Published online Jun 7, 2017. doi: 10.3748/wjg.v23.i21.3890
Peer-review started: March 11, 2017
First decision: March 30, 2017
Revised: April 5, 2017
Accepted: May 4, 2017
Article in press: May 4, 2017
Published online: June 7, 2017
Processing time: 88 Days and 20 Hours
To identify demographic, clinical, metabolomic, and lifestyle related predictors of relapse in adult ulcerative colitis (UC) patients.
In this prospective pilot study, UC patients in clinical remission were recruited and followed-up at 12 mo to assess a clinical relapse, or not. At baseline information on demographic and clinical parameters was collected. Serum and urine samples were collected for analysis of metabolomic assays using a combined direct infusion/liquid chromatography tandem mass spectrometry and nuclear magnetic resolution spectroscopy. Stool samples were also collected to measure fecal calprotectin (FCP). Dietary assessment was performed using a validated self-administered food frequency questionnaire.
Twenty patients were included (mean age: 42.7 ± 14.8 years, females: 55%). Seven patients (35%) experienced a clinical relapse during the follow-up period. While 6 patients (66.7%) with normal body weight developed a clinical relapse, 1 UC patient (9.1%) who was overweight/obese relapsed during the follow-up (P = 0.02). At baseline, poultry intake was significantly higher in patients who were still in remission during follow-up (0.9 oz vs 0.2 oz, P = 0.002). Five patients (71.4%) with FCP > 150 μg/g and 2 patients (15.4%) with normal FCP (≤ 150 μg/g) at baseline relapsed during the follow-up (P = 0.02). Interestingly, baseline urinary and serum metabolomic profiling of UC patients with or without clinical relapse within 12 mo showed a significant difference. The most important metabolites that were responsible for this discrimination were trans-aconitate, cystine and acetamide in urine, and 3-hydroxybutyrate, acetoacetate and acetone in serum.
A combination of baseline dietary intake, fecal calprotectin, and metabolomic factors are associated with risk of UC clinical relapse within 12 mo.
Core tip: This was a pilot prospective cohort study to evaluate the determinants of clinical relapse in adult ulcerative colitis patients. We found that different dietary, anthropometric, and metabolomic factors at baseline were associated with the risk of disease relapse within 12 mo of follow-up.
- Citation: Keshteli AH, van den Brand FF, Madsen KL, Mandal R, Valcheva R, Kroeker KI, Han B, Bell RC, Cole J, Hoevers T, Wishart DS, Fedorak RN, Dieleman LA. Dietary and metabolomic determinants of relapse in ulcerative colitis patients: A pilot prospective cohort study. World J Gastroenterol 2017; 23(21): 3890-3899
- URL: https://www.wjgnet.com/1007-9327/full/v23/i21/3890.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i21.3890
Ulcerative colitis (UC), a subtype of the inflammatory bowel diseases (IBD), is a chronic relapse-remitting inflammatory condition that affects the colon in a diffuse, continuous, and superficial pattern. It often presents in young adulthood and is more common in developed countries. UC affects both genders equally and its presenting symptoms are usually rectal bleeding, urgency, and tenesmus, with diarrhea. UC prevalence is estimated at 5-500 people per 100000 worldwide[1]. In addition, the incidence of UC is increasing and its health-care burden is considerable[2]. The incidence and prevalence of IBD (including UC and Crohn’s disease (CD) in Canada are amongst the highest in the world[3]. The pathogenesis of IBD is largely unknown. Current evidence suggests that environmental factors and microbial dysbiosis may interact to trigger a dysregulated immune response which induces chronic intestinal inflammation in genetically susceptible hosts[4].
Patients with UC can experience multiple disease relapses in spite of receiving adequate standard treatment. It has also been shown that poor disease control and multiple relapses result in deteriorated quality of life[5] and an increased probability of colitis-associated colorectal cancer[6]. Although determinants of UC relapse have not been fully elucidated, a variety of demographic, clinical, endoscopic, psychosocial, serologic and fecal biomarkers have been investigated in several studies with inconsistent findings[7-13].
Metabolomics is the systemic identification and quantitation of all metabolites in a given organism or set of biological samples[14]. Similar to other “omic” approaches that are used to study the pathophysiology of different diseases, metabolomics has the potential to reveal the underlying multifactorial mechanisms of diseases, including IBD[15], especially if measured before disease relapse occurs. Other investigators have shown that urinary, serum, and fecal metabolomic profiles of IBD patients differ from healthy controls[15,16]. In addition, it has been suggested that metabolomics has the potential to identify novel biomarkers that could be useful for surveillance and early detection of IBD relapse[15].
Understanding predictors of UC relapse is of great importance for both patients and healthcare providers and only few prospective studies have been done in this regard. Therefore, the aim of this study was to examine the roles of multiple clinical, demographic, dietary and metabolomic factors that may predict UC relapse.
This pilot prospective cohort study was performed in Edmonton, Alberta, Canada. Using a convenience non-probability sampling method, adult UC patients who were able to read and write in English were recruited consecutively from the IBD clinic at the University of Alberta. The diagnosis of UC was confirmed using a combination of clinical, endoscopic and histological criteria. All patients were included if they were in clinical remission at the time of enrollment determined by a validated partial Mayo score of less than 3[17]. Subjects were excluded if they had used oral corticosteroids in the previous four weeks, used corticosteroids within the previous two weeks before enrollment, used any biological agents for UC management within 3 mo before the enrollment, or had a history of colectomy. Written informed consent was obtained from all participants and the study protocol was approved by the Health Research Ethics Board-Biomedical Panel, University of Alberta, Edmonton, Canada (Pro00032213).
Participants were asked to come to the research clinic for the first visit (Baseline). At baseline visit participants’ demographic and clinical information was obtained and participants completed a food frequency questionnaire (FFQ) that assessed their food intake in the past 12 mo. Anthropometric assessments (as described below), clinical information, and urine and blood samples were collected for metabolomics analyses, and stool was collected for fecal calprotectin (FCP). Twelve months after the baseline visit, patients were followed-up by a telephone interview and their clinical files were reviewed to determine if they had experienced a clinical UC relapse (partial Mayo score of 3 or more) during these 12 mo. Comparisons were made between patients who remained in clinical remission versus those who experienced a clinical relapse.
At baseline, demographic (age, gender), and clinical information was collected. Long-term dietary intake was assessed using the National Cancer Institute’s self-administered Diet History Questionnaire II (DHQ)[18,19]. This validated semi-quantitative FFQ included questions about 134 food items and accounts for seasonal intake of a variety of foods, portion size and frequency of intake for each food item. Body weight was measured to the nearest 0.01 kg (Health o Meter Professional 752KL medical scale) and height was measured (HM200P Portstad portable stadiometer, Charder Electronic Co, Ltd) without shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Waist circumference was measured at the narrowest part of abdomen over light clothing using a non-stretch measuring tape and recorded to the nearest 0.1 cm. Waist to height ratio was calculated as the ratio of waist circumference/height. Body composition (i.e., total fat mass and body fat percentage) was determined by air displacement plethysmography (BodPod) (COSMED Concord, CA, United States).
Subjects were provided appropriate materials and instructions to collect morning urine and stool samples. In addition, fasting blood samples were collected from each participant at baseline. Urine and serum samples were assayed using a combined direct infusion (DI-)/liquid chromatography (LC-) tandem mass spectrometry (MS/MS) (AbsolutIDQ p180 kit, Biocrates Life Sciences AG, Innsbruck, Austria) and nuclear magnetic resolution (NMR) spectroscopy, using the previously described protocol[20] in order to identify and quantify metabolites. All metabolomic assays were performed at the Metabolomics Innovation Centre (Edmonton, Canada). Fecal calprotectin (FCP) was measured in stool samples using an enzyme-linked immunosorbent assay with monoclonal antibodies specific to calprotectin (Bühlmann Laboratories AG, Basel, Switzerland). FCP levels above 150 μg/g stool were used to define “high FCP” due to its association with increased risk of UC relapse[21].
For the metabolomic analysis, concentrations of urinary metabolites (μmol/L) were normalized by creatinine (mmol/L) and reported as a ratio (μmol/mmol). Concentrations of identified metabolites were normalized using logarithmic transformation and pareto scaling. Metabolites with a P value less than 0.1 in the univariate analyses were selected for generating the logistic regression model. Multivariate statistical analysis was performed using partial least squares discriminant analysis (PLS-DA). A 10-fold cross-validation technique was used to ensure that the logistic regression models were robust. Permutation analysis using random resampling (n = 2000) of the two groups of patients (i.e., clinical relapse versus remission) was conducted to determine the probability that the observed separation was a result of chance or not, and a P value that represents the probability of a random finding was generated. To identify the major metabolites that were responsible for the discrimination between patients with clinical relapse and patients in clinical remission variable importance in projection (VIP) values were used. The VIP value indicates the contribution of each feature to the regression model. MetaboAnalyst 3.0[22] was used for the metabolomic statistical analysis.
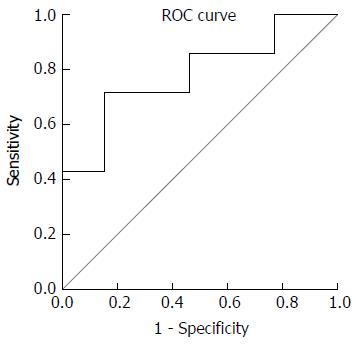
Categorical and numerical variables are presented as percentage and median (interquartile range (IQR), respectively. Fisher’s exact test and Mann-Whitney U test were used to compare categorical and numerical variables between two groups of UC patients (i.e., clinical relapse vs remission), respectively. To test the relationship between overweight/obesity and disease relapse, we used binary logistic regression analysis after adjusting for age and gender. A receiver operating characteristic (ROC) curve was constructed in order to calculate the accuracy of FCP in predicting UC patients who developed a clinical relapse (partial Mayo score > 2[17]) versus those who remained in clinical remission during the 12-mo follow-up. Statistical Package for the Social Sciences, version 16.0 (SPSS Inc, Chicago, IL, United States) was used for statistical analysis. A two-tailed P value of less than 0.05 was considered to be statistically significant.
Twenty UC patients in clinical remission were recruited with a mean age of 42.7 ± 14.8 years; 11 (55%) were females. Two (10%) patients were current smokers. Eleven patients (55%) were diagnosed to have pancolitis and 13 (65%) subjects were on either oral or rectal 5-aminosalicylic acid (5-ASA) medications. Twenty three percent of patients were on no UC-related medication (Table 1).
| Remission (n = 13) | Relapse (n = 7) | P value | |
| Age, yr | 46.0 (32.5-56.5) | 33.0 (28.0-52.0) | 0.18 |
| Females | 7 (53.8) | 4 (57.1) | 1.00 |
| Current smoker | 2 (15.4) | 0 (0.0) | 0.52 |
| Body mass index, kg/m2 | 28.1 (25.3-32.7) | 22.0 (20.3-22.8) | < 0.01 |
| Overweight/obese | 10 (76.9) | 1 (14.3) | 0.02 |
| Waist circumference, cm | 99.1 (84.4-105.3) | 82.8 (70.1-89.0) | 0.03 |
| Waist to height ratio | 0.6 (0.5-0.6) | 0.5 (0.4-0.5) | 0.02 |
| Body fat percentage | 35.8 (27.0-47.6) | 29 (20.4-34.1) | 0.16 |
| Fat mass, kg | 34.1 (20.4-45.5) | 20.1 (16.3-24.6) | 0.04 |
| Age at diagnosis, yr | 26.0 (22.5-44.5) | 25.0 (17-29) | 0.39 |
| Months since last relapse | 12.0 (4.5-33) | 11.0 (6.0-40) | 0.76 |
| UC subtype | |||
| Proctitis | 1 (7.7) | 1 (14.3) | |
| Left-sided | 4 (30.8) | 3 (42.9) | 0.71 |
| Pancolitis | 8 (61.5) | 3 (42.9) | |
| Medication | |||
| 5-ASA | 9 (69.2) | 4 (57.1) | 0.65 |
| Immunosuppressants | 4 (30.8) | 2 (28.6) | 1.00 |
| No medication | 3 (23.1) | 1 (14.3) | 1.00 |
Patients were followed for 12.1 ± 1.9 mo and during this time 7 (35%) patients experienced a clinical relapse. The comparison between different demographic, anthropometric, and clinical characteristics of patients (at the time of recruitment) with clinical relapse and those who were still in clinical remission at the time of follow-up is presented in Table 1. There was no significant difference between these two groups of patients in terms of age, gender, and UC-related factors (age at diagnosis, months since last relapse, UC subtype, and UC medication) at baseline. However, UC patients who developed a clinical relapse within 12 mo had significantly lower BMI, waist circumference, waist to height ratio, and fat mass compared to patients with no clinical relapse. Six out of 9(66.7%) patients with normal BMI (18.5-24.9 kg/m2) had a clinical relapse, whereas 1 out of 11(9.1%) patients with overweight/obesity (BMI > 25 kg/m2) at baseline relapsed during the follow-up (RR = 7.3, 95%CI: 1.1-50.3, P = 0.02) and this was still statistically significant (P = 0.03) after adjusting for age and gender.
There was no statistically significant difference between intake of different macro-, micronutrients as well as food groups at baseline in patients with clinical relapse versus remission within 12 mo of follow-up, except for poultry and maltose intake which were significantly higher in patients who remained in remission (Table 2). There was a positive correlation between maltose intake and total grain (r = 0.50, P = 0.03) and whole grain intake (r = 0.47, P = 0.04) suggesting that the main source of maltose in our patients was grain or grain products.
| Patient groups | P value3 | ||
| Remission (n = 13) | Relapse (n = 7) | ||
| Energy (Kcal/d) | 1530.8 (1258.3-2121.4) | 1820.2 (1430.8-2337.1) | 0.70 |
| Nutrients | |||
| Carbohydrate (g/d) | 229.6 (217.0-253.4) | 201.5 (156.4-237.1) | 0.21 |
| Protein (g/d) | 61.6 (55.0-74.9) | 62.0 (40.6-70.0) | 0.70 |
| Fat (g/d) | 59.0 (50.7-61.6) | 62.3 (46.2-76.0) | 0.21 |
| Cholesterol (g/d) | 198.0 (155.9-275.4) | 166.8 (117.6-207.4) | 0.18 |
| SFA (g/d) | 18.5 (16.4-20.3) | 20.4 (11.9-23.8) | 0.52 |
| MUFA (g/d) | 21.3 (17.3-23.1) | 21.7 (15.0-30.4) | 0.64 |
| PUFA (g/d) | 14.4 (12.9-14.8) | 12.9 (9.1-15.5) | 0.70 |
| TFA (g/d) | 3.3 (2.8-3.5) | 3.5 (1.7-4.5) | 0.97 |
| Vitamin C (mg/d) | 112.3 (101.2-166.7) | 100.1 (93.6-124.3) | |
| Vitamin B6 (mg/d) | 1.8 (1.5-2.5) | 1.7 (1.3-1.9) | 0.52 |
| Total folate (mcg/d) | 401.3 (289.5-540.9) | 402.3 (222.8-474.9) | 0.77 |
| Vitamin B12 (mcg/d) | 3.8 (3.3-5.7) | 4.6 (2.3-5.7) | 0.90 |
| Vitamin E (IU) | 14.8 (8.7-20.8) | 10.2 (4.4-14.4) | 0.13 |
| Calcium (mg/d) | 832.0 (730.8-1234.0) | 719.9 (551.7-924.0) | 0.21 |
| Iron (mg/d) | 13.4 (10.4-25.6) | 11.7 (7.4-15.0) | 0.42 |
| Zinc (mg/d) | 9.7 (9.4-16.2) | 9.8 (6.9-10.2) | 0.64 |
| Sodium (mg/d) | 2558.6 (2175.3-2900.6) | 2521.9 (1818.8-2810.8) | 0.77 |
| Potassium (mg/d) | 2948.8 (2216.0-3624.5) | 2951.9 (1890.8-3330.1) | 0.83 |
| Fibre (g/d) | 16.1 (13.5-26.0) | 14.1 (9.7-22.2) | 0.32 |
| Sucrose (g/d) | 40.2 (29.9-47.3) | 27.6 (20.6-40.4) | 0.11 |
| Fructose (g/d) | 31.1 (18.4-42.3) | 25.5 (11.7-38.5) | 0.64 |
| Lactose (g/d) | 13.6 (9.4-19.0) | 9.8 (1.8-17.5) | 0.32 |
| Maltose (g/d) | 3.9 (3.1-4.3) | 1.6 (1.1-3.0) | < 0.01 |
| Food groups | |||
| Fruit (cup equivalents/d) | 2.1 (0.8-2.6) | 1.4 (0.7-2.0) | 0.28 |
| Vegetable (cup equivalents/d) | 1.8 (1.5-2.4) | 1.8 (1.0-3.1) | 0.77 |
| Whole grain (oz equivalents/d) | 1.0 (0.5-1.3) | 0.7 (0.2-1.1) | 0.32 |
| Meat (oz/d) | 1.0 (0.6-1.3) | 1.0 (0.3-1.8) | 1.00 |
| Processed meat (oz/d) | 0.3 (0.1-0.5) | 0.2 (0.0-1.0) | 0.83 |
| Fish (oz/d) | 0.3 (0.2-0.8) | 0.5 (0.2-2.2) | 0.64 |
| Poultry (oz/d) | 0.9 (0.1-1.3) | 0.2 (0.1-0.3) | < 0.01 |
| Dairy (cup equivalents/d) | 1.4 (1.0-1.8) | 1.2 (0.3-2.0) | 0.52 |
| Eggs (oz equivalents/d) | 0.4 (0.2-0.8) | 0.2 (0.2-0.4) | 0.42 |
| Alcohol (drink/d) | 0.6 (0.0-0.8) | 0.6 (0.0-3.5) | 0.90 |
The median (IQR) level of fecal calprotectin (FCP)at baseline in UC patients with clinical relapse and remission at 12 mo of follow-up was 195.9 (41.2-347.9) and 23.3 (12.9-84.5) μg/g, respectively (P = 0.05). Five (71.4%) patients with high FCP versus 2 (15.4%) patients with normal FCP at baseline relapsed during the follow-up (RR = 4.6, 95%CI: 1.2-18.1, P = 0.02). ROC curves for FCP as a predictor of clinical relapse in UC is presented in Figure 1. An FCP concentration of 124 μg/g resulted in a sensitivity of 71.4%, a specificity of 84.6%, a positive predictive value (PPV) of 71.4, and a negative predictive value (NPV) of 84.6% in predicting UC clinical relapse.
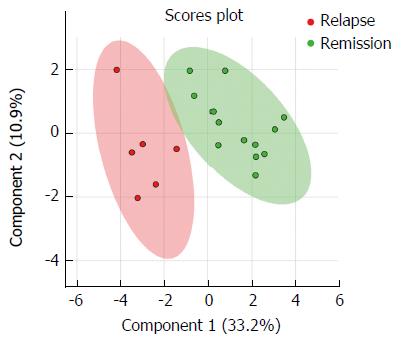
Using the described metabolomic assays, we identified and quantified 216 and 247 metabolites in serum and urine samples, respectively. After conducting univariate analysis, 16 candidate metabolites were candidate for further statistical analysis based on the P value of < 0.1. As presented in Figure 2, UC patients who experienced clinical relapse or stayed in clinical remission during follow-up could be discriminated in different clusters from each other by their metabolomic profile at baseline. Using the permutation testing, we showed that this separation was statistically significant (P = 0.04). The R2 and Q2 of the model was 0.84 and 0.59, respectively. VIP values of six metabolites were above 1.0, showing their important role in the discrimination between metabolomic profiles of the two UC groups. The median (IQR) levels and VIP scores of these metabolites are presented in Table 3. In comparison to UC patients who were still in remission during follow-up, those study patients with clinical relapse had significantly higher levels of trans-aconitate (urine), 3-hydroxybutyrate (serum), acetoacetate (serum), acetone (serum), and lower levels of acetamide (urine) and cystine (urine).
| UC groups | VIP score | Fold change (remission/relapse) | P value3 | ||
| Remission | Relapse | ||||
| Trans-aconitate (urine) | 0.5 (0.5-1.6) | 3.6 (1.9-5.5) | 1.9 | 0.2 | 0.02 |
| 3-hydroxybutyrate (serum) | 21.0 (15.0-33.9) | 127.8 (37.5-232.0) | 1.4 | 0.2 | < 0.01 |
| Cystine (urine) | 5.3 (4.0-8.6) | 3.3 (1.4-5.9) | 1.4 | 2.7 | 0.07 |
| Acetamide (urine) | 4.9 (2.7-7.1) | 2.6 (0.8-3.8) | 1.3 | 2.4 | 0.05 |
| Acetoacetate (serum) | 7.8 (5.8-13.0) | 25.6 (10.5-60.2) | 1.2 | 0.3 | 0.02 |
| Acetone (serum) | 5.3 (4.2-8.7) | 11.9 (6.0-24.7) | 1.1 | 0.4 | 0.06 |
In this small pilot study, we identified potential predictors of clinical relapse in UC patients. We found that a history of higher dietary poultry and maltose intake, and high BMI, body fat mass, and waist circumference at baseline were associated with UC clinical remission during a 12-mo follow-up. Of significant interest, we found that the baseline serum and urinary metabolomic profile of patients who relapsed during follow-up was significantly different from those patients who did not develop a relapse.
The clinical course of UC includes periods of remission and relapse. Although mucosal healing (macroscopic or microscopic) as determined by endoscopic and histologic evaluation is shown to be a strong predictor of long-term remission[11], due to the invasive nature of colonoscopy and its burden on healthcare system there has been considerable interest in identifying non-invasive predictors of disease relapse in UC. However, so far, only a limited number of clinical, lifestyle-related factors or biomarkers have been identified in relation to UC relapse[7-13].
Interestingly, we found that baseline serum and urine metabolomic profiles of UC patients who developed UC relapse versus those who did not, were significantly different from each other. Metabolomics which is the science of studying metabolites in the different biological samples, has recently been used in IBD-related research. However, the focus of most previous studies was to identify “biomarkers” in urine, serum, or stool[15,16] samples of IBD patients that could discriminate them from non-IBD cohorts. To date, only one study has used a metabolomic approach to find metabolites in relation to risk of clinical relapse in UC patients. In a prospective cohort study, Hisamatsu et al[23] measured plasma levels of nineteen amino acids and found that decreased histidine level in plasma free amino acids was associated with increased risk of relapse in UC patients during a one-year follow-up. To the best of our knowledge, our study is the first one that used NMR and DI- LC- MS/MS methods to identify metabolites in urine and serum samples that had the potential to predict UC relapse. Although we could identify and quantify specific serum and urine amino acids using NMR, we did not find any statistically significant difference in serum histidine levels between the two UC groups which might be due to sampling from a different population of UC patients or the result of small sample size.
In the present study, higher levels of trans-aconitate (urine), 3-hydroxybutyrate (serum), acetoacetate (serum), and acetone (serum) were found in the patients who relapsed within 12 mo. In a previous study by Stephens et al[24], it was reported that urinary trans-aconitate, which is a tricarboxylic acid, was decreased in IBD patients in comparison to non-IBD controls. Previously, 3-hydroxybutyrate (ketone body) was shown to be higher in serum samples of UC[25] or IBD[26] patients than in controls. Elevated serum levels of acetoacetate (ketone body) were also reported in IBD patients in comparison to controls[26]. The large increase in concentration of ketone bodies (acetoacetate, acetone and 3-hydroxybutyrate) was previously reported in DSS-induced colitis mice which may reflect the higher demand of the body for energy[27] and changes in cellular energy metabolism that occur in IBD patients[26], in our population even before disease relapse. In addition, we noticed a negative correlation between some of these ketone bodies with BMI which highlights the role of energy-related metabolic alterations before UC relapse (data not shown).
In our study, we also found that relatively lower levels of cystine and acetamide in urine were associated with increased risk of relapse. Cystine is an oxidized dimeric form of cysteine (a semi-essential proteinogenic amino acid). Cystine and cysteine are limiting substrates in the biosynthesis of tripeptide glutathione (GSH), which is known to be the most important intracellular antioxidant. It was shown that low plasma cysteine and cystine levels were associated with decreased mucosal synthesis of GSH, increased oxidative damage, and presence of inflammation in UC and CD patients[28]. In the present study, decreased cystine levels were associated with disease relapse possibly through reduction in GSH synthesis and increased oxidative damage. Acetamide is the amide of acetic acid. It has been shown that acetamide has antimicrobial, anti-inflammatory, and antibiotic functions[29,30]. Acetamide has dietary sources. A significant increase in urinary acetamide level was reported in rats that were fed a diet enriched with sweet potato residue as dietary fibre[31]. In another study, rats on a wheat bran fibre diet had significantly higher urinary acetamide than the control group[32]. Interestingly, in the present study a history of high dietary intake of non-whole grain products, and thus less fibre, was inversely correlated with urinary acetamide levels (data not shown). These findings suggest a role for specific dietary components in the pathophysiology of UC relapse and should be examined in future perspective larger cohort studies and clinical trials.
In the present study, we indicated that FCP at baseline could predict UC clinical relapse. We found that UC patients who had elevated FCP levels at baseline had 4.6 times higher risk of developing clinical relapse during follow-up than patients with low FC. This finding is in agreement with several previous studies[9,17,21,33].
Our small pilot study indicated that overweight/obesity at baseline was protective for development of clinical relapse. In addition, waist circumference, waist to height ratio, and fat mass was also higher in patients who stayed in remission 12 mo after the initiation of the study. The relationship between body weight and IBD is controversial. Although obesity is associated with a pro-inflammatory state[34] and increased intestinal permeability[35], increased BMI was not related to incidence of UC in EPIC study[36]. In contrast, in a large prospective cohort of US women higher indicators of adiposity were associated with an increased risk of CD, but not UC[37]. In a recent study by Flores et al[38] it was found that obese UC patients were significantly less likely to receive anti-TNF treatment or experience a hospitalization for their UC. The authors concluded that obesity is a marker of less aggressive or less severe UC[38].
Interestingly, a higher intake of poultry and maltose was found to be related to decreased risk of UC clinical relapse in the present study. However, we did not find any association between intake of other macro/micronutrients and development of relapse. So far, there have been only a few prospective cohort studies to investigate the dietary determinants of relapse in IBD patients. In a prospective cohort study by Jowett et al[13], 191 UC patients in remission where followed for one year. The authors reported that consumption of meat, red meat and processed meat, protein, alcohol, sulphur and sulphate were related to increased risk of relapse. However, similar to our pilot study, they did not find any association between consumption of dairy products, fibre, carbohydrate, and fat and increased risk of UC relapse. In another recent study of 489 UC patients, Brotherton et al[39] did not find any association between fibre intake and disease relapse. In another recent study, higher intake of lactose, alpha linolenic fatty acid, and myristic fatty acid were related to increased risk of relapse in UC patients[40]. In comparison to red meat, poultry has less saturated fat and heme iron, both being inducers of oxidative stress and DNA damage[41]. In addition, poultry consumption was shown to be inversely related to inflammation[42] and is suggested to be a healthier source of animal protein than red meat[43].
We also found maltose consumption decreases risk of UC relapse. Maltose is a disaccharide derived from two units of glucose and is found largely in vegetables, fruits and grains. There are scarce data on the beneficial effects of maltose intake for human health in comparison to other types of sugars. However, maltose is among the preferred carbohydrate energy sources for specific colonic bacteria that have beneficial properties[44,45]. It should also be mentioned that in the present study we also observed a positive correlation between maltose intake and total grain and whole grain intake (data not shown) which suggests that the main source of maltose in our patients could have been grain or grain products. Although fruit and whole grain intake in our study was numerically higher at baseline in patients with clinical remission than patients who developed a UC clinical relapse, this difference was not statistically significant.
Age, gender, UC subtype, UC medication, age at diagnosis, and months since last clinical relapse were not related to UC clinical relapse over a 12-mo period in our study. Similarly, Zenlea et al[46] did not find any relationship between age, gender, type of medication, and increased risk of UC relapse. Also, median duration of remission before study and disease extent was not related to increased risk of UC relapse in another prospective cohort study by Bessissow et al[47] which is in agreement with our findings. However, younger age, shorter duration of remission before study, and greater number of prior relapses were associated with earlier time to relapse in[7]. In addition, relapse was more frequent in females during a 5-year follow-up in IBSEN study[48]. Our finding of no relationship between smoking status and UC relapse was also shown in previous studies[7,46-48]. However, Höie et al[49] showed that UC patients who were current smokers had a lower relapse rate than nonsmokers during a 10-year follow-up.
Although in our pilot prospective cohort study we tried to investigate several potential contributors of relapse in UC patients which makes the findings valuable, our study has several limitations as well. The major limitation of the study is the small sample size. Thus, for several parameters between two groups of patients our study did not have the enough statistical power to detect true differences. Due to this limitation, we could not perform more complex statistical analysis (e.g., comparison of relapse between tertiles of dietary intake for each nutrient and adjusting for several confounding variables). In addition, since we did not correct our analyses for multiple testing, some of the findings in this study might have been “false positive” findings which needs to be considered before interpreting our results. However, despite this limitation, the separate clusters with significant difference between urinary and serum metabolomics as a predictor for clinical relapse versus remission remains striking. In addition, using a FFQ to assess dietary intake during the past 12 mo is subject to recall bias which is another limitation of this epidemiological study. However, we have tried to overcome this limitation by using a validated tool which has been shown to assess long-term dietary intake among adult population in several settings. Since this project is considered to be a pilot study, we believe that these findings should be regarded as hypothesis-generating findings, deserving further evaluation in future studies.
In conclusion, we identified several metabolites, as well as dietary parameters that are related to the development of clinical relapse in UC patients within 12 mo. Due to the importance of this topic in the management of UC patients, we suggest that further well-designed prospective cohort studies studying these parameters with larger sample size should be performed.
We wish to thank all participants of this study for their excellent cooperation.
In spite of adequate standard medical treatment, a large number of ulcerative colitis (UC) patients experience disease relapse. Currently, determinants of UC relapse including lifestyle-related factors are largely unknown and there few biomarkers to predict its occurrence.
A few studies with controversial results have suggested that long-term dietary intake may play a role in the development of UC relapse. So far there has been one prospective study that used a metabolomic approach to identify metabolites in plasma samples in relation to UC relapse.
The novel finding of this study was the identification of some metabolites in urine and serum samples of UC patients at the time of clinical remission that were related to development of disease clinical relapse within a 12-mo follow-up. Assessment of dietary intake and anthropometric measurements along with metabolomic evaluations, enabled us investigate how lifestyle factors are related to UC relapse.
The authors have identified and quantified a number of metabolites in serum and urine samples that can be used as novel biomarkers for prediction of relapse in adult UC patients after being validated in future studies. In addition, findings from this study confirms that patients’ dietary intake at the time of clinical remission is related to risk of experiencing UC relapse. Therefore, modifications of diet can be used as an adjunctive approach to prevent future disease relapse.
Scientific terms that have been used in this manuscript have are familiar with most readers and have been described comprehensively in different section of the manuscript.
This is a prospective pilot study which assessed a number of factors able to predict relapse in UC patients followed during a 12-mo period. The study is well conducted and the authors identified factors which have not been previously reported using urinary and serum metabolomics profiling. This pilot study deserves to be repeated in a larger cohort of patients.
| 1. | Ford AC, Moayyedi P, Hanauer SB. Ulcerative colitis. BMJ. 2013;346:f432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (2)] |
| 2. | Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1157] [Cited by in RCA: 2023] [Article Influence: 183.9] [Reference Citation Analysis (1)] |
| 3. | Rocchi A, Benchimol EI, Bernstein CN, Bitton A, Feagan B, Panaccione R, Glasgow KW, Fernandes A, Ghosh S. Inflammatory bowel disease: a Canadian burden of illness review. Can J Gastroenterol. 2012;26:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 4. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2256] [Article Influence: 132.7] [Reference Citation Analysis (10)] |
| 5. | Casellas F, Arenas JI, Baudet JS, Fábregas S, García N, Gelabert J, Medina C, Ochotorena I, Papo M, Rodrigo L. Impairment of health-related quality of life in patients with inflammatory bowel disease: a Spanish multicenter study. Inflamm Bowel Dis. 2005;11:488-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 201] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2128] [Article Influence: 85.1] [Reference Citation Analysis (2)] |
| 7. | Bitton A, Peppercorn MA, Antonioli DA, Niles JL, Shah S, Bousvaros A, Ransil B, Wild G, Cohen A, Edwardes MD. Clinical, biological, and histologic parameters as predictors of relapse in ulcerative colitis. Gastroenterology. 2001;120:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 355] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 8. | Bitton A, Sewitch MJ, Peppercorn MA, deB Edwardes MD, Shah S, Ransil B, Locke SE. Psychosocial determinants of relapse in ulcerative colitis: a longitudinal study. Am J Gastroenterol. 2003;98:2203-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Theede K, Holck S, Ibsen P, Kallemose T, Nordgaard-Lassen I, Nielsen AM. Fecal Calprotectin Predicts Relapse and Histological Mucosal Healing in Ulcerative Colitis. Inflamm Bowel Dis. 2016;22:1042-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 10. | Hosseini SV, Safarpour AR, Taghavi SA. Developing a novel risk-scoring system for predicting relapse in patients with ulcerative colitis: A prospective cohort study. Pak J Med Sci. 2015;31:1511-1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Liverani E, Scaioli E, Digby RJ, Bellanova M, Belluzzi A. How to predict clinical relapse in inflammatory bowel disease patients. World J Gastroenterol. 2016;22:1017-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 12. | Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 650] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 13. | Jowett SL, Seal CJ, Pearce MS, Phillips E, Gregory W, Barton JR, Welfare MR. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut. 2004;53:1479-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 343] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 14. | Idle JR, Gonzalez FJ. Metabolomics. Cell Metab. 2007;6:348-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 214] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | De Preter V, Verbeke K. Metabolomics as a diagnostic tool in gastroenterology. World J Gastrointest Pharmacol Ther. 2013;4:97-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 16. | Bjerrum JT, Wang Y, Hao F, Coskun M, Ludwig C, Günther U, Nielsen OH. Metabonomics of human fecal extracts characterize ulcerative colitis, Crohn’s disease and healthy individuals. Metabolomics. 2015;11:122-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 17. | De Vos M, Louis EJ, Jahnsen J, Vandervoort JG, Noman M, Dewit O, Dʼhaens GR, Franchimont D, Baert FJ, Torp RA. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Dis. 2013;19:2111-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (3)] |
| 18. | Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America’s Table Study. Am J Epidemiol. 2001;154:1089-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 983] [Cited by in RCA: 1166] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 19. | National Institutes of Health, Epidemiology and Genomics Research Program, National Cancer Institute. Diet History Questionnaire II, Version 2.0. 2010. Available from: http://www.epi.grants.cancer.gov/dhq2. |
| 20. | Bouatra S, Aziat F, Mandal R, Guo AC, Wilson MR, Knox C, Bjorndahl TC, Krishnamurthy R, Saleem F, Liu P. The human urine metabolome. PLoS One. 2013;8:e73076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1023] [Cited by in RCA: 1052] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 21. | Costa F, Mumolo MG, Ceccarelli L, Bellini M, Romano MR, Sterpi C, Ricchiuti A, Marchi S, Bottai M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 426] [Article Influence: 20.3] [Reference Citation Analysis (1)] |
| 22. | Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0--making metabolomics more meaningful. Nucleic Acids Res. 2015;43:W251-W257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2063] [Cited by in RCA: 2155] [Article Influence: 195.9] [Reference Citation Analysis (0)] |
| 23. | Hisamatsu T, Ono N, Imaizumi A, Mori M, Suzuki H, Uo M, Hashimoto M, Naganuma M, Matsuoka K, Mizuno S. Decreased Plasma Histidine Level Predicts Risk of Relapse in Patients with Ulcerative Colitis in Remission. PLoS One. 2015;10:e0140716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Stephens NS, Siffledeen J, Su X, Murdoch TB, Fedorak RN, Slupsky CM. Urinary NMR metabolomic profiles discriminate inflammatory bowel disease from healthy. J Crohns Colitis. 2013;7:e42-e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 25. | Zhang Y, Lin L, Xu Y, Lin Y, Jin Y, Zheng C. 1H NMR-based spectroscopy detects metabolic alterations in serum of patients with early-stage ulcerative colitis. Biochem Biophys Res Commun. 2013;433:547-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Dawiskiba T, Deja S, Mulak A, Ząbek A, Jawień E, Pawełka D, Banasik M, Mastalerz-Migas A, Balcerzak W, Kaliszewski K. Serum and urine metabolomic fingerprinting in diagnostics of inflammatory bowel diseases. World J Gastroenterol. 2014;20:163-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 129] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 27. | Schicho R, Nazyrova A, Shaykhutdinov R, Duggan G, Vogel HJ, Storr M. Quantitative metabolomic profiling of serum and urine in DSS-induced ulcerative colitis of mice by (1)H NMR spectroscopy. J Proteome Res. 2010;9:6265-6273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Sido B, Hack V, Hochlehnert A, Lipps H, Herfarth C, Dröge W. Impairment of intestinal glutathione synthesis in patients with inflammatory bowel disease. Gut. 1998;42:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 197] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Jawed H, Shah SU, Jamall S, Simjee SU. N-(2-hydroxy phenyl) acetamide inhibits inflammation-related cytokines and ROS in adjuvant-induced arthritic (AIA) rats. Int Immunopharmacol. 2010;10:900-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Muri EM, Williamson JS. Anti-Helicobacter pylori agents. An update. Mini Rev Med Chem. 2004;4:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Liu G, Yang G, Fang T, Cai Y, Wu C, Wang J, Huang Z, Chen X. NMR-based metabolomic studies reveal changes in biochemical profile of urine and plasma from rats fed with sweet potato fiber or sweet potato residue. RSC Advances. 2014;4:23749-23758. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Liu G, Xiao L, Fang T, Cai Y, Jia G, Zhao H, Wang J, Chen X, Wu C. Pea fiber and wheat bran fiber show distinct metabolic profiles in rats as investigated by a 1H NMR-based metabolomic approach. PLoS One. 2014;9:e115561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Sipponen T, Kolho KL. Fecal calprotectin in diagnosis and clinical assessment of inflammatory bowel disease. Scand J Gastroenterol. 2015;50:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 34. | Greenfield JR, Samaras K, Jenkins AB, Kelly PJ, Spector TD, Gallimore JR, Pepys MB, Campbell LV. Obesity is an important determinant of baseline serum C-reactive protein concentration in monozygotic twins, independent of genetic influences. Circulation. 2004;109:3022-3028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Gummesson A, Carlsson LM, Storlien LH, Bäckhed F, Lundin P, Löfgren L, Stenlöf K, Lam YY, Fagerberg B, Carlsson B. Intestinal permeability is associated with visceral adiposity in healthy women. Obesity (Silver Spring). 2011;19:2280-2282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 36. | Chan SS, Luben R, Olsen A, Tjonneland A, Kaaks R, Teucher B, Lindgren S, Grip O, Key T, Crowe FL. Body mass index and the risk for Crohn’s disease and ulcerative colitis: data from a European Prospective Cohort Study (The IBD in EPIC Study). Am J Gastroenterol. 2013;108:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 37. | Khalili H, Ananthakrishnan AN, Konijeti GG, Higuchi LM, Fuchs CS, Richter JM, Chan AT. Measures of obesity and risk of Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2015;21:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 38. | Flores A, Burstein E, Cipher DJ, Feagins LA. Obesity in Inflammatory Bowel Disease: A Marker of Less Severe Disease. Dig Dis Sci. 2015;60:2436-2445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (4)] |
| 39. | Brotherton CS, Martin CA, Long MD, Kappelman MD, Sandler RS. Avoidance of Fiber Is Associated With Greater Risk of Crohn’s Disease Flare in a 6-Month Period. Clin Gastroenterol Hepatol. 2016;14:1130-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 40. | Barnes EL, Nestor MA, Onyewadume L, De Silva PS, Korzenik JR. A prospective study: the role of diet in exacerbations of patients with ulcerative colitis in remission on monotherapy with mesalamine. Gastroenterology. 2016;150:S5-S6. [DOI] [Full Text] |
| 41. | Tappel A. Heme of consumed red meat can act as a catalyst of oxidative damage and could initiate colon, breast and prostate cancers, heart disease and other diseases. Med Hypotheses. 2007;68:562-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | van Bussel BC, Henry RM, Ferreira I, van Greevenbroek MM, van der Kallen CJ, Twisk JW, Feskens EJ, Schalkwijk CG, Stehouwer CD. A healthy diet is associated with less endothelial dysfunction and less low-grade inflammation over a 7-year period in adults at risk of cardiovascular disease. J Nutr. 2015;145:532-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Ley SH, Sun Q, Willett WC, Eliassen AH, Wu K, Pan A, Grodstein F, Hu FB. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am J Clin Nutr. 2014;99:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 44. | Scott KP, Martin JC, Duncan SH, Flint HJ. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol Ecol. 2014;87:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 336] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 45. | Charalampopoulos D, Pandiella SS, Webb C. Evaluation of the effect of malt, wheat and barley extracts on the viability of potentially probiotic lactic acid bacteria under acidic conditions. Int J Food Microbiol. 2003;82:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 138] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Zenlea T, Yee EU, Rosenberg L, Boyle M, Nanda KS, Wolf JL, Falchuk KR, Cheifetz AS, Goldsmith JD, Moss AC. Histology Grade Is Independently Associated With Relapse Risk in Patients With Ulcerative Colitis in Clinical Remission: A Prospective Study. Am J Gastroenterol. 2016;111:685-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 47. | Bessissow T, Lemmens B, Ferrante M, Bisschops R, Van Steen K, Geboes K, Van Assche G, Vermeire S, Rutgeerts P, De Hertogh G. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am J Gastroenterol. 2012;107:1684-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 48. | Henriksen M, Jahnsen J, Lygren I, Sauar J, Kjellevold Ø, Schulz T, Vatn MH, Moum B; IBSEN Study Group. Ulcerative colitis and clinical course: results of a 5-year population-based follow-up study (the IBSEN study). Inflamm Bowel Dis. 2006;12:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 49. | Höie O, Wolters F, Riis L, Aamodt G, Solberg C, Bernklev T, Odes S, Mouzas IA, Beltrami M, Langholz E. Ulcerative colitis: patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol. 2007;102:1692-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Daniel F, Henderson P, Lakatos PL, Lankarani KB S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF