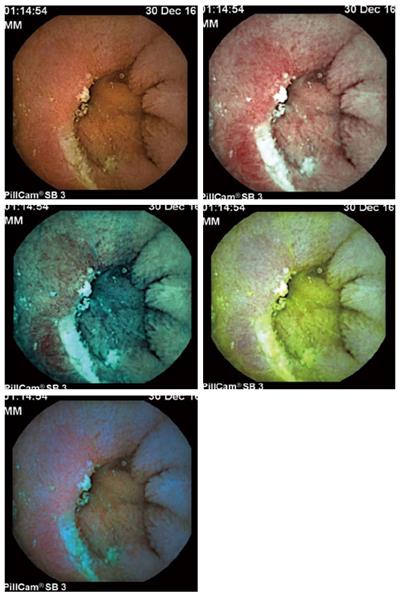
Recently, FICE has been increasingly used to improve videocapsule endoscopy (VCE) image quality and increase the detection of lesions of the small bowel, particularly erosions/ulcerations (Figure 3).
This method is useful for better categorizing difficult-to-interpret small-bowel mucosal ulcerative lesions and can increase the diagnostic yield for Crohn’s disease. However, care must be taken, patients should be selected, and the individual images should be evaluated only as part of a recording sequence because this technology can also result in the interpretation of artifacts as ulcerative lesions[25]. At the moment, in Crohn’s disease, pan-enteric endoscopy using the colon capsule might be used to monitor patients and to stratify disease activity (due to the well-known limitations of clinical assessment) and can also be paramount in the guidance of therapeutic modifications. The combination of FICE and blue mode can result in the detection of even subtle lesions and therefore reduce the risk of recurrence[26].
An analysis of the literature did not reveal a superiority of the new methods over classical chromoendoscopy. Although the potential benefits of the newer optical and digital dye-less chromoendoscopy techniques are substantial, only the dye-based chromoendoscopy can currently be recommended for improving dysplasia detection in long-standing IBD patients[27]. However, digital chromoendoscopy has the potential to quantify disease activity and mucosal healing in IBD[27].
Other endoscopic imaging technologies for dysplasia identification are currently available. One such technology is confocal laser endomicroscopy (CLE), which offers microscopic imaging of the colonic mucosa in real-time[28]. Compared to CME, CLE with targeted biopsies detected almost twice as many intraepithelial neoplasias in a controlled trial of UC patients[29]. Moreover, CLE has the capacity to evaluate disease activity and identify characteristics that can be predictive of disease relapse[30]. Although it appears to be a promising method for detecting dysplasias and assessing IBD activity, CLE requires is time-consuming, requires perfect training, and requires larger studies to evaluate its efficacy; thus, for now, it remains research tool. Based on the same functional principle, endocytoscopy has the same deficiencies as CLE[31].
NBI results in similar dysplastic lesion detection rates as WLE, but it seems that NBI identifies more adenoma-like lesions, whereas WLE with random biopsies identifies more non-adenoma-like lesions[21]. The combination of these two techniques, which is used instinctively by many endoscopists, might improve the dysplasia detection rate. Inflammation in patients with IBD makes dysplasia diagnosis difficult. FICE can help differentiate dysplastic and non-dysplastic lesions[32] and can also predict the risk of recurrence, which allows us to modulate the treatment to gain better control of the disease.
When evaluating a new endoscopic imaging method, we look for the following criteria: efficiency, low cost, the ability to identify lesions, and being easy to learn. CME meets these criteria, although there is a small increase in the procedure duration. Compared with WLE with random biopsies, CME has been demonstrated to increase the rate of dysplasia detection. For CME to become the new standard surveillance strategy for dysplasia in IBD patients, additional clinical studies have to be performed. Because CME increases the rates of dysplasia detection and early recurrence, the benefits of this new technique are obvious. However, what are the limitations? The increase in procedure time and the decrease in accessibility (training is needed before newer endoscopic techniques can be used in our daily activity) seem to be the main restraining motifs that limit the use of these newer endoscopic methods mainly to tertiary centers. Regarding cost, we should note that, on one hand, there are greater costs associated with training and new endoscopes; on the other hand, the number of biopsies is lower, and the diagnostic yield is higher. Thus, the overall balance hangs on the side of new techniques.
In addition to endoscopy techniques, stool and blood biomarkers provide a helping hand for disease progress reduction and disease monitoring. When used wisely and combined with endoscopy, these methods exhibit promise in terms of the selection of patients for the early detection of dysplastic lesions and for the prevention of inflammatory relapse and can allow a personalized approach. We recommend the routine use of careful white light examination combined with NBI or FICE to target biopsies in all IBD patients.
Although we still do not have the perfect method, the available new endoscopic techniques offer promising results. The identification of a gold standard method for the evaluating disease evolution and preventing colon cancer in our IBD patients is only a matter of time.
| 1. | Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther. 2006;23:1097-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. 2013;19:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Jess T, Simonsen J, Jørgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143:375-381.e1; quiz e13-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Askling J, Dickman PW, Karlén P, Broström O, Lapidus A, Löfberg R, Ekbom A. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology. 2001;120:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48-54. [PubMed] [DOI] [Full Text] |
| 9. | Farraye FA, Odze RD, Eaden J, Itzkowitz SH, McCabe RP, Dassopoulos T, Lewis JD, Ullman TA, James T, McLeod R. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Lutgens MW, Oldenburg B, Siersema PD, van Bodegraven AA, Dijkstra G, Hommes DW, de Jong DJ, Stokkers PC, van der Woude CJ, Vleggaar FP. Colonoscopic surveillance improves survival after colorectal cancer diagnosis in inflammatory bowel disease. Br J Cancer. 2009;101:1671-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Watanabe T, Ajioka Y, Mitsuyama K, Watanabe K, Hanai H, Nakase H, Kunisaki R, Matsuda K, Iwakiri R, Hida N. Comparison of Targeted vs Random Biopsies for Surveillance of Ulcerative Colitis-Associated Colorectal Cancer. Gastroenterology. 2016;151:1122-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | McGill SK, Kaltenbach T, Friedland S, Soetikno RM. The learning curve for detection of nonpolypoid (flat and depressed) colorectal neoplasms. Gastrointest Endosc. 2012;75:AB177-AB178. |
| 13. | Kiesslich R, Fritsch J, Holtmann M, Koehler HH, Stolte M, Kanzler S, Nafe B, Jung M, Galle PR, Neurath MF. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Marion JF, Waye JD, Present DH, Israel Y, Bodian C, Harpaz N, Chapman M, Itzkowitz S, Steinlauf AF, Abreu MT. Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol. 2008;103:2342-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Subramanian V, Mannath J, Ragunath K, Hawkey CJ. Meta-analysis: the diagnostic yield of chromoendoscopy for detecting dysplasia in patients with colonic inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Guagnozzi D, Lucendo AJ. Colorectal cancer surveillance in patients with inflammatory bowel disease: What is new? World J Gastrointest Endosc. 2012;4:108-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Dekker E, van den Broek FJ, Reitsma JB, Hardwick JC, Offerhaus GJ, van Deventer SJ, Hommes DW, Fockens P. Narrow-band imaging compared with conventional colonoscopy for the detection of dysplasia in patients with longstanding ulcerative colitis. Endoscopy. 2007;39:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 19. | van den Broek FJ, Fockens P, van Eeden S, Stokkers PC, Ponsioen CY, Reitsma JB, Dekker E. Narrow-band imaging versus high-definition endoscopy for the diagnosis of neoplasia in ulcerative colitis. Endoscopy. 2011;43:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Ignjatovic A, East JE, Subramanian V, Suzuki N, Guenther T, Palmer N, Bassett P, Ragunath K, Saunders BP. Narrow band imaging for detection of dysplasia in colitis: a randomized controlled trial. Am J Gastroenterol. 2012;107:885-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Leifeld L, Rogler G, Stallmach A, Schmidt C, Zuber-Jerger I, Hartmann F, Plauth M, Drabik A, Hofstädter F, Dienes HP. White-Light or Narrow-Band Imaging Colonoscopy in Surveillance of Ulcerative Colitis: A Prospective Multicenter Study. Clin Gastroenterol Hepatol. 2015;13:1776-1781.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Pellisé M, López-Cerón M, Rodríguez de Miguel C, Jimeno M, Zabalza M, Ricart E, Aceituno M, Fernández-Esparrach G, Ginès A, Sendino O. Narrow-band imaging as an alternative to chromoendoscopy for the detection of dysplasia in long-standing inflammatory bowel disease: a prospective, randomized, crossover study. Gastrointest Endosc. 2011;74:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Naymagon S, Marion JF. Surveillance in inflammatory bowel disease: chromoendoscopy and digital mucosal enhancement. Gastrointest Endosc Clin N Am. 2013;23:679-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Hoffman A, Sar F, Goetz M, Tresch A, Mudter J, Biesterfeld S, Galle PR, Neurath MF, Kiesslich R. High definition colonoscopy combined with i-Scan is superior in the detection of colorectal neoplasias compared with standard video colonoscopy: a prospective randomized controlled trial. Endoscopy. 2010;42:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Rimbaş M, Negreanu L, Ciobanu L, Benguş A, Spada C, Băicuş CR, Costamagna G. Is virtual chromoendoscopy useful in the evaluation of subtle ulcerative small-bowel lesions detected by video capsule endoscopy? Endosc Int Open. 2015;3:E615-E620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Boal Carvalho P, Rosa B, Dias de Castro F, Moreira MJ, Cotter J. PillCam COLON 2 in Crohn’s disease: A new concept of pan-enteric mucosal healing assessment. World J Gastroenterol. 2015;21:7233-7241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Tontini GE, Vecchi M, Neurath MF, Neumann H. Review article: newer optical and digital chromoendoscopy techniques vs. dye-based chromoendoscopy for diagnosis and surveillance in inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:1198-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Kantsevoy SV, Adler DG, Conway JD, Diehl DL, Farraye FA, Kaul V, Kethu SR, Kwon RS, Mamula P, Rodriguez SA. Confocal laser endomicroscopy. Gastrointest Endosc. 2009;70:197-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Hurlstone DP, Kiesslich R, Thomson M, Atkinson R, Cross SS. Confocal chromoscopic endomicroscopy is superior to chromoscopy alone for the detection and characterisation of intraepithelial neoplasia in chronic ulcerative colitis. Gut. 2008;57:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Neumann H, Vieth M, Atreya R, Grauer M, Siebler J, Bernatik T, Neurath MF, Mudter J. Assessment of Crohn’s disease activity by confocal laser endomicroscopy. Inflamm Bowel Dis. 2012;18:2261-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Maeda Y, Ohtsuka K, Kudo SE, Wakamura K, Mori Y, Ogata N, Wada Y, Misawa M, Yamauchi A, Hayashi S. Endocytoscopic narrow-band imaging efficiency for evaluation of inflammatory activity in ulcerative colitis. World J Gastroenterol. 2015;21:2108-2115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Matsumura T, Arai M, Sato T, Nakagawa T, Maruoka D, Tsuboi M, Hata S, Arai E, Katsuno T, Imazeki F. Efficacy of computed image modification of capsule endoscopy in patients with obscure gastrointestinal bleeding. World J Gastrointest Endosc. 2012;4:421-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Romania
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Amornyotin S, Kouraklis G S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF