Published online May 7, 2017. doi: 10.3748/wjg.v23.i17.3150
Peer-review started: December 22, 2016
First decision: January 10, 2017
Revised: February 13, 2017
Accepted: April 13, 2017
Article in press: April 13, 2017
Published online: May 7, 2017
Processing time: 137 Days and 14.5 Hours
To determine the clinical effectiveness of nutritional counseling on reduction of non-alcoholic fatty liver disease (NAFLD) severity, weight loss, metabolic and anthropometric indexes and liver enzymes.
Forty-six adults with NAFLD received a 6-mo clinical and a dietary intervention (based on Mediterranean diet) carried out respectively by a gastroenterologist and a nutritionist with counseling license. The counseling process consisted of monthly meeting (about 45 min each). The effect of the treatment was evaluated monitoring liver enzymes, metabolic parameters, cardiovascular risk indexes, NAFLD severity [assessed by ultrasound (US)] and related indexes. All parameters were assessed at baseline. Biochemistry was also assessed at mid- and end-interventions and US was repeated at end-intervention.
The percentage of patients with steatosis grade equal or higher than 2 was reduced from 93% to 48% and steatosis regressed in 9 patients (20%). At the end of the treatment the end-point concerning the weight (i.e., a 7% weight reduction or achievement/maintenance of normal weight) was accomplished by 25 out of 46 patients (i.e., 54.3%). As far as the liver enzymes is concerned, all three liver enzymes significantly decrease during the treatment the normalization was particularly evident for the ALT enzyme (altered values reduced from 67% down to 11%). Several parameters, i.e., BMI, waist circumference, waist-to-hip ratio, AST, ALT, GGT, HDL, serum glucose, Tot-Chol/HDL, LDL/HDL, TG/HDL, AIP, HOMA, FLI, Kotronen index, VAI, NAFLD liver fat score and LAP, showed a significant improvement (P < 0.01) between baseline and end-treatment.
Outcomes of this study further strengthen the hypothesis that MedDiet and more active lifestyle can be considered a safe therapeutic approach for reducing risk and severity of NAFLD and related disease states. The proposed approach may be proposed as a valid and recommended approach for improving the clinical profile of NAFLD patients.
Core tip: Most recent advances in the management and treatment of Non-alcoholic fatty liver disease (NAFLD) show that a multifaceted approach is likely to achieve the best outcomes. Our study evaluates the effectiveness of 6-mo multidisciplinary approach jointly carried out by a gastroenterologist and a nutritionist with counseling license. The approach was effective on the reduction of NAFLD severity, weight, anthropometric indexes, cardiovascular disease risk factors and the normalization of metabolic index, as well as liver enzymes. This study strengthens the hypothesis that MedDiet and more active lifestyle can be considered a safe therapeutic approach for reducing severity of NAFLD.
- Citation: Gelli C, Tarocchi M, Abenavoli L, Di Renzo L, Galli A, De Lorenzo A. Effect of a counseling-supported treatment with the Mediterranean diet and physical activity on the severity of the non-alcoholic fatty liver disease. World J Gastroenterol 2017; 23(17): 3150-3162
- URL: https://www.wjgnet.com/1007-9327/full/v23/i17/3150.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i17.3150
Non-alcoholic fatty liver disease (NAFLD) is a relevant issue in public health because represents a major cause of chronic liver diseases worldwide. Approximately 20% to 30% of adult population has NAFLD, making it the most common liver disease in developed countries[1,2]. NAFLD is the result of fat accumulation in the liver (with fat representing more than 5% of whole liver weight) in absence of excessive alcohol consumption (usually evaluated using thresholds of 20 and 30 g/d for women and men respectively[3].
NAFLD includes two pathologically distinct conditions with different prognoses: non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH); the latter covers a wide spectrum of disease severity, including fibrosis and cirrhosis and promoting hepatocellular carcinoma development[4].
NAFLD is a multifactorial disease and the exact mechanism is unknown. It is usually associated to one or more conditions that contribute to the metabolic syndrome (MS) (diabetes mellitus, obesity, hypertension, and hyperlipidemia) and therefore is considered as its hepatic manifestation[5-9].
Several therapeutic interventions for NAFLD have been proposed over the last decades but any of these proved to be safe and effective[10,11]. Among therapeutic interventions there are weight reduction (e.g., low-calorie diet, exercise and bariatric surgery), insulin sensitizer agents and incretins, lipid lowering drugs (e.g., statins), antioxidants (e.g., vitamin E) and treatment of vitamin D3 deficiency[12]. Pharmacological therapies have not been always successful in reducing liver steatosis or inflammation[12,13]. On the other hand, other drugs, such as thiazolidinediones, have some success in improving liver histology but have undesirable side effects such as weight gain[14].
Epidemiological evidence suggests a tight relationship between unhealthy lifestyle (mainly related to diet and physical inactivity) and NAFLD (Zelber-Sagi et al[15], 2011) and therefore there is a general consensus on considering healthy diet and regular physical activity as the cornerstones in the treatment of NAFLD[4,12,16]. In addition, these interventions have been demonstrated also effective for improving conditions of MS and the related risk of cardiovascular disease (CVD)[17].
So far several dietary models have been proposed for reducing severity and preventing NAFLD. Among these, the Mediterranean diet (MedDiet) can be considered one of the healthiest as its benefits are attributed to a lower incidence of chronic disease including CVD, obesity, cancers, and overall mortality demonstrating its safety, palatability and sustainability[18]. According to several studies carried out during the last few years[19,20], the MedDiet resulted as the most effective for inducing weight loss together with beneficial effects on all the risk factors associated with MS and NAFLD as demonstrated in several[21] state that higher adherence to MedDiet seems to have a beneficial effect on the severity of disease in patients with NAFLD.
Increased routinary physical activity demonstrated to be beneficial in reducing the severity of NAFLD both associated to weight loss[22] or per se[23] and is well known for its beneficial effects on reducing risks of developing components of MS[24]. Some studies also showed that increased routinary physical activity[25] and cardiorespiratory fitness[26] are inversely associated with NAFLD.
For all these reasons, a structured programme aimed at lifestyle changes towards healthy diet (with a 7%-10% weight loss in overweight/obese patients) and routinary physical activity is the most advisable for the treatment of NAFLD[4].
Nonetheless, lifestyle modification is usually difficult to achieve and maintain, above all in the long term, for a variety of reasons[27,28]. In particular, many patients have difficulty to find motivation to change their unhealthy lifestyle although most of them are well aware of the importance of healthy diet and habitual physical activity. Centis et al[29] report NAFLD cases have scarce readiness to physical activity change, and 50% of cases were classified in either the precontemplation or contemplation stage of change, i.e., refractory to increase exercise.
For these reasons there, in order to increase the success of the approach laying on lifestyle change towards a healthy diet and habitual physical activity, there is the need to move from the traditional prescriptive approach to an individualized, multidisciplinary, empowerment-based intervention, tailored on patients’ preferences.
In this study we tested the effectiveness of nutritional counseling on reduction of NAFLD severity, weight loss, metabolic and anthropometric indexes and liver enzymes. The approach was jointly carried out by a gastroenterologist and a nutritionist with counseling patent and was aimed at supporting patients to move toward a lifestyle including MedDiet-based dietary habits and regular physical activity.
This observational study proposes a 6-mo interventions for treating patients with NAFLD. The approach is based on a clinical and a MedDiet-based dietary intervention carried out respectively by a gastroenterologist and a nutritionist with counseling license. The innovation of this approach is the use of the nutritional counseling to strengthen participants’ motivation and increase adherence to healthy lifestyle recommendations and, at the same time, educating and promoting lifelong healthy eating habits.
Prospective participants underwent a thorough screening visit and a detailed analysis to asses clinical and health-related conditions, biochemistry, dietary habits, physical activity level, lifestyle and anthropometry. The nutritionist also provided patients with indications for drafting the food and physical activity diaries (including 3 weekdays and 1 weekend).
Eligible participants underwent a second meeting (after 1 wk) with the nutritionist who gather drafted food and physical activity diaries (then used to check and further refine the outcomes of the first interview) and deliver to the participants written resources containing the food pyramid, healthy eating guidelines and tips, basic information on portion size, examples to reduce food intake (maximum calorie reduction, 500 kcal/d) and dietary recommendations. These latter were specifically designed and tailored taking into account nutritional needs, diseases (if any), and other information and/or needs communicated during the first meeting and consisted of daily and weekly meal plans and suggestion for meals composition stimulating patients to self-arrange their diet according to the MedDiet. Within this meeting also starts the counseling process (see below).
During monthly meetings, carried out for the counseling process, the nutritionist also evaluates the adherence to the dietary recommendations, makes eventual adjustments, assesses anthropometry and assists participants in maintaining motivations.
Biochemistry was assessed at mid- and end-interventions (3 and 6 mo) and ultrasonography was repeated at end-intervention (6 mo).
The following end-points were set to assess the efficacy of the proposed approach: (1) Reduction of at least 1 unit of steatosis grade; (2) A 7% weight reduction (in accordance to EASL-EASD-EASO, 2016[4]) or achievement/maintenance of normal weight; (3) Normalization or improvement of metabolic indexes [Total cholesterol (Tot-Chol), HDL-cholesterol, LDL-cholesterol, serum glucose, triglycerides (TG)]; and (4) normalization or improvement of ALT, AST, GGT.
For this study were recruited 46 adult (26-71 years old) patients with recent (within the previous 6 mo) diagnosis of NAFLD, who visited the outpatient liver clinics of the at Clinical Gastroenterology Unit, University Hospital Careggi between February 2016 and August 2016.
The diagnosis was based on clinical, ultrasonographic and laboratory values, after exclusion of any other cause of chronic liver disease (hepatitis B and C virus, autoimmune and metabolic disease) and alcohol intake not exceeding 20 g/d (confirmed by relatives).
Participants were excluded if they: (1) refused or were unable to give informed consent to participate in the study; (2) had evidence of advanced liver disease (cirrhosis, hepatocellular carcinoma); (3) had clinically relevant pathologies (e.g., pulmonary, gastro-intestinal, renal, metabolic, haematological, neurological, psychiatric, systemic or any acute infectious disease or signs of acute illness); and (4) had contraindications included bulimia nervosa, substance abuse, clinically significant depression, or current psychiatric care.
The study was approved by the appropriate Ethics Committee (“Centro, RC” 30.11.02.2016) and was carried out in accordance with the declaration of Helsinki[30]. All patients gave their written informed consent before the recruitment.
The dietary intervention was based on the MedDiet which was firstly introduced and described by Keys et al in “The Seven Countries Study”. After that study several other studies confirmed the beneficial effects and outcomes of the MedDiet on the health mainly reducing mortality, cardiovascular disease risk factors and cancer (Sofi et al[18], 2010).
The MedDiet is based on a balanced use of foods rich in fiber, antioxidants and unsaturated fats, a healthy approach designed to reduce the consumption of animal fats and cholesterol in a diet with an appropriate balance between energy intake and expenditure[31]. Therefore the MedDiet is characterized by high consumption of vegetables, fruits, non-refined cereals, legumes and potatoes, moderate consumption of fish and poultry and low consumption of full fat dairies, red meat and its products and homemade sweets. Olive oil is the basic source of fat used for food preparation and during consumption. Meals are often accompanied by low-to-moderate amounts of wine. The relationships between the macronutrient in the MedDiet is 55%-60% of carbohydrates of which 80% complex carbohydrates (bread, pasta, rice), 10%-15% of proteins about 60% of animal origin (especially white meat, fish), 25%-30% fat (mostly olive oil)[31].
The MedDiet has also a graphic representation which is typically a pyramid whose most updated version is described by[32]. The graphic representation follows the previous pattern: at the base, food items that should sustain the diet and provide the highest energy intake, and at the upper levels, foods to be eaten in moderate amounts such as those of animal origin and/or rich in sugars and fats that should be eaten in moderation and some of them left for special occasions[32].
Along with recommendations regarding the proportion and frequency of food consumption, the incorporation of cultural and lifestyle elements is one of the innovations of the latest version of the pyramid. These concepts represented outside of the pyramid, but at its base, are: moderation, socialization, culinary activities, physical activity, adequate rest, seasonality and traditional, local, eco-friendly and biodiverse products.
Weight, height, waist circumference, hip circumference, BMI were taken using standard procedures by the nutritionist.
Liver enzymes (AST, ALT, alkaline phosphatase, GGT), Tot-Chol, HDL-cholesterol, LDL-cholesterol, serum glucose, TG, insulin resistance index HOMA-IR and QUICKI were acquired or calculated from medical records delivered by participants.
The following indexes related to NAFLD were also calculated: the Kotronen index and NAFLD liver fat score[33], the Fatty Liver Index (FLI)[34] and the Visceral Adipose Index (VAI)[35]. Kotronen index is used as predictor of liver fat percentage. NAFLD liver fat score allows identification of NAFLD using easily available clinical and laboratory data. FLI measures the probability of significant steatosis (values < 30 rule out and values ≥ 60 rule in fatty liver). VAI is an indicator of visceral fat associated with cardiometabolic risk.
In order to make an enlarged clinical and nutritional evaluation of participants were estimated some cardiovascular risk indexes [Tot-Chol/HDL, LDL/HDL, TG/HDL and atherogenic index of plasma (AIP)], systemic inflammation indexes [platelet to lymphocyte ratio (PLR) and neutrophil to lymphocyte ratio (NLR)], a marker of MS [lipid accumulation product (LAP)] and two indexes used to assess advanced liver fibrosis in patients with NAFLD (BARD and NAFLD).
The level of adherence to the Mediterranean dietary pattern was evaluated using the Mediterranean Diet Score (MedDietScore) as defined by Panagiotakos et al[36]. Briefly, for the score calculation, the consumption of food items from 9 food groups (non-refined starchy food, potatoes, fruit, vegetables, legumes, fish, meat and meat products, poultry, and full fat dairy products), as well as olive oil, and alcoholic beverages, have to be taken into account. For each food item a score is assigned on the basis of the frequency of consumption. For food items close to the MedDiet the higher the frequency the higher the score and viceversa for food items that are away from the MedDiet. For further information, refer to Panagiotakos et al[36].
The counseling is an ongoing process taking all the intervention’s duration and in each meeting the nutritionist and the patient discuss the opportunity to reevaluate goals and strategies for achieving set goals. During the counseling process the nutritionist and the patient work together to identify areas where change is needed, prioritize changes, and problem-solve as to how to make the changes.
In this study the counseling process was individually-tailored and consisted of 6 monthly 30-45 min face-to face meetings aimed to enhance internal motivation and facilitate behavior change concerning both diet and lifestyle (in terms of physical activity level). The techniques used were mainly: motivational interviewing, active listening and Rogers/PNL/Gestalt techniques.
As far as dietary habits, patients were stimulated in order to strengthen their capability to successfully follow and adhered to the recommendations contained in written resources delivered and dietary intervention as described in the previous section.
The nutritionist initially explains to the patient that making dietary change should be a gradual process and that the truth challenge is not in making the initial dietary changes, but in maintaining them over the long term. The process may start with one or two easier dietary changes in the first few weeks and gradually make additional or more difficult changes over several weeks or months. In addition, factors affecting food decisions (ethnic background, religion, group affiliation, socioeconomic status) are taken into account during meetings.
The nutritionist and patient also set behavior-oriented goals together focusing on the behaviors needed to achieve the desired dietary change, not on an absolute value, such as achieving a certain body weight. Once the needed changes and goals have been identified, the patient and nutrition counselor think through potential problems that may arise (e.g., purchasing different foods, planning ahead for social events, or bringing special foods to work).
As far as the physical activity is concerned, the counseling approach was mainly used to stimulate patients to increase their physical activity. A motivational method instead of the common prescriptive one, was used to favor a more active lifestyle taking an incremental approach i.e., trying to increase the general level of activity throughout the day and every day up to a level sustainable and compatible with physical conditions and time available.
During the meetings patients and the nutritional counselor tried to set achievable goals tailored on the physical conditions of single patient. People with (very) limited physical activity level were stimulated to start identifying and putting in practice ways to increase their physical activity. For example, elder people and obese were stimulated to identify daily activities that could increase their physical activity (e.g., walking, riding the bike, get off the bus one stop earlier, walk up the stairs instead of taking the elevator, walk the dog, walk with their children, etc.). All patients without any physical constrain were stimulated to identify and take up/maintain regular physical activity. For these latter patients, the final goal was a habitual physical activity workload > 20 METs/h per week (corresponding to 3-h/wk moderate-intense physical activity) which was used in the study of Montesi et al[37].
Severity of NAFLD was assessed by ultrasound (US) (Ultrasound alpha 7, Hitachi Aloka Medical America, Inc.). The US results were interpreted always by the same investigator well experienced in performing and interpreting hepatic US and blinded to the clinical data. The sonographic findings that were specifically evaluated included the hepato-renal contrast, bright hepatic echoes, deep attenuation, vessel blurring and non-specific findings of heterogeneous echoes. The degree of steatosis during US was graded as absent (0), mild (1), moderate (2) and severe (3). The liver image was assessed to be normal if the texture was homogenous, exhibited fine level echoes and isoechoic compared to the renal cortex and adequate visualization of the hepatic vessels and diaphragm (grade 0). Criteria for determining the degree of liver steatosis included: presence of bright echoes or increased hepato-renal contrast indicative of mild steatosis (grade 1); presence of both bright echoes and increased hepato-renal contrast as well as vessel blurring indicative of moderate steatosis (grade 2); severe steatosis was considered to be present when in addition to the criteria for moderate steatosis, there was evidence of posterior beam attenuation and non-visualization of the diaphragm (grade 3)[38].
Microsoft Office Excel 2007 and IBM SPSS Statistics for Windows 20.0.0 were used for all the statistical calculations. Continuous variables are presented as mean values ± SD, while categorical variables are presented as frequencies. Unpaired t-test (2-tail) was used to test differences between baseline, mid-treatment (3 mo) and end-treatment (6 mo). Fisher’s distribution was used to assess significance of linear regression between steatosis grade and anthropometric, clinical and metabolic parameters at baseline (BL), mid-treatment (3 mo) and end-treatment (6 mo). All reported P values were based on two-sided tests and compared to a significance level of 5% (P < 0.05).
The screening visit was carried out on 82 potential participants, out of which 36 excluded and 46 recruited. All patients were Italian and Caucasian ethnicity. Table 1 summarizes characteristics of patients recruited for the study in terms of gender, age, anthropometry, BMI and steatosis grade, components of MS[39], MedDietScore, physical activity and smoking habits.
| Parameter | Value (mean ± SD or frequency) |
| Population n (%) | |
| Total | 46 |
| M | 29 (63%) |
| F | 17 (37%) |
| Age (yr) | |
| Range | 26-71 |
| mean ± SD | 47.5 ± 12 |
| BMI (kg/m2) | |
| Range | 18.9-45.3 |
| mean ± SD | 29.3 ± 6.1 |
| BMI (kg/m2) | |
| Obesity grade | |
| 18.5-24.9 (normal weight) | 23.90% |
| 25-29.9 (overweight) | 43.50% |
| 30-34.9 (class I obesity) | 17.40% |
| 35-39.9 (class II obesity) | 8.70% |
| ≥ 40 (class III obesity) | 6.50% |
| Waist circumference (cm) | |
| M | 100.88 ± 12.97 |
| F | 101.88 ± 15.07 |
| Obesity central (Waist circumference) | |
| M (> 102 cm) | 48.30% |
| F (> 88 cm) | 82.40% |
| Waist-to-hip ratio | |
| Android obesity | |
| M (≥ 1) | 41.40% |
| F (≥ 0.85) | 70.60% |
| Gynoid obesity | |
| M (≤ 0.94) | 20.70% |
| F (≤ 0.78) | 0.00% |
| Components of MS2 | |
| 0 | 4 (8.7%) |
| 1 | 20 (43.5%) |
| 2 | 10 (21.7%) |
| 3 | 5 (10.9%) |
| 4 | 5 (10.9%) |
| 5 | 2 (4.3%) |
| Liver steatosis grade | |
| 1 | 3 (6.5%) |
| 2 | 19 (41.3%) |
| 3 | 24 (52.2%) |
| MedDiet Score | |
| Range | 21-41 |
| mean ± SD | 28.4 ± 3.9 |
| Physical activity | |
| No | 24 (52.2%) |
| Yes | 22 (47.8%) |
| Times1/wk | |
| 1 | 6 (13%) |
| 2 | 7 (15.2%) |
| 3 | 7 (15.2%) |
| > 3 | 2 (4.3%) |
| Smoking | |
| No | 35 (76.1%) |
| Ex | 3 (6.5%) |
| Yes | 8 (17.4%) |
Table 2 focuses on 12 patients with MS showing the number of patients affected by each component. At the moment of the inclusion in the study, 5 out of 12 patients with MS were under treatment for one or more components of the MS (5 for hypertension and 2 also for dyslipidaemia) and 4 patients without MS were under treatment for the type 2 diabetes. In all cases the treatment was maintained unaltered throughout the study.
| Component | Hypertension | Blood pressure | TG | HDL | Serum glucose |
| n | 12 | 6 | 12 | 7 | 8 |
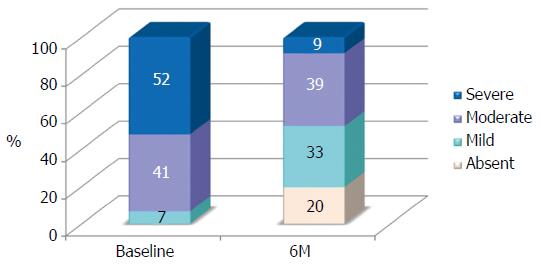
At the baseline steatosis was grade 1 for 3 patients (7%), grade 2 for 19 patients (41%) and grade 3 for 24 patients (52%). At the end of the treatment steatosis was regressed in 9 patients (20%), 15 patients had a grade 1 (33%), 18 patients a grade 2 (39%) and only 4 patients still had a grade 3 (9%). Distribution of patients among steatosis grade at baseline and end-treatment is shown in Figure 1. Percentage of patients with severe steatosis was slightly higher than 50% at baseline and below 10% after the treatment.
At baseline 35 patients were overweight (BMI > 25) and 11 had normal weight. At the end of the treatment patients with a normal weight increased to 20. Among patients overweight at baseline, 12 achieved a weight reduction of at least 7% and in 7 cases it was reached a normal weight. At the end of the treatment the end-point concerning the weight was accomplished by 25 out of 46 patients (i.e., 54.3%).
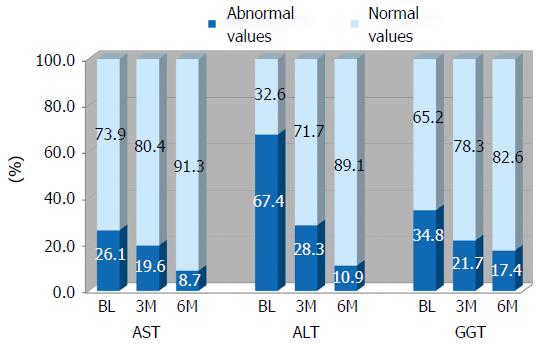
As far as the liver enzymes is concerned, Figure 2 shows the percentage of abnormal values at BL, at mid-intervention (3M) and end-intervention (6M). All three liver enzymes significantly decrease during the treatment (see Table 3) and the reduction of abnormal values was elevated. The normalization was particularly evident for the ALT enzyme whose altered values reduced from 67% at baseline down to 11% after the treatment.
| Parameter | mean ± SD, BL | mean ± SD, 3M | mean ± SD, 6M | P value | ||
| BL vs 3M | 3M vs 6M | BL vs 6M | ||||
| BMI (kg/m2) | 29.3 ± 6.11 | 28.0 ± 6.21 | 27.5 ± 6.21 | < 0.01a | < 0.01a | < 0.01a |
| Waist circumference (cm) | 101.25 ± 13.63 | 97.50 ± 14.07 | 96.24 ± 13.64 | < 0.01a | < 0.01a | < 0.01a |
| Hip-to-waist ratio | 0.95 ± 0.08 | 0.94 ± 0.07 | 0.93 ± 0.08 | < 0.01a | < 0.01a | < 0.01a |
| AST (U/L) | 36.16 ± 21.11 | 28.73 ± 11.28 | 24.84 ± 10.70 | < 0.01a | < 0.01a | < 0.01a |
| ALT (U/L) | 65.66 ± 31.92 | 48.37 ± 33.44 | 37.33 ± 19.53 | < 0.01a | < 0.01a | < 0.01a |
| GGT (U/L) | 60.19 ± 61.43 | 41.35 ± 36.49 | 39.73 ± 35.36 | < 0.01a | 0.545 | < 0.01a |
| FA (U/L) | 81.48 ± 37.19 | 79.54 ± 32.04 | 80.04 ± 35.02 | 0.489 | 0.787 | 0.574 |
| Total cholesterol (mg/dL) | 215.24 ± 40.78 | 205.04 ± 41.75 | 207.18 ± 38.48 | 0.044a | 0.598 | 0.097 |
| LDL cholesterol (mg/dL) | 137.70 ± 32.87 | 131.25 ± 35.88 | 130.16 ± 32.65 | 0.071 | 0.941 | 0.050a |
| HDL cholesterol (mg/dL) | 49.72 ± 10.59 | 50.17 ± 11.64 | 53.09 ± 11.86 | 0.550 | < 0.01a | < 0.01a |
| TG (mg/dL) | 133.39 ± 67.77 | 123.65 ± 71.31 | 118.24 ± 65.14 | 0.112 | 0.358 | 0.017a |
| Serum glucose (mg/dL) | 98.33 ± 15.87 | 94.52 ± 13.07 | 95.76 ± 13.46 | < 0.01a | 0.133 | < 0.01a |
| Chol Tot/HDL | 4.50 ± 1.27 | 4.23 ± 1.07 | 3.95 ± 1.15 | 0.013a | 0.010a | < 0.01a |
| LDL/HDL | 2.89 ± 0.95 | 2.67 ± 0.93 | 2.56 ± 0.84 | 0.028a | 0.015a | < 0.01a |
| TG/HDL | 2.87 ± 1.76 | 2.66 ± 1.89 | 2.39 ± 1.66 | 0.152 | 0.037a | < 0.01a |
| AIP | 0.03 ± 0.25 | -0.01 ± 0.23 | -0.06 ± 0.25 | 0.097 | 0.089 | < 0.01a |
| HOMA- IR (mU/L) | 3.25 ± 1.96 | 2.96 ± 1.73 | 2.94 ± 1.67 | < 0.01a | 0.841 | < 0.01a |
| QUICKI | 0.33 ± 0.04 | 0.34 ± 0.04 | 0.34 ± 0.03 | < 0.01a | 0.330 | < 0.017a |
| PLR | 115.37 ± 46.60 | 109.89 ± 51.77 | 113.65 ± 43.77 | 0.195 | 0.281 | 0.816 |
| NLR | 1.98 ± 1.12 | 1.88 ± 1.10 | 1.79 ± 0.89 | 0.287 | 0.509 | 0.185 |
| FLI | 61.55 ± 26.76 | 50.54 ± 29.37 | 48.83 ± 31.01 | < 0.01a | 0.286 | < 0.01a |
| Kotronen index (%) | 6.05 ± 5.02 | 4.10 ± 2.22 | 3.92 ± 2.40 | < 0.01a | 0.277 | < 0.01a |
| VAI | 1.85 ± 1.17 | 1.55 ± 0.71 | 1.32 ± 0.65 | 0.011 | 0.010 | < 0.01a |
| NAFLD Liver Fat Score | 0.12 ± 1.63 | -0.02 ± 1.42 | -0.70 ± 1.20 | 0.418 | < 0.01a | < 0.01a |
| LAP (male) | 48.41 ± 28.14 | 37.01 ± 19.95 | 33.18 ± 17.49 | < 0.01a | 0.166 | < 0.01a |
| LAP (female) | 84.15 ± 63.06 | 78.92 ± 70.51 | 74.13 ± 58.89 | 0.290 | 0.452 | < 0.01a |
| BARD | 1.09 ± 1.26 | 1.46 ± 1.17 | 1.37 ± 1.04 | 0.011a | 0.486 | 0.091 |
| NAFLD | -2.29 ± 0.93 | -2.13 ± 1.01 | -2.28 ± 1.00 | 0.015a | 0.032a | 0.986 |
Table 3 summarizes results of the statistical analysis carried out in order to assess the effect of the treatment on anthropometric, clinical and metabolic parameters considered in this study.
Several parameters, i.e., BMI, waist circumference, waist-to-hip ratio, AST, ALT, GGT, LDL, HDL, TG, serum glucose, Tot-Chol/HDL, LDL/HDL, TG/HDL, AIP, HOMA, QUICKI, FLI and LAP, showed a significant improvement between baseline and end-treatment. Among parameters positively affected by the treatment after 6 mo, most of them, i.e., BMI, waist circumference, waist-to-hip ratio, AST, ALT, GGT, serum glucose, Tot-Chol/HDL, LDL/HDL, HOMA, QUICKI, FLI and LAP (men) showed a significant improvement also after 3 mo from the start of the treatment.
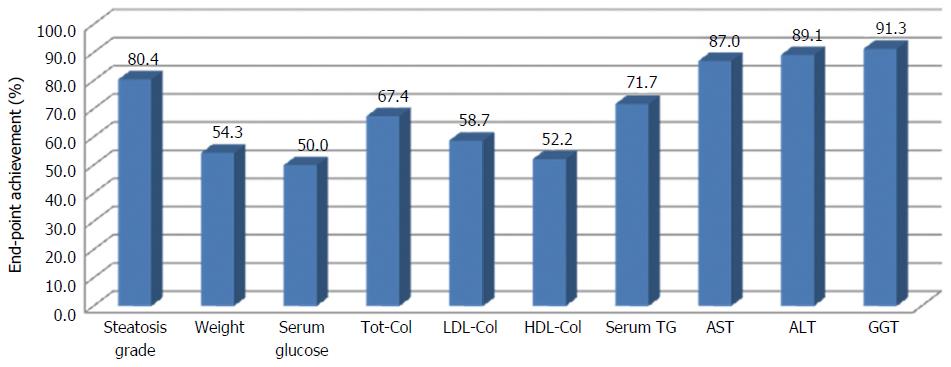
In order to evaluate the efficacy of the approach proposed in this study for the management of NAFLD, the achievement of set end-points was evaluated. Figure 3 shows the percentage of patients that achieved the target, singularly for each end-points.
All criteria were achieved for at least 50% of patients. The percentage of success is particularly high (more than 80%) for steatosis, ALT, AST and GGT.
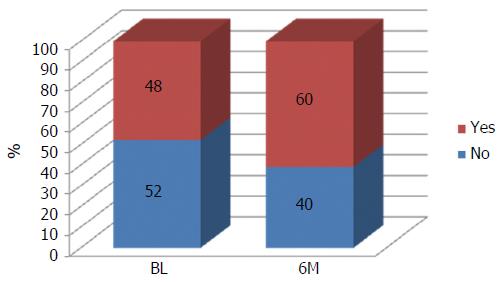
The intervention was also successful in increasing the percentage of participants carrying out physical activity on a regular basis (about 49% at baseline and 60% at end-intervention, Figure 4). Moreover, also people not able to carry out physical activity due to physical constraints declared their engagement in increasing their physical activity through simple daily activities indicated in the section concerning the counseling process.
Table 4 shows the correlations between steatosis grade and anthropometric, clinical and metabolic parameters considered in this study.
| Parameter | Grade 0 (n = 9) | Grade I (n = 15) | Grade 2 (n = 18) | Grade 3 (n = 4) | P value | |
| mean ± STD | mean ± STD | mean ± STD | mean ± STD | |||
| BMI (kg/m2) | BL | - | 30.19 ± 2.15 | 28.05 ± 6.47 | 30.11 ± 6.14 | 0.499 |
| 6M | 23.39 ± 3.52 | 25.94 ± 5.60 | 31.36 ± 6.53 | 25.77 ± 1.14 | 0.013a | |
| Waist circumference (cm) | BL | - | 103.00 ± 1.73 | 96.05 ± 12.94 | 105.15 ± 13.91 | 0.122 |
| 6M | 86.44 ± 11.98 | 90.73 ± 9.24 | 106.03 ± 12.64 | 94.88 ± 9.37 | < 0.01a | |
| Hip-to-waist ratio (-) | BL | - | 0.94 ± 0.05 | 0.91 ± 0.08 | 0.98 ± 0.07 | 0.020a |
| 6M | 0.88 ± 0.06 | 0.91 ± 0.06 | 0.97 ± 0.08 | 0.93 ± 0.09 | < 0.01a | |
| Tot Chol (mg/dL) | BL | - | 260.67 ± 27.21 | 210.95 ± 31.20 | 212.96 ± 46.24 | 0.266 |
| 6M | 215.89 ± 39.78 | 196.27 ± 62.92 | 212.06 ± 34.35 | 154.75 ± 44.90 | 0.078 | |
| HDL (mg/dL) | BL | - | 47.00 ± 3.46 | 52.05 ± 13.39 | 48.21 ± 8.39 | 0.532 |
| 6M | 142.16 ± 33.53 | 130.60 ± 22.94 | 131.33 ± 35.27 | 96.25 ± 38.88 | 0.070 | |
| LDL (mg/dL) | BL | - | 186.67 ± 37.63 | 133.52 ± 19.00 | 134.88 ± 37.05 | 0.119 |
| 6M | 54.33 ± 12.97 | 53.73 ± 14.08 | 53.56 ± 10.74 | 45.75 ± 1.89 | 0.399 | |
| TG (mg/dL) | BL | - | 134.00 ± 43.28 | 125.21 ± 42.18 | 139.79 ± 85.63 | 0.597 |
| 6M | 100.22 ± 31.69 | 119.20 ± 40.40 | 138.22 ± 88.51 | 65.25 ± 42.94 | 0.807 | |
| AST | BL | - | 0.72 ± 0.28 | 0.87 ± 0.33 | 0.95 ± 0.67 | 0.435 |
| 6M | 0.55 ± 0.15 | 0.63 ± 0.19 | 0.67 ± 0.37 | 0.53 ± 0.17 | 0.641 | |
| ALT | BL | - | 0.91 ± 0.20 | 1.32 ± 0.68 | 1.35 ± 0.64 | 0.419 |
| 6M | 0.56 ± 0.14 | 0.79 ± 0.37 | 0.82 ± 0.50 | 0.67 ± 0.23 | 0.334 | |
| AST/ALT | BL | - | 0.76 ± 0.15 | 0.71 ± 0.19 | 0.66 ± 0.20 | 0.276 |
| 6M | 0.95 ± 0.15 | 0.76 ± 0.22 | 0.73 ± 0.26 | 0.78 ± 0.11 | 0.058 | |
| GGT | BL | - | 58.67 ± 64.53 | 51.89 ± 54.69 | 42.21 ± 25.60 | 0.392 |
| 6M | 17.00 ± 4.12 | 43.53 ± 25.67 | 44.39 ± 0.10 | 27.25 ± 0.50 | 0.147 | |
| Blood gluc. (mg/dL) | BL | - | 96.67 ± 9.29 | 94.16 ± 15.71 | 101.83 ± 16.26 | 0.181 |
| 6M | 88.00 ± 1.94 | 91.27 ± 7.33 | 104.33 ± 17.03 | 91.50 ± 8.19 | 0.018a | |
| SM (n) | BL | - | 2.67 ± 1.15 | 1.32 ± 1.00 | 2.17 ± 1.43 | 0.350 |
| 6M | 1.44 ± 1.13 | 1.73 ± 1.28 | 2.28 ± 1.49 | 1.25 ± 0.50 | 0.415 | |
| Tot-Chol/HDL | BL | - | 5.60 ± 1.03 | 4.29 ± 1.27 | 4.53 ± 1.26 | 0.637 |
| 6M | 4.07 ± 0.84 | 3.84 ± 1.55 | 4.09 ± 0.94 | 3.41 ± 1.09 | 0.479 | |
| LDL/HDL | BL | - | 4.03 ± 1.14 | 2.72 ± 0.82 | 2.88 ± 0.96 | 0.355 |
| 6M | 2.70 ± 0.82 | 2.60 ± 0.90 | 2.54 ± 0.82 | 2.12 ± 0.91 | 0.333 | |
| TG/HDL | BL | - | 2.83 ± 0.78 | 2.63 ± 1.36 | 3.07 ± 2.11 | 0.516 |
| 6M | 1.92 ± 0.73 | 2.44 ± 1.25 | 2.79 ± 2.24 | 1.46 ± 1.02 | 0.698 | |
| AIP | BL | - | 0.44 ± 0.11 | 0.37 ± 0.21 | 0.39 ± 0.29 | 0.983 |
| 6M | 0.25 ± 0.19 | 0.34 ± 0.21 | 0.35 ± 0.27 | 0.08 ± 0.31 | 0.780 | |
| HOMA-IR (mU/L) | BL | - | 2.77 ± 0.22 | 2.37 ± 1.37 | 4.01 ± 2.18 | 0.015a |
| 6M | 1.50 ± 0.84 | 2.47 ± 1.06 | 4.12 ± 1.71 | 2.67 ± 1.51 | < 0.01a | |
| QUICKI | BL | - | 0.33 ± 0.00 | 0.35 ± 0.04 | 0.32 ± 0.03 | 0.082 |
| 6M | 0.37 ± 0.05 | 0.34 ± 0.02 | 0.32 ± 0.02 | 0.34 ± 0.03 | < 0.01a | |
| PLR | BL | - | 120.89 ± 28.83 | 107.31 ± 32.51 | 121.06 ± 57.08 | 0.467 |
| 6M | 93.44 ± 22.18 | 106.95 ± 48.08 | 127.33 ± 47.95 | 122.70 ± 30.46 | 0.058 | |
| NLR | BL | - | 1.36 ± 0.37 | 1.79 ± 0.80 | 2.21 ± 1.34 | 0.112 |
| 6M | 1.32 ± 0.21 | 1.67 ± 1.08 | 2.21 ± 0.85 | 1.44 ± 0.50 | 0.097 | |
| FLI | BL | - | 98.97 ± 0.30 | 98.84 ± 0.39 | 98.70 ± 0.75 | 0.612 |
| 6M | 98.48 ± 0.72 | 98.79 ± 0.40 | 98.75 ± 0.67 | 97.08 ± 1.82 | 0.143 | |
| Kotronen index (%) | BL | - | 4.63 ± 2.14 | 4.3 ± 2.0 | 7.6 ± 6.4 | 0.047a |
| 6M | 2.15 ± 0.83 | 3.5 ± 1.0 | 5.3 ± 3.2 | 3.1 ± 0.6 | 0.017a | |
| VAI | BL | - | 3.5 ± 1.8 | 1.9 ± 1.0 | 1.6 ± 1.1 | 0.041a |
| 6M | 1.44 ± 0.64 | 1.38 ± 0.65 | 1.29 ± 0.67 | 0.9 ± 0.67 | 0.224 | |
| NAFLD fat liver score | BL | - | -0.42 ± 0.9 | -0.6 ± 1.1 | 0.7 ± 1.8 | 0.016a |
| 6M | 1.88 ± 0.71 | -0.84 ± 0.78 | 0.07 ± 1.27 | -0.99 ± 0.63 | < 0.01a | |
| LAP (male) | BL | - | 52.00 ± 0.00 | 44.27 ± 19.98 | 50.51 ± 32.82 | 0.694 |
| 6M | 25.67 ± 11.38 | 31.11 ± 14.62 | 42.38 ± 19.13 | 26.11 ± 22.32 | 0.386 | |
| LAP (female) | BL | - | 72.10 ± 32.56 | 56.13 ± 31.50 | 130.21 ± 83.11 | 0.377 |
| 6M | 24.42 ± 16.25 | 52.21 ± 23.70 | 109.22 ± 67.63 | - | 0.147 | |
| BARD | BL | - | 2.33 ± 1.15 | 0.79 ± 1.08 | 1.17 ± 1.34 | 0.733 |
| 6M | 1.89 ± 0.33 | 1.07 ± 1.03 | 1.56 ± 1.15 | 0.50 ± 1.00 | 0.192 | |
| NAFLD | BL | - | -3.41 ± 0.48 | -2.25 ± 0.73 | -2.17 ± 1,02 | 0.112 |
| 6M | -2.44 ± 0.93 | -2.68 ± 0.87 | -1.88 ± 1.07 | -2.27 ± 0.91 | 0.144 | |
| MedDiet Score | BL | - | 30.0 ± 3.0 | 29.6 ± 4.6 | 27.2 ± 3.1 | 0.044a |
| 6M | NA | NA | NA | NA | NA |
Only waist-to-hip ratio and HOMA correlated with steatosis grade both at baseline and at end-treatment. Some parameters, e.g., BMI, waist circumference, serum glucose and QUICKI, didn’t show any significant correlation at baseline but the correlation was observed at end-treatment. Other parameters, e.g., Total Cholesterol, HDL, AST/ALT and PLR, showed a tendency only at the end- treatment.
Table 5 shows the correlations between BMI and anthropometric, clinical and metabolic parameters considered in this study.
| Parameter | P value | Parameter | P value | ||
| Steatosis grade | BL | 0.499 | TG/HDL | BL | 0.370 |
| 6M | 0.013a | 6M | 0.416 | ||
| Waist circumf. (cm) | BL | < 0.01a | AIP | BL | 0.194 |
| 6M | < 0.01a | 6M | 0.307 | ||
| Hip-to-waist ratio (-) | BL | 0.231 | HOMA-IR (mU/L) | BL | < 0.01a |
| 6M | 0.020a | 6M | < 0.01a | ||
| Tot Chol (mg/dL) | BL | 0.257 | QUICKI | BL | < 0.01a |
| 6M | < 0.01a | 6M | < 0.01a | ||
| HDL (mg/dL) | BL | 0.255 | PLR | BL | 0.213 |
| 6M | < 0.01a | 6M | 0.645 | ||
| LDL (mg/dL) | BL | 0.211 | NLR | BL | 0.066 |
| 6M | 0.768 | 6M | 0.167 | ||
| TG (mg/dL) | BL | 0.352 | FLI | BL | < 0.01a |
| 6M | 0.229 | 6M | 0.137 | ||
| AST | BL | 0.244 | Kotronen index (%) | BL | 0.038a |
| 6M | 0.093 | 6M | < 0.01a | ||
| ALT | BL | 0.504 | VAI | BL | 0.479 |
| 6M | 0.154 | 6M | 0.373 | ||
| AST/ALT | BL | 0.195 | NAFLD fat liver score | BL | < 0.01a |
| 6M | 0.114 | 6M | < 0.01a | ||
| GGT | BL | 0.075 | LAP (male) | BL | < 0.01a |
| 6M | 0.176 | 6M | < 0.01a | ||
| MS (n) | BL | 0.025a | LAP (female) | BL | 0.075 |
| 6M | 0.025a | 6M | 0.176 | ||
| Blood gluc. (mg/dL) | BL | 0.182 | BARD | BL | < 0.01a |
| 6M | 0.142 | 6M | 0.079 | ||
| Tot-Chol/HDL | BL | 0.218 | NAFLD | BL | 0.058 |
| 6M | 0.090 | 6M | 0.167 | ||
| LDL/HDL | BL | 0.185 | MedDiet Score | BL | 0.049a |
| 6M | 0.087 | 6M | NA |
Some parameters, i.e., waist circumference, MS, HOMA-IR, QUICKI, Kotronen index, NAFLD liver fat score, and LAP (male) correlated with BMI both at baseline and at end-treatment. Some parameters, i.e., hip-to-waist ratio, Tot. Chol., HDL didn’t show any significant correlation at baseline but the correlation was observed at end-treatment. On the other hand, FLI and BARD, showed a significant correlation only at baseline. Correlation between BMI and steatosis grade was previously reported, while correlation between BMI and MedDiet score is significant but is available only at baseline.
Most recent advances in the management and treatment of NAFLD show that a multifaceted approach, using a multidisciplinary team is likely to achieve the best outcome[40]. Combination of physical activity, medications, and dietetic interventions can be more effective than each prescription alone[24]. In addition, intervention tailored and aimed at encouraging self-empowerment and behavioral change are regarded as essential for increasing adhesion to treatments[40].
This observational study is set in this research trend and was aimed at evaluating the effectiveness of a 6-mo multidisciplinary approach jointly carried out by a gastroenterologist and a nutritionist with counseling license for treating patients with NAFLD. The nutritional counseling was used as tool for encouraging patients to improve their lifestyle (MedDiet-based dietary habits and regular physical activity).
According to our results, the approach clearly demonstrated its effectiveness on reduction of NAFL severity, weight loss and anthropometric indexes as well as normalization of metabolic index and liver enzymes.
Some patients’ characteristics at baseline are of relevance for discussing the results obtained. Firstly, it is interesting to point out that the MedDiet score is similar to that reported by Kontogianni et al[21] (28.4 ± 3.9 in this study and 32.5 ± 5.0 in the other study). According to our results the MedDietScore was significantly and inversely correlated with steatosis grade (P = 0.044) (Table 4).
Also, a significant percentage of patients (about 25%) had normal weight confirming that, even if positive energy balance and increased BMI substantially increase the risk of NAFLD, this latter may develop in individuals with normal BMI as well[41] e.g., as a consequence of an uncorrected lifestyle.
It is also interesting to underline that BMI and waist circumference does not correlate with NAFLD grade at baseline but correlation is significant at end-treatment for both parameters. At baseline, patients were also characterized by altered insulin resistance (IR) and insulin sensitivity, respectively estimated by HOMA-IR and QUICKI, as could be expected considering that IR is usually associated to NAFLD[12] and recently identified as the key aspect in the pathophysiology of both NAFLD and MS[42].
The reduction of NAFLD severity of patients is the main and relevant outcome of the treatment. In fact, the effects of MedDiet and increased physical activity, both associated or separated, have been investigated in several studies in terms of NAFLD-related indexes[37], hepatic enzymes[43], reduction of cardiovascular risk[44] or improvement in insulin sensitivity[45]. However, the present observational study is the first specifically examining effects of a multidisciplinary approach aimed at promoting the adherence to MedDiet and an active lifestyle directly on NAFLD severity.
According to Figure 3, in more than 80% of patients at least one grade reduction of NAFLD severity was observed. Our results confirm the positive outcomes of the study carried out by Ryan et al[46] who observed a positive effect of MedDiet on NAFLD. However, results cannot be directly compared due to the different method used to assess NAFLD severity.
The treatment showed positive effects on anthropometric indexes. In fact BMI decreased on average from 29.3 ± 6.11 to 27.5 ± 6.21, waist circumference decreased on average from 101.25 ± 13.63 to 96.24 ± 13.64 and hip-to-waist ratio decreased on average from 0.95 ± 0.08 to 0.93 ± 0.08 (Table 3). The improvement of previous anthropometric indexes is of relevance considering that all of them impact on CVD and MS related states and risks.
Excellent results of the treatment was confirmed also by the significant reduction of the Kotronen index (by 35% of the average and P < 0.01), the VAI (by 28% of the average and P < 0.01), NAFLD liver fat score (by 18% of the average and P < 0.01) and FLI (by 21% of the average and P < 0.01) (Table 3). Steatosis grade resulted significantly correlated with Kotronen index and NAFLD liver fat score both at baseline and end-treatment while correlation of steatosis grade with VAI was significant only at baseline. Absence of correlation at end-treatment could be due to the significant number of patients which showed regression of the disease. On the contrary the index FLI did not correlate with steatosis grade neither at baseline nor at end-treatment.
During the 6-mo of the treatment was also observed an improvement of the lipid profile of several patients (Figure 3). In fact, considering mean values, it was observed a reduction of Tot-Chol (215.24 ± 40.78 vs 207.18 ± 38.48), LDL (137.70 ± 32.87 vs 130.16 ± 32.65) and TG (133.39 ± 67.77 vs 118.24 ± 65.14) while an increase was observed for HDL (49.72 ± 10.59 vs 53.09 ± 11.86) (Table 3).
The treatment had also positive effects on liver enzymes with improvements or normalization of AST (36.16 ± 21.11 at baseline and 24.84 ± 10.70 at end-treatment), ALT (65.66 ± 31.92 at baseline and 37.33 ± 19.53 at end-treatment) and GGT (60.19 ± 61.43 at baseline and 39.73 ± 35.36 at end-treatment) (Table 3 and Figure 3). Of particular relevance was the effect on ALT with altered values reduced from almost 70% at baseline to just 10% at end-treatment. Normalization of values was significant also for AST (altered values at baseline were three-fold those at end treatment) and GGT (altered values were halved at end-treatment).
Positive effects of the MedDiet on liver enzymes were already observed by Fraser et al[43] in obese patients with type 2 diabetes and in the ATTICA study, that evaluated the prevalence of MS among over 3000 Greek adults[47]. On the other hand it is interesting to point out that Ryan et al[46] observed a positive effect of MedDiet on reduction in liver fat content (assessed with magnetic resonance imaging) but no significant differences in AST and ALT values were observed.
The treatment also showed positive effects on insulin resistance and insulin sensitivity: HOMA-IR significantly reduce at end-treatment with respect to baseline (3.25 ± 1.96 vs 2.94 ± 1.67, P < 0.01) while QUICKI increase (0.33 ± 0.04 vs 0.34 ± 0.03). Considering the reduction observed for the NAFLD grade, this result was expected, as NAFLD worsens the state of IR and may lead on to type 2 diabetes in the predisposed subjects[48]. Improvement of IR was expected considering that it was already observed in previous studies as a consequence of treatment centered either on MedDiet[46] or increased physical activity[37].
In conclusions, outcomes of this study further strengthen the hypothesis that MedDiet and more active lifestyle can be considered a safe therapeutic approach for reducing risk and severity of NAFLD and related disease states such as MS and CVD. This suggests that the proposed approach, carried out by a multidisciplinary team composed of a gastroenterologist and a nutritionist with counseling license, may be proposed as a valid and recommended approach for improving the clinical profile of NAFLD patients.
Although this study does not allow to assess the effect of nutritional counseling, excellent results in terms end-points achievement (Figure 3) suggest that the adherence to the proposed approach was very successful.
Replication and improvement of the study design, by a control arm and a follow-up after the end-treatment, are advisable to confirm the outcomes obtained so far and to assess the importance of multidisciplinary and counseling in modern approach for clinical management of NAFLD.
In this study we did not distinguish between simple fatty liver and NASH. In addition, despite MR-S is considered gold standards for assessing the severity of NAFLD using non-invasive techniques, in this study was used US which is considered the preferred first-line diagnostic procedure for imaging (EASL-EASD-EASO, 2016). According to previous studies (inter alia Ghaemi et al[49], 2013) we also supported the diagnosis with US with serum biomarkers and scores.
Non-alcoholic fatty liver disease (NAFLD) is a relevant issue in public health because represents a major cause of chronic liver diseases worldwide. Approximately 20% to 30% of adult population has NAFLD, making it the most common liver disease in developed countries. Most recent advances in the management and treatment of NAFLD show that a multifaceted approach to improve lifestyle is likely to achieve the best outcomes.
These results indicate the possibility of using lifestyle change, based on MetDiet and constant physical activity, as a powerful tool for treating dismetabolic diseases and only in some cases is required pharmacological treatments.
In this work the authors demonstrate that MedDiet and more active lifestyle can be considered a safe therapeutic approach for reducing risk and severity of NAFLD and related disease states. The real novelty was the double approach carried out respectively by a gastroenterologist and a nutritionist with counseling license.
This research focuses on the improvement of the therapy of patients with NAFLD but our results indicate that this double approach can be used also to increase adherence to diet or therapy of general patients.
NAFLD is an important liver disease worldwide generally associated with the Metabolic Syndrome. In this study to achieve an improvement in these patients an important treatment method based on life-style modification and Mediterranean diet (MetDiet) was achieved through a multidisciplinary approach jointly carried out by a gastroenterologist and a nutritionist with counseling license.
The study discusses an approach for treatment NAFLD which is an important liver disease worldwide. The study is well written and interesting. The study focused on an important treatment method based on lifestyle modification and diet control.
| 1. | Wah-Kheong C, Khean-Lee G. Epidemiology of a fast emerging disease in the Asia-Pacific region: non-alcoholic fatty liver disease. Hepatol Int. 2013;7:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Papamiltiadous ES, Roberts SK, Nicoll AJ, Ryan MC, Itsiopoulos C, Salim A, Tierney AC. A randomised controlled trial of a Mediterranean Dietary Intervention for Adults with Non Alcoholic Fatty Liver Disease (MEDINA): study protocol. BMC Gastroenterol. 2016;16:14. [PubMed] |
| 3. | Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 796] [Article Influence: 49.8] [Reference Citation Analysis (1)] |
| 4. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3280] [Article Influence: 328.0] [Reference Citation Analysis (6)] |
| 5. | Levene AP, Goldin RD. The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology. 2012;61:141-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Moore JB. Non-alcoholic fatty liver disease: the hepatic consequence of obesity and the metabolic syndrome. Proc Nutr Soc. 2010;69:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. 2015;47:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 522] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 8. | Rector RS, Thyfault JP, Wei Y, Ibdah JA. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World J Gastroenterol. 2008;14:185-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 213] [Cited by in RCA: 229] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 9. | Tarantino G, Finelli C. What about non-alcoholic fatty liver disease as a new criterion to define metabolic syndrome? World J Gastroenterol. 2013;19:3375-3384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1637] [Article Influence: 109.1] [Reference Citation Analysis (1)] |
| 11. | Kaser S, Ebenbichler CF, Tilg H. Pharmacological and non-pharmacological treatment of non-alcoholic fatty liver disease. Int J Clin Pract. 2010;64:968-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Del Ben M, Polimeni L, Baratta F, Pastori D, Loffredo L, Angelico F. Modern approach to the clinical management of non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:8341-8350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 439] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 14. | Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 506] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 15. | Zelber-Sagi S, Ratziu V, Oren R. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World J Gastroenterol. 2011;17:3377-3389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 210] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 16. | Sofi F, Casini A. Mediterranean diet and non-alcoholic fatty liver disease: new therapeutic option around the corner? World J Gastroenterol. 2014;20:7339-7346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 17. | Loria P, Adinolfi LE, Bellentani S, Bugianesi E, Grieco A, Fargion S, Gasbarrini A, Loguercio C, Lonardo A, Marchesini G. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the Italian Association for the Study of the Liver (AISF) Expert Committee. Dig Liver Dis. 2010;42:272-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (2)] |
| 18. | Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1250] [Cited by in RCA: 1107] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 19. | Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health. Biofactors. 2013;39:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Kastorini CM, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol. 2011;57:1299-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 756] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 21. | Kontogianni MD, Tileli N, Margariti A, Georgoulis M, Deutsch M, Tiniakos D, Fragopoulou E, Zafiropoulou R, Manios Y, Papatheodoridis G. Adherence to the Mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clin Nutr. 2014;33:678-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (3)] |
| 22. | St George A, Bauman A, Johnston A, Farrell G, Chey T, George J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2009;50:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 224] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 23. | Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 379] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 24. | Oliveira CP, de Lima Sanches P, de Abreu-Silva EO, Marcadenti A. Nutrition and Physical Activity in Nonalcoholic Fatty Liver Disease. J Diabetes Res. 2016;2016:4597246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Perseghin G, Lattuada G, De Cobelli F, Ragogna F, Ntali G, Esposito A, Belloni E, Canu T, Terruzzi I, Scifo P. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care. 2007;30:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 26. | Church TS, Kuk JL, Ross R, Priest EL, Biltoft E, Blair SN. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130:2023-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, Bowman JD, Pronk NP. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107:1755-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 1068] [Article Influence: 56.2] [Reference Citation Analysis (8)] |
| 28. | Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 993] [Article Influence: 66.2] [Reference Citation Analysis (15)] |
| 29. | Centis E, Moscatiello S, Bugianesi E, Bellentani S, Fracanzani AL, Calugi S, Petta S, Dalle Grave R, Marchesini G. Stage of change and motivation to healthier lifestyle in non-alcoholic fatty liver disease. J Hepatol. 2013;58:771-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 872] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 31. | Altomare R, Cacciabaudo F, Damiano G, Palumbo VD, Gioviale MC, Bellavia M, Tomasello G, Lo Monte AI. The mediterranean diet: a history of health. Iran J Public Health. 2013;42:449-457. [PubMed] |
| 32. | Bach-Faig A, Berry EM, Lairon D, Reguant J, Trichopoulou A, Dernini S, Medina FX, Battino M, Belahsen R, Miranda G. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14:2274-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1356] [Cited by in RCA: 1141] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 33. | Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, Lundbom N, Rissanen A, Ridderstråle M, Groop L. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137:865-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 622] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 34. | Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1238] [Cited by in RCA: 2217] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 35. | Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, Galluzzo A. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33:920-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 778] [Cited by in RCA: 1278] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 36. | Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med. 2007;44:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 498] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 37. | Montesi L, Caselli C, Centis E, Nuccitelli C, Moscatiello S, Suppini A, Marchesini G. Physical activity support or weight loss counseling for nonalcoholic fatty liver disease? World J Gastroenterol. 2014;20:10128-10136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51:1061-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 499] [Article Influence: 29.4] [Reference Citation Analysis (3)] |
| 39. | Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8720] [Cited by in RCA: 10945] [Article Influence: 643.8] [Reference Citation Analysis (0)] |
| 40. | Mahady SE, George J. Exercise and diet in the management of nonalcoholic fatty liver disease. Metabolism. 2016;65:1172-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | Margariti A, Deutsch M, Manolakopoulos S, Tiniakos D, Papatheodoridis GV. The severity of histologic liver lesions is independent of body mass index in patients with nonalcoholic fatty liver disease. J Clin Gastroenterol. 2013;47:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Abenavoli L, Milic N, Di Renzo L, Preveden T, Medić-Stojanoska M, De Lorenzo A. Metabolic aspects of adult patients with nonalcoholic fatty liver disease. World J Gastroenterol. 2016;22:7006-7016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (1)] |
| 43. | Fraser A, Abel R, Lawlor DA, Fraser D, Elhayany A. A modified Mediterranean diet is associated with the greatest reduction in alanine aminotransferase levels in obese type 2 diabetes patients: results of a quasi-randomised controlled trial. Diabetologia. 2008;51:1616-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599-2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2876] [Cited by in RCA: 3093] [Article Influence: 134.5] [Reference Citation Analysis (1)] |
| 45. | Martínez-González MA, de la Fuente-Arrillaga C, Nunez-Cordoba JM, Basterra-Gortari FJ, Beunza JJ, Vazquez Z, Benito S, Tortosa A, Bes-Rastrollo M. Adherence to Mediterranean diet and risk of developing diabetes: prospective cohort study. BMJ. 2008;336:1348-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 329] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 46. | Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, O’Dea K, Desmond PV, Johnson NA, Wilson AM. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 560] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 47. | Tzima N, Pitsavos C, Panagiotakos DB, Chrysohoou C, Polychronopoulos E, Skoumas J, Stefanadis C. Adherence to the Mediterranean diet moderates the association of aminotransferases with the prevalence of the metabolic syndrome; the ATTICA study. Nutr Metab (Lond). 2009;6:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Walle P, Takkunen M, Männistö V, Vaittinen M, Lankinen M, Kärjä V, Käkelä P, Ågren J, Tiainen M, Schwab U. Fatty acid metabolism is altered in non-alcoholic steatohepatitis independent of obesity. Metabolism. 2016;65:655-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 49. | Ghaemi A, Taleban FA, Hekmatdoost A, Rafiei A, Hosseini V, Amiri Z, Homayounfar R, Fakheri H. How Much Weight Loss is Effective on Nonalcoholic Fatty Liver Disease? Hepat Mon. 2013;13:e15227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Esmat SM, Hekmatdoost A, Kobyliak NK S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF