Published online May 21, 2017. doi: 10.3748/wjg.v23.i19.3546
Peer-review started: November 4, 2016
First decision: January 10, 2017
Revised: January 21, 2017
Accepted: February 17, 2017
Article in press: February 17, 2017
Published online: May 21, 2017
Processing time: 197 Days and 15.6 Hours
To compare the outcomes between laparoscopic Nissen fundoplication (LNF) and proton pump inhibitors (PPIs) therapy in patients with laryngopharyngeal reflux (LPR) and type I hiatal hernia diagnosed by oropharyngeal pH-monitoring and symptom-scale assessment.
From February 2014 to January 2015, 70 patients who were diagnosed with LPR and type I hiatal hernia and referred for symptomatic assessment, oropharyngeal pH-monitoring, manometry, and gastrointestinal endoscopy were enrolled in this study. All of the patients met the inclusion criteria. All of the patients underwent LNF or PPIs administration, and completed a 2-year follow-up. Patients’ baseline characteristics and primary outcome measures, including comprehensive and single symptoms of LPR, PPIs independence, and satisfaction, and postoperative complications were assessed. The outcomes of LNF and PPIs therapy were analyzed and compared.
There were 31 patients in the LNF group and 39 patients in the PPI group. Fifty-three patients (25 in the LNF group and 28 in the PPI group) completed reviews and follow-up. Oropharyngeal pH-monitoring parameters were all abnormal with high acid exposure, a large amount of reflux, and a high Ryan score, associated reflux symptom index (RSI) score. There was a significant improvement in the RSI and LPR symptom scores after the 2-year follow-up in both groups (P < 0.05), as well as typical symptoms of gastroesophageal reflux disease. Improvement in the RSI (P < 0.005) and symptom scores of cough (P = 0.032), mucus (P = 0.011), and throat clearing (P = 0.022) was significantly superior in the LNF group to that in the PPI group. After LNF and PPIs therapy, 13 and 53 patients achieved independence from PPIs therapy (LNF: 44.0% vs PPI: 7.14%, P < 0.001) during follow-up, respectively. Patients in the LNF group were more satisfied with their quality of life than those in the PPI group (LNF: 62.49 ± 28.68 vs PPI: 44.36 ± 32.77, P = 0.004). Body mass index was significantly lower in the LNF group than in the PPI group (LNF: 22.2 ± 3.1 kg/m2vs PPI: 25.1 ± 2.9 kg/m2, P = 0.001).
Diagnosis of LPR should be assessed with oropharyngeal pH-monitoring, manometry, and the symptom-scale. LNF achieves better improvement than PPIs for LPR with type I hiatal hernia.
Core tip: Laryngopharyngeal reflux disease is often associated with hiatal hernia and gastroesophageal reflux disease. Although the role of oropharyngeal pH-monitoring in the diagnosis of laryngopharyngeal reflux is clear, little is known regarding the anti-acid and anti-reflux therapeutic outcome by pH-monitoring and symptom-scale diagnosis. Laparoscopic Nissen fundoplication and proton pump inhibitors (PPIs) are effective in patients with laryngopharyngeal reflux and type I hiatal hernia. Nissen fundoplication shows better symptom relief than PPIs administration, and it also controls body mass index of patients. Our findings shed new insight into diagnosis and management for patients with laryngopharyngeal reflux disease.
- Citation: Zhang C, Hu ZW, Yan C, Wu Q, Wu JM, Du X, Liu DG, Luo T, Li F, Wang ZG. Nissen fundoplication vs proton pump inhibitors for laryngopharyngeal reflux based on pH-monitoring and symptom-scale. World J Gastroenterol 2017; 23(19): 3546-3555
- URL: https://www.wjgnet.com/1007-9327/full/v23/i19/3546.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i19.3546
Laryngopharyngeal reflux (LPR) is a common condition in patients with gastroesophageal reflux disease (GERD). The effect of GERD on the upper aero-digestive tract seriously affects the quality of life of patients, with symptoms such as hoarseness, rhinitis, pharyngalgia, foreign body sensation, throat clearing, chronic cough, and laryngospasm[1,2]. All of these clinical presentations of LPR are considered as extraesophageal symptoms for distinguishing typical symptoms of GERD, such as heartburn and regurgitation. The incidence rate of reflux-induced laryngitis ranges from 18%-80%[3,4]. The association between GERD and hiatal hernia has been well confirmed, including sliding hernia (type I), paraesophageal hernia (type II), and mixed hernia (types III and IV)[5]. Recently, evidence has suggested that hiatal hernia is one of the major risk factors for the occurrence of LPR in patients with GERD[4]. The management strategies for LPR, GERD, and type I hiatal hernia are similar, which involve controlling the occurrence of reflux and reducing reflux-induced symptoms. Common management methods include lifestyle modification, anti-acid therapy, and anti-reflux surgery. To the best of our knowledge, few studies have focused on the treatment outcome of patients with LPR and type I hiatal hernia, especially regarding comparison of anti-acid therapy and anti-reflux surgery with lifestyle modification.
Currently, diagnosis of LPR mainly includes empirical therapeutic trials of proton pump inhibitors (PPIs), the reflux symptom index (RSI) score, pH-monitoring and laryngoscopy[6]. Among these diagnostic methods, PPIs do not have objective evidence for diagnosis of LPR. Additionally, the placebo effect of anxiety in patients without LPR cannot be excluded. Laryngoscopic findings, such as erythema and edema, are also nonspecific signs of LPR[7]. The reflux finding score, a clinical severity rating scale based on laryngoscopic findings, has poor reliability in detecting LPR[8,9]. Monitoring of pH can directly detect increased esophageal or laryngopharyngeal acid exposure by a pH probe. Therefore, it is regarded as the best evidence for diagnosis of LPR[10]. Some studies have documented LPR using a new pH sensor[9,11] and others have investigated the pH threshold for identifying patients with an abnormal pharyngeal pH environment[12,13]. However, few studies have shown the therapeutic outcome in patients with LPR who were diagnosed by oropharyngeal pH-monitoring. Moreover, abnormal laryngopharyngeal acid exposure can indicate the presence of pathological reflux, but it does not provide proof of causality for symptoms of LPR. Therefore, evidence of LPR should be combined with pH-monitoring and special symptom-scales, such as the RSI score[14] and single symptom score[15].
Therefore, in this study, we investigated two different therapeutic strategies for LPR with hiatal hernia: anti-reflux surgery and anti-acid therapy both with lifestyle modifications. We assessed the postoperative 6-mo and 2-year outcomes based on diagnosis by oropharyngeal pH-monitoring and the symptom-scales. In particular, we analyzed the integrated results of pH-monitoring, manometry, and endoscopy, which may demonstrate the characteristics of patients with LPR and hiatal hernia.
Medical records of 70 patients with LPR and type I hiatal hernia who manifested laryngopharyngeal symptoms, who underwent laparoscopic Nissen fundoplication (LNF), or were administered PPIs therapy alone between February 2014 and January 2015, were obtained. The following criteria were met before enrolment: Patients complaint with laryngopharyngeal symptoms (hoarseness, globus, throat clearing/pain, mucus, and chronic cough) were suspected by the otolaryngologist, the LPR symptom occurred at least once a week, and lasted at least 6 mo; RSI score ≥ 13; type I hiatal hernia (increase of the squamo-columnar junction by > 2 cm, and without paraesophageal hernia); absence of significant esophagitis (Los Angeles grades A and B esophagitis); abnormal Ryan score during 24-h oropharyngeal pH-monitoring; and abnormal lower esophageal sphincter (LES) pressure as detected by esophageal manometry. Patients were symptomatically stable and generally medically fit for anti-acid or surgical anti-reflux treatments. Patients with broncho-pulmonary disease, central nervous system diseases, connective tissue diseases, previous pharyngolaryngeal, esophageal or gastric surgery, esophageal stricture, a shortened esophagus, impaired distal esophageal peristalsis, Barrett’s esophagus, autoimmune diseases, collagen vascular disease, and/or coagulation disorders were excluded. This prospective, observational study was approved by the Institutional Review Board at the Second Artillery General Hospital of Chinese People’s Liberation Army and Xuanwu Hospital. Informed consent was obtained from each participant according to the Helsinki Declaration.
The diagnosis of LPR was confirmed using the 24-h oropharyngeal Restech pH recorder system (Respiratory Technology Corp., San Diego, CA, United States) and LPR symptom-scale (outcome assessment). Patients were instructed to stop taking any anti-acid medications at least 1 wk before insertion of the probe. The pH probe was inserted through the patient’s nose and advanced slowly to its destination in the back of the oropharynx just 5 mm below the tip of the uvula. Placement of the probe in the oropharynx was verified by observing the flashing light at the tip of the probe. Patients were asked to keep a diary indicating the time of meals and the time spent in the supine and upright positions. Meal periods were excluded in the final analyses. The data recorder was downloaded to a dedicated software program (DataView Lite V3; Respiratory Technology Corp.) and correlated with the patient’s diary. Tracings were all manually evaluated by a single operator. Thresholds for the detection of acidic reflux were 5.5 for the upright position and 5.0 for the supine position. The percentage of time spent below these thresholds was then calculated. The Ryan score was also calculated using the same pH thresholds for the upright and supine positions. This score was obtained by combining the following three different parameters: (1) the number of reflux episodes; (2) the duration of the longest reflux episode; and (3) the percentage of time spent below the defined threshold. A score greater than 9.41 in the upright position and/or 6.81 in the supine position was regarded as LPR[12,16].
A solid-state manometric catheter assembly with 36 circumferential sensors spaced in 1-cm intervals was used (Sierra Scientific Instruments, Los Angeles, CA, United States). Before the recording, the transducers were calibrated, and a thermal compensation program was applied using external pressure. The catheter was passed via the nose and positioned to provide simultaneous recordings from the hypopharynx and the esophagus to the stomach. Ten 5-mL water swallows were provided to evaluate peristalsis. Upper esophageal sphincter (UES) and LES pressure, the size of hiatal hernia, and esophageal body contractions were recorded for data analysis. Moreover, hiatal hernia, reflux esophagitis, and esophageal metaplasia were determined by gastrointestinal (GI) endoscopy, which was independent of pH-monitoring and manometry. If esophagitis was present, it was graded according to the Los Angeles classification[17].
Patients were allocated to the PPI or LNF group according to their own preference and physical conditions after the following instructions: PPIs medication focused on the anti-acid, which need life-long medication but could not cause other damage or complication on upper gastrointestinal, whereas, LNF was an invasive operation, aiming to make a one-way flap by fundus for anti-reflux, with more possibility of injury and complications, but a lower recurrence rate. Of the 70 patients, 39 were treated with esomeprazole 40 mg every day for 61-96 d (mean, 78 d). LNF was carried out in the remaining 31 patients. Briefly, LNF was performed with five ports under general anesthesia. After dissecting the gastrohepatic ligament with a harmonic scalpel, a widow was created behind the lower esophagus. The diaphragmatic crura were then carefully dissected and the distal esophagus was mobilized at approximately 5 cm. In all cases, the gastric fundus was dissected by dividing short gastric vessels. The diaphragmatic crura were sewn behind the esophagus with 1-2 non-absorbable sutures. A posterior 360° with a 2-cm-long fundoplication was constructed with 2-3 interrupted non-absorbable stitches. After operation, omeprazole 40mg i.v. was administered once for gastric mucosal protection.
We also suggested that lifestyle modifications (head elevation during bedtime, no fatty foods and eating close to bedtime, eating more frequently with smaller meals, and reduction of cigarettes, alcohol, or caffeine) should be adopted for all of the patients. Body mass index [BMI, body weight (kg) divided by the square of standing height (m)] was calculated before treatment and after treatment at a 2-year follow-up.
Comprehensive symptom LPR was evaluated on the basis of symptom scoring using the RSI. The RSI accurately documents symptoms with LPR with a nine-item self-administered outcome instrument. An RSI score greater than 13 is considered to indicate LPR[18,19]. The single symptom score was used to measure the frequency and severity of each symptom, including heartburn, regurgitation, cough, globus, mucus, hoarseness, throat pain and clearing. Data on these outcome measures were collected through a standardized questionnaire as previously described[20,21]. More specifically, the total of the frequency score (5 points) and the severity score (5 points) for each of these measures was designed as the symptom score out of 10 points. The questionnaires were prepared in simplified Chinese and administered to the patients before and aftertreatment. Other outcome measures included PPI independence (PPIs was prescribed and administered continually over 3 d for recurrent GERD and LPR symptoms that were excluded from PPI independence in LNF or PPI group), satisfaction, and complications.
Data are expressed as mean ± SD or number (%) unless specified otherwise. For statistical analyses, normality was assessed by the Kolmogorov-Smirnov test. Data were analyzed by the independent-/paired-sample Student’s t test (Table 1) or nonparametric tests (Tables 2-4, Figures 1 and 2) based on the normality of data distribution. Independent-sample t-test and Mann-Whitney U test were performed for independent samples in LNF and PPI groups (Figures 1 and 2, Tables 2-4), whereas paired-sample t test and the Wilcoxon test for within-group paired samples (Table 4). The statistical analysis software, SPSS-17.0 (SPSS Inc., Chicago, IL, United States), was used. Statistical review of the study was performed by a professional statistician. Differences were considered significant when P < 0.05.
Consecutive patients who were diagnosed with LPR with type I hiatal hernia and met our inclusion criteria were enrolled between February 2014 and January 2015 in this study. Patients were divided into two groups based on the patients’ choice to undergo an LNF surgery or PPIs administration. A total of 39 patients were included in the PPI group and 31 patients were included in the LNF group. A total of 61 patients were still in the study at the 6-mo follow-up, and 53 patients (25 patients in the LNF group and 28 patients in the PPI group) completed the 2-year follow-up (follow-up time ranged from 1.7 to 2.5 years; average of 2 years). The demographic data for each group are listed in Table 1. Baseline demographic data were similar between the LNF and PPI groups, including the mean age, sex distribution, and pre-treatment values for the RSI and BMI. And 66.7%-74.2% patients also suffered from typical GERD symptoms (regurgitation and/or heartburn) in the LNF and PPI groups. The number of presenting complaints and the RSI were not significantly different between the two groups, except for globus (LNF group: 27/31 vs PPI group: 20/39, P = 0.003).
| Characteristic/parameter | LNF | PPI | P value |
| No. | 31 | 39 | |
| Age (yr) | 47.2 ± 10.7 | 51.3 ± 12.5 | 0.218 |
| Sex | 0.670 | ||
| Male | 14 (45.2) | 19 (48.7) | |
| Female | 17 (54.8) | 20 (51.3) | |
| BMI (kg/m2) | 23.9 ± 3.8 | 25.0 ± 3.1 | 0.285 |
| RSI score | 15.3 ± 3.5 | 14.2 ± 4.0 | 0.759 |
| Presenting complaint | |||
| Regurgitation1 | 21 (67.7) | 26 (66.7) | 0.926 |
| Heartburn1 | 23 (74.2) | 28 (71.8) | 0.826 |
| Cough | 18 (58.1) | 21 (53.8) | 0.729 |
| Mucus | 14 (45.1) | 12 (30.7) | 0.222 |
| Globus | 27 (87.1) | 20 (51.3) | 0.0032 |
| Hoarseness | 12 (38.7) | 10 (25.6) | 0.248 |
| Throat pain | 12 (38.7) | 10 (25.6) | 0.248 |
| Throat clearing | 10 (32.2) | 9 (23.1) | 0.398 |
The results of diagnostic examinations are summarized in Table 2, including 24-h oropharyngeal pH-monitoring, high-resolution manometry, and GI endoscopy. Almost all of the patients demonstrated more reflux events and a longer duration of acid exposure in the upright position, with a much higher Ryan value than the standard upright-threshold (Ryan score = 9.41), regardless of the groups. The mean supine Ryan score still exceeded 6.81, which was the upper limit of the normal value. Although the presenting complaints varied, there was no significant difference in pH-monitoring between the two groups. For manometric investigation, 13 of 70 patients presented with ineffective or abnormal esophageal motility, with a significant difference between the two groups (LNF group: 3/31 vs PPI group: 10/39, P = 0.090). LES pressure values ranged from 11.78 to 14.84 mmHg, which were lower than the normal value[22,23]. UES pressure was lower in the LNF group than in the PPI group (P = 0.045). Hiatal hernia was assessed by high-resolution manometry and GI endoscopy. Manometry showed hiatal hernia in 57/70 patients (LNF group: 24/31, PPI group: 33/39). However, endoscopy showed endoscopic hiatal hernia in 46/70 patients (LNF group: 16/31, PPI group: 30/39). Additionally, 13 patients in the LNF group and 12 in the PPI group had esophagitis as shown by endoscopy (Table 2).
| Characteristic/examination parameter | LNF | PPI | P value |
| Oropharyngeal pH-monitoring | |||
| Acid exposure (upright, %) | 11.77 ± 18.95 | 8.49 ± 15.66 | 0.416 |
| Acid exposure (supine, %) | 4.25 ± 12.79 | 3.28 ± 7.92 | 0.182 |
| Number of reflux events (upright) | 53.84 ± 97.48 | 34.93 ± 65.35 | 0.195 |
| Number of reflux events (supine) | 7.44 ± 18.42 | 5.14 ± 9.22 | 0.232 |
| Ryan score (upright) | 335.13 ± 491.08 | 274.57 ± 459.10 | 0.617 |
| Ryan score (supine) | 8.98 ± 16.18 | 7.23 ± 7.81 | 0.217 |
| High-resolution Manometry | |||
| LES pressure (mmHg) | 11.78 ± 8.07 | 14.84 ± 9.73 | 0.236 |
| UES pressure (mmHg) | 43.8 ± 28.33 | 67.08 ± 42.51 | 0.0451 |
| Dysperistalsis | 3 (9.7) | 10 (25.6) | 0.0901 |
| Hiatal hernia | 24 (77.4) | 33 (84.6) | 0.449 |
| GI endoscopy | |||
| Esophagitis (grade A) | 10 (32.2) | 10 (25.6) | 0.549 |
| Esophagitis (grade B) | 3 (9.7) | 2 (5.1) | 0.470 |
| Hiatal hernia | 16 (51.6) | 30 (76.9) | 0.0271 |
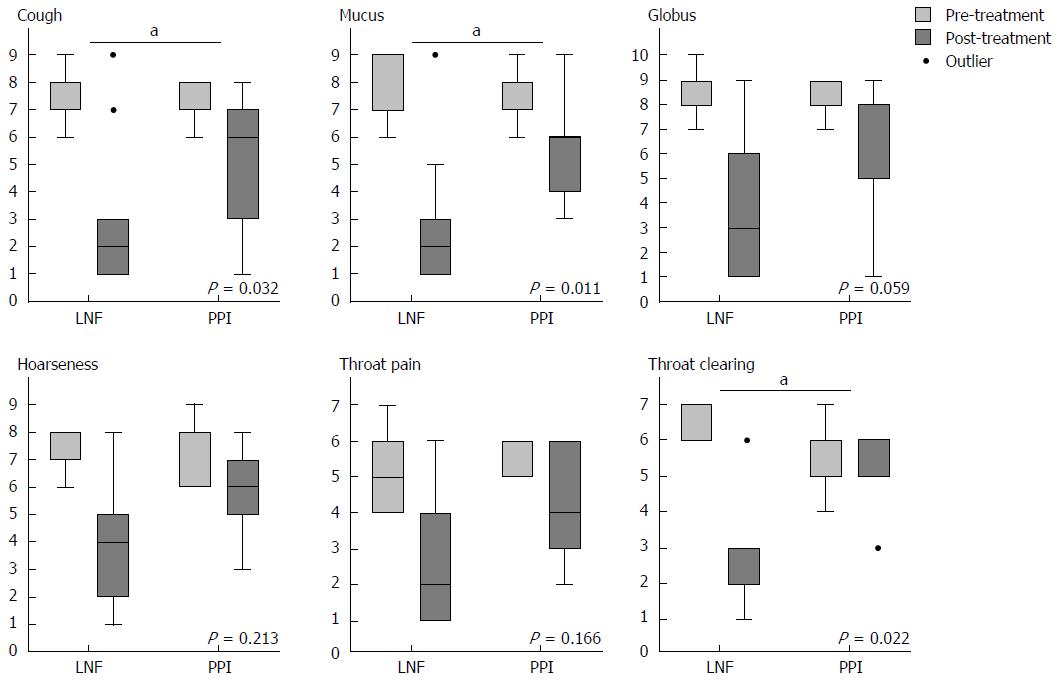
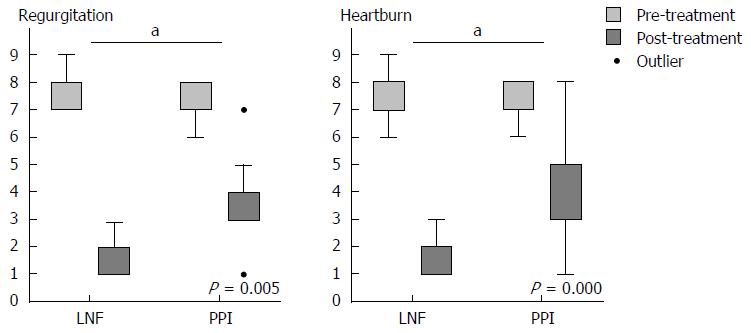
When we completed the 2-year follow-up, all of the data were collected to assess the efficiency of controlling symptoms of LPR. To assess relief from symptoms, we evaluated each symptom via a questionnaire that was scaled by frequency and severity. There were no significant differences in the pre-treatment symptom scores between patients in the PPI and LNF groups. The LPR and GERD typical symptom scores for cough, mucus, globus, hoarseness, and throat pain and clearing improved in both groups at the 6-mo and 2-year follow-up. The overall mean value of the symptom score decreased from 7.71 to 1.12 after both treatments (Table 3). Evaluation at the 2-year follow-up showed significantly better improvement in cough, mucus, and throat clearing of the LNF group than in the PPI group (Figure 1), as well as typical symptoms of GERD, including regurgitation and heartburn (Figure 2). The symptom scores for globus, hoarseness and throat pain were not significantly different between the two groups. However, the post-treatment symptom score for globus was lower in the LNF group than in the PPI group (LNF group: 2.95 ± 2.75 vs PPI group: 5.43 ± 2.50, P = 0.013 at 6 mo and LNF group: 2.77 ± 2.87 vs PPI group: 5.28 ± 2.86, P = 0.017 at 2 years, Table 3). We also observed no improvement in the LPR and GERD symptom scores in a few patients in both groups. This finding indicated no effect of LNF or PPI in three patients (Figures 1 and 2).
| Characteristic/symptom score (No. of LNF/PPI) | Baseline (n = 70) | 6-mo follow-up (n = 61) | 2-yr follow-up (n = 53) | ||||||
| LNF | PPI | P value | LNF | PPI | P value | LNF | PPI | P value | |
| Regurgitation2 (17/20) | 6.47 ± 0.62 | 6.24 ± 0.56 | 0.385 | 1.41 ± 1.69 | 2.53 ± 1.15 | 0.0221 | 1.12 ± 1.47 | 2.53 ± 1.26 | 0.0051 |
| Heartburn2 (19/20) | 6.33 ± 0.68 | 6.30 ± 0.59 | 0.100 | 1.33 ± 1.06 | 2.60 ± 1.69 | 0.0081 | 0.94 ± 1.10 | 3.05 ± 2.20 | 0.0011 |
| Cough (15/16) | 7.71 ± 0.82 | 7.67 ± 0.46 | 0.804 | 2.34 ± 2.37 | 4.40 ± 2.10 | 0.0221 | 2.28 ± 2.12 | 5.00 ± 2.28 | 0.0121 |
| Mucus (11/9) | 7.09 ± 0.83 | 7.22 ± 0.44 | 0.824 | 2.82 ± 2.24 | 4.77 ± 1.85 | 0.0551 | 3.27 ± 2.18 | 5.29 ± 1.78 | 0.0201 |
| Globus (23/14) | 6.10 ± 0.66 | 7.01 ± 0.88 | 0.268 | 2.95 ± 2.75 | 5.43 ± 2.50 | 0.0131 | 2.77 ± 2.87 | 5.28 ± 2.86 | 0.0171 |
| Hoarseness (10/7) | 7.30 ± 2.78 | 7.33 ± 2.55 | 0.954 | 3.80 ± 2.69 | 5.00 ± 2.65 | 0.409 | 3.50 ± 2.76 | 4.33 ± 2.53 | 0.546 |
| Throat pain (11/4) | 7.20 ± 1.03 | 7.50 ± 0.58 | 0.552 | 3.70 ± 2.98 | 5.50 ± 2.65 | 0.100 | 3.20 ± 3.46 | 4.25 ± 2.06 | 0.166 |
| Throat clearing (6/7) | 7.67 ± 0.52 | 7.14 ± 0.52 | 0.063 | 3.33 ± 2.06 | 6.00 ± 1.53 | 0.0341 | 2.83 ± 2.40 | 6.28 ± 1.51 | 0.0201 |
The comprehensive LPR diagnostic scale of the RSI score was assessed in this study. All 70 patients reported one or more symptoms that were included in the RSI scale. Forty-three (81.1%) patients had an RSI score ≥ 13 during the 2-year follow-up. The RSI score decreased after treatment in the LNF and PPI groups. Importantly, the mean RSI score in patients who had LNF surgery was significantly lower (9.7 ± 4.1) than that in patients who had PPIs administration (12.8 ± 3.1) at the 2-year follow-up (P = 0.004). Similar results were observed in the rate of a positive RSI score between the two groups (P = 0.003). Interestingly, 11 (44.0%) patients in the LNF group achieved independence of PPIs at the 2-year follow-up. However, only two (7.1%) patients were completely weaned off of PPIs in the PPI group (P < 0.001). Moreover, we found that the mean BMI was significantly decreased after LNF compared with before LNF (24.9 kg/m2vs 22.2 kg/m2, P < 0.001). However, the mean BMI of the PPI group did not significantly change before and after treatment (25.0-25.1 kg/m2, P = 0.991). There was a significant difference in BMI between the LNF and PPI groups at the 2-year follow-up (LNF group: 22.2 ± 3.1 vs PPI group: 25.1 ± 2.9, P = 0.001), but not at pre-treatment (Table 4).
| Characteristic/parameter | LNF (n = 25) | PPI (n = 28) | P value |
| BMI (kg/m2) | |||
| Pre-treatment | 24.9 ± 3.8 | 25.0 ± 3.1 | 0.285 |
| Post-treatment | 22.2 ± 3.12 | 25.1 ± 2.9 | 0.0011 |
| RSI score (value) | |||
| Pre-treatment | 15.3 ± 3.5 | 14.2 ± 4.0 | 0.759 |
| Post-treatment | 9.7 ± 4.12 | 12.8 ± 3.12 | 0.0041 |
| RSI score (≥ 13), n (%) | |||
| Pre-treatment | 19 (76.0) | 24 (85.7) | 0.284 |
| Post-treatment | 7 (28.0)2 | 19 (67.9)2 | 0.0031 |
| PPI independence, n (%) | 11(44.0) | 2 (7.14) | 0.0001 |
| Satisfaction, n (%) | 62.49 ± 28.68 | 44.36 ± 32.77 | 0.0041 |
From baseline to the 2-year follow-up, the mean satisfaction score of patients improved by 62.49 ± 28.68 in the LNF group and 44.36 ± 32.77 in the PPI group. Patients were more satisfied with their quality of life after undergoing LNF than with PPI therapy (P = 0.004, Table 4). However, three (12%) patients suffered from severe dysphagia after LNF surgery, and this was relieved after bougie dilation treatment. No patients experienced perforation, infection, or death.
LPR remains a controversial issue with inconsistent data on epidemiology, etiology, diagnosis, and management, even though LPR and GERD are both caused by reflux of stomach contents. The association between GERD and hiatal hernia is well known; hiatal hernia is present in 83% of patients with GERD. Additionally, the prevalence of GERD is 68% in patients with hiatal hernia[24,25]. Recent studies have shown that LPR is found in 70% of patients with GERD, 53% patients with GERD and LPR have hiatal hernia, and approximately 50% of patients with GERD and hernia have common symptoms of LPR[4,26]. In our study, we found that 67.7%-74.2% of patients with LPR and type I hiatal hernia had typical GERD symptoms, similar to a pervious study[26]. We also found that esophagitis was present in 35.7% of patients with LPR. Indeed, hiatal hernia appears to be an important risk factor for occurrence of LPR as GERD. A method of treating hernia or GERD might also improve symptoms of LPR. Therefore, this study was designed to focus on the comparison of diagnosis and treatment for LPR with hiatal hernia.
Currently, diagnosis of LPR mainly includes empirical therapeutic trials of PPIs, the RSI scale, laryngoscopy, and pH-monitoring[6]. Among these diagnostic methods, PPI trials and the RSI scale are subjective methods, which cannot provide direct pathophysiological evidence of LPR. The reflux finding score, which is based on laryngoscopic findings, also has poor reliability in detecting LPR[8,9]. Recent evidence has suggested no relationship between clinical findings of LPR, laryngoscopy, and the reflux finding score[26]. Monitoring of pH can directly detect increased esophageal or laryngopharyngeal acid exposure by a pH probe, and is thus regarded as the best evidence for diagnosis of LPR. Advances in oropharyngeal pH-monitoring have been made, such as the oropharyngeal pH-monitoring system. This system is a sensitive and minimally invasive device for determining acid reflux in oropharynx[10,13]. Our study was designed to diagnose LPR by combining a comprehensive RSI scale/single symptom score and pH-monitoring to assess the consistent reflux events and the occurrence of symptoms. Monitoring of pH showed that all patients had positive pH-monitoring (Ryan score) with an RSI score ≥ 13. This finding suggested that oropharyngeal pH-monitoring and the RSI have the same diagnostic value for LPR. Moreover, oropharyngeal pH-monitoring showed more reflux events and a longer duration of acid exposure in the upright position than in the supine position. The potential reasons for these findings could be due to the following: (1) increased abdominal pressure induces high-level reflux and acid has direct laryngeal contact in the upright position; (2) feeding and acid secretion stimulate the vagal afferents to promote irregular contraction of the distal/proximal esophagus in the daytime; and (3) activity of the laryngopharynx in the daytime affects the nozzle structure[27], as hypothesized by our group. A recent study proposed that, in the upright position, intragastric air rushes proximally with the assistance of increased intra-abdominal pressure, and the resultant gastric distension triggers relaxation of the intra-thoracic portion of the LES via stretch receptors in the stomach[28]. Our result of a higher Ryan score in the upright position than in the supine position is consistent with the LPR characteristics of aerosol of acidic contents.
The primary determinants of severity of GERD are a dysfunctional anti-reflux barrier and impaired esophageal clearance[29]. Disruption of the anti-reflux barrier can be related to a hypotensive LES (< 10 mmHg), dysperistalsis, and hiatal hernia[29-31]. All of these factors may contribute to the occurrence of symptoms of LPR. Our study showed that LES pressure was reduced to approximately 10-15 mmHg. Additionally, a high incidence of dysperistalsis and hiatal hernia was shown by esophageal high-resolution manometry and GI endoscopy. High-resolution manometry had a greater sensitivity than endoscopy for diagnosis of hiatal hernia in this study. Additionally, we analyzed the UES pressure of patients with LPR. Unfortunately, we obtained a different baseline of UES pressure before treatment, and no studies have focused on normal values of UES pressure or the relationship between UES pressure and LPR. However, our center has proposed a mechanism of LPR by using a special pharyngeal nozzle structure, and measured hypertensive UES pressure in a rat model[32,33].
Hiatal hernia impairs LES function by reducing its length and pressure, and appears to be an important risk factor for the occurrence of LPR. LPR symptoms are likely to be cured by surgery for hernia repair and fundoplication. However, the guideline for management of GERD[34] suggests that the strength of evidence is insufficient, with no consistent benefit attributed to surgery for LPR. PPIs therapy is the first choice for LPR in patients who also have typical symptoms of GERD or objective evidence of GERD by endoscopy or reflux monitoring.
The efficacy of empirical PPIs therapy for suspected LPR has been previously investigated. PPIs therapy reduced the incidence rate of LPR symptoms by 50.3% in one study (range: 38%-90%)[35]. Another study specified that abnormal pH testing was an inclusion criterion, and the rate for responding to PPIs was 59.1%[36]. In our study, comprehensive symptoms (RSI scale) markedly improved in 10 patients, and the mean RSI score significantly decreased after PPIs treatment. However, the rate of independence from PPIs (2 patients, 7.14%) was low. Additionally, some symptoms were not significantly relieved in some patients in the PPI group, such as globus, hoarseness, and throat pain and clearing. Some studies have suggested that symptoms persisted or recurred in the long-term follow-up, even though some patients used a double dose of PPI twice daily[37,38].
LNF has become the surgical gold standard for GERD treatment. LNF can repair diaphragmatic crura to correct the anatomical problem of hiatus, and establish a warp to provide an anti-reflux barrier. Nevertheless, the guideline[34] suggests that controlling symptoms of LPR is not satisfied by Nissen fundoplication. In our study, we focused on patients with LPR with a clear diagnosis of hiatal hernia, and compared LNF with PPIs administration, which is the first recommended choice for LPR. We found that LNF was effective in reducing the RSI score, and the frequency and severity of LPR and typical GERD symptoms. Additionally, LNF was superior to empirical PPIs therapy in all aspects, especially in independence from PPIs, and patients’ satisfaction with LNF tended to be better than that with PPIs therapy. These findings demonstrated that anti-reflux surgery could be as effective for LPR as for GERD. However, the accuracy of diagnosis of LPR could be a problem. With strict screening via multichannel intraluminal impedance-pH[39,40] or oropharyngeal pH-monitoring, symptoms of LPR are likely to demonstrate improvement following anti-reflux surgery.
Another important factor related to the occurrence of GERD is BMI, which affects the efficacy of anti-reflux surgery for LPR[41]. In our study, BMI ranged from 21.1 to 28.1 kg/m2, with no difference between the LNF and PPI groups. However, all of the patients were instructed to adopt lifestyle modifications. BMI was remarkably decreased only in the LNF group during the 2-year follow-up. Some studies have suggested that obesity is associated with GERD[42] and an increased BMI is associated with increased esophageal acid exposure[43]. Therefore, LNF could play a role in maintaining or reducing BMI, which affects the progress of GERD, as well as symptoms of LPR. However, this effect of LNF still needs be observed in a long-term investigation.
A limitation of this study is that it was an uncontrolled, nonrandomized study, which made it impossible to control for baseline demographics. Although all of the patients underwent either anti-reflux or anti-acid therapy, the methods of therapy were not randomly chosen. Oropharyngeal pH-monitoring is a costly and time-consuming technique, which is still not widely available for use in patients for follow-up. Only improvement of symptoms was used to evaluate the effect of treatment, such as the RSI and specific symptoms score. Another limitation of the study is its small sample size and the loss of follow-up. Only 75.7% of patients finally completed the 2-year follow-up. A multicenter, randomized, controlled trial with more samples is required to reach a conclusion regarding the superiority of anti-reflux surgery for controlling LPR.
In conclusion, current knowledge on diagnosis of LPR needs to be expanded with multiple diagnostic strategies, including oropharyngeal pH-monitoring and high-resolution manometry. These strategies should be combined with classical techniques, such as GI endoscopy and symptom-scale assessment. Anti-reflux surgery and anti-acid therapy are effective in patients with LPR and type I hiatal hernia. LNF shows better improvement than PPIs administration, and it also controls BMI of patients in short time. Our findings shed new insights into the diagnosis and management for patients with LPR.
Laryngopharyngeal reflux (LPR) is considered as extraesophageal symptoms for distinguishing typical symptoms of gastroesophageal reflux disease (GERD). Evidence has suggested hiatal hernia is a risk factor for the occurrence of LPR as well as GERD. Common diagnosis and management for LPR depend on empirical proton pump inhibitors (PPIs) therapy; however, it cannot solve the hiatal hernia. Few studies have focused on the outcome of LPR patients following anti-reflux surgery, like laparoscopic Nissen fundoplication. The current trial was designed to evaluate anti-acid and anti-reflux therapy for LPR with type I hiatal hernia.
LPR symptoms are much harder to be improved than GERD typical symptoms, such as heartburn and regurgitation. GERD could be cured by anti-reflux surgery, thus increasing studies focused on the outcome of laparoscopic fundoplication to find the best therapeutic strategy for LPR. Meanwhile, oropharyngeal pH-monitoring is still in debate for diagnosing LPR, the integrated results of symptom-scale and oropharyngeal pH-monitoring could demonstrate the characteristics of LPR.
The literature suggests that the LPR patient should be assessed with oropharyngeal pH-monitoring and symptom-scale, combination of manometry and endoscopy, which could avoid the misdiagnosis of hiatal hernia. LNF achieves better improvement than PPIs for LPR with type I hiatal hernia, and could play a role in controlling BMI in short-term.
This study provides clinical evidence to support the effect of laparoscopic Nissen fundoplication on LPR patients diagnosed by oropharyngeal pH-monitoring and symptom-scale.
Oropharyngeal pH-monitoring: A pH probe is inserted in laryngopharyngeal to detect acid exposure for continual 24 h. The pH-monitoring system includes a pH sensor with a teardrop shape to avoid becoming covered with food or mucus, a recorder to store the message and a new parameter calculation system (Ryan score) with different pH thresholds for upright and supine positions. Reflux symptom index (RSI): the scale accurately documents laryngopharyngeal symptoms with 9 item self administered outcome instrument, ranges from 0 to 45 (worst possible score). RSI more than 13 is considered as LPR diagnosis.
The authors have performed an interesting single-centre, comparative study. The outcomes of interest are well described and defined in the manuscript.
| 1. | Koufman JA, Aviv JE, Casiano RR, Shaw GY. Laryngopharyngeal reflux: position statement of the committee on speech, voice, and swallowing disorders of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg. 2002;127:32-35. [PubMed] |
| 2. | Vaezi MF, Hicks DM, Abelson TI, Richter JE. Laryngeal signs and symptoms and gastroesophageal reflux disease (GERD): a critical assessment of cause and effect association. Clin Gastroenterol Hepatol. 2003;1:333-344. [PubMed] |
| 3. | Gatta L, Vaira D, Sorrenti G, Zucchini S, Sama C, Vakil N. Meta-analysis: the efficacy of proton pump inhibitors for laryngeal symptoms attributed to gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2007;25:385-392. [PubMed] |
| 4. | Saruç M, Aksoy EA, Vardereli E, Karaaslan M, Ciçek B, Ince U, Oz F, Tözün N. Risk factors for laryngopharyngeal reflux. Eur Arch Otorhinolaryngol. 2012;269:1189-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Kahrilas PJ, Pandolfino JE. Hiatus hernia. 4th ed. Philadelphia: Lippincott Williams & Wilkins, 2004: 389-407. . |
| 6. | Friedman M, Hamilton C, Samuelson CG, Kelley K, Taylor R, Darling R, Taylor D, Fisher M, Maley A. The value of routine pH monitoring in the diagnosis and treatment of laryngopharyngeal reflux. Otolaryngol Head Neck Surg. 2012;146:952-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Qadeer MA, Swoger J, Milstein C, Hicks DM, Ponsky J, Richter JE, Abelson TI, Vaezi MF. Correlation between symptoms and laryngeal signs in laryngopharyngeal reflux. Laryngoscope. 2005;115:1947-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Musser J, Kelchner L, Neils-Strunjas J, Montrose M. A comparison of rating scales used in the diagnosis of extraesophageal reflux. J Voice. 2011;25:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Mesallam TA, Malki KH, Farahat M, Bukhari M, Alharethy S. Voice problems among laryngopharyngeal reflux patients diagnosed with oropharyngeal pH monitoring. Folia Phoniatr Logop. 2013;65:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Merati AL, Lim HJ, Ulualp SO, Toohill RJ. Meta-analysis of upper probe measurements in normal subjects and patients with laryngopharyngeal reflux. Ann Otol Rhinol Laryngol. 2005;114:177-182. [PubMed] |
| 11. | Andrews TM, Orobello N. Histologic versus pH probe results in pediatric laryngopharyngeal reflux. Int J Pediatr Otorhinolaryngol. 2013;77:813-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Ayazi S, Lipham JC, Hagen JA, Tang AL, Zehetner J, Leers JM, Oezcelik A, Abate E, Banki F, DeMeester SR. A new technique for measurement of pharyngeal pH: normal values and discriminating pH threshold. J Gastrointest Surg. 2009;13:1422-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Sun G, Muddana S, Slaughter JC, Casey S, Hill E, Farrokhi F, Garrett CG, Vaezi MF. A new pH catheter for laryngopharyngeal reflux: Normal values. Laryngoscope. 2009;119:1639-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice. 2002;16:274-277. [PubMed] |
| 15. | Yan C, Liang WT, Wang ZG, Hu ZW, Wu JM, Zhang C, Chen MP. Comparison of Stretta procedure and toupet fundoplication for gastroesophageal reflux disease-related extra-esophageal symptoms. World J Gastroenterol. 2015;21:12882-12887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Chheda NN, Seybt MW, Schade RR, Postma GN. Normal values for pharyngeal pH monitoring. Ann Otol Rhinol Laryngol. 2009;118:166-171. [PubMed] |
| 17. | Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172-180. [PubMed] |
| 18. | Patigaroo SA, Hashmi SF, Hasan SA, Ajmal MR, Mehfooz N. Clinical manifestations and role of proton pump inhibitors in the management of laryngopharyngeal reflux. Indian J Otolaryngol Head Neck Surg. 2011;63:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Dymek A, Dymek L, Starczewska-Dymek L, Bożek A, Dymek T, Nowak K. Laryngopharyngeal Reflux (LPR) in patients with persistent hoarseness. Otolaryngol Pol. 2012;66:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Liang WT, Wang ZG, Wang F, Yang Y, Hu ZW, Liu JJ, Zhu GC, Zhang C, Wu JM. Long-term outcomes of patients with refractory gastroesophageal reflux disease following a minimally invasive endoscopic procedure: a prospective observational study. BMC Gastroenterol. 2014;14:178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Zhang C, Wu J, Hu Z, Yan C, Gao X, Liang W, Liu D, Li F, Wang Z. Diagnosis and Anti-Reflux Therapy for GERD with Respiratory Symptoms: A Study Using Multichannel Intraluminal Impedance-pH Monitoring. PLoS One. 2016;11:e0160139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Richter J, Wu W, Johns D, Blackwell J, Nelson J, III , Castell J, Castell D. Esophageal manometry in 95 healthy adult volunteers. Digest Dis Sci. 1987;32:583-592. [RCA] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 340] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Swoger J, Ponsky J, Hicks DM, Richter JE, Abelson TI, Milstein C, Qadeer MA, Vaezi MF. Surgical fundoplication in laryngopharyngeal reflux unresponsive to aggressive acid suppression: a controlled study. Clin Gastroenterol Hepatol. 2006;4:433-441. [PubMed] |
| 24. | Savas N, Dagli U, Sahin B. The effect of hiatal hernia on gastroesophageal reflux disease and influence on proximal and distal esophageal reflux. Dig Dis Sci. 2008;53:2380-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | de Vries DR, van Herwaarden MA, Smout AJ, Samsom M. Gastroesophageal pressure gradients in gastroesophageal reflux disease: relations with hiatal hernia, body mass index, and esophageal acid exposure. Am J Gastroenterol. 2008;103:1349-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Vardar R, Varis A, Bayrakci B, Akyildiz S, Kirazli T, Bor S. Relationship between history, laryngoscopy and esophagogastroduodenoscopy for diagnosis of laryngopharyngeal reflux in patients with typical GERD. Eur Arch Otorhinolaryngol. 2012;269:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Wang Z, Hu Z, Wu J, Ji F, Wang H, Lai Y, Gao X, Ning Y, Zhang C, Li Z. Insult of gastroesophageal reflux on airway: clinical significance of pharyngeal nozzle. Front Med. 2015;9:117-122. [PubMed] |
| 28. | Hoppo T, Komatsu Y, Nieponice A, Schrenker J, Jobe B. Toward an Improved Understanding of Isolated Upright Reflux: Positional Effects on the Lower Esophageal Sphincter in Patients with Symptoms of Gastroesophageal Reflux. World J Surg. 2012;36:1623-1631. |
| 29. | Pandolfino JE, Roman S. High-resolution manometry: an atlas of esophageal motility disorders and findings of GERD using esophageal pressure topography. Thorac Surg Clin. 2011;21:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Sloan S, Rademaker AW, Kahrilas PJ. Determinants of gastroesophageal junction incompetence: hiatal hernia, lower esophageal sphincter, or both? Ann Intern Med. 1992;117:977-982. [PubMed] |
| 31. | Pandolfino JE, Kim H, Ghosh SK, Clarke JO, Zhang Q, Kahrilas PJ. High-resolution manometry of the EGJ: an analysis of crural diaphragm function in GERD. Am J Gastroenterol. 2007;102:1056-1063. [PubMed] |
| 32. | Zhu G, Wang Z, Hu Z, Gao X, Ji F, Liang W. Pharyngeal nozzle and its spray: gastroesophageal reflux indult on airway. Zhonghua Shiyan Waike Zazhi. 2014;2:3330-3332. |
| 33. | Wang Z, Hu Z, Wu J, Ji F, Wang H, Lai Y, Gao X, Ning Y, Zhang C, Li Z. Insult of gastroesophageal reflux on airway: clinical significance of pharyngeal nozzle. Front Med. 2015;9:117-122. |
| 34. | Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328; quiz 329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1136] [Cited by in RCA: 1148] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 35. | Qadeer MA, Phillips CO, Lopez AR, Steward DL, Noordzij JP, Wo JM, Suurna M, Havas T, Howden CW, Vaezi MF. Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2006;101:2646-2654. [PubMed] |
| 36. | Vailati C, Mazzoleni G, Bondi S, Bussi M, Testoni PA, Passaretti S. Oropharyngeal pH monitoring for laryngopharyngeal reflux: is it a reliable test before therapy? J Voice. 2013;27:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Mehta S, Bennett J, Mahon D, Rhodes M. Prospective trial of laparoscopic nissen fundoplication versus proton pump inhibitor therapy for gastroesophageal reflux disease: Seven-year follow-up. J Gastrointest Surg. 2006;10:1312-1316; discussion 1316-1317. [PubMed] |
| 38. | Bruley des Varannes S, Coron E, Galmiche JP. Short and long-term PPI treatment for GERD. Do we need more-potent anti-secretory drugs? Best Pract Res Clin Gastroenterol. 2010;24:905-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 39. | Carroll TL, Nahikian K, Asban A, Wiener D. Nissen Fundoplication for Laryngopharyngeal Reflux After Patient Selection Using Dual pH, Full Column Impedance Testing: A Pilot Study. Ann Otol Rhinol Laryngol. 2016;125:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Weber B, Portnoy JE, Castellanos A, Hawkshaw MJ, Lurie D, Katz PO, Sataloff RT. Efficacy of anti-reflux surgery on refractory laryngopharyngeal reflux disease in professional voice users: a pilot study. J Voice. 2014;28:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Mazzini Gda S, Gurski RR. Impact of laparoscopic fundoplication for the treatment of laryngopharyngeal reflux: review of the literature. Int J Otolaryngol. 2012;2012:291472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Choi CW, Kim GH, Song CS, Wang SG, Lee BJ, I H, Kang DH, Song GA. Is obesity associated with gastropharyngeal reflux disease? World J Gastroenterol. 2008;14:265-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Feretis M S- Editor: Yu J L- Editor: Ma JY E- Editor: Wang CH