Published online Jun 14, 2017. doi: 10.3748/wjg.v23.i22.4102
Peer-review started: February 8, 2017
First decision: March 16, 2017
Revised: March 29, 2017
Accepted: May 9, 2017
Article in press: May 9, 2017
Published online: June 14, 2017
Processing time: 136 Days and 11.9 Hours
To analyze 1-year liver injury burden in inflammatory bowel disease (IBD) patients.
During a 6-mo inclusion period, consecutive IBD cases having a control visit at IBD center were included. Basic demographics, IBD phenotype and IBD treatment were recorded on entry. Aminotransferase (AT) activities of ALT, AST, ALP and gamma-glutamyl transpeptidase (GGT) were measured at baseline, 3 mo prior to study entry and prospectively every 3 mo for 1 year. Liver injury patterns were predefined as: Grade 1 in ALT 1-3 × upper limit of normal (ULN), grade 2 in ALT > 3 × ULN, hepatocellular injury in ALT > 2 × ULN, cholestatic injury in simultaneous GGT and ALP elevation > ULN. Persisting injury was reported when AT elevations were found on > 1 measurement. Risk factors for the patterns of liver injury were identified among demographic parameters, disease phenotype and IBD treatment in univariate and multivariate analysis. Finally, implications for the change in IBD management were evaluated in cases with persisting hepatocellular or cholestatic injury.
Two hundred and fifty-one patients were included having 917 ALT and 895 ALP and GGT measurements. Over one year, grade 1 injury was found in 66 (26.3%), grade 2 in 5 (2%) and hepatocellular injury in 16 patients (6.4%). Persisting hepatocellular injury was found in 4 cases. Cholestasis appeared in 11 cases (4.4%) and persisted throughout the entire study period in 1 case. In multivariate analysis, hepatocellular injury was associated with BMI (OR = 1.13, 1.02-1.26), liver steatosis (OR = 10.61, 2.22-50.7), IBD duration (1.07, 1.00-1.15) and solo infliximab (OR = 4.57, 1.33-15.7). Cholestatic liver injury was associated with prior intestinal resection (OR = 32.7, 3.18-335), higher CRP (OR = 1.04, 1.00-1.08) and solo azathioprine (OR = 10.27, 1.46-72.3). In one case with transient hepatocellular injury azathioprine dose was decreased. In 4 cases with persisting hepatocellular injury, fatty liver or alcohol were most likely causes and IBD treatment was pursued without change. In the case with persisting cholestatic injury, no signs of portal hypertension were identified and treatment with infliximab continued.
Liver injury was frequent, mostly transient and rarely changed management. Infliximab or azathioprine were confirmed as its risk factors indicating the need for regular AT monitoring.
Core tip: We evaluated liver injury in consecutive inflammatory bowel disease (IBD) patients followed for one year in whom aminotransferase activities (AT) were measured at baseline and at 3 mo intervals. We found AT elevations frequently, but they were mostly mild and transient. Even persisting abnormalities had rarely an effect on IBD management. However, ALT elevations and cholestasis appeared more commonly among patients treated with infliximab (ALT) or azathioprine (cholestasis). This finding points to their potential for hepatotoxicity and the need for regular AT monitoring.
- Citation: Koller T, Galambosova M, Filakovska S, Kubincova M, Hlavaty T, Toth J, Krajcovicova A, Payer J. Drug-induced liver injury in inflammatory bowel disease: 1-year prospective observational study. World J Gastroenterol 2017; 23(22): 4102-4111
- URL: https://www.wjgnet.com/1007-9327/full/v23/i22/4102.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i22.4102
Inflammatory bowel disease (IBD) is a chronic inflammatory condition of the digestive tract. Over past decades, significant changes in the treatment of this condition have occurred. Most patients are currently treated with long-term immunosuppression, which has been shown to be effective in improving patients’ symptoms and quality of life[1]. The treatment has a potential for various adverse events including a drug-induced liver injury (DILI). Recently, several reports have raised a concern that hepatotoxicity of IBD treatment might be underestimated. The DILI network has listed infliximab and azathioprine in category A, with more than one hundred well documented cases of hepatotoxicity[2]. A population-based study from Iceland reported that azathioprine and infliximab were among five most common drugs causing liver injury[3]. Furthermore, chronic drug induced liver injury is increasingly being recognized[4]. Drug induced liver injury may range from mild aminotransferase elevations to symptomatic hepatitis or acute liver failure. Finding causes of mild or persisting aminotransferase (AT) elevations in immunosuppressed IBD patients is challenging. Apart from DILI, many other comorbid conditions and therapies could be involved[5]. Analogically to other causes of liver injury, such abnormalities might indicate an ongoing liver injury[6]. However, clinical relevance and evolution of these findings remain unclear. We are lacking studies from a real-life setting reporting on how often this potential risk actually interferes with IBD management.
We aimed to analyze a real-life burden of liver related adverse events in IBD patients over one year. First, we aimed to estimate the prevalence of liver injury among treated IBD patients. Second, to assess its prevalence according to IBD treatment. Third, to analyze evolution of liver injury and its independent risk factors. Fourth, to evaluate its implications on further IBD management.
Our study was carried out in a single IBD center where we prospectively included all consecutive IBD patients having a control visit between January 2nd 2014 and June 30th 2014. Inclusion criteria for the study population were the diagnosis of ulcerative colitis or Crohn’s disease and a control visit at the center during the inclusion period. All studied parameters were prospectively entered into an electronic hospital database and patient folders according to the pre-defined protocol. At entry to the study, we recorded basic demographics (age, gender, body mass index), characteristics of IBD phenotype [IBD duration, IBD type, diagnosis of primary sclerosing cholangitis (PSC)], past surgeries (intestinal resections), current inflammatory activity (serum CRP and fecal calprotectin), quality of life (short IBDQ questionnaire) and current IBD therapy and dosing.
Patients with the history of known chronic liver disease other than PSC and patients with the history of known cirrhosis were not included in the study. Alcohol abuse was estimated during the baseline visit. In cases with significant AT elevations [> 3 times upper limit of normal (ULN)] it was also estimated in the process of evaluation for possible DILI. All patients were tested for HBs antigen and antibodies against HCV prior to any IBD treatment with negative results in all cases. Previous or incident infections with other hepatotropic viruses as well as other possible hepatotoxic drug were not assessed in the data analysis.
Serum activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP) and serum bilirubin concentration were used as markers of liver injury as recommended by the regulatory authorities[7]. Blood sampling for AT activities was carried out on entry to the study and was pre-planned at 3 mo intervals for up to 12 mo. We also recorded aminotransferase activities from electronic records 3 mo prior to the study entry. This allowed us to evaluate the evolution of AT elevations even in cases having abnormal baseline AT values. Overall, each patient had a baseline AT measurement, four prospective AT and bilirubin measurements and one retrospective AT measurement from electronic records.
Liver injury was graded as defined by the Common terminology criteria for adverse events v. 4.03[8]. For hepatocellular injury enzymes ALT and AST, a grade 1 injury was defined as an increase in activity up to three times the upper limit of normal (3 × ULN), grade 2 injury as an increase superior to 3 × ULN. We also evaluated a more conservative and clinically relevant ALT cut-off of > 2 × ULN further referred to as “hepatocellular injury”. For cholestatic enzymes GGT and ALP, a grade 1 injury was defined as an increase in activity up to 2.5 × ULN and Grade 2 injury as an increase superior to 2.5 × ULN. Since GGT elevation is not considered a specific marker for liver injury, we used it in parallel with alkaline phosphatase. An event of cholestasis was defined as an increase in GGT and ALP > ULN. An increase in bilirubin concentration was recorded when superior to 2 × ULN (> 34 μmol/L). A subgroup patients (n = 155) had undergone abdominal ultrasound for the presence or absence of liver steatosis, but severity of liver steatosis was not assessed. The following liver injury events were pre-defined: grade 1 and 2 ALT and AST increase, hepatocellular injury with ALT increase > 2 × ULN, grade 1 and 2 ALP increase, cholestasis and bilirubin increase > 2 × ULN.
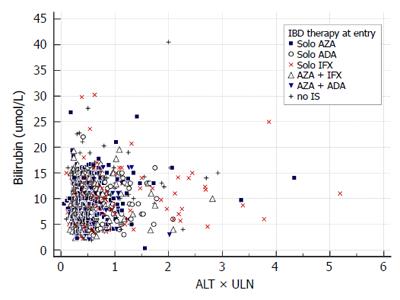
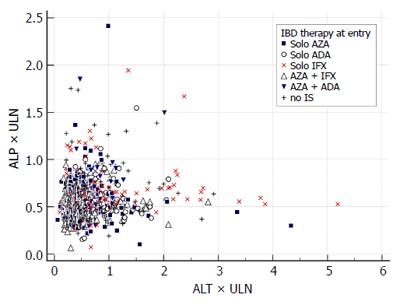
We report the prevalence of liver injury by counting all particular events of an enzyme increase among all measurements of the enzyme. Second, we report the injury events separately for each of the following treatment groups: no immunosuppression, solo azathioprine, solo infliximab, solo adalimumab, azathio-prine and infliximab, azathioprine and adalimumab. These treatment groups were defined according to the IBD treatment received at baseline. The prevalence of the liver event in the treatment group was compared to the prevalence of the same event in the entire study population. For graphical illustration of the findings we constructed an evaluation of drug induced serious hepatotoxicity (eDISH) plot[9]. Third, an evolution of liver injury is reported by counting how many times a liver injury event was observed in patients (from once up to five times). Two patterns of evolution were pre-defined: a transient increase was defined as one grade 1 event, a persisting increase as two or more grade 1 events.
We assessed risk factors for the following events and patterns of liver injury: persisting grade 1 ALT and AST increase, hepatocellular injury with ALT increase > 2 × ULN and any event of cholestasis. Finally, all hepatocellular injury cases (ALT > 2 × ULN) were analyzed for further evolution and change of treatment.
All fasting blood samples were analyzed in a single local laboratory by the standard automated analyzers. All suspected DILI events were investigated for possible alternative causes of increased AT activities and were followed more closely.
Data were assessed by the statistical software package MedCalc v. 14, Ostende, Belgium. For normality testing we used Kolmogorov-Smirnov test and variables are expressed as a mean ± SD, median and interquartile range or as a relative count and percentage. AT activities were expressed as multiples of an upper limit of normal. We used chi-square test to compare a relative proportion of an event in the treatment group with its proportion in the entire study population. Risk factors for liver injury events and patterns were identified using a logistic regression. All available parameters were assessed as independent variables and the liver injury event or pattern as the dependent variable. Results are given as odds ratios (OR) with 95% confidence intervals and P values. Risk factors were considered significant when P values were inferior to 0.05. All identified risk factors were entered into a stepwise multivariate logistic regression model to identify independently associated risk factors.
Our study was non-interventional and was carried out in accordance with the Helsinki declaration. Data acquisition was approved by our University hospital ethics committee. Due to the noninterventional nature of our study, patients were exempted from signing an informed consent form.
We included 251 IBD patients fulfilling the inclusion criteria. Summary statistics of the entire study population is displayed in Table 1. One hundred and fifty-four cases had Crohn’s disease and ninety-seven had ulcerative colitis. The prevalence of all predefined liver injury events is displayed in Table 2.
| n = 251 | Median; IQR, n (%) |
| Age | 39; 30.0-52.75 |
| Female gender | 129 (51.4) |
| Body mass index | 24.298; 21.19-27.34 |
| Crohn's disease | 154 (61.4) |
| Ulcerative colitis | 97 (38.6) |
| Inflammatory bowel disease duration (yr) | 8 (5-13 |
| Primary sclerosign cholangitis | 2 (0.8) |
| Prior intestinal resection | 73 (29.4) |
| Short IBDQ questionaire score | 58 (50-64) |
| Fecal calprotectin on study entry (mg/g) | 83.195 (23.45-331.8) |
| C-reactive protein on study entry (mg/L) | 5 (2.4-8.2) |
| Inflammatory bowel disease therapy on entry | |
| Mesalamine | 173 (69.5) |
| Antibiotics | 17 (6.9) |
| No immunosupression | 66 (26.3) |
| Steroids | 42 (16.9) |
| Azathioprine solo | 47 (18.7) |
| anti TNF therapy (all) | 138 (55) |
| anti TNF therapy solo | 74 (29.5) |
| Infliximab solo | 41 (16.3) |
| Adalimumab solo | 33 (13.1) |
| Combination therapy (anti-TNF and azathioprine) | 64 (25.5) |
| Days between 1st and 5th AT sampling | 382 (353-439.8) |
| Liver steatosis on ultrasound (n = 155) | 34 (21.9) |
| Liver injury event | All measurements | Abnormal |
| n | Number of events | |
| ALT increase Grade 1 (0-3 × ULN) | 917 | 112 (12.21) |
| ALT increase > 2 × ULN (hepatocellular injury) | 917 | 26 (2.84) |
| ALT increase Grade 2 (> 3 × ULN) | 917 | 6 (0.65) |
| AST increase Grade 1 (0-3 × ULN) | 917 | 55 (6.0) |
| AST increase Grade 2 (> 3 × ULN) | 917 | 8 (0.87) |
| GGT increase Grade 1 (0-2.5 × ULN) | 895 | 80 (8.94) |
| GGT increase Grade 2 (> 2.5 × ULN) | 895 | 24 (2.68) |
| ALP increase Grade 1 (0-2.5 × ULN) | 897 | 34 (3.79) |
| ALP increase Grade 2 (> 2.5 × ULN) | 897 | 0 (0) |
| Cholestasis (parallel ALP and GGT elevation) | 895 | 11 (1.23) |
| Total bilirubin > 2 × ULN | 370 | 1 (0.27) |
We assessed 917 ALT and AST measurements. Grade 1 ALT and AST elevation was observed in 112 and 55 measurements (12.2% and 6%). Hepatocellular injury with ALT superior to 2 × ULN was observed 26 times (2.84%), Grade 2 ALT and AST increase was observed 6 and 8 times (0.65 and 0.87%). We did not observe any grade 3 or 4 liver injury.
We assessed 897 ALP and 895 GGT measurements. Grade 1 and 2 ALP elevation was observed in 34 and 0 measurements (3.79 and 0%). Cholestasis was observed 11 times (1.23%).
Among 251 study patients, 66 had no maintenance therapy, 47 were on solo azathioprine, 74 on solo anti TNF therapy (infliximab 41, adalimumab 33) and 64 had combination therapy of anti-TNF (infliximab 48, adalimumab 16) with azathioprine. Proportions of liver injury according to IBD treatment are displayed in Table 3. Grade 1 ALT increase was more common in patients treated with solo adalimumab (18.5% vs 12.2%) compared with the entire study population. In patients treated with solo infliximab: grade 2 ALT increase (2.5% vs 0.65%), hepatocellular injury with ALT > 2 × ULN (9.2% vs 2.84%) and grade 1 AST increase (12.3% vs 6.0%) were more common. The DISH plot showing ALT/total bilirubin and ALT/ALP relationship for all measurements and treatment groups is shown in Figures 1 and 2. Cholestasis was more common among patients treated with solo azathioprine (3.4% vs 1.23 %). There were no differences among other treatment groups (combination therapy and no-immunosuppression) for other liver injury events.
| Liver injury event | IBD treatment | |||||
| Solo azathioprine | Solo adalimumab | Solo infliximab | ||||
| All measurements | Abnormal | All measurements | Abnormal | All measurements | Abnormal | |
| n | n (%) | n | n (%) | n | n (%) | |
| ALT increase Grade 1 (0-3 × ULN) | 154 | 16 (10.4) | 135 | 25 (18.5)a | 163 | 26 (16) |
| ALT > 2 × ULN (hepatocellular injury) | 3 (1.9) | 2 (1.5) | 15 (9.2)a | |||
| ALT increase Grade 2 (> 3 × ULN) | 2 (1.3) | 0 (0) | 4 (2.5)a | |||
| AST increase Grade 1 (0-3 × ULN) | 154 | 6 (3.9) | 135 | 7 (5.2) | 162 | 20 (12.3)a |
| AST increase Grade 2 (> 3 × ULN) | 0 (0) | 1 (0.1) | 2 (1.2) | |||
| ALP increase Grade 1 (0-2.5 × ULN) | 148 | 6 (4.1) | 132 | 2 (1.5) | 163 | 11 (6.7) |
| ALP increase Grade 2 (> 2.5 × ULN) | 0 (0) | 0 (0) | 0 (0) | |||
| Cholestasis (parallel ALP and GGT > ULN) | 146 | 5 (3.4)a | 132 | 1 (0.1) | 162 | 2 (1.2) |
| Azathioprine + infliximab | Azathioprine + adalimumab | No immunosupression | ||||
| All measurements | Abnormal | All measurements | Abnormal | All measurements | Abnormal | |
| ALT increase Grade 1 (0-3 × ULN) | 199 | 15 (7.5) | 59 | 8 (13.6) | 207 | 22 (10.6) |
| ALT > 2 × ULN (hepatocellular injury) | 2 (1) | 1 (1.7) | 3 (1.4) | |||
| ALT increase Grade 2 (> 3 × ULN) | 0 (0) | 0 (0) | 0 (0) | |||
| AST increase Grade 1 (0-3 × ULN) | 200 | 11 (5.5) | 59 | 2 (3.4) | 207 | 9 (4.3) |
| AST increase Grade 2 (> 3 × ULN) | 1 (0.5) | 2 (3.4) | 2 (0.1) | |||
| ALP increase Grade 1 (0-2.5 × ULN) | 195 | 0 (0) | 58 | 3 (5.2) | 201 | 12 (6) |
| ALP increase Grade 2 (> 2.5 × ULN) | 0 (0) | 0 (0) | 0 (0) | |||
| Cholestasis (parallel ALP and GGT > ULN) | 195 | 0 (0) | 58 | 1 (1.7) | 201 | 2 (1) |
ALT elevation was the most common liver injury event. Grade 1 increase was observed in 66 (26.3%) patients, it was unique in 38 patients and persisted in 28 cases (11.2%). Grade 2 increase was found in 5 cases, it was unique in 4 cases. Hepatocellular injury (ALT > 2 × ULN) was observed in 16 (6.3%) cases and was unique in 12 patients.
For AST, grade 1 increase was found in 34 (13.5%) cases, it was unique in 25 and persisted in 9 cases (3.6%). Grade 2 increase was observed in 6 cases, it was unique in 4 cases.
For ALP, grade 1 increase was found in 19 (7.6%) patients, it was unique in 11 cases. We recorded no case of grade 2 ALP increase. Cholestasis was found in 11 (4.4%) cases, and was unique in 7 cases. Total bilirubin superior to 2 × ULN was observed once and it was not paralleled with AT elevation suggesting a Gilbert syndrome. Numbers of cases for each liver injury event and pattern of evolution are summarized Table 4.
| Liver injury event | Numbers of cases with liver injury events | ||||||
| Transient | Persisting injury | ||||||
| No event | Any event | 1 event | 2 events | 3 events | 4 events | 5 events | |
| ALT increase Grade 1 (0-3 × ULN) | 185 (73.7) | 66 (26.3) | 38 (15.1) | 16 (6.4) | 6 (2.4) | 6 (2.4) | 0 (0) |
| ALT increase > 2 × ULN (hepatocellular injury) | 235 (93.63) | 16 (6.4) | 12 (4.8) | 1 (0.4) | 1 (0.4) | 1 (0.4) | 1 (0.4) |
| ALT increase Grade 2 (> 3 × ULN) | 246 (98.0) | 5 (2) | 4 (1.6) | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) |
| AST increase Grade 1 (0-3 × ULN) | 217 (86.45) | 34 (13.5) | 25 (10) | 4 (1.6) | 1 (0.4) | 1 (0.4) | 3 (1.2) |
| AST increase Grade 2 (> 3 × ULN) | 245 (97.6) | 6 (2.3) | 4 (1.6) | 2 (0.8) | 0 (0) | 0 (0) | 0 (0) |
| ALP increase Grade 1 (0-2.5 × ULN) | 232 (92.43) | 19 (7.5) | 11 (4.4) | 3 (1.2) | 4 (1.6) | 0 (0) | 1 (0.4) |
| ALP increase Grade 2 (> 2.5 × ULN) | 251 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Cholestasis (parallel ALP and GGT elevation) | 240 (95.61) | 11 (4.4) | 7 (2.8) | 2 (0.8) | 1 (0.4) | 0 (0) | 1 (0.4) |
| Total bilirubin > 2 × ULN | 250 (99.6) | 1 (0.4) | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Univariate regression identified five risk factors for persisting grade 1 ALT increase: female gender (OR = 0.22), body mass index (OR = 1.13), solo infliximab (OR = 2.8) and liver steatosis (OR = 7.77). For hepatocellular injury (ALT > 2 × ULN): body mass index (OR = 1.13), duration of IBD (OR = 1.08), solo infliximab (OR = 3.43) and liver steatosis (HR = 7.14). The exact same pattern of risk factors was observed for persisting AST increase. Finally, risk factors for cholestasis were prior intestinal resection (HR = 7.06), the level of CRP on entry (HR = 1.04) and solo azathioprine (HR = 3.93). IBD phenotype was not found to be a risk factor for any type of liver injury. Odds ratios for all demographic parameters, IBD related phenotype, inflammatory activity, the quality of life and IBD treatment are displayed in Table 5.
| Risk factors for selected liver injury events and patterns | ||||||||
| Persisting ALT increase | ALT > 2 × ULN | Persisting AST increase | Cholestasis | |||||
| OR; 95%CI | P value | OR; 95%CI | P value | OR; 95%CI | P value | OR; 95%CI | P value | |
| Age1 | 1.01; 0.98-1.04 | 0.628 | 1.02; 0.98-1.05 | 0.317 | 1.03; 0.99-1.08 | 0.185 | 1.02; 0.98-1.06 | 0.300 |
| Female gender | 0.22; 0.87-0.57 | 0.002 | 0.55; 0.19-1.55 | 0.260 | 0.75; 0.20-2.86 | 0.672 | 2.62; 0.68-10.13 | 0.160 |
| Body mass index1 | 1.13; 1.04-1.23 | 0.004 | 1.13; 1.03-1.23 | 0.011 | 1.18; 1.06-1.32 | 0.003 | 0.95; 0.82-1.08 | 0.413 |
| Crohn's disease | 2.03; 0.83-4.97 | 0.122 | 2.89; 0.80-10.41 | 0.105 | 1.27; 0.31-5.20 | 0.739 | 2.95; 0.62-13.94 | 0.173 |
| Ulcerative colitis | 0.49; 0.20-1.21 | 0.122 | 0.35; 0.10-1.25 | 0.105 | 0.79; 0.19-3.22 | 0.739 | 0.34; 0.07-1.60 | 0.173 |
| Inflammatory bowel disease duration (yr)1 | 1.04; 0.98-1.09 | 0.180 | 1.08; 1.02-1.15 | 0.007 | 1.10; 1.03-1.18 | 0.006 | 1.04; 0.97-1.11 | 0.295 |
| Prior intestinal resection | 0.95; 0.40-2.28 | 0.915 | 1.10; 0.37-3.27 | 0.869 | 0.68; 0.14-3.33 | 0.631 | 7.06; 1.81-27.41 | 0.005 |
| Short IBDQ questionaire score1 | 1.00; 0.97-1.04 | 0.935 | 0.99; 0.95-1.04 | 0.703 | 0.99; 0.94-1.06 | 0.925 | 0.98; 0.93-1.04 | 0.545 |
| Fecal calprotectin on study entry (mg/g)1 | 0.999; 0.99-1.00 | 0.909 | 1.00; 0.99-1.00 | 0.895 | 0.99; 0.99-1.00 | 0.424 | 1.00; 0.99-1.00 | 0.346 |
| C-reactive protein on study entry (mg/L)1 | 0.98; 0.94-1.03 | 0.520 | 0.98; 0.93-1.04 | 0.590 | 1.00; 0.95-1.06 | 0.907 | 1.04; 1.01-1.07 | 0.024 |
| Inflammatory bowel disease therapy on entry | ||||||||
| Mesalamine | 0.64; 0.29-1.45 | 0.288 | 0.54; 0.19-1.51 | 0.241 | 0.34; 0.09-1.29 | 0.112 | 0.76; 0.22-2.67 | 0.667 |
| No immunosupression | 0.58; 0.21-1.59 | 0.287 | 0.38; 0.08-1.73 | 0.211 | 0.34; 0.41-2.77 | 0.314 | 0.61; 0.13-2.9 | 0.535 |
| Steroids | 0.16; 0.02-1.22 | 0.078 | 0.69; 0.15-3.13 | 0.627 | 0.60; 0.07-4.96 | 0.640 | 1.90; 0.48-7.50 | 0.357 |
| Azathioprine solo | 0.49; 0.14-1.69 | 0.258 | 1.00; 0.27-3.67 | 0.998 | 0.53; 0.07-4.36 | 0.557 | 3.93; 1.15-13.47 | 0.030 |
| Anti-TNF therapy solo | 2.72; 1.22-6.03 | 0.014 | 2.56; 0.923-7.10 | 0.071 | 5.11; 1.24-21.05 | 0.024 | 0.89; 0.23-3.46 | 0.870 |
| Infliximab solo | 2.80; 1.18-6.80 | 0.020 | 3.43; 1.17-10.03 | 0.030 | 4.43; 1.14-17.28 | 0.032 | 1.15; 0.24-5.50 | 0.866 |
| Adalimumab solo | 1.51; 0.53-4.31 | 0.437 | 0.94; 0.20-4.33 | 0.937 | 1.94; 0.39-9.79 | 0.420 | 0.65; 0.08-5.25 | 0.686 |
| Combination therapy (anti-TNF and azathioprine) | 0.776; 0.3-2.01 | 0.601 | 0.66; 0.18-2.39 | 0.525 | 0.36; 0.04-2.90 | 0.330 | 0.28; 0.04-2.24 | 0.231 |
| Liver steatosis on ultrasound (n = 155) | 7.77; 3.03-19.9 | < 0.0001 | 7.14; 2.15-23.59 | 0.001 | 6.78; 1.53-30.03 | 0.012 | 1.45; 0.27-7.83 | 0.666 |
Multivariate logistic regression identified the following independent risk factors for persisting grade 1 ALT increase: female gender (OR = 0.221; 95%CI: 0.07-0.67), BMI (OR = 1.15, 1.05-1.27), and liver steatosis (OR = 31.0, 6.76-142.1), for hepatocellular injury (ALT > 2 × ULN): IBD duration (OR = 1.07, 1.00-1.15), BMI (OR = 1.13, 1.02-1.26), solo infliximab (OR = 4.57, 1.33-15.7) and steatosis (OR = 10.61, 2.22-50.7), for cholestasis: prior IBD resection (OR = 32.7, 3.18-335); CRP (1.04, 1.00-1.08) and solo azathioprine (OR = 10.266, 1.46-72.3).
Sixteen patients (6.3%) with observed hepatocellular injury (ALT > 2 × ULN) were closely managed by a treating physician. ALT normalized in 12 cases with subsequent follow-up. In 4 cases, ALT elevation persisted. Analysis of possible causes of this persisting elevation identified other possible conditions: alcohol abuse in one case, type 2 diabetes with liver steatosis twice, obesity with liver steatosis once. Fifteen subjects had no change in IBD treatment, azathioprine dose was halved in one patient. Cholestasis was transient in 7 from 11 cases. One patient had persisting cholestasis at all measurements and was evaluated for possible NRH. Upper endoscopy and ultrasound did not suggest signs of portal hypertension and the patient continues the treatment with infliximab. We did not observe worsening of liver injury among subjects pursuing the treatment.
Our prospective study reports on the liver injury burden among treated IBD patients over one year. We found mild ALT elevation in 26.3% of patients, hepatocellular injury 6.4% and persisting elevation in 11.2% of patients. Events of hepatocellular injury were more common with higher BMI and steatosis, with longer duration of IBD and on treatment with solo infliximab. Cholestasis was observed in 4.4% of patients and was more common after intestinal resection, higher inflammatory activity at baseline and on therapy with solo azathioprine. Most events of liver injury were transient and rarely required any change in management.
Clinical interpretation of hepatocellular injury occurring in IBD patients remains a challenge. In most cases, competing etiologies do not allow identification of a single one. Alcohol abuse, fatty liver disease, de-novo viral infection, concomitant medication and comorbidities could all be involved. Specific etiological models such as RUCAM model[10], might help in confirming or excluding DILI. However, AT abnormalities are frequently transient and stopping IBD therapy is not feasible at the moment of first appearance of liver injury. Therefore, this model does not help in cases with mild elevation. Moreover, it has been shown that several causes occurring in one patient might have a synergistic effect. Schröder et al[11] reported that patients with fatty liver were more susceptible to liver injury when treated with non-anti TNF immunosuppression. For now, it is not clear whether this finding also applies to anti-TNF therapy. In an observation study, Cappello et al[12] reported mild hepatocellular injury in 20.9% of 335 IBD patients. Liver injury was transient and most commonly due to fatty liver or DILI. Authors did not give details on IBD treatment and the most commonly observed pattern of injury was mild cholestasis. Parisi et al[13] report abnormal ALT in 39.2% and grade 2 increase in 7.9% of 176 patients treated with infliximab. In this study, authors identified several risk factors for liver injury: previous abnormal ALT suggesting a preexisting liver disease, immunomodulatory use and duration of infliximab therapy. Shelton et al[14] report a new transient increase in ALT > 2 × ULN following anti-TNF induction in 102 (6%) of 1753 patients. Liver injury could be linked to alternative etiologies in 54 of these cases (antibiotics, thiopurines, alcohol, fatty liver), leaving 48 (2.7%) directly linked to anti-TNF therapy. In 34 cases, ALT abnormalities were transient and anti-TNF therapy could continue. Fourteen patients with persisting abnormalities had to stop therapy. In our study, we included patients regardless of the timing of IBD induction and we did not exclude patients with fatty liver or preexisting ALT elevation. Nevertheless, our results appear consistent with previous reports showing ALT elevations in roughly the same proportion of cases. It appears, that transient liver injury occurs during induction, but also during maintenance therapy with a thiopurine as well as anti-TNF[13-15].
In our study, BMI, fatty liver, solo infliximab and longer duration of therapy have been identified as independent risk factors for hepatocellular injury. For BMI and fatty liver, the findings are consistent with the previous reports. However, ALT elevation was also more frequent among patients on solo infliximab compared to other treatment groups (adalimumab, azathioprine, combination therapy, no immunosuppression). This observation might be interpreted as a specific safety signal for solo infliximab therapy[5,7]. It has been previously shown that severe liver injury occurs on the background of less severe ALT elevations occurring much more commonly. Björnsson et al[16] showed that solo infliximab therapy had higher risk of DILI compared to adalimumab or combination therapy. In theory, solo infliximab therapy could be more immunogenic than a combination therapy in triggering an immune response. Parisi et al[13] also found that immunomodulatory therapy increased the risk of infliximab induced liver injury. This might be explained by hepatotoxicity of the immunomodulatory therapy itself, but the true reason for this finding is unclear. Observed normalization of ALT in most cases does not appear to exclude drug-induced immune mediated injury. It might actually support it, since presumed induction of immune tolerance to the drug might lead to the resolution of liver injury. Normalization of ALT in cases of fatty liver without any intervention appears less likely. For now, there appears to be a background of common causes of hepatocellular injury (steatosis, obesity, diabetes, alcohol). In our study, it might be observed in patients with no immunosuppression. On top of this, there is likely a higher risk of the injury in cases with solo infliximab therapy. Concomitant immunomodulator and liver steatosis probably serve as modulators, but we might also anticipate other influencing factors: genetic, ethnic, co-treatment and its dosing, infliximab administration protocol etc. We also report that longer duration of IBD increases the risk of hepatocellular injury. Some studies have found a similar association with the duration of infliximab therapy.
In view of the observed safety signals and the fact that liver injury occurs at distinct time points, our results support the need for regular AT monitoring regardless of the treatment duration. There is apparently a growing need for predictive models being able to identify patients at risk for DILI[17]. However, to date there are no clinically usable biomarkers that could preclude IBD patients from aminotransferase monitoring.
Cholestasis, a parallel ALP and GGT elevation, was observed in 4.4% of patients. Cholestasis was more frequently observed in cases treated with solo azathioprine compared with other treatment groups. Moreover, independent risk factors for cholestasis were solo azathioprine therapy, prior intestinal resection and higher CRP. Clinical interpretation of cholestasis in IBD patients should start with excluding possible biliary obstruction and the primary sclerosing cholangitis[18]. In our cohort, two cases had diagnosed PSC, but none had cholestasis during the study period. Drug induced cholestatic liver injury has been described in IBD patients treated with azathioprine or less commonly by anti-TNF therapy[19,20]. Symptomatic cholestatic hepatitis has been reported in patients on high-dose azathioprine[21]. Milder liver injuries might also present as cholestasis. In fact, a very recent study shows that mild transient cholestasis is actually the most common pattern of azathioprine induced liver injury[22]. Our findings are consistent with the report, since we found transient cholestasis in 7 of 11 cases. In contrast, persisting cholestasis during azathioprine therapy could be caused by PSC, IBD drugs and nodular regenerative hyperplasia (NRH). NRH is asymptomatic in most subjects and no specific NRH markers have been identified. The exact risk of this condition has not been accurately established[23,24]. Nevertheless, thiopurine therapy, prior intestinal resection and male gender have been previously reported as risk factors for NRH[25,26].
Another interesting finding of our study is that prior intestinal resection was an independent risk factor for cholestasis. Seventy-three patients in our study cohort had prior intestinal resection, 68 of whom had Crohn’s disease. Azathioprine therapy was equally distributed between groups with or without resection, excluding the effect of the drug itself. It was therefore likely, that cholestasis was a consequence of shortening the terminal ileum. Extensive intestinal resection with intestinal failure have been shown to cause severe cholestasis[27]. Moreover, terminal ileum resection in Crohn’s disease decreases the amount of biliary acids reabsorbed into the enterohepatic pool[28], causing diarrhea due to bile acid malabsorption. One proposed mechanism of this condition could be associated with cholestasis. Impaired bile acid reabsorption decreases farnesoid X receptor signaling (FXR) with decreased production of fibroblast growth factor 19 (FGF19). This might have an stimulatory effect on bile acid synthesis in the liver[29]. Indeed, levels of FGF19 have been reported decreased in patients with diarrhea due to bile acid malabsorption[30]. The role of FGF19 in cases with NRH remains unclear. Furthermore, cholestasis in patients with prior resection could also point to the NRH, as discussed above. For now, it appears that azathioprine treated patients are at risk for cholestatic hepatitis and NRH and should be monitored for liver adverse events while on treatment.
Our study has several strengths. We report a real-life burden of liver injury, not excluding patients with preexisting liver disease or other comorbidities. Our study observed various well defined liver injury events, their severities and patterns. On entry to the study, the study cohort was well defined for IBD phenotype and IBD therapy. We also included patients not treated with immunosuppression. This enabled us to identify the background of liver injury in IBD and the comparison among treatment groups. Our study completes the mosaic of reports on the burden of liver injury in IBD patients coming from clinical trials, registries and cohort studies on azathioprine or infliximab induction.
There are several limitations of our study. The size our cohort was not designed to capture the prevalence of severe DILI. Not surprisingly, we have not observed a single case of severe liver injury. However, in accordance with previous reports, some of our results could be viewed as safety signals for solo infliximab or azathioprine. Our study cohort consisted of patients regardless of the treatment timing. Although most patients were on stable maintenance therapy, some patients could have induction treatment. Our treatment groups were assigned according to the treatment at baseline. Later changes in therapy due to treatment efficacy on IBD were possible, but were infrequent. Inclusion of one retrospective value of AT 3 mo prior to study entry was designed to minimize an effect of this change. Finally, cases with incident mild AT elevations were not investigated for other possible hepatotoxic drugs taken, nor changes in alcohol intake or infections with various hepatotropic viruses. By missing these factors, the real DILI prevalence could have been overestimated.
In conclusion, our study shows that aminotransferase abnormalities are common in IBD patients. They are caused by preexisting liver diseases as well as by the IBD treatment. We found no case of severe DILI, but we observed higher risk of hepatocellular injury in patients on solo infliximab and cholestasis in patients on solo azathioprine. These findings could be viewed as safety signals. The real burden of liver injury on IBD appeared low with most cases resolving spontaneously without any change in disease management. However, our findings point to the potential for hepatotoxicity indicating the need for regular aminotransferase monitoring.
There is a growing concern, that drug-induced liver injury of inflammatory bowel disease (IBD) therapies might be underestimated. Therefore the authors aimed to analyze a real-life burden of liver injury and its impact of IBD management in a prospective observation during 1 year.
There are no valid predictive models or biomarkers which would allow us to predict liver injury in IBD treated patients. Therefore, all patients should be monitored for liver tests during treatment. Liver test monitoring during long-term therapy is costly and time consuming. The authors observation was designed to estimate the burden of liver injury and to identify its risk factors (from demographics, IBD phenotype and the particular treatment). Possibly, to identify patients with high-risk of liver injury in which the liver test monitoring should be close. Or, to identify a low risk group in which monitoring would not be necessary. This observation study was the first step in this research project.
They showed that liver injury was common, but in the great majority of cases it was mild and did not require any change in IBD management. However, patients with longer IBD duration, on solo immunosupressive therapy with infliximab or solo therapy with azathioprine had higher risk of liver injury. It appears therefore, that this group of patients would require regular monitoring of liver tests. These observations might lead in the future, to the development of a predictive model for liver injury in IBD.
For now, all patients on IBD therapy should be monitored. However, the risk is significantly higher in patients on solo immunosuppressive therapy, with prior intestinal resection and with longer IBD duration and these cases should be closely monitored for liver injury. In contrast, they have no arguments which would allow us to preclude other patients from liver test monitoring. Predictive models based on the current finding would probably allow us to identify low risk groups in the future.
They use common terms and definitions. Hepatocellular injury was defined as ALT elevation superior to 2 x the upper limit of normal (ULN) and cholestasis as ALP and GGT elevation above the ULN.
The paper is well written and provides new insights about drug-induced liver injury occurrence and features in IBD patients.
| 1. | Miehsler W, Novacek G, Wenzl H, Vogelsang H, Knoflach P, Kaser A, Dejaco C, Petritsch W, Kapitan M, Maier H. A decade of infliximab: The Austrian evidence based consensus on the safe use of infliximab in inflammatory bowel disease. J Crohns Colitis. 2010;4:221-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Björnsson ES, Hoofnagle JH. Categorization of drugs implicated in causing liver injury: Critical assessment based on published case reports. Hepatology. 2016;63:590-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 196] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 3. | Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425, 1425.e1-3; quiz e19-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 606] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 4. | Stine JG, Chalasani N. Chronic liver injury induced by drugs: a systematic review. Liver Int. 2015;35:2343-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 5. | Kaplowitz N. Drug-Induced Liver Injury. In: Drug-induced liver disease. Third edition Elsevier Inc 2013; 3-14. |
| 6. | Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol. 2017;112:18-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 789] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 7. | United States Departement of Health and Human Services F. Guidance for Industry Drug-Induced Liver Injury: Premarketing Clinical Evaluation. Drug Saf 2009; 28. Available from: https://www.fda.gov/downloads/Drugs/.../Guidances/UCM174090.pdf. |
| 8. | Common Terminology Criteria for Adverse Events (CTCAE) 4.03. USDEPARTMENT Heal Hum Serv Natl Institutes Heal Natl Cancer Inst 2010. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. |
| 9. | Watkins PB, Desai M, Berkowitz SD, Peters G, Horsmans Y, Larrey D, Maddrey W. Evaluation of drug-induced serious hepatotoxicity (eDISH): application of this data organization approach to phase III clinical trials of rivaroxaban after total hip or knee replacement surgery. Drug Saf. 2011;34:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Lewis JH, Larrey D, Olsson R, Lee WM, Frison L, Keisu M. Utility of the Roussel Uclaf Causality Assessment Method (RUCAM) to analyze the hepatic findings in a clinical trial program: evaluation of the direct thrombin inhibitor ximelagatran. Int J Clin Pharmacol Ther. 2008;46:327-339. [PubMed] |
| 11. | Schröder T, Schmidt KJ, Olsen V, Möller S, Mackenroth T, Sina C, Lehnert H, Fellermann K, Büning J. Liver steatosis is a risk factor for hepatotoxicity in patients with inflammatory bowel disease under immunosuppressive treatment. Eur J Gastroenterol Hepatol. 2015;27:698-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Cappello M, Randazzo C, Bravatà I, Licata A, Peralta S, Craxì A, Almasio PL. Liver Function Test Abnormalities in Patients with Inflammatory Bowel Diseases: A Hospital-based Survey. Clin Med Insights Gastroenterol. 2014;7:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Parisi I, O‘Beirne J, Rossi RE, Tsochatzis E, Manousou P, Theocharidou E, Hamilton M, Murray C, Epstein O, Burroughs AK. Elevated liver enzymes in inflammatory bowel disease: the role and safety of infliximab. Eur J Gastroenterol Hepatol. 2016;28:786-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Shelton E, Chaudrey K, Sauk J, Khalili H, Masia R, Nguyen DD, Yajnik V, Ananthakrishnan AN. New onset idiosyncratic liver enzyme elevations with biological therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Gisbert JP, González-Lama Y, Maté J. Thiopurine-induced liver injury in patients with inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2007;102:1518-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Björnsson ES, Gunnarsson BI, Gröndal G, Jonasson JG, Einarsdottir R, Ludviksson BR, Gudbjörnsson B, Olafsson S. Risk of Drug-Induced Liver Injury From Tumor Necrosis Factor Antagonists. Clin Gastroenterol Hepatol. 2014;Internet. [RCA] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Chen M, Borlak J, Tong W. A Model to predict severity of drug-induced liver injury in humans. Hepatology. 2016;64:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Yarur AJ, Czul F, Levy C. Hepatobiliary manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:1655-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Gisbert JP, Chaparro M, Gomollón F. Common misconceptions about 5-aminosalicylates and thiopurines in inflammatory bowel disease. World J Gastroenterol. 2011;17:3467-3478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Shaye OA, Yadegari M, Abreu MT, Poordad F, Simon K, Martin P, Papadakis KA, Ippoliti A, Vasiliauskas E, Tran TT. Hepatotoxicity of 6-mercaptopurine (6-MP) and Azathioprine (AZA) in adult IBD patients. Am J Gastroenterol. 2007;102:2488-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Chertoff J, Alam S, Black M, Elgendy IY. Azathioprine-induced hepatitis and cholestasis occurring 1 year after treatment. BMJ Case Rep. 2014;2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Björnsson ES, Gu J, Kleiner DE, Chalasani N, Hayashi PH, Hoofnagle JH. Azathioprine and 6-Mercaptopurine-induced Liver Injury: Clinical Features and Outcomes. J Clin Gastroenterol. 2017;51:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Jharap B, van Asseldonk DP, de Boer NK, Bedossa P, Diebold J, Jonker AM, Leteurtre E, Verheij J, Wendum D, Wrba F. Diagnosing Nodular Regenerative Hyperplasia of the Liver Is Thwarted by Low Interobserver Agreement. PLoS One. 2015;10:e0120299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Morris JM, Oien KA, McMahon M, Forrest EH, Morris J, Stanley AJ, Campbell S. Nodular regenerative hyperplasia of the liver: survival and associated features in a UK case series. Eur J Gastroenterol Hepatol. 2010;22:1001-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Musumba CO. Review article: the association between nodular regenerative hyperplasia, inflammatory bowel disease and thiopurine therapy. Aliment Pharmacol Ther. 2013;38:1025-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Vernier-Massouille G, Cosnes J, Lemann M, Marteau P, Reinisch W, Laharie D, Cadiot G, Bouhnik Y, De Vos M, Boureille A. Nodular regenerative hyperplasia in patients with inflammatory bowel disease treated with azathioprine. Gut. 2007;56:1404-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Mutanen A, Lohi J, Heikkilä P, Koivusalo AI, Rintala RJ, Pakarinen MP. Persistent abnormal liver fibrosis after weaning off parenteral nutrition in pediatric intestinal failure. Hepatology. 2013;58:729-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Nolan JD, Johnston IM, Pattni SS, Dew T, Orchard TR, Walters JR. Diarrhea in Crohn’s disease: investigating the role of the ileal hormone fibroblast growth factor 19. J Crohns Colitis. 2015;9:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Walters JR, Appleby RN. A variant of FGF19 for treatment of disorders of cholestasis and bile acid metabolism. Ann Transl Med. 2015;3:S7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 30. | Pattni SS, Brydon WG, Dew T, Johnston IM, Nolan JD, Srinivas M, Basumani P, Bardhan KD, Walters JR. Fibroblast growth factor 19 in patients with bile acid diarrhoea: a prospective comparison of FGF19 serum assay and SeHCAT retention. Aliment Pharmacol Ther. 2013;38:967-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Slovakia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Harmanci O, Pompili M, Lakatos PL S- Editor: Qi Y L- Editor: A E- Editor: Wang CH