Published online Apr 28, 2017. doi: 10.3748/wjg.v23.i16.2928
Peer-review started: September 22, 2016
First decision: January 10, 2017
Revised: January 24, 2017
Accepted: February 16, 2017
Article in press: February 16, 2017
Published online: April 28, 2017
Processing time: 219 Days and 19 Hours
To observe whether there are differences in the effects of electro-acupuncture (EA) and moxibustion (Mox) in rats with visceral hypersensitivity.
EA at 1 mA and 3 mA and Mox at 43 °C and 46 °C were applied to the Shangjuxu (ST37, bilateral) acupoints in model rats with visceral hypersensitivity. Responses of wide dynamic range neurons in dorsal horns of the spinal cord were observed through the extracellular recordings. Mast cells (MC) activity in the colons of rats were assessed, and 5-hydroxytryptamine (5-HT), 5-hydroxytryptamine 3 receptor (5-HT3R) and 5-HT4R expressions in the colons were measured.
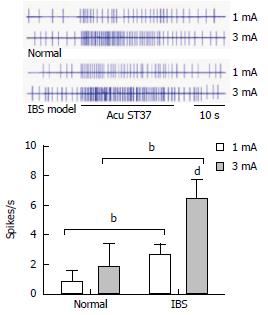
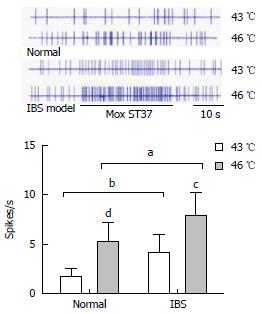
Compared with normal control group, responses of wide dynamic range neurons in the dorsal horn of the spinal cord were increased in the EA at 1 mA and 3 mA groups (1 mA: 0.84 ± 0.74 vs 2.73 ± 0.65, P < 0.001; 3 mA: 1.91 ± 1.48 vs 6.44 ± 1.26, P < 0.001) and Mox at 43 °C and 46 °C groups (43 °C: 1.76 ± 0.81 vs 4.14 ± 1.83, P = 0.001; 46 °C: 5.19 ± 2.03 vs 7.91 ± 2.27, P = 0.01). MC degranulation rates and the expression of 5-HT, 5-HT3R and 5-HT4R in the colon of Mox 46 °C group were decreased compared with model group (MC degranulation rates: 0.47 ± 0.56 vs 0.28 ± 0.78, P < 0.001; 5-HT: 1.42 ± 0.65 vs 7.38 ± 1.12, P < 0.001; 5-HT3R: 6.62 ± 0.77 vs 2.86 ± 0.88, P < 0.001; 5-HT4R: 4.62 ± 0.65 vs 2.22 ± 0.97, P < 0.001).
The analgesic effects of Mox at 46 °C are greater than those of Mox at 43 °C, EA 1 mA and EA 3 mA.
Core tip: Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder, and the visceral hypersensitivity is considered to be one of the most important factors in its pathogenesis. Both acupuncture and moxibustion can regulate visceral hypersensitivity in IBS; however, the underlying mechanisms remain unclear. The present study is designed to observe whether there are differences in the effects of electro-acupuncture with different current intensities and moxibustion at varying temperatures on visceral hypersensitivity and to explore the potential analgesic mechanisms of these two stimulations.
- Citation: Zhao JM, Li L, Chen L, Shi Y, Li YW, Shang HX, Wu LY, Weng ZJ, Bao CH, Wu HG. Comparison of the analgesic effects between electro-acupuncture and moxibustion with visceral hypersensitivity rats in irritable bowel syndrome. World J Gastroenterol 2017; 23(16): 2928-2939
- URL: https://www.wjgnet.com/1007-9327/full/v23/i16/2928.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i16.2928
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder characterized by abdominal discomfort or pain associated with abnormal bowel movements. The pathogenesis of IBS is complex and has not been fully elucidated. However, current data suggest that it is associated with visceral hypersensitivity, altered gut motility and dysfunction of the brain-gut axis[1-4]. Recently, visceral hypersensitivity was considered to be one of the specific indicators for IBS, and it can, to some extent, induce symptoms, including urgent defecation, flatulence and abdominal pain[5]. Studies have shown that acupuncture can greatly regulate visceral hypersensitivity in IBS[6-8], but the underlying mechanism has not been identified. Other research has indicated that the stimulation from acupuncture and moxibustion can converge with visceral nociceptive afferent impulses in the spinal cord[9-11]. To provide experimental evidence for electro-acupuncture (EA) and moxibustion (Mox) in treating IBS with visceral hypersensitivity, this study attempted to observe the convergence of afferent impulse induced by EA with different current intensities and Mox at varying temperatures in wide dynamic range (WDR) neurons in the dorsal horns of the spinal cord, and the subsequent influence on activation of mast cells and changes in expression of 5-HT, 5-HT3R and 5-HT4R in the colon.
Sprague-Dawley (SD) rats (male, Specific Pathogen Free, 250-300 g) were supplied by the Experimental Animal Center of the Chinese Academy of Traditional Chinese Medicine. Animals used in this study were treated according to Beijing Administration Rule of Laboratory Animal. All efforts were made to minimize the number of animals utilized and their suffering. All animal experiments in this study were performed under the guidelines approved by the Animal Ethics Committee of the China Academy of Traditional Chinese Medicine.
An experimental rat model of visceral hypersensitivity was established as previously described[12]. On the second day after the rats were fasted, the experiment was begun. Rats in the normal group were stimulated through manual manipulation around the anus, while rats in the other groups were stimulated by distending the colorectum (CRD). Daily CRD using an inflatable balloon (constructed from a latex glove finger, length 4 cm, inflated with air) attached to an intravenous line connected via a Y connector to a manual pump and a sphygmomanometer. The balloon was inserted into the colon while the animal was sedated, and CRD was induced while animals were fully awake. The distention was repeated twice daily at a 30-min interval. The rats were reared until they reached adulthood (at least 6 week old), and behavioral responses to visceral pain induced by acute CRD were then examined.
Fifty D-IBS rats were randomly assigned to five groups as follows: (1) EA 1 mA group (n = 10): needles (0.22 mm diameter, 13 mm length, Hwato, Suzhou Medical Appliance Factory, Ltd, Suzhou, China) were inserted 3-5 mm into the skin at the ST37 (Shangjuxu, bilateral) acupoints, and each acupuncture needle was connected to a HANS-100 pain relieving apparatus (Nanjing Jisheng Medical Science and Technology, Ltd, Nanjing, China) with a stimulation frequency of 2.0 Hz and a stimulation intensity of 1.0 mA; (2) EA 3 mA group (n = 10): the treatment was the same as for the EA 1 mA group with a stimulation intensity of 3 mA; (3) Mox 43 °C group (n = 10): fine moxibustion made for animal experiments was ignited and placed 20 mm ± 5 mm above the acupoints. A surface thermometer (Testo 905-T2, Testo, Germany) was used to confirm the temperature (43 °C ± 1 °C); (4) Mox 46 °C group (n = 10): the treatment was the same as for the Mox 43 °C, with a temperature confirmed to be 46 °C ± 1 °C; and (5) Model control (MC) group (n = 10): no treatment was applied, but they were monitored similarly to the experimental treatment groups. Another ten normal rats were used for the normal control (NC) group.
The acupoints of bilateral ST37 were selected, and EA and Mox treatments were each applied for a total of 10 min, once daily, for seven consecutive days. The ST37 acupoints of rats are located in the hind legs, approximately 10 mm below the head of the fibula at the distolateral aspect of the knee. Positions of rat acupoints were determined according to Experimental Acupuncture (Li Z, Chief Editor, Chinese Medicine Press, 2007).
After anesthetizing with an intraperitoneal injection of urethane (10%, 1.0-1.2 g/kg), SD rats were fixed on the operating table in a prone position. The spinal cord at L1-L3 was exposed by removing the overlying musculature along the dorsal midline, and then spinal clamps were fixed on the vertebral plate. Spinal dura mater was carefully removed under a microscope to prevent damage to the medulla. After the surgery, a groove was sutured with the flap and then covered with liquid paraffin at 38 °C in order to keep the medulla wet. Following the insertion of micropipettes, 2% agar gel was placed over the surface of the medulla, not only to prevent the medulla from drying but also to stabilize the recordings by keeping the medulla fixed during respiration.
The positions of the recorded sites were as follows: 0.5-1.5 mm lateral to the posterior median fissure of the spinal cord and 500-1500 μm beneath the surface of the medulla. During the recording, glass micropipettes (cusp: 5 μm, impedance: 8-12 MΩ) were inserted into nuclear groups to target WDR neurons. Through the micropipettes, single-unit activities were channeled into an 8301-micropipette amplifier (MEZ8301, Nihon Kohden) and Power Labdata collection system (PL4097, AD Instruments, Australia) for the amplification of cell discharge and signal management. The standard recording procedures were as follows: after neuronal discharges were stabilized, background activity was first recorded for 10 s. After 30 s of EA and Mox stimulation, the background activity was recorded again for 20 s.
Each acupuncture needle (0.25 mm × 25 mm) was connected to a HANS-100 pain relieving apparatus with a stimulation frequency of 2.0 Hz and a stimulation intensity of 1 mA and 3 mA. Mox stimulation at the temperatures of 43 °C ± 1 °C and 46 °C ± 1 °C were randomly applied. Both of EA and Mox were applied to Shangjuxu (ST37, bilateral) acupoints on the same side of the receptor field.
The abdominal withdrawal reflex (AWR) scoring criteria are shown in Table 1, using a described procedure[12]. Rats in the treatment group were fasted beginning from the afternoon of the previous day. Vaseline was smeared on the surface of the balloon which was then slowly inserted into 5 cm of the rat anus according to the physical curve of the colorectum, and retained for 5 min. The test was initiated when the rats became adapted. After air was added into the balloon with a syringe, the rat rectum was stimulated and different degrees of contraction reactions were observed. The pressure (mmHg) during behavior responses, scored as 1, 2, 3, and 4, was recorded and expressed as the threshold of sensitivity (Table 1). Each score was tested three times, and each rat was tested by two persons who had not participated in the research design. Means were calculated (6 values in total). Three-minute intervals were used between each of the two tests to allow for full adaptation of rats.
| Score 0 No behavioral response to colorectum |
| Score 1 Immobile during distension of CR and occasional clicking the head at onset of the stimulus |
| Score 2 A mild contraction of abdominal muscles, but no lifting of abdomen off the plattorm |
| Score 3 A strong contraction of abdominal muscles and lifting of abdomen off the platform, no lifting of pelvic structure off the platform |
| Score 4 Arching body and lifting of pelvic structure and scrotum |
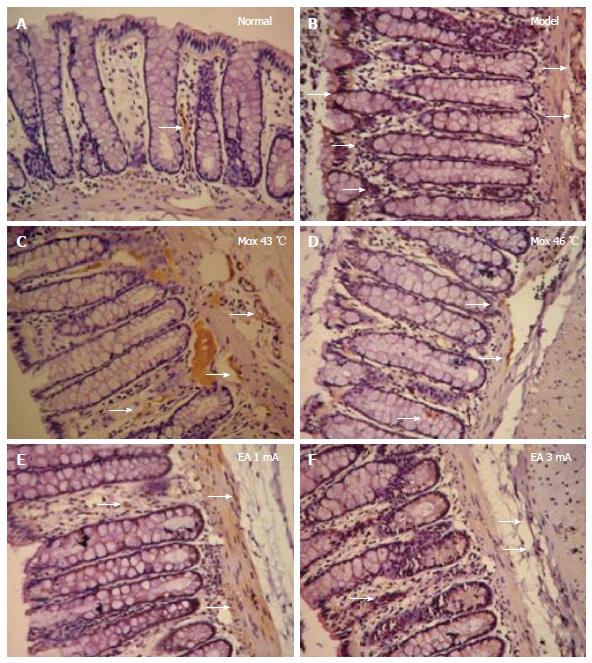
Toluidine blue staining was used to stain MCs. First, de-paraffinization and hydration of 4-μm paraffin-embedded sections was achieved by soaking samples in xylenes I and II for 20 min each and then anhydrous graded ethanol was applied (90%, 80%, and 70% for 5 min each). Toluidine was then added to the tissue sections and stained for 20 min. Samples were washed in distilled water for 10 s and 0.5% acetic acid was added to differentiate color. Slides were observed under a light microscope until the cytoplasm turned purple-red. Samples were dehydrated with 95% ethanol for 1 min and then with anhydrous ethanol for 1 min two times. Tissues were cleared twice with xylene for 20 min each. The appropriate amount of neutral resin was added and sections were sealed. The sample slides (× 400) and counted MCs were observed. Cytoplasmic granules were stained purple-red and nuclei were stained blue. Clear, smooth, and intact cytoplasmic membranes with clear indicated stable MCs. Broken cytoplasmic membranes with purple-red granules around cells indicated degranulated MCs. MCs were counted in three non-overlapping random views and means were obtained for total and degranulated MCs. Degranulation rates were calculated as follows: degranulation rate (%) = degranulated MC count/total MC count × 100%.
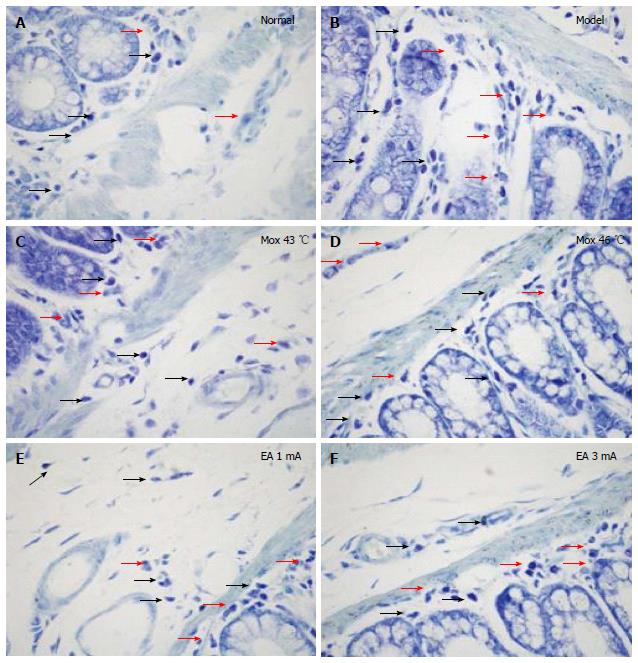
The expressions of 5-HT, 5-HT3R and 5-HT4R in the colonic tissue of D-IBS rats was detected by immunohistochemical (IHC) staining. The sections were exposed to 0.01 mol/L citrate buffer (pH 6.0), microwaved at 30% power for 20 min for heat fixation, and then cooled to room temperature. The sections were washed 3 times with phosphate buffered saline (PBS) for 3 min and exposed to 0.3% H2O2 for 20 min at room temperature to inhibit endogenous peroxidases. Following a final PBS wash (3 min × 3 min), the samples were exposed to 20% normal goat serum and incubated for 30 min. Antibodies were added drop-wise (5-HT 1:100, 5-HT3R 1:50, 5-HT4R 1:80, Santa Cruz, CA, United States), and the sections were incubated at 37 °C for 2 h. The sections were washed with PBS 3 times for 3 min, incubated in horseradish peroxidase/rabbit reagent at 37 °C for 30 min, and PBS washed 3 times for 3 min. The sections were then incubated in 3,3-diaminobenzidine (DAB) chromogenic reagent for 8-12 min and dyed with hematoxylin lining and blue in the presence of hot water. After drying, the sections were sealed with neutral gum for further observation under a light microscope. A semi-quantitative analysis of the staining was performed using the medical image quantitative analysis system (MIQAS, Shanghai Qiuwei Biomedical Technology Co., Shanghai, China). Positive results were indicated by the presence of brown or tan particles in the stained colonic tissue cells. In each slice, three positive areas were counted and assessed for optical density (OD) in a high power field to calculate an IHC positive index (positive area × OD/total area) for 5-HT, 5-HT3R and 5-HT4R.
All statistical analyses were performed using SPSS 19.0 (SPSS Inc. Chicago, IL, United States). AWR scores for rats are presented as interquartile ranges. Differences in means were compared by one-way ANOVA testing. Non-normal data were compared using a non-parametric test. All two-sided P values < 0.05 were considered statistically significant.
Ten WDR neurons were recorded in the dorsal horn of the spinal cord for 11 control rats and 12 model rats, and the effects of EA with different current intensities on the WDR neurons were observed. It was found that the background activities of these neurons were activated by EA. Within the range of 1 mA and 3 mA, the degree of neuronal activation was increased concurrently with the increase in current intensity. When EA with 1 mA was applied, the degree of activation in the WDR neurons of normal rats was 0.84 ± 0.74 spikes/s, while that of model rats increased to 2.73 ± 0.65 spikes/s. There were statistically significant differences between the two groups of rats (P < 0.001). When the intensity increased to 3 mA, the degree of activation in the WDR neurons of normal rats was 1.91 ± 1.48 spikes/s, while that of model rats increased to 6.44 ± 1.26 spikes/s. There were also statistically significant differences between the two groups (P < 0.001). It is evident that EA with different intensities on ST37 acupoints has increased activation effects on WDR neurons in the dorsal horn of the spinal cord in model rats with visceral hypersensitivity, compared to normal rats (Figure 1).
Ten WDR neurons in the dorsal horn of the spinal cords of 11 normal rats and 13 model rats were recorded, respectively, and the effects of Mox at different temperatures on the WDR neurons were observed. The background activities of these neurons were activated by Mox. At a range of 43 °C - 46 °C, the degree of neuronal activation was increased concurrently with the increase in temperature. When Mox at 43 °C was applied, the degree of activation in the WDR neurons of normal rats was 1.76 ± 0.81 spikes/s, while that of model rats increased to 4.14 ± 1.83 spikes/s. There were statistically differences between the two groups (P = 0.001). When the temperature increased to 46 °C, the degree of activation in the WDR neurons of normal rats was 5.19 ± 2.03 spikes/s, while that of model rats increased to 7.91 ± 2.27 spikes/s. There were also statistically differences between the two groups (P = 0.01). It is evident that Mox applied at different temperatures to ST37 acupoints has increased activation effects on WDR neurons in the dorsal horn of the spinal cord in model rats with visceral hypersensitivity, as compared with normal rats (Figure 2).
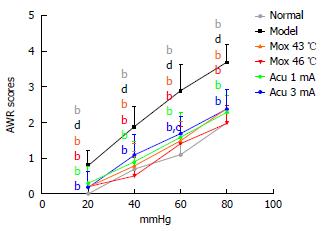
AWR scores of the Mox at 43 °C, Mox at 46 °C, EA 1 mA groups and the EA 3 mA group were all significantly different from the model group at CRD pressure of 20, 40, 60, and 80 mmHg (P < 0.01). AWR scores of the NC group were not significantly different from the Mox at 43 °C, Mox at 46 °C and EA 1mA groups at CRD pressure of 20, 40, 60, and 80 mmHg (20 mmHg: PMox43 °C = 0.265, PMox46 °C = 0.265, PAcu1mA = 0.097; 40 mmHg: PMox43 °C = 0.693, PMox46 °C = 0.401, PAcu1mA = 0.416; 60 mmHg: PMox43 °C = 0.119, PMox46 °C = 0.262, PAcu1mA = 0.069; 80 mmHg: PMox43 °C = 0.063, PMox46 °C = 1.000, PAcu1mA = 0.170). AWR scores of the EA 3 mA group at 20mmHg, 40 mmHg and 80 mmHg were not significantly different from the NC group (20 mmHg: P = 0.265, 40 mmHg: P = 0.107, 80 mmHg: P = 0.063), whereas AWR scores of the EA 3mA group at 60 mmHg were different from the NC group (60 mmHg: P = 0.016). These data indicated that Mox at 46 °C and EA at 1 mA treatments were able to decrease visceral hypersensitivity or increase the pain threshold significantly, and they were superior to the EA 3 mA group (Figure 3).
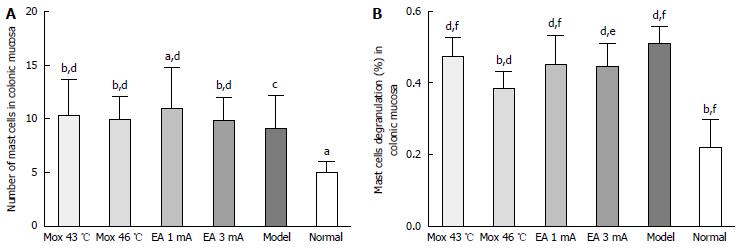
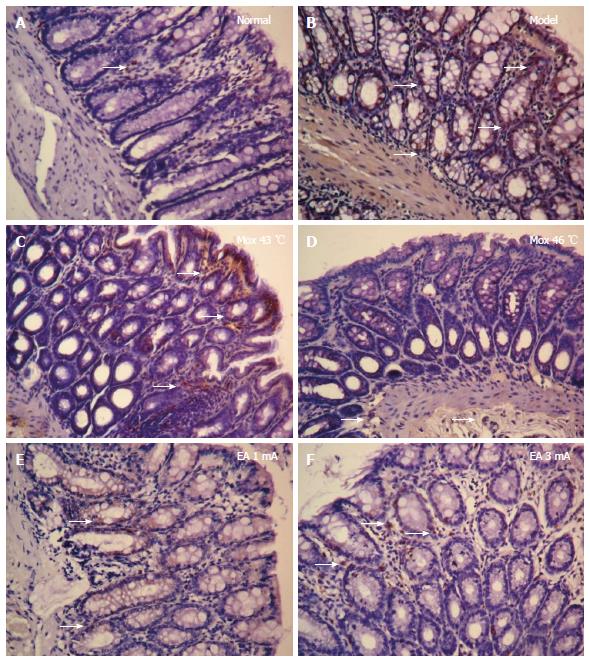
Compared with the NC group, the MC counts in the colon were significantly increased in the Mox at 43 °C and 46 °C groups, and EA at 1 mA and 3 mA groups (PMox43 °C < 0.001, PMox46 °C < 0.001, PAcu1mA = 0.007, PAcu3mA < 0.001). The MC degranulation rates in the colon were also significantly increased in the Mox at 43 °C and Mox at 46 °C and EA at 1 mA and 3 mA groups compared with the NC group (P < 0.001). Compared with the MC group, the MC degranulation rates in the colon were significantly decreased in the Mox at 46 °C group (P < 0.001), and there were differences in MC degranulation rates between the Mox at 46 °C group and the Mox at 43 °C group (P = 0.005) (Figures 4 and 5).
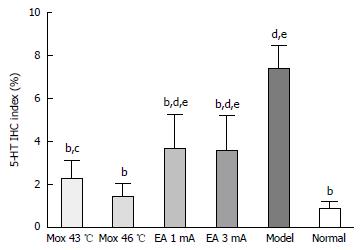
Compared with the MC group, 5-HT expressions in the colon were significantly decreased in the NC group, the Mox at 43 °C and 46 °C groups and the EA at 1 mA, 3 mA groups (P < 0.001), and the expressions in the Mox at 43 °C and 46 °C groups were significantly lower than in the EA at 1 mA, 3 mA groups (PMox43 °CvsAcu1mA = 0.007, PMox43 °CvsAcu3mA = 0.014, PMox46 °CvsAcu1mA < 0.001, PMox46 °CvsAcu3mA < 0.001). 5-HT expression in the colon was not significantly different between the Mox 46 °C group and the NC group (P = 0.332) (Figures 6 and 7).
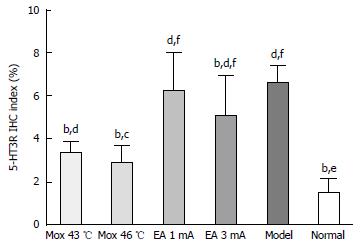
Compared with the MC group, 5-HT3R expressions in the colon were significantly decreased in the NC group, the Mox at 43 °C and 46 °C groups and the EA at 3 mA group (PNC < 0.001, PMox43 °C < 0.001, PMox46 °C < 0.001, PAcu3mA = 0.008), and the expressions in the Mox at 43 °C and 46 °C groups were significantly reduced compared to the EA at 1 mA and 3 mA groups (PMox43 °CvsAcu1mA < 0.001, PMox43°CvsAcu3mA = 0.002, PMox46 °CvsAcu1mA < 0.001, PMox46 °CvsAcu3mA < 0.001)(Figures 8 and 9).
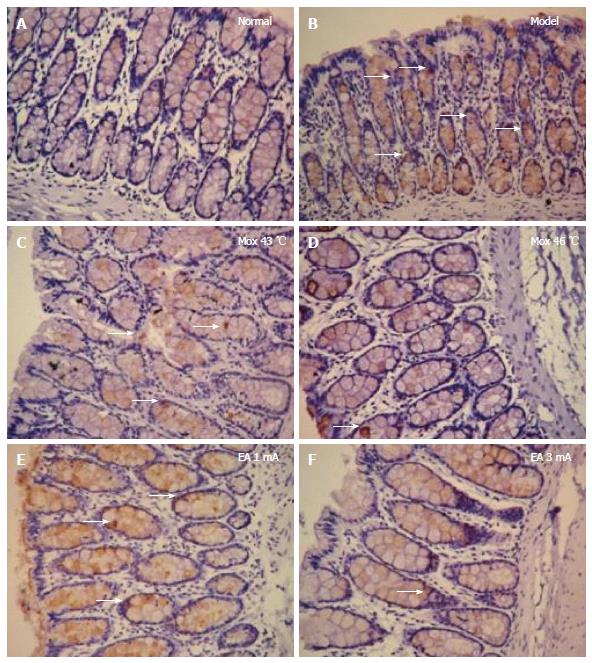
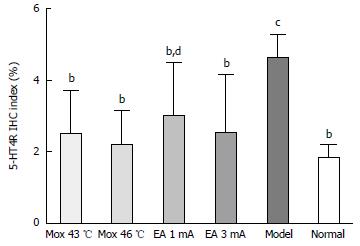
Compared with the MC group, 5-HT4R expressions in the colon were significantly decreased in the NC group, the Mox at 43 °C and 46 °C groups and the EA at 1 mA and 3 mA groups (PMox43 °C < 0.001, PMox46 °C < 0.001, PAcu1mA = 0.003, PAcu3mA < 0.001). 5-HT4R expressions in the colon were not significantly different between the NC group and the Mox at 43 °C and 46 °C groups and the EA at 3 mA group (PMox43 °C = 0.184, PMox46 °C = 0.448, PAcu3mA = 0.178), whereas there was a statistically significant between the NC group and EA 1 mA group (PAcu1mA = 0.025)(Figures 10 and 11).
Studies have proven that acupuncture and moxibustion have analgesic effects[13,14]. Receptors in skin or muscles are activated by the mechanical stimulation of acupuncture and the heat stimulation of moxibustion, which affects nervous system and the biological activity of tissues and cells to relieve pains[15,16]. With the help of the supraspinal cord, afferent impulses induced by acupuncture and moxibustion can transmit along peripheral A or C fibers to the dorsal horn of the spinal cord, where they will converge and interact with the visceral nociceptive afferent impulse. Therefore, acupuncture and moxibustion can inhibit the response of neurons in the dorsal horn of the spinal cord activated by visceral nociceptive afferent impulses[11,17]. Afferent impulses of organs or deep tissues can sensitize related segmental neurons, which makes the impulse responses from the body surface more intense. Meanwhile, the size and function of acupoints can change with the visceral functions[18], and in pathological conditions, the function of an acupoint area in reflecting and treating disease can be greatly enhanced to reach the status of acupoints area sensitization[19]. Visceralgia and sensitization interact with each other. Therefore, the dorsal horn of the spinal cord is the key to regulation of visceral hypersensitivity. The function of acupoints is not only related to the sensitization in the condition of visceral hyperalgesia, but is also associated with the convergence of body surface afferent and visceral nociceptive afferents in the spinal cord or supraspinal cord[20]. Acupuncture and moxibustion transmit signals through the activation of receptors in acupoint areas or through bioactive substances, and the stimulation of acupuncture in the body surface can converge with visceral nociceptive stimuli in the spinal cord to regulate the body functions.
Our study found that while stimulating ST37 in model rats with visceral hypersensitivity, with the increase of EA intensities from 1 mA to 3 mA and Mox temperature from 43 °C to 46 °C, the activation reaction of WDR neurons in the dorsal horn of the spinal cord was increased. Meanwhile, the intestinal pain threshold of model rats with visceral hypersensitivity was markedly inhibited. The abnormal activation of mast cells that induce gut motility and secretion in the colon and abnormal expression of 5-HT, 5-HT3R and 5-HT4R were also significantly inhibited. This indicates that the analgesic effects of EA and/or Mox on the target tissues of model rats with visceral hypersensitivity not only have a positive correlation with their intensities, but are also associated with the fact that WDR neurons can converge and integrate the stimulation induced by EA and Mox. It has been reported that needling the acupoints near the pain spot or the adjacent vertebrae would achieve better analgesic effects[21]. Furthermore, the analgesic effects were achieved when Aδ fibers were activated, and they became stronger when type-C fibers were activated[22]. In this study, ST37 was selected as the location for EA and Mox application, and ST37 was located in the receptive field of WDR neurons that involved convergence and integration of visceral nociceptive afferent impulses[23]. We applied EA with 1 mA and 3 mA intensities, and Mox at 43 °C and 46 °C, to ST37 of normal rats and model rats respectively. It was found that EA with 3 mA and Mox at 46 °C can achieve more significant activation of WDR neurons in the dorsal horn of the spinal cord. Stimulation with low intensities, such as 1 mA EA and 43 °C Mox, can only activate type A neural fibers, whereas stimulation with high intensities, such as 3 mA EA and 46 °C Mox, can also activate type C fibers, thereby inducing greater analgesic effects.
Abnormal activation of mass cells in the colon is one of the main pathological characteristics of visceral hypersensitivity (including gut motility, abnormal secretion and visceral pain)[24,25]. In this study, the MC counts and degranulation rates in the colons of all model rats (model group, 1 mA EA group, 3 mA EA group, 43 °C Mox group, 46 °C Mox group) were greatly increased compared with those of normal rats, which indicates that with visceral hypersensitivity, the counts of MCs in the colon are not only significantly increased, but also present abnormal activation (degranulation). Furthermore, degranulated MCs may release large quantities of active substances, such as histamine and 5-HT. According to several studies, as an important agent of regulating the brain-gut axis and the intestinal neural system, 5-HT can regulate visceral perception and visceral neural reflex[26]. The activation of 5-HT3R can induce cellular the pathways for non-specific positive ion movement, allowing the entry of Na+ and Ca2+ and the presence of K+. These pathways of positive ion movement can depolarize postsynaptic neurons, transmit signals around and induce the release of excitatory or inhibitory neurotransmitters. In this way, smooth muscles may be contracted and relaxed abnormally[27]. In addition, 5-HT can influence gastrointestinal mobility through 5-HT4R, as activated 5-HT4R can play an active role in regulating gut mobility and visceral hyperalgesia through the induction of a release of acetylcholine or neurotransmitters for P motor nerves[28,29].
In our study, we observed that after applying EA with different intensities and Mox at varying temperatures, degranulation rate of MC in the colons of rats with visceral hypersensitivity was reduced in varying degrees, which demonstrates that EA and Mox, especially the Mox at 46 °C, have an inhibitory function towards the abnormally activated MCs in areas with visceral hypersensitivity. At the same time, it was also found that the expression levels of 5-HT, 5-HT3R and 5-HT4R in model rats were much higher than those in normal group, as were the AWR scores. After stimulation of EA with 1 mA and 3 mA and Mox at 43 °C and 46 °C was performed, compared to the model group, the expression of 5-HT, 5-HT3R and 5-HT4R was decreased to varying degrees. Mox was more effective than EA in reducing the expression. Meanwhile, AWR scores for all treatment groups (especially the Mox at 46 °C) were decreased markedly compared with the model group. These results indicate that in colons of rats with visceral hypersensitivity, MCs are activated abnormally and 5-HT, 5-HT3R and 5-HT4R have increased expression. Stimulating ST37 with EA with different intensities and Mox at varying temperatures can activate the WDR neurons in the dorsal horn of the spinal cord, thus relieving pains in the target organs (colons). Among four different stimulations, Mox at 46 °C has the best analgesic effects. Additionally, we also found that the analgesic effects were achieved by EA and Mox through WDR neurons of the spinal cord, which may affect the abnormal MC activation and high expression of 5-HT, 5-HT3R and 5-HT4R by converging and integrating the visceral nociceptive afferent impulse with stimulation induced by EA and Mox at the ST37 acupoints. These results may reveal one of the mechanisms of analgesic effects of acupuncture in treating IBS with visceral hypersensitivity.
There are differences between stimulation by EA with different current intensities and Mox at different temperatures in the relief of visceral hypersensitivity. The analgesic effects of Mox at 46 °C are much greater than those of Mox at 43 °C, EA at 1 mA and EA at 3 mA. A potential mechanism is that WDR neurons from the dorsal horn of the spinal cord may converge and integrate the stimulation induced by EA and Mox in the segmental receptor field with visceral nociceptive afferent impulses and then influence the MC activation reaction of the colon and the high expression of 5-HT, 5-HT3R and 5-HT4R.
We are grateful to Bing Zhu, Pei-Jing Rong, Xin-Yan Gao from Institute of Acu-Mox, China Academy of Chinese Medical Sciences for their guidance on the electrophysiological experiments.
The pathogenesis of irritable bowel syndrome is complex and has not been fully elucidated, recently visceral hypersensitivity was considered to be one of the specific indicators for irritable bowel syndrome (IBS), and it can, to some extent, induce the symptoms, including urgent defecation, flatulence and abdominal pain. Acupuncture and moxibustion both can regulate visceral hypersensitivity in IBS, however, the underlying mechanisms remain unclear.
Previous studies have shown that acupuncture can greatly regulate visceral hypersensitivity in IBS, this study attempted to provide experimental evidence for electro-acupuncture (EA) and moxibustion (Mox) in treating IBS with visceral hypersensitivity.
This study attempted to observe the convergence of afferent impulse induced by EA with different current intensities and Mox at varying temperatures in wide dynamic range neurons in the dorsal horn of the spinal cord, and its influence on activation of mast cells and expression changes of 5-HT, 5-HT3R and 5-HT4R in colon.
To provide experimental evidence for EA and Mox in treating IBS with visceral hypersensitivity, and the results may reveal one of the mechanisms of analgesic effects of acupuncture in treating IBS with visceral hypersensitivity.
This manuscript is a well-conducted study with moderate relevance and novelty.
| 1. | Blomhoff S, Spetalen S, Jacobsen MB, Vatn M, Malt UF. Intestinal reactivity to words with emotional content and brain information processing in irritable bowel syndrome. Dig Dis Sci. 2000;45:1160-1165. [PubMed] |
| 2. | Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, Shoji T, Karahashi K, Hongo M, Fukudo S. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958-964. [PubMed] |
| 3. | Sinhamahapatra P, Saha SP, Chowdhury A, Chakrabarti SK, Ghosh A, Maiti B. Visceral afferent hypersensitivity in irritable bowel syndrome--evaluation by cerebral evoked potential after rectal stimulation. Am J Gastroenterol. 2001;96:2150-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Rössel P, Pedersen P, Niddam D, Arendt-Nielsen L, Chen AC, Drewes AM. Cerebral response to electric stimulation of the colon and abdominal skin in healthy subjects and patients with irritable bowel syndrome. Scand J Gastroenterol. 2001;36:1259-1266. [PubMed] |
| 5. | Zhou Q, Price DD, Caudle RM, Verne GN. Visceral and somatic hypersensitivity in a subset of rats following TNBS-induced colitis. Pain. 2008;134:9-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Liu HR, Yang Y, Wu HG. Clinical study on acupuncture in treating diarrhea-predominant irritable bowel syndrome. Zhenjiu Tuina Yixue. 2008;6:360-362. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Schneider A, Weiland C, Enck P, Joos S, Streitberger K, Maser-Gluth C, Zipfel S, Bagheri S, Herzog W, Friederich HC. Neuroendocrinological effects of acupuncture treatment in patients with irritable bowel syndrome. Complement Ther Med. 2007;15:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 8. | Chu WC, Wu JC, Yew DT, Zhang L, Shi L, Yeung DK, Wang D, Tong RK, Chan Y, Lao L. Does acupuncture therapy alter activation of neural pathway for pain perception in irritable bowel syndrome?: a comparative study of true and sham acupuncture using functional magnetic resonance imaging. J Neurogastroenterol Motil. 2012;18:305-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Yu LL, Li L, Rong PJ, Zhu B, Qin QG, Ben H, Huang GF. Changes in responses of neurons in spinal and medullary subnucleus reticularis dorsalis to acupoint stimulation in rats with visceral hyperalgesia. Evid Based Complement Alternat Med. 2014;2014:768634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Li L, Yu L, Rong P, Ben H, Li X, Zhu B, Chen R. Visceral nociceptive afferent facilitates reaction of subnucleus reticularis dorsalis to acupoint stimulation in rats. Evid Based Complement Alternat Med. 2013;2013:931283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Rong PJ, Zhu B, Huang QF, Gao XY, Ben H, Li YH. [Acupuncture inhibiting responses of spinal dorsal dorsal horn neurons induced by noxious dilation rectum and colon]. Zhongguo Zhen Jiu. 2005;25:645-650. [PubMed] |
| 12. | Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276-1285. [PubMed] |
| 13. | Palecek J, Paleckova V, Willis WD. The roles of pathways in the spinal cord lateral and dorsal funiculi in signaling nociceptive somatic and visceral stimuli in rats. Pain. 2002;96:297-307. [PubMed] |
| 14. | Liu HR, Qi L, Wu LY, Ma XP, Qin XD, Huang WY, Dong M, Wu HG. Effects of moxibustion on dynorphin and endomorphin in rats with chronic visceral hyperalgesia. World J Gastroenterol. 2010;16:4079-4083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Zhang J, Zhang N. Study on mechanisms of acupuncture analgesia. Zhongguo Zhen Jiu. 2007;27:72-75. |
| 16. | Huang R, Zhao J, Wu L, Dou C, Liu H, Weng Z, Lu Y, Shi Y, Wang X, Zhou C. Mechanisms underlying the analgesic effect of moxibustion on visceral pain in irritable bowel syndrome: a review. Evid Based Complement Alternat Med. 2014;2014:895914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Bing Z, Villanueva L, Le Bars D. Acupuncture and diffuse noxious inhibitory controls: naloxone-reversible depression of activities of trigeminal convergent neurons. Neuroscience. 1990;37:809-818. [PubMed] |
| 18. | Yu XC, Zhu B, Gao JH, Fu WX, Lu B, Cui HF, Qin LP. The scientific basis of the dynamic process of acupoints. Zhongyi Zazhi. 2007;48:971-973. |
| 19. | Ziegler EA, Magerl W, Meyer RA, Treede RD. Secondary hyperalgesia to punctate mechanical stimuli. Central sensitization to A-fibre nociceptor input. Brain. 1999;122:2245-2257. [PubMed] |
| 20. | Yu LL, Li L, Qin QG, Ben H, Rong PJ, Zhu B. Colorectal nociceptive signal input facilitates impact of acupoint stimulation of “Zusanli” (ST36) on electrical activities of wide dynamic range neurous in lumbar spinal cord in rats. Zhenci Yanjiu. 2014;39:390-395. |
| 21. | Zhu B, Rong PJ, Ben H, Gao XY, Li YQ. Study on the mechanism of acupuncture analgesia of segmental and systemic character. Zhenci Yanjiu. 2007;32:1. |
| 22. | Rong PJ. Convergence and interaction between acupuncture signals and the inputs of visceral nociception. Beijing: Beijing University of Traditional Chinese Medicine 2004; . |
| 23. | Zhu B. Systematic acupuncture and moxibustion. Beijing: People’s Medical Publishing House 2015; 115. |
| 24. | Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693-702. [PubMed] |
| 25. | Shibata S. Novel cardiostimulant polypeptides (anthopleurin-A, B and C) isolated from sea anemone. Jpn J Pharmacol. 1980;30:7P-9P. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 597] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 26. | Grundy D. 5-HT system in the gut: roles in the regulation of visceral sensitivity and motor functions. Eur Rev Med Pharmacol Sci. 2008;12 Suppl 1:63-67. [PubMed] |
| 27. | Camilleri M. Serotonergic modulation of visceral sensation: lower gut. Gut. 2002;51 Suppl 1:i81-i86. [PubMed] |
| 28. | Yan C, Xin-Guang L, Hua-Hong W, Jun-Xia L, Yi-Xuan L. Effect of the 5-HT4 receptor and serotonin transporter on visceral hypersensitivity in rats. Braz J Med Biol Res. 2012;45:948-954. [PubMed] |
| 29. | Mader R, Kocher T, Haier J, Wieczorek G, Pfannkuche HJ, Ito M. Investigation of serotonin type 4 receptor expression in human and non-human primate gastrointestinal samples. Eur J Gastroenterol Hepatol. 2006;18:945-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): C
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Garg P S- Editor: Ma YJ L- Editor: Ma JY E- Editor: Wang CH