Published online Apr 28, 2017. doi: 10.3748/wjg.v23.i16.2957
Peer-review started: January 14, 2017
First decision: February 23, 2017
Revised: March 13, 2017
Accepted: March 20, 2017
Article in press: March 20, 2017
Published online: April 28, 2017
Processing time: 104 Days and 16.9 Hours
To verify the value of Gutuo Jiejiu decoction in improving the survival of patients with severe alcoholic hepatitis (SAH).
We performed a retrospective cohort study in consecutive patients diagnosed with SAH at the Teaching Hospital of Chengdu University of Traditional Chinese Medicine and Shuguang Hospital, Shanghai University of Traditional Chinese Medicine. The traditional Chinese medicine formula Gutuo Jiejiu decoction was employed as an exposure factor. Patients from the Teaching Hospital of Chengdu University of Traditional Chinese Medicine who had been treated with Gutuo Jiejiu decoction + prednisone were assigned to an observation group, and patients from Shuguang Hospital, Shanghai University of Traditional Chinese Medicine who had been treated with prednisone alone were selected as a control group. A retrospective analysis was performed by comparing age, alcohol intake, and clinical parameters of liver injury before and after treatment. Additionally, the 3- and 12-mo survival rates and the occurrence of complications were analyzed.
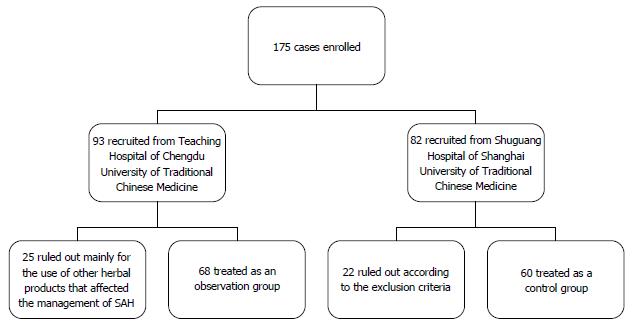
One hundred and twenty-eight eligible patients were selected from 175 cases with SAH, of which 68 were assigned to the observation group and the other 60 to the control group. No significant difference was found in the patients’ baseline characteristics (P > 0.05). However, significant improvements of 90-d survival rate [56/68 (82.4%) vs 27/60 (45.0%), P = 0.0000] and 365-d survival rate [48/68 (70.6%) vs 13/60 (21.7%), P = 00000] were observed in the observation group after treatment. After the first 3 mo of treatment, more improvements in the clinical parameters and scoring systems related to liver injury occurred in the observation group than in the control group (P < 0.05). After treatment for 12 mo, the differences in the clinical parameters and scoring systems related to liver injury between the two groups were more significant (P < 0.05). No significant differences in complications and adverse effects were found between the two groups.
Gutuo Jiejiu decoction could improve the survival rates and clinical parameters of liver injury in patients with SAH, and may represent a new option for treating SAH.
Core tip: Severe alcoholic hepatitis (SAH) has high mortality, regardless of the standard therapy. Finding new effective and safe therapeutic options is frequently necessary. With this aim, we reviewed SAH cases from January 2013 to July 2015 at the Teaching Hospital of Chengdu University of Traditional Chinese Medicine and Shuguang Hospital, Shanghai University of Traditional Chinese Medicine and performed a retrospective analysis. We found that Gutuo Jiejiu decoction + prednisone improved the 3- and 12-mo survival rates and the clinical parameters of liver injury in patients with SAH more significantly than prednisone alone.
- Citation: Mou HY, Nie HM, Hu XY. Gutuo Jiejiu decoction improves survival of patients with severe alcoholic hepatitis: A retrospective cohort study. World J Gastroenterol 2017; 23(16): 2957-2963
- URL: https://www.wjgnet.com/1007-9327/full/v23/i16/2957.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i16.2957
Alcohol use disorders affect millions of individuals worldwide. Alcohol consumption is directly associated with liver disease mortality and accounts for elevated social and economic costs[1]. Alcoholic liver disease (ALD) encompasses a spectrum of injury, ranging from simple steatosis to frank cirrhosis[2]. Severe alcoholic hepatitis (SAH) is characterized by a Maddrey’s discriminant function (MDF) score higher than 32, which implies a 2-mo mortality near 50% if untreated[3-5]. Although several therapeutic measures are now available to improve survival rates in patients with SAH, the overall prognosis remains dismal[6]. The pathophysiology of SAH is complicated. Activation of Kupffer cells (KCs), the production of pro-inflammatory cytokines, and increases in the level of reactive oxygen species (ROS) are implicated in liver damage. Furthermore, mitochondrial glutathione (m-GS-H), which is the principal antioxidant agent in hepatocytes, is severely depleted[7].
Corticosteroids have become a first-line therapy for biopsy-proven, severe alcoholic steatohepatitis (ASH)[6]. A recent study carefully examined the effects of prednisone on liver injury and regeneration in several experimental models of alcoholic liver injury[8]. Generally, corticosteroids suppress inflammatory and immune-mediated hepatic destruction, but their marked anti-anabolic effect may suppress regeneration and slow healing by inhibiting the expression of genes (e.g., pSTAT3) that regulate the proliferation and repair of hepatocytes[8]. In a recent meta-analysis, patients allocated to a corticosteroid treatment group (40 mg/d for 28 d) had a higher 28-d survival rate than those allocated to a non-corticosteroid treatment group[9]. Although prednisolone is widely used and is considered the standard treatment for severe acute alcoholic hepatitis with an MDF score ≥ 32, this treatment is not free of adverse effects and has been considered controversial[10].
Numerous studies have reported the effects of traditional Chinese medicine (TCM) for alcoholic hepatitis, including improvement of symptoms, reduction of the degree of liver fibrosis, amelioration of liver function, and reduction of the risk of adverse reactions. Gutuo Jiejiu decoction is an empirical formula from Professor Xiao-Yu Hu, which has been being used effectively for a long term in the Department of Infectious Diseases, Teaching Hospital of Chengdu University of Traditional Chinese Medicine. The purpose of this study was to retrospectively analyze the effectiveness of Gutuo Jiejiu decoction in patients with SAH.
The present investigation was a retrospective study executed at the Teaching Hospital of Chengdu University of Traditional Chinese Medicine and the Shuguang Hospital of Shanghai University of Traditional Chinese Medicine from January 2013 to July 2015.
Patients aged between 31 and 70 years who met the clinical and biochemical criteria for SAH and were characterized by a history of chronic and heavy alcohol consumption (> 30 g/d within the previous 5 years) and the presence of clinical and or biochemical abnormalities suggestive of liver injury were included in the study[11]. The criteria for inclusion were a rapid onset of jaundice unrelated to biliary tract obstruction based on ultrasound, painful hepatomegaly and ascites, transaminases levers more than two times the normal values, an aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio exceeding two times of the normal ratio, leukocytosis caused by an elevated neutrophil count, total bilirubin > 5 mg/dL, and MDF score > 32 (calculated using the formula [4.6 × (patient prothrombin time (PT) - control PT, in seconds) + total bilirubin, in mg/dL]).
Patients with the following concomitant diseases or conditions were excluded from the study: acquired immunodeficiency syndrome, cancer, autoimmune diseases, psychiatric disorders different from alcoholism, such as depression and anxiety according to the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition criteria; a history of atopy or asthma, diabetes, obesity, pregnancy, tuberculosis, hepatitis C virus infection and gastrointestinal bleeding. Additionally, patients who had taken illicit drugs, antioxidant supplements, or other herbal products were not included in this study. Finally, patients who did not adhere to the formula were removed from this study either.
A review of all cases diagnosed with SAH between January 2013 and July 2015 was performed at the Teaching Hospital of Chengdu University of Traditional Chinese Medicine and Shuguang Hospital, Shanghai University of Traditional Chinese Medicine. TCM therapy with Gutuo Jiejiu decoction was employed as an exposure factor. Patients who had been treated by using TCM therapy with Gutuo Jiejiu decoction + prednisone at the Teaching Hospital of Chengdu University of Traditional Chinese Medicine were considered an observation group. Patients from Shuguang Hospital, Shanghai University of Traditional Chinese Medicine who had been treated with prednisone alone were selected as a control group. The following variables were used for analysis: age, alcohol intake, leukocyte count, neutrophil count, haemoglobin level, platelet count, blood urea-nitrogen (BUN), sodium (Na), albumin, total bilirubin, ALT, AST, gamma glutamyl transpeptidase, prothrombin time, international normalized ratio, the Child-Turcotte-Pugh and MDF scores before and after treatment for 3 mo and 12 mo, the 3- and 12-mo survival rates, and the occurrence of complications. The screening procedures are shown in Figure 1.
Prednisone was administered based on the guidelines of the American Association for the Study of Liver Diseases (AASLD) (40 mg/d for 4 wk, then tapered over 2-4 wk, or stopped, depending on the clinical situation)[2]. The composition of Gutuo Jiejiu decoction consisted of Renshen (Panax ginseng), Gegen (Pueraria lobata), Huangqin (Hovenia acerba), etc. The control group was treated with prednisone alone, and the observation group was treated with Gutuo Jiejiu decoction + prednisone. TCM clinicians tailored the formula based on changes in the syndrome and the patients’ conditions. Additionally, all of the patients were given standard comprehensive medical treatment.
Adverse events such as unexpected symptoms or feelings of discomfort were recorded. As for each adverse event, the start date, end date and degree were recorded and a causative relationship with the medicine was considered. The complications were recorded in detail. Patients with any adverse events or complications received appropriate treatment. Additionally, TCM clinicians made required modifications to the formula based on the patients’ conditions.
Overall survival was the only endpoint used in the analysis. Overall survival was defined as the time elapsed from the date of diagnosis to either the date of death or 365 d after diagnosis. Univariate survival curves were estimated using the Kaplan-Meier method and compared using the log rank test. The observed death rates at 90 and 365 d for each group are reported. To compare the baseline characteristics and the outcomes after treatment between groups, Student’s t-test, the rank test, the χ2 test or Ridit analysis was applied based on the variable type (case deletion was employed in the management of missing data). The level of significance was set at P < 0.05. All analyses were performed with SPSS 21.0 statistical software.
A total of 175 cases were collected from the two hospitals. All of the patients were male. One hundred and twenty-eight patients were eligible for our study, of whom 68 were included in the observation group and the other 60 in the control group.
The baseline characteristics of the patients are given in Table 1. No significant differences were found in these parameters. After treatment for 3 mo, greater improvements in the clinical parameters and scoring systems in relation to liver injury were observed in the observation group (P < 0.05). After treatment for 12 mo, the differences in the clinical parameters and scoring systems related to liver injury between the two groups were more significant (P < 0.05; Tables 2 and 3).
| Characteristic | Observation group | Controlgroup | P value |
| Age (yr) | 50.50 ± 9.84 | 52.18 ± 10.31 | 0.347 |
| Alcohol intake (g/d) | 142.60 ± 46.00 | 140.72 ± 42.95 | 0.994 |
| Leukocytes (× 109/L) | 6.36 ± 3.35 | 5.55 ± 1.86 | 0.207 |
| Neutrophils (× 109/L) | 4.21 ± 2.60 | 3.58 ± 1.35 | 0.158 |
| Hemoglobin (g/L) | 102.49 ± 23.90 | 107.75 ± 16.46 | 0.249 |
| Platelets (× 109/L) | 88.00 ± 39.91 | 83.35 ± 28.20 | 0.613 |
| Blood urea nitrogen (mmol/L) | 11.86 ± 2.19 | 12.26 ± 2.20 | 0.232 |
| Sodium (mmol/L) | 135.93 ± 8.31 | 136.18 ± 5.56 | 0.616 |
| Albumin (g/L) | 29.27 ± 5.97 | 30.34 ± 5.75 | 0.303 |
| Total bilirubin (μmol/L) | 89.73 ± 33.50 | 95.49 ± 47.97 | 0.727 |
| Alanine aminotransferase (U/L) | 76.41 ± 27.06 | 85.72 ± 28.86 | 0.055 |
| Aspartate aminotransferase (U/L) | 173.04 ± 74.21 | 185.15 ± 71.61 | 0.225 |
| Gamma glutamyl transpeptidase (U/L) | 148.40 ± 88.04 | 159.42 ± 84.30 | 0.301 |
| Prothrombin time (s) | 21.69 ± 1.50 | 21.46 ± 1.76 | 0.173 |
| International normalized ratio | 1.92 ± 0.13 | 1.95 ± 0.16 | 0.173 |
| Maddrey’s discriminant function | 40.63 ± 6.69 | 39.97 ± 7.72 | 0.226 |
| Child-Turcotte-Pugh | 10.56 ± 1.36 | 10.40 ± 1.26 | 0.555 |
| Characteristic | Observation group | Controlgroup | P value |
| Leukocytes (× 109/L) | 5.60 ± 1.03 | 5.52 ± 0.98 | 0.748 |
| Neutrophils (× 109/L) | 4.13 ± 0.68 | 4.20 ± 0.75 | 0.652 |
| Hemoglobin (g/L) | 116.57 ± 12.81 | 111.22 ± 13.39 | 0.087 |
| Platelets (× 109/L) | 107.82 ± 17.45 | 108.37 ± 12.13 | 0.521 |
| Blood urea nitrogen (mmol/L) | 5.95 ± 1.52 | 7.57 ± 1.76 | 0.000 |
| Sodium (mmol/L) | 126.15 ± 8.74 | 125.64 ± 10.32 | 0.828 |
| Albumin (g/L) | 34.86 ± 4.36 | 32.79 ± 4.39 | 0.048 |
| Total bilirubin (μmol/L) | 56.11 ± 19.13 | 67.49 ± 19.70 | 0.016 |
| Alanine aminotransferase (U/L) | 63.79 ± 17.16 | 77.81 ± 16.75 | 0.001 |
| Aspartate aminotransferase (U/L) | 83.93 ± 19.92 | 92.48 ± 20.24 | 0.076 |
| Gamma glutamyl transpeptidase (U/L) | 78.29 ± 15.14 | 86.04 ± 14.96 | 0.032 |
| Prothrombin time (s) | 17.46 ± 1.85 | 18.59 ± 2.26 | 0.038 |
| International normalized ratio | 1.59 ± 0.17 | 1.69 ± 0.21 | 0.038 |
| Maddrey’s discriminant function | 19.22 ± 8.84 | 25.07 ± 10.59 | 0.014 |
| Child-Turcotte-Pugh | 8.52 ± 1.50 | 9.89 ± 1.97 | 0.002 |
| Characteristic | Observation group | Control group | P value |
| Leukocytes (× 109/L) | 5.18 ± 0.99 | 5.89 ± 0.95 | 0.029 |
| Neutrophils (× 109/L) | 3.52 ± 0.74 | 3.98 ± 0.54 | 0.018 |
| Hemoglobin (g/L) | 125.54 ± 12.38 | 118.69 ± 10.36 | 0.045 |
| Platelets (× 109/L) | 120.65 ± 19.10 | 105.23 ± 20.31 | 0.009 |
| Blood urea nitrogen (mmol/L) | 4.39 ± 0.87 | 5.74 ± 2.09 | 0.049 |
| Sodium (mmol/L) | 134.12 ± 5.43 | 128.72 ± 5.36 | 0.005 |
| Albumin (g/L) | 38.77 ± 4.76 | 34.10 ± 4.55 | 0.005 |
| Total bilirubin (μmol/L) | 37.71 ± 14.53 | 47.44 ± 13.44 | 0.015 |
| Alanine aminotransferase (U/L) | 49.23 ± 18.41 | 58.77 ± 15.00 | 0.040 |
| Aspartate aminotransferase (U/L) | 42.10 ± 11.96 | 51.31 ± 17.61 | 0.042 |
| Gamma glutamyl transpeptidase (U/L) | 53.75 ± 15.95 | 66.38 ± 17.87 | 0.022 |
| Prothrombin time (s) | 16.41 ± 1.81 | 18.23 ± 2.77 | 0.047 |
| International normalized ratio | 1.50 ± 0.17 | 1.66 ± 0.25 | 0.047 |
| Maddrey’s discriminant function | 13.90 ± 8.65 | 22.24 ± 12.77 | 0.017 |
| Child-Turcotte-Pugh | 7.46 ± 1.35 | 9.15 ± 1.63 | 0.001 |
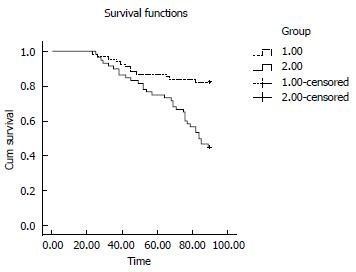
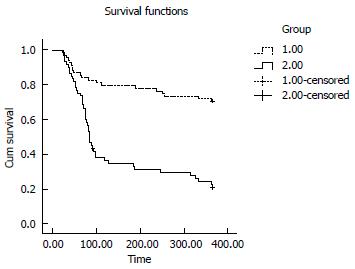
At day 90, the survival rate was higher in the observation group than in the control group [56/68 (82.4%) vs 27/60 (45%), respectively; P = 0.0000]. At day 365, the difference in the survival rate between the two groups was more significant [(48/68 (70.6%) in the observation group vs 13/60 (21.7%) in the control group, P = 0.0000]. The Kaplan-Meier curves of liver-related mortality according to the response to the treatments are shown in Figures 2 and 3 (P = 0.0000 by log-rank test).
Regarding the development or progression of complications, 25 patients in the observation group developed hepatic encephalopathy (HE), compared with 21 patients in the control group (36.8% vs 35.0%, P = 0.8550). Eleven patients in the observation group experienced venous bleeding compared with 8 patients in the control group (16.2% vs 13.3%, P = 0.6520). Twenty-two patients in the observation group and 20 patients in the control group developed hepatorenal syndrome (32.4% vs 33.3%, P = 0.906). No significant difference was found in the development of infections between the two groups (8.8% vs 15.0%, P = 0.2780).
Patients in both groups reported adverse effects including epigastric burning, nausea, and vomiting. Six patients in the control group and 8 patients in the observation group complained of these adverse effects.
Up to 40% of patients with SAH die within 6 months after the onset of the clinical syndrome[12]. Nonetheless, only a few reports have investigated SAH. The most important approaches for the treatment of SAH in these studies are abstinence, nutrition therapy, corticosteroids, anticytokine therapy, and antioxidants. In one study involving the treatment of SAH patients, Ramond et al[13] reported a 12.5% mortality among patients receiving prednisolone and a 55% mortality among patients receiving a placebo (P = 0.001). A subgroup analysis performed in trials with a low risk of bias revealed a significantly reduced mortality in corticosteroid-treated patients with either an MDF ≥ 32 or hepatic encephalopathy (RR = 0.33, 95%CI: 0.11-0.97)[14]. Carithers et al[15] found that patients with SAH treated with methylprednisolone (32 mg/d, for 28 d) had a lower mortality than patients who received a placebo (6% vs 35%, P = 0.006). Additionally, corticosteroids (specifically prednisone) have been shown to significantly reduce mortality for at least 1 year[16]. However, SAH still has a high mortality. Thus, the discovery of a new effective therapy in clinical practice is vital for the management of SAH. The experiences of Chinese clinicians provide an important source of effective TCM therapies. The TCM treatment approach works at different levels and on different targets, whereas Western drugs target very specific pathways[17]. Additionally, Western and TCM approaches have been used together worldwide to exert a greater effect[18]. In a clinical trial conducted by Li et al[19], the outcomes showed that the effective rate of TCM therapy + prednisone was higher than that of prednisone alone (88.9% vs 56.7%, P < 0.05). A clinical analysis performed by Wang[20] showed that TCM improved the symptoms of acute-on alcoholic liver failure including abdominal distension, loss of appetite, insomnia, diarrhea or coprostasis.
In TCM, SAH is categorized as “alcoholophilia”. Disharmony between the liver and spleen results from the internal retention of Qi, blood and water attributed to the prolonged illness and not maintaining abstinence. Additionally, the kidney will be ultimately impaired with the depletion of both Yin and Yang[21]. Thus, supplementing Qi to prevent collapse and relieving alcoholism are the most significant approach for SAH treatment. According to these principles, Professor Xiao-Yu Hu prescribed a Chinese herbal formula called Gutuo Jiejiu decoction in clinical practice. This formula has been being used effectively for a long term in the Department of Infectious Diseases, Teaching Hospital of Chengdu University of Traditional Chinese Medicine. Therefore, we present a retrospective study involving a group of SAH cases treated with the Chinese herbal formula Gutuo Jiejiu decoction + prednisone or prednisone alone at two hospitals, with the aim of verifying the value of Gutuo Jiejiu decoction in patients with SAH.
In our study, the formula Gutuo Jiejiu decoction consists of Renshen (Panax ginseng), Gegen (Pueraria lobata), Huangqin (Hovenia acerba), etc. The indication for the formula is disharmony between the liver and spleen and depletion of both Yin and Yang. Thus, the formula has the effect of supplementing the Qi to prevent collapse and relieving alcoholism. In particular, Panax ginseng is applied to tonify essential Qi, supplement the Qi to prevent collapse and replenish spleen Qi, Pueraria lobata is applied to relive alcoholism and activate spleen Qi and regulate stomach, and Hovenia acerba is applied to clear away heat-dampness and heat-toxin. Therefore, the compatibility of the herbs in the formula can achieve the effect of supplementing the Qi to prevent collapse and relieve alcoholism. Additionally, in pharmacology, Panax ginseng can enhance the enzyme activity of various substances involved in liver metabolism to increase the detoxification capacity of the liver, and thereby enhance the body’s tolerance to a variety of chemical substances. Studies have shown that Pueraria lobata can affect the gastrointestinal absorption and metabolism of ethanol, the saponin constituents in the flowers of Pueraria lobata have a hepatoprotective effect, and isoflavones can decrease the blood ethanol and acetaldehyde concentrations and reduce the BUN level[22]. Baicalin significantly reduced the ALT and AST levels as well as the MDA and TNF-α contents, and improved the SOD and GSH-Px in sera of rats with alcoholic liver injury[23].
The clinical characteristics of the participants in this study have been described previously. Our data showed that Gutuo Jiejiu decoction could enhance the effect of prednisone in reducing mortality and ameliorating the clinical parameters of liver injury. This study may provide an effective and safe option for patients with SAH.
The present study had several limitations. For instance, the retrospective nature of this study may have represented a potential source of selection bias due to the lack of availability of criteria for listing these 128 patients among the thousands of cases of SAH that are seen and managed in China annually. Additionally, the study was only a retrospective study, and employing the prospective clinical study standard of the double-blind, random method was impossible.
In summary, our study showed that the survival rates and clinical parameters of patients with SAH treated with Gutuo Jiejiu decoction + prednisone were better than those of patients treated with prednisone alone. In view of the non-availability of better options for the treatment of SAH and the absence of evidence of encouraging data for liver transplantation in patients with SAH, we propose that the Chinese herbal formula Gutuo Jiejiu decoction may be considered an option for patients with this disease. Henceforth, well-designed prospective studies with longer follow-up periods are needed to assess the long-term efficacy and survival rates in patients with treated by traditional Chinese methods.
All cases were obtained from the Teaching Hospital of Chengdu University of Traditional Chinese Medicine and Shuguang Hospital, Shanghai University of Traditional Chinese Medicine. The authors are particularly grateful for the excellent professional assistance from their colleagues in the Department of Infectious Diseases, Teaching Hospital of Chengdu University of Traditional Chinese Medicine and the Department of Liver Diseases, Shuguang Hospital, Shanghai University of Traditional Chinese Medicine.
Regardless of its standard therapy, severe alcoholic hepatitis (SAH) has a high mortality. Numerous studies have reported the effect of traditional Chinese medicine (TCM) for alcoholic liver hepatitis, such as improvement of symptoms, reduction of the degree of liver fibrosis, amelioration of liver function, and reduction of the risk of adverse reactions. Nonetheless, there are only a few reports involving the therapy of SAH.
Novel therapeutic targets to restore altered gut mucosal integrity, suppress inflammation based on cytokine profiles, promote hepatic regeneration, and limit innate immune responses have been the recent research focus in SAH.
In the current study, the authors found that Chinese herbal formula Gutuo Jiejiu decoction is an effective therapy for SAH; the patients treated with Gutuo Jiejiu decoction had better survival at 3 and at 12 mo compared with those treated with standard therapy with steroids.
The data from this study suggest that Gutuo Jiejiu decoction could be used as an effective therapy for patients with SAH.
SAH is a condition characterized by a rapid onset of jaundice in the absence of biliary tract obstruction, painful hepatomegaly and ascites, transaminases ≥ two times above the normal values, an aspartate aminotransferase/alanine aminotransferase ratio ≥ 2, neutrophilia, total bilirubin > 5 mg/dL, and a Maddrey’s discriminant function score > 32 (calculated with the formula [4.6 × (patient prothrombin time (PT) control PT, in seconds) + total bilirubin in mg/dL]), which occurs in patients with a history of chronic and heavy alcohol intake.
The authors analyzed the effect of Chinese herbal formula Gutuo Jiejiu decoction on alcoholic hepatitis patients. Although the effect of formula is not a break-through kind of thing, there is some improvement in the clinical profile of the patients.
| 1. | Bruha R, Dvorak K, Petrtyl J. Alcoholic liver disease. World J Hepatol. 2012;4:81-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (2)] |
| 2. | O’Shea RS, Dasarathy S, McCullough AJ; Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology. 2010;51:307-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 862] [Article Influence: 53.9] [Reference Citation Analysis (2)] |
| 3. | Haber PS, Warner R, Seth D, Gorrell MD, McCaughan GW. Pathogenesis and management of alcoholic hepatitis. J Gastroenterol Hepatol. 2003;18:1332-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Mathurin P, Abdelnour M, Ramond MJ, Carbonell N, Fartoux L, Serfaty L, Valla D, Poupon R, Chaput JC, Naveau S. Early change in bilirubin levels is an important prognostic factor in severe alcoholic hepatitis treated with prednisolone. Hepatology. 2003;38:1363-1369. [PubMed] |
| 5. | McCullough AJ, O’Connor JF. Alcoholic liver disease: proposed recommendations for the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2022-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Kim W, Kim DJ. Severe alcoholic hepatitis-current concepts, diagnosis and treatment options. World J Hepatol. 2014;6:688-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 7. | Higuera-de la Tijera F, Servín-Caamaño AI, Cruz-Herrera J, Serralde-Zúñiga AE, Abdo-Francis JM, Gutiérrez-Reyes G, Pérez-Hernández JL. Treatment with metadoxine and its impact on early mortality in patients with severe alcoholic hepatitis. Ann Hepatol. 2014;13:343-352. [PubMed] |
| 8. | Kwon HJ, Won YS, Park O, Feng D, Gao B. Opposing effects of prednisolone treatment on T/NKT cell- and hepatotoxin-mediated hepatitis in mice. Hepatology. 2014;59:1094-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Mathurin P, O’Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, Ramond MJ, Naveau S, Maddrey WC, Morgan TR. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 310] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 10. | Christensen E. Alcoholic hepatitis--glucocorticosteroids or not? J Hepatol. 2002;36:547-548. [PubMed] |
| 11. | European Association for the Study of the Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012;57:399-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 464] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 12. | Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 699] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 13. | Ramond MJ, Poynard T, Rueff B, Mathurin P, Théodore C, Chaput JC, Benhamou JP. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med. 1992;326:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis--a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther. 2008;27:1167-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 15. | Carithers RL, Herlong HF, Diehl AM, Shaw EW, Combes B, Fallon HJ, Maddrey WC. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Ann Intern Med. 1989;110:685-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 325] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Mathurin P, Duchatelle V, Ramond MJ, Degott C, Bedossa P, Erlinger S, Benhamou JP, Chaput JC, Rueff B, Poynard T. Survival and prognostic factors in patients with severe alcoholic hepatitis treated with prednisolone. Gastroenterology. 1996;110:1847-1853. [PubMed] |
| 17. | Shi ZX. Optimal approach in treating and controlling hypertension-Train of thought about treatment of high blood pressure with integrative traditional and Western medicine. Chin J Integr Med. 2004;10:2-9. |
| 18. | Robinson N. Integrative medicine - traditional Chinese medicine, a model ? Chin J Integr Med. 2011;17:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Li YF, Jiao Y. 36 cases with alcohol-related severe jaundice treated by the combination of Traditional Chinese Medicine and glucocorticoid. Zhongxiyi Jiehe Ganbing Zazhi. 2012;22:346-347. |
| 20. | Wang XJ. Clinical Characteristics, Prognosis, Preliminary Study on Traditional Chinese Medicine Therapy of 65 Patients with Acute-on Alcoholic Liver Failure. Beijing: Beijing university of Chinese Medicine 2013; . |
| 21. | Zhan ZY, Sun MY. Traditional Chinese Medicine Treatment and Research Progress of Alcoholic Liver Disease. Sichuan Zhongyi Zazhi. 2014;32:182-186. |
| 22. | Tan DH. Practical Chinese Medicine Dictionary. Beijing: People’s Medical Publishing House 2012; 1897. |
| 23. | Li HY, Li X, Jin XQ. Effect of Baicalin on chronic alcoholic liver injury in rats. Zhongguo Shiyan Fangjixue Zazhi. 2008;14:58-60. |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Waheed Y S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH