Published online Apr 28, 2017. doi: 10.3748/wjg.v23.i16.2912
Peer-review started: January 12, 2017
First decision: February 9, 2017
Revised: February 28, 2017
Accepted: March 15, 2017
Article in press: March 15, 2017
Published online: April 28, 2017
Processing time: 106 Days and 19.8 Hours
To investigate the antioxidant and hepatoprotective effects of Cortex Dictamni aqueous extract (CDAE) in carbon tetrachloride (CCl4)-induced liver damage in rats.
The in vitro antioxidant effect of CDAE was investigated using α,α-diphenyl-β-picrylhydrazyl (DPPH), 2,2’-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), β-carotene bleaching, reducing power, and thiobarbituric acid reactive substance assays. A linoleic acid system, including ferric thiocyanate (FTC) and thiobarbituric acid (TBA) assays, was used to evaluate the inhibition of lipid peroxidation. The in vivo hepatoprotective and antioxidant effects of CDAE against CCl4-induced liver damage were evaluated in Sprague-Dawley rats. Silymarin was used as a positive control. Liver damage was assessed by determining hepatic histopathology and liver marker enzymes in serum. Enzyme and non-enzyme antioxidant levels and lipid peroxide content were measured in the liver. Cytochrome P450 2E1 (CYP2E1) protein expression was measured via immunohistochemical staining. Nuclear factor E2-related factor (Nrf2), heme oxygenase-1 (HO-1), NAD(P)H quinine oxidoreductase 1 (NQO1), and γ-glutamylcysteine synthetase catalytic subunit (γ-GCSc) protein expression was measured by Western blot.
Our results showed that CDAE exhibited a strong antioxidant activity in vitro. CDAE scavenged DPPH and ABTS radicals in a dose-dependent manner. CDAE inhibited lipid peroxidation with a lipid peroxide inhibition rate of 40.6% ± 5.2%. In the FTC and TBA assays, CDAE significantly inhibited lipid peroxidation (P < 0.01). In vivo histopathological studies indicated that CCl4-induced liver injury was alleviated following CDAE treatment in rats of both sexes. CDAE (160 and 320 mg/kg) significantly prevented CCl4-induced elevations of alkaline phosphatase, glutamate pyruvate transaminase, aspartate aminotransferase, and total bilirubin levels in rats of both sexes (P < 0.05, 0.01, or 0.001). Moreover, CDAE restored the decreased activities of hepatic antioxidant enzymes, including superoxide dismutase, catalase, and glutathione peroxidase, as well as non-enzyme antioxidant glutathione, which were induced by CCl4 treatment. CDAE significantly suppressed the up-regulation of CYP2E1 and promoted Nrf2, HO-1, NQO1, and γ-GCSc protein expression.
CDAE exhibits good antioxidant performance in vitro, with marked radical-scavenging and anti-lipid peroxidation activities. CDAE is effective in preventing CCl4-induced hepatic damage in rats of both sexes. The hepatoprotective activity of CDAE may be attributable to its antioxidant activity, which may involve Keap1-Nrf2-mediated antioxidant regulation.
Core tip: This study is the first to systematically investigate the antioxidant activity of Cortex Dictamni aqueous extract (CDAE) in vitro, especially its anti-lipid perioxidation activity, which is important for physiological function and pathological processes. In traditional Chinese medicine, Cortex Dictamni has been used to treat hepatic disease, but has lacked sufficient support and research. Therefore, to the best of our knowledge, our study is the first to demonstrate the effects of CDAE in CCl4-induced hepatic injury in rats.
- Citation: Li L, Zhou YF, Li YL, Wang LL, Arai H, Xu Y. In vitro and in vivo antioxidative and hepatoprotective activity of aqueous extract of Cortex Dictamni. World J Gastroenterol 2017; 23(16): 2912-2927
- URL: https://www.wjgnet.com/1007-9327/full/v23/i16/2912.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i16.2912
The liver is a critical organ in the human body that is responsible for the detoxification of exogenous and endogenous compounds. Hepatic injury can be caused by toxic chemicals, drugs, and viral infiltration via ingestion or infection[1,2]. Notably, synthetic drugs used to treat hepatic injury have limited therapeutic effects and are sometimes associated with adverse effects[3]. Therefore, it is necessary to explore alternative treatments for hepatic injury.
Acute liver injury is common and can be easily triggered by various toxicants[4]. Carbon tetrachloride (CCl4), a well-known environmental biohazard, has been widely used for the experimental induction of acute liver injury. CCl4 primarily accumulates in hepatic parenchymal cells and is catalysed by the phase I metabolic enzyme cytochrome P450 2E1 (CYP2E1) to produce unstable free trichloromethyl radicals (CCl▪ 3). These radicals then react with oxygen to form trichloromethyl-peroxyl (CCl3O▪ 2) radicals and reactive oxygen species (ROS)[5]. Hepatocytes contain an antioxidant system that helps the liver eliminate excessive ROS. However, once a burst of ROS formation occurs, ROS accumulation facilitates oxidative stress. Excessive ROS are capable of binding to proteins or lipids and inducing lipid peroxidation and oxidative damage[6,7]. Therefore, antioxidant compounds are believed to ameliorate oxidative stress during CCl4-induced acute liver injury[6].
The genus Dictamnus includes approximately five species, which are distributed across Europe and Asia. Of these, Dictamnus dasycarpus Turcz, whose root bark (Cortex Dictamni) has been used in Chinese folk medicine to treat jaundice, chronic hepatitis, cough, rheumatism and some skin diseases[8-10], is widely distributed throughout China[11]. Limonoids, furoquinoline alkaloids, and flavonoids isolated from Cortex Dictamni have proven to be antioxidant[12,13]. A recent study of in vitro antioxidant capacity found that Cortex Dictamni acetone extract is a good scavenger of free radicals[14]. Additionally, our previous in vivo study showed that Cortex Dictamni aqueous extract (CDAE) can inhibit lipid peroxidation by increasing antioxidant enzyme activity in ApoE-/- mice and in myocardial ischemia-reperfusion rats[15,16]. Based on these findings, we hypothesized that CDAE may exhibit antioxidant properties in vitro. Therefore, in the present study, the antioxidant effect of CDAE was systematically evaluated in vitro, including its radical-scavenging activity and anti-lipid peroxidation activity.
In recent years, many studies have reported that traditional herbal medicines and their extracts effectively inhibit hepatic pathologies[17]. CDAE has also been demonstrated to improve the hepatic injury induced by delayed-type hypersensitivity by inhibiting the immune response[18]. Moreover, Cortex Dictamni ethanol extract protects mice from hepatic fibrosis[19]. However, the protective effect of CDAE against CCl4-induced hepatic injury in rats has yet to be addressed.
Hence, the present study aimed to systematically evaluate the antioxidant activity of CDAE in vitro, including its radical-scavenging activity and anti-lipid peroxidation activity, and to investigate the hepatoprotective effect of CDAE, as well as its mechanism, on CCl4-induced acute liver injury in rats.
α,α-Diphenyl-β-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), thiobarbituric acid (TBA), ascorbic acid (Vc), and β-carotene were purchased from Sigma-Aldrich (St. Louis, MO, United States). Linoleic acid was purchased from Alfa Aesar (Ward Hill, MA, United States). Butylated hydroxyltoluene (BHT), potassium ferricyanide, and trichloroacetic acid were obtained from the National Medicine Group Chemical Reagent Co., LTD (Beijing, China). The kits for determination of glutamate-pyruvic transaminase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP), total protein (TP), and albumin (ALB) were purchased from Beckman (Atlanta, GA, United States). The kits for determination of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), glutathione (GSH), malondialdehyde (MDA), and total bilirubin (TBIL) were obtained from Nanjing Jiancheng Institute of Biological Engineering (Nanjing, China). Nuclear factor E2-related factor (Nrf2) and heme oxygenase-1 (HO-1) antibodies were obtained from Abcam (Cambridge, MA, United States). NAD(P)H quinine oxidoreductase 1 (NQO1), γ-glutamylcysteine synthetase catalytic subunit (γ-GCSc), and cytochrome P450 2E1 (CYP2E1) antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, United States).
The Chinese herb Bai-Xian-Pi was purchased from Beijing Qiancao Chinese Herbal Pieces Co., Ltd (Beijing, China) and identified by Prof. Zhongmei Zou (Pharmacology and Toxicology Research Center, Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College) as Dictamnus dasycarpus Turcz. A voucher specimen (No. 010001850001) was deposited in the National Compound Bank of Traditional Chinese Medicines of the Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Beijing.
Standardized aqueous extract was prepared as previously reported[20]. Briefly, 5 kg of Cortex Dictamni decoction pieces were boiled in 50 L of distilled water under reflux for 2 h. The decoction was then filtered and the filtrates were concentrated using a vacuum evaporator. The concentrated solution was lyophilized under reduced pressure. The yield of this extract was approximately 19% (w/w). The dried residue was stored at 4 °C and was dissolved in distilled water at the time of use.
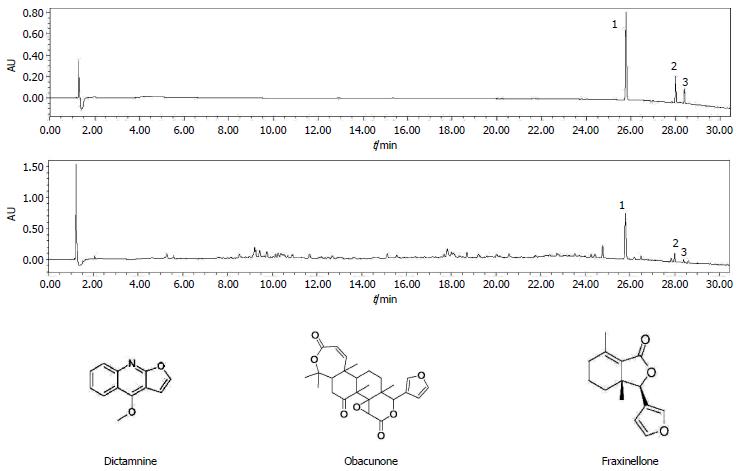
Ultra-performance liquid chromatography (UPLC) analysis was performed on a Waters AcquityTM Ultra Performance LC system (Waters Corporation, Milford, MA, United States) equipped with a Waters HSS T3TM (150 mm × 2.1 mm, 1.8 μm) column. The method and chromatographic condition were as described previously[20]. Stock solutions of three reference substances (dictamnine, obacunone and fraxinellone) were prepared at 2 mg/mL in methanol. CDAE (200 mg) was dissolved in 10 mL of deionized water and extracted three times with water-saturated butanol. The filtrates were then combined and concentrated under vacuum. The final extract was dissolved in 5 mL of methanol and filtered through a 0.22-μm syringe filter before use. Finally, 2 μL of the resulting solutions were injected into the LC instrument for UPLC analysis.
The mobile phase with a flow rate of 0.3 mL/min consisted of aqueous with 0.1% formic acid (A) and acetonitrile (B). The following solvent gradient system was used: 1% B from 0 to 2 min, 1%-16% B from 2 to 8 min, 16% B from 8 to 12 min, 16%-28% B from 12 min to 20 min, 28%-50% B from 20 min to 25 min, and 50%-99% B from 25 min to 30 min. The re-equilibration duration was 5 min between individual runs. All of the samples were kept at 4 °C during the analysis.
Total alkaloid, limonoid and flavonoid compounds were determined according to previously described methods[21-23].
Free radical scavenging activity: Free radical scavenging activity was determined by DPPH radical scavenging[24,25], ABTS radical scavenging[26,27], and β-carotene bleaching[28] assays.
Total antioxidant activity: Total antioxidant activity was measured using reducing power[29] and ferric reducing antioxidant power assay (FRAP) assays according to Jessica Nilsson’s methods[30].
Anti-lipid peroxidation activity: Anti-lipid peroxidation activity was assessed with a linoleic acid system using the ferric thiocyanate (FTC)[31] and thiobarbituric acid (TBA) assays[32].
Test animals: Adult male (150 ± 10 g) and female Sprague-Dawley (SD) rats (130 ± 10 g), 5 wk of age, were obtained from Beijing Vital River Laboratory Animal Technology Co. Ltd. These rats were kept at the animal room of the Institute of Medicinal Plant Development for 5 d prior to oral administration to allow for their acclimatization to the laboratory conditions. The animal room was ventilated with a 12-h cycle of day and night light conditions and the temperature was maintained at approximately 25 °C. The animals were fed with a standard rodent pellet diet and water ad libitum. All interventions and animal care procedures were performed in accordance with the Guidelines and Policies for Animal Surgery provided by our institute (Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China) and were approved by the Institutional Animal Use and Care Committee.
The rats were randomly divided into the following seven groups, each containing 6 male and 6 female animals, based on their body weight: (1) Group I (control group): distilled water (1 mL/kg body weight, p.o.) daily for 7 d and olive oil (2 mL/kg body weight, i.p.) on days 2 and 5; (2) Group II (CDAE control group): CDAE (320 mg/kg body weight, p.o.) daily for 7 d and olive oil (2 mL/kg body weight, i.p.) on days 2 and 5; (3) Group III (CCl4 control group): distilled water (1 mL/kg bodyweight, p.o.) for 7 d and a 1:1 mixture of CCl4 and olive oil (2 mL/kg bodyweight, i.p.) on days 2 and 5; (4) Group IV (positive control): silymarin (100 mg/kg body weight, p.o.) daily for 7 d and a 1:1 mixture of CCl4 and olive oil (2 mL/kg bodyweight, i.p.) on days 2 and 5; and (5) Groups V, VI and VII (test groups): CDAE (80, 160, and 320 mg/kg body weight, respectively, p.o.) for 7 d and a 1:1 mixture of CCl4 and olive oil (2 mL/kg bodyweight, i.p.) on days 2 and 5.
According to the 2015 Chinese Pharmacopoeia, the maximum daily intake of Cortex Dictamni is 6-10 g/kg per day in human[33]. Clinical application of the Cortex Dictamni complex prescription at a therapeutic dose of 10-15 g/kg per day has been suggested[34]. Assuming that the weight of an adult is 60 kg and that our extraction yield of CDAE is 19% w/w, the 1 × human equivalent dose (10 g/kg per day) of CDAE is 160 mg/kg for rats. Doses of 80 and 320 mg/kg of CDAE, which equate to 1/2 × and 2 × the human equivalent doses, respectively, were used in this study.
At the end of the experiment, the animals were anaesthetized by inhalation of ethyl ether. Blood was collected and allowed to clot. Serum was separated for the assessment of enzyme activity. The weights of the livers were assessed soon after the animals were sacrificed. The liver index, which was used to evaluate hepatomegaly, is expressed as follows: (liver weight/body weight) × 100%. Liver samples were dissected, and subsequently transferred into 10% buffer formalin for histopathological investigation and immunohistochemical determination and then stored in a refrigerator for biochemical index determination and Western blot.
Serum biochemical markers, including ALT, AST, and ALP were measured using a standard commercial kit provided by Beckman Coulter (Atlanta, GA, United States) and an automatic biochemical analyzer (Beckman Coulter). Serum TBIL was measured using a commercial kit obtained from Nanjing Jiancheng Institute of Biological Engineering (Nanjing, China).
A portion of the left lobe of the liver was preserved in 10% buffer formalin for at least 24 h and then processed and embedded in paraffin according to the standard protocol. Sections were cut into 5 μm thick portions, transferred onto glass slides, and stained with haematoxylin and eosin (HE) for histopathological examination.
Liver homogenates (10%, w/v) were prepared in ice-cold physiological saline, and the suspension was centrifuged at 2500 rpm for 10 min at 4 °C. The protein content of the homogenates was determined using a standard commercial kit provided by the Nanjing Jiancheng Institute of Biological Engineering (Nanjing, China). The clear supernatant was used for the determination of antioxidant markers, including SOD, CAT, GSH-Px, and GSH, as well as lipid peroxidation production of MDA. Assays were conducted using the kits obtained from Jiancheng Institute of Biotechnology (Nanjing, China) according to the protocol of the manufacturer and the results are expressed in mg per g protein.
For immunohistochemistry, deparaffinized sections were immersed in a vessel containing 0.01 mol/L citrate (pH = 6.0) and microwaved for 15 min, followed by incubation with 3% H2O2 for 30 min. Goat serum was used to block nonspecific protein binding for 30 min. The sections were then incubated overnight at 4 °C with primary antibody (rabbit anti-CYP2E1) diluted 1:100, followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit IgG. The sections were then washed three times with PBS, color-developed using DAB, and subsequently counterstained with hematoxylin. Images were obtained via digital imaging light microscopy (Olympus, Japan). Positive staining was analyzed using ImagePro Plus 6.0 software.
Total soluble protein was homogenized and extracted from the liver tissues using extraction buffer supplemented with 1 mmol/L phenylmethanesulfonyl fluoride. The homogenates were centrifuged at 15000 g for 15 min at 4 °C and the supernatant was collected. Nuclear soluble protein was homogenized and extracted using a standard commercial kit provided by ComWin Biotech (Beijing, China). After protein quantitation, equal amounts of protein were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Pall, Ann Arbor, MI). The membranes were blocked with 5% non-fat dry milk and incubated overnight at 4 °C with the corresponding primary antibodies. After three washes in TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies at a 1:5000 dilution for 2 h at room temperature. The signals were detected using an electrochemiluminescence (ECL) system. The relative intensities of the bands were quantified using AlphaEaseFCTM software. β-actin was used as an internal standard.
Values are expressed as the mean ± SD and were analyzed via one-way analysis of variance (ANOVA) followed by the LSD test and the Dunnett’s test, using SPSS 18.0 software. Statistical significance was set at P < 0.05.
CDAE was analyzed using UPLC and matched by the Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine (Version 2004A). Dictamnine, obacunone, and fraxinellone are active compounds and quality control markers of Cortex Dictamnus[33]. The components were identified as dictamnine (peak 1), obacunone (peak 2), and fraxinellone (peak 3) by comparing the retention times of standard substances, which are shown in Figure 1. The amounts of dictamnine, obacunone, and fraxinellone in CDAE were 0.011%, 0.073%, and 0.003%, respectively.
Alkaloids, limonoids and flavonoids isolated from Cortex Dictamni are thought to be the biologically active agents[12,13]. The total amounts of alkaloid, limonoid, and flavonoid compounds in CDAE were measured in this study. The amounts of total alkaloid (dictamnine equivalents), limonoid (obacunone equivalents), and flavonoid (quercetin equivalents) compounds from CDAE were 36.79 ± 2.23, 145.868 ± 10.72, and 79.23 ± 2.60 mg/g extract, respectively.
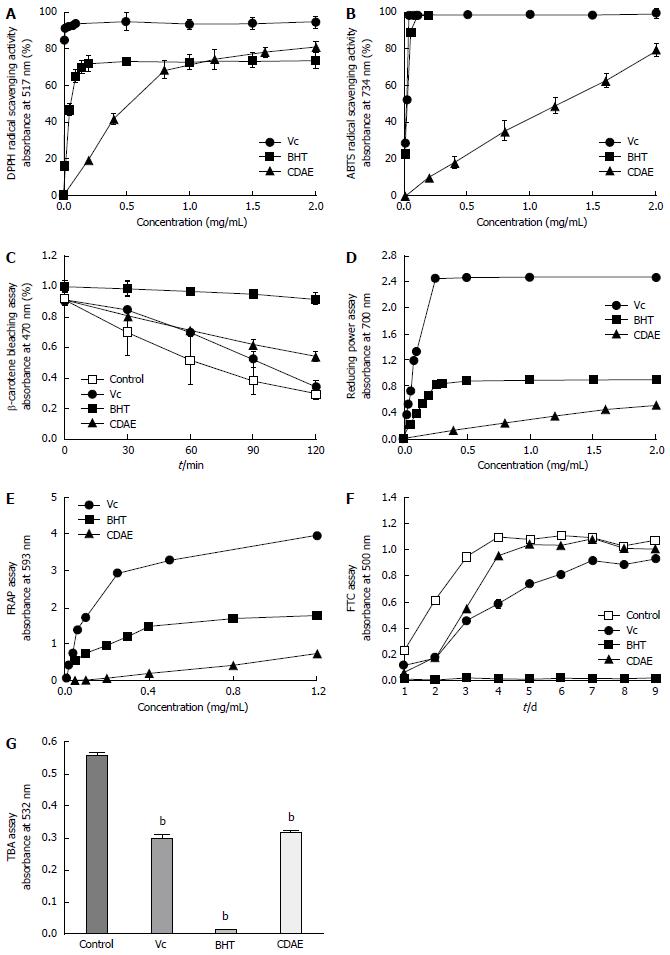
DPPH and ABTS free radical scavenging activity: DPPH and ABTS are synthetic radicals that are used to evaluate the free radical scavenging activity of herbal medicines. The antioxidant activity of CDAE was determined based on its capacity to scavenge DPPH and ABTS radicals (Figure 2A and B). CDAE scavenged DPPH and ABTS radicals in a concentration-dependent manner. Vc showed the highest scavenging activity, while the DPPH radical scavenging activity of CDAE was equal to BHT at 1 mg/mL.
β-Carotene bleaching assay: The β-carotene bleaching assay is based on the discoloration of β-carotene, owing to its reaction with linoleic acid-generated free radicals[35]. By means of scavenging these free radicals, antioxidants can suppress β-carotene bleaching. In the control group, the color decreased rapidly over time, but decreased much more slowly in the presence of CDAE and BHT (Figure 2C), indicating that CDAE and BHT possess free radical scavenging activity. Compared with the control treatment, CDAE scavenged free radicals and inhibited lipid peroxidation (40.6% ± 5.2%) significantly better than Vc (7.9% ± 3.0%).
Reducing power and FRAP assays: The reducing power assay indirectly assesses total antioxidant capacity, while the FRAP assay directly assesses total antioxidant capacity. The absorbance of CDAE in both assays increased with increasing concentration, but did not differ substantially from Vc and BHT (Figure 2D and E).
Antioxidant activity in a linoleic acid system using the FTC and TBA assays: The FTC and TBA methods were used to evaluate anti-lipid peroxidation activity in a linoleic acid system. In the FTC assay, the absorbance of the control group increased over time and peaked on day 6 (Figure 2F). BHT showed a high capacity to inhibit linoleic acid peroxidation, whereas CDAE exhibited comparable activity with Vc. In the TBA assay, the absorbance values obtained for BHT, CDAE, and Vc were significantly lower than those obtained for the control (P < 0.01) (Figure 2G). The inhibition rates of BHT, Vc, and CDAE were 97.6% ± 0.1%, 46.8% ± 2.0%, and 43.3% ± 0.4%, respectively. Thus, CDAE and Vc exhibited comparable anti-lipid peroxidation activities.
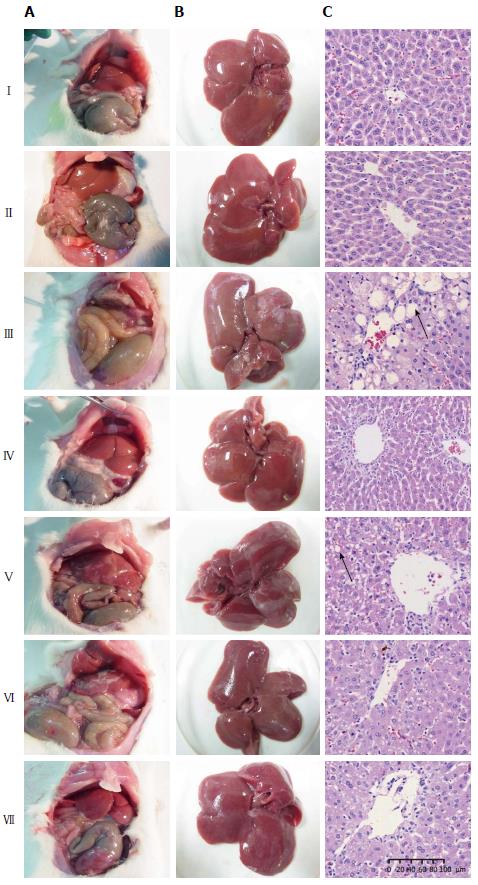
Effect of CDAE on histopathological changes in the liver of rats with CCl4-induced liver damage: The liver tissues of the control group (I) were normal (Figure 3A and B). In addition, compared with the control treatment, after CDAE treatment, no major abnormalities were found in the liver tissues (320 mg/kg). Liver adhesion (Figure 3A) and hepatomegaly (Figure 3B) were observed after CCl4 administration, which were attenuated after CDAE treatment.
Based on the histopathological observations of the liver sections, the control group exhibited normal cellular architecture, with distinct hepatocytes and no histological abnormalities (Figure 3C). However, CCl4 administration caused hepatocyte fat deposition, massive fatty changes, hepatocyte necrosis and swelling, vacuole formation and loss of cellular boundaries, especially in the centrilobular area. After CDAE treatment, the histological features of hepatocytes were restored to some extent. High-dose CDAE (320 mg/kg) yielded more effective rescue, almost comparable to the normal control group.
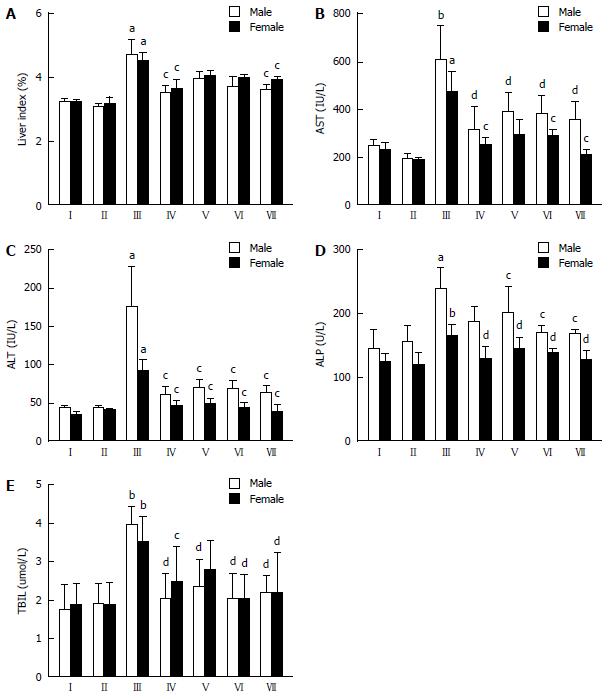
Effect of CDAE on the liver index of rats with CCl4-induced liver damage: Hepatic biotransformation systems and, consequently, drug toxicity measurements, display well-known sex differences[36,37]. Therefore, to investigate the hepatoprotective and antioxidant activity of CDAE, we separated both sexes of rats during the analysis. Treating normal rats with a high dose of CDAE (320 mg/kg) did not affect the liver index in either sex compared with that in the control group (I) (Figure 4A), indicating that high doses of CDAE may have no liver toxicity in rats of both sexes. Compared with the control treatment, after CCl4 administration, the liver index significantly increased in rats of both sexes (P < 0.05), indicating serious hepatomegaly that was markedly suppressed by a high dose of CDAE (320 mg/kg) (P < 0.05), which is consistent with the liver histopathological observations.
Effect of CDAE on hepatic markers in CCl4-induced liver damage in rats: ALT, AST, and ALP are liver enzyme markers, and elevated levels of these markers in serum indicate the loss of hepatocyte structural integrity and liver injury. Similarly, TBIL is an index of normal hepatic metabolism. As shown in Figure 4B-E, no marked changes of AST, ALT, ALP, and TBIL levels were detected in normal rats of either sex treated with a high dose of CDAE (320 mg/kg), which confirmed the safety of CDAE, at least in a dose range below 320 mg/kg. Compared with their levels in the control group (I), AST, ALT, ALP, and TBIL levels were significantly increased in male rats in the CCl4 group (III) (P < 0.05 or P < 0.001) and female rats in the CCl4 group (III) (P < 0.05, P < 0.01 or P < 0.001). However, CDAE treatment markedly decreased the CCl4-induced AST, ALT, ALP, and TBIL release into serum in male rats in a dose-dependent manner, similar to silymarin treatment. In female rats, the serum levels of these markers were also significantly inhibited after CDAE treatment in a dose-dependent manner. These results indicated a marked protective effect of CDAE on CCl4-induced liver injury in rats of both sexes.
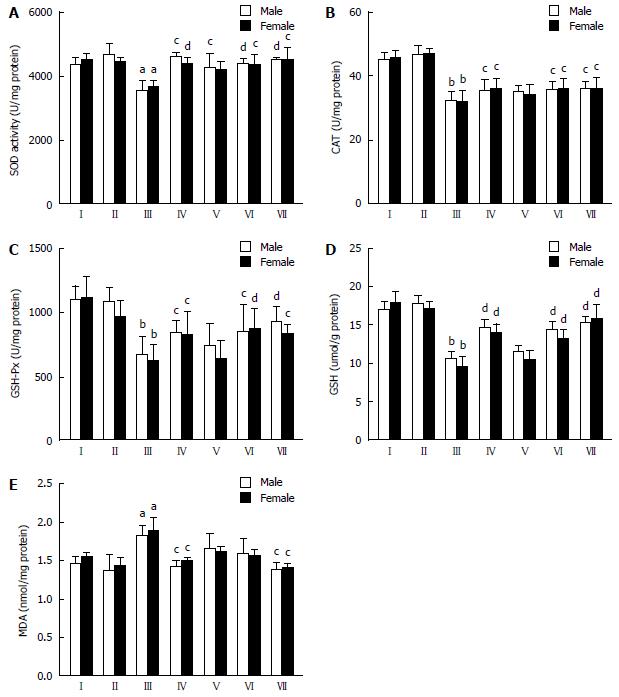
Effect of CDAE on antioxidant markers and lipid peroxidation production in the liver of rats with CCl4-induced liver damage: To evaluate the protective effect of CDAE against oxidative stress and lipid peroxidation, SOD activity, CAT activity, GSH-Px activity, GSH content, and MDA content were measured in liver homogenates (Figure 5). In male rats, CCl4 treatment significantly decreased SOD activity, CAT activity, GSH-Px activity, and GSH content (P < 0.05 or P < 0.001) but significantly increased MDA content (P < 0.05) compared with the normal control group (I). The middle and high doses of CDAE (160 and 320 mg/kg) significantly inhibited the CCl4-induced decrease in SOD activity (P < 0.05), CAT activity (P < 0.05), GSH-Px activity (P < 0.05 or P < 0.01), and GSH content (P < 0.001). Moreover, the high dose of CDAE (320 mg/kg) significantly suppressed the formation of MDA (P < 0.05) induced by CCl4 treatment. In female rats, CCl4 treatment similarly induced a significant decrease in SOD activity, CAT activity, GSH-Px activity, and GSH content (P < 0.001) but induced an increase of MDA content (P < 0.05), which were markedly inhibited by CDAE in a dose-dependent manner. Taken together, CDAE significantly increased enzyme antioxidant activity and non-enzyme antioxidant content in rats of both sexes, which contributed to the inhibition of lipid peroxidation.
Effect of CDAE on Nrf2, HO-1, NQO1, and γ-GCSc protein expression in CCl4-induced liver damage in rats: Nrf2 is an essential transcription factor that contributes to drug detoxification and antioxidant mechanisms. To assess the effect of CDAE on Nrf2 regulation, Nrf2 protein levels were measured in nuclear extracts. First, we investigated potential sex differences in Nrf2 protein expression. Figure 6A shows that Nrf2 protein levels were similar in male and female rats in both the control and CCl4 groups. Moreover, we examined the protein level of HO-1, which participates in antioxidant defense mechanisms and whose expression is mediated by Nrf2. Similar to Nrf2 protein expression, no significant differences in HO-1 protein expression were detected between male and female rats in both the control and CCl4 groups, consistent with the antioxidant markers (Figure 5). Based on these results, we further investigated the effect of CDAE on Nrf2 protein expression and the related antioxidant proteins.
As shown in Figure 6B, CCl4 treatment increased relative Nrf2 protein levels in nuclear extracts but with no significant differences compared with the control group (I). However, CDAE treatment (160 and 320 mg/kg) significantly enhanced Nrf2 protein expression by 2.61- and 5.57-fold compared with that in the CCl4 group (P < 0.01 and P < 0.001). Similarly, CDAE treatment (160 and 320 mg/kg) significantly increased the relative HO-1 (P < 0.001 and P < 0.001) and γ-GCSc (P < 0.01 and P < 0.001) protein expression compared with that in the CCl4 group (III). Relative NQO1 protein expression significantly increased after CDAE treatment in a dose-dependent manner (P < 0.01 and P < 0.001). These results indicated that CDAE protects the liver from CCl4-induced oxidative damage by regulating Nrf2-mediated antioxidant protein expression.
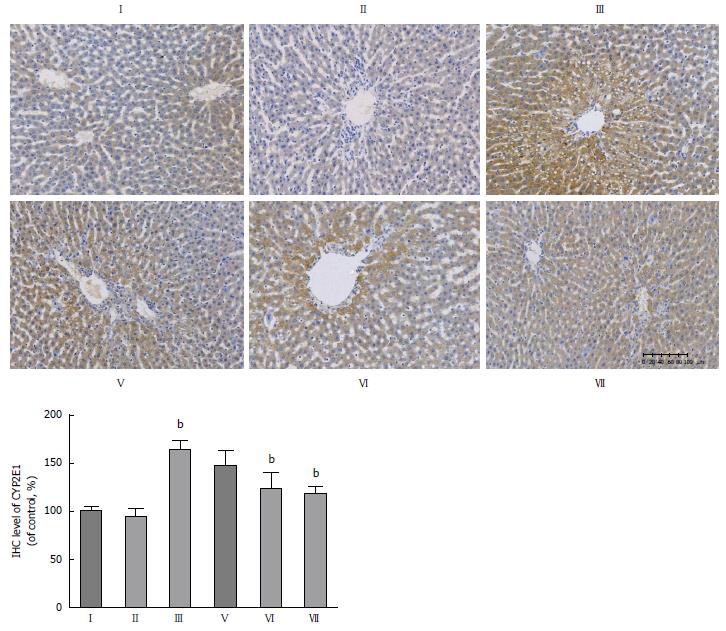
Effect of CDAE on CYP2E1 expression in CCl4-induced liver damage in rats: CYP2E1 is one of the most potent microsome cytochromes to generate ROS and is a major isozyme for the biotransformation of CCl4[7,38]. Immunohistochemical analysis revealed increased CYP2E1 expression after CCl4 administration, especially in the centrilobular area (Figure 7). CDAE treatment (160 and 320 mg/kg) significantly decreased the CYP2E1 expression by 0.71- and 0.72-fold compared with that in the CCl4 group (III) (P < 0.01), indicating that CDAE inhibited CYP2E1 expression and the subsequent biotransformation of CCl4.
Oxidative stress, which induces hepatocyte dysfunction, contributes to the pathogenesis of acute liver diseases. Herbs and their active compounds with antioxidant activity have attracted considerable interest as a source for the development of liver-curing drugs[39]. In the present study, the antioxidant activity of CDAE was investigated by assessing total antioxidant activity, radical scavenging activity, and the inhibition of lipid peroxidation in vitro. The hepatoprotective effects were evaluated in vivo using CCl4-induced rats.
The DPPH and ABTS free radical scavenging assays, as well as the β-carotene bleaching assay, are often used to evaluate the free-radical-scavenging activity of pure compounds or crude plant extracts[40]. The reducing power and FRAP assays are often used for routine analyses of the total antioxidant activity of plant extracts[41,42]. In our study, CDAE exhibited an obvious radical scavenging and antioxidant activity in a dose-dependent manner. Excessive reactive radicals promote lipid peroxidation, which is considered to be an important mechanism in the pathogenesis of cell damage[43]. Therefore, it is important to investigate the inhibition of lipid peroxidation in vitro. Linoleic acid, a polyunsaturated fatty acid, is one of the primary fatty acids in the membrane that is easily oxidized via dehydrogenation[44]. During dehydrogenation, the formation of linoleic acid radicals in turn initiates lipid peroxidation. The in vitro linoleic acid test system, including the FTC and TBA assays, is widely used to evaluate the inhibition of lipid peroxidation. The FTC method is widely used to determine the amount of peroxide at the initial stage of lipid peroxidation[45]. During the acid-heat treatment in the FTC method, lipid peroxides decompose to MDA, which can react with TBA to produce a red complex that can be measured by its absorbance[46]. In the present study, CDAE inhibited lipid peroxidation even better than vitamin C. Taken together, the results of the in vitro antioxidant study indicated that CDAE showed good antioxidant activity and inhibition of lipid peroxidation, which may be attributable to its direct function in scavenging free radicals. Alkaloids, limonoids and flavonoids isolated from Cortex Dictamni have been identified as potential antioxidants[12,13]. All three types of compounds were found in CDAE in the present study, of which the amount of limonoids was 145.868 ± 10.72 mg/g extract, indicating that limonoids are the main component of CDAE and may be the contributing factor to its good antioxidant activity. However, the exact chemical components that determine the antioxidant effect of CDAE require further exploration.
Our in vitro antioxidant study and previous evidence[15,16] suggest that CDAE may exert a protective effect against reactive free radical-induced oxidative stress injury. CCl4 is conventionally used to induce liver toxicity and oxidative stress injury in vivo, followed by the testing of plant-based drugs for their liver protective properties[47]. Although CCl4-induced liver damage is rare, the same pathogenic factors underlying CCl4-induced oxidative stress exist in other liver diseases, such as virus- and medicine-induced liver injury. In CCl4-induced liver damage, CCl4 primarily accumulates in hepatic parenchymal cells and is metabolized by the phase I metabolic enzyme CYP2E1 to produce free radicals, which are capable of binding to proteins or lipids, and subsequently initiate oxidative stress injury, lipid peroxidation, and the loss of hepatocyte structural integrity[5], resulting in hepatocyte necrosis, vacuole formation and loss of cellular boundaries, especially in the centrilobular area. Elevated serum ALT, AST, ALP, and TBIL levels are also indicative of cellular leakage[48,49].
Current evidence reveals that herbal products may have safety problems and cause liver injury. To evaluate the safety of CDAE, a previous study investigated its acute and sub-chronic toxicity, yielding a median lethal dose of 48.2 g/kg, which is more than 150-fold higher than the high dosage used in the present study[20]. Furthermore, to confirm the safety of CDAE, the high dose of CDAE was administered to normal rats in the present study, and the results showed that normal rats treated with the high dose of CDAE did not exhibit hepatotoxicity. Consequently, we further investigated the hepatoprotective effect of CDAE on CCl4-induced liver injury.
Sex differences have been observed in hepatic biotransformation systems and, consequently, in drug toxicity[36,37]. To investigate the hepatoprotective effect of CDAE, we separated male and female rats in our data analysis. CDAE treatment effectively protected rats of both sexes against CCl4-induced liver damage by reducing elevated serum ALT, AST and ALP activities, as well as liver pathological changes. Similarly, the stabilization of serum TBIL levels upon CDAE administration also indicated improvement in the functional status of the hepatic cells. These results indicated that CDAE can protect against CCl4-induced liver damage.
Biological systems protect themselves against the damaging effects of activated species by several methods, including free radical scavengers and chain reaction terminators, such as SOD, CAT, and GSH-Px[50]. SOD catalyses the dismutation of superoxide anion (▪O- 2) into O2 and hydrogen peroxide (H2O2), which can be reduced to H2O by GSH-Px in the cytosol or by CAT in peroxisomes. SOD, CAT, and GSH-Px are easily induced by oxidative stress, and the activity levels of these enzymes have been used to quantify oxidative stress in cells[51]. As a non-enzymatic antioxidant, GSH becomes depleted in response to oxidative stress and subsequently enhances the peroxidation process[52]. In the present study, CDAE markedly elevated SOD, CAT, and GSH-Px activity and GSH levels to different extents in injured livers in CCl4-induced liver-injury rats of both sexes.
ROS are known to attack polyunsaturated fatty acids in cell membranes. In CCl4-induced hepatotoxicity, ROS produced by CCl4 metabolism cause lipid peroxidation in the cell membrane. MDA is a lipid peroxidation product that has been used as a biomarker of lipid peroxidation[53]. In the present study, CDAE markedly inhibited the increase in MDA levels to different extents in CCl4-induced liver-injury rats of both sexes, indicating that the hepatoprotective effect of CDAE may be associated with the inhibition of lipid peroxidation, which is consistent with the in vitro linoleic acid assay.
The centrilobular zone contains abundant CYP2E1, an enzyme responsible for generating trichloromethyl peroxy radicals, which trigger hepatocellular damage by covalent binding. Numerous studies have used CCl4 to induce liver injury because it is mainly metabolized into highly reactive trichloromethyl free radicals by CYP2E1[54], which subsequently induces oxidative stress and liver injury. Our study demonstrated that CDAE was effective in protecting the liver from CYP2E1 overexpression. CYP2E1 induction has been shown to occur via the inhibition of CYP2E1 protein degradation[55,56], which contributes to CYP2E1 overexpression. Therefore, the mechanism by which CDAE mediates CYP2E1 protein overexpression merits further exploration.
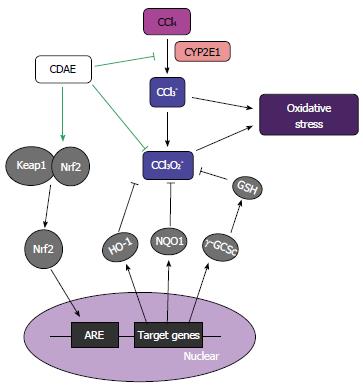
Oxidative stress is caused by highly reactive metabolites and is mediated by the Nrf2 pathway in CCl4-induced hepatic injury. Nrf2 is a transcription factor that regulates the expression of antioxidant phase II detoxifying enzymes by activating the antioxidant-reactive element (ARE). Nrf2 is located in both the cytoplasm and the nucleus. Only when Nrf2 accumulates in the nucleus after dissociation from Keap 1 in the cytoplasm does it recognize and bind to AREs on antioxidant genes[57], which subsequently increases the expression of phase II detoxifying enzymes, including HO-1, NQO1, and γ-GCSc. In the present study, CDAE treatment markedly augmented Nrf2 levels in the nucleus. HO-1 is the rate-limiting enzyme in the catabolism of heme into billiverdin, which is a potent antioxidant. NQO1 is a highly inducible enzyme with superoxide-scavenging properties[58]. Furthermore, γ-GCSc participates in de novo GSH synthesis[59]. GSH replenishment is followed by the recovery of cellular redox status, with decreased oxidative stress in γ-GCSc-overexpressing cells. In the present study, CDAE treatment augmented HO-1, NQO1, and γ-GCSc expression levels, indicating that CDAE might protect against CCl4-induced hepatic injury regulating the Keap1-Nrf2 antioxidant pathway (Figure 8). The PI3K-PKB/Akt-GSK-3 pathway has been shown to regulate Nrf2[60,61]. In particular, Nrf2 is repressed by the GSK-3/β-TrCP axis. Therefore, further work is required to identify the regulatory effect of CDAE on factors upstream of Nrf2 and related antioxidant proteins.
In summary, the present study demonstrated that CDAE exhibits marked antioxidant activity, including free radical scavenging activity and the inhibition of lipid peroxidation, both in vitro and in vivo. Moreover, CDAE effectively prevented CCl4-induced hepatic damage. This effect may be attributable to its antioxidant activity, which is regulated by Keap1-Nrf2.
The liver is a vital organ in the body that is responsible for the maintenance of metabolic functions and the detoxification of exogenous and endogenous compounds. Hepatic injury can be caused by toxic chemicals, drugs, and viral infiltration via ingestion or infection. However, synthetic drugs used to treat hepatic injury are sometimes associated with adverse effects. Therefore, it is necessary to explore alternative treatments for hepatic injury.
This study is the first to show the inhibition of lipid peroxidation by Cortex Dictamni aqueous extract (CDAE) using a linoleic acid system in vitro.
From the perspective of inhibiting oxidative stress, in vivo experiments were conducted to study the protective effects of CDAE on CCl4-induced hepatic injury.
This study suggested that CDAE has a strong antioxidant activity and may be a potential treatment for hepatic injury.
The manuscript is very well written. Both abstract and introduction give a good background for the reader to study the methods used and the results obtained. The discussion brings the data obtained in context with earlier studies and presents an excellent analysis of the methods used. The very thorough discussion leads to a relatively reliable conclusion: CDAE exhibits a marked antioxidant activity and is effective for the prevention of CCl4-induced hepatic damage in rats of both sexes.
| 1. | Lee CP, Shih PH, Hsu CL, Yen GC. Hepatoprotection of tea seed oil (Camellia oleifera Abel.) against CCl4-induced oxidative damage in rats. Food Chem Toxicol. 2007;45:888-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Mihailović V, Mihailović M, Uskoković A, Arambašić J, Mišić D, Stanković V, Katanić J, Mladenović M, Solujić S, Matić S. Hepatoprotective effects of Gentiana asclepiadea L. extracts against carbon tetrachloride induced liver injury in rats. Food Chem Toxicol. 2013;52:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36:S237-S244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 208] [Article Influence: 8.7] [Reference Citation Analysis (2)] |
| 4. | Oumi N, Taniguchi KA, Kanai AM, Yasunaga M, Nakanishi T, Sato K. A crucial role of bone morphogenetic protein signaling in the wound healing response in acute liver injury induced by carbon tetrachloride. Int J Hepatol. 2012;2012:476820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Shenoy KA, Somayaji SN, Bairy KL. Evaluation of hepatoprotective activity of Ginkgo biloba in rats. Indian J Physiol Pharmacol. 2002;46:167-174. [PubMed] |
| 6. | Balogun FO, Ashafa AOT. Antioxidant and hepatoprotective activities of Dicoma anomala Sond. Aqueous root extract against carbon tetrachloride-induced liver damage in Wistar rats. J Tradit Chin Med. 2016;36:504-513. |
| 7. | Recknagel RO, Glende EA, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 803] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 8. | Lei J, Yu J, Yu H, Liao Z. Composition, cytotoxicity and antimicrobial activity of essential oil from Dictamnus dasycarpus. Food Chem. 2008;107:1205-1209. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Wu TS, Wang ML, Shyur HJ, Leu YL, Chan , YY . Chemical constituents and bioactive principles from the root bark of Dictamnus dasycarpus. Chin Pharmaceut J. 1994;46:447-455. |
| 10. | Yoon JS, Sung SH, Kim YC. Neuroprotective limonoids of root bark of Dictamnus dasycarpus. J Nat Prod. 2008;71:208-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Fan XW, Zhang X, Wang SM. A Survey of Studies on the Chemical Constituents and Pharmacological Activity of Dictamnus L. Special Wild Economic Animal and Plant Research. 2003;25:50-52. |
| 12. | Jiang Y, Li SP, Chang HT, Wang YT, Tu PF. Pressurized liquid extraction followed by high-performance liquid chromatography for determination of seven active compounds in Cortex Dictamni. J Chromatogr A. 2006;1108:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Jovanovic SV, Steenken S, Tosic M, Marjanovic B, Simic M. Flavonoids as antioxidants. J Am Chem Soc. 1994;116:4846-4851. [RCA] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 819] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 14. | Chen R, Su W, Li PY, Huo LN, Lu RM, Peng MP. Free radical-scavenging and antioxidant activity of skin of Dictamnus dasycarpus. Asian J Chem. 2013;25:1753-1754. |
| 15. | Qin M, Guo HB, Xu Y. Cortex Dictamni inhibits formation of early aortic atherosclerotic lesions in ApoE-Deficient mice. Acta Labor Animalis Scientia Sinica. 2010;18:191-195. |
| 16. | Xu ML, Wang LL, Li L, Li ZQ, Qin M, Li JM, Xu Y. Protective effects of aqueous extract of Cortex dictamni on myocardial ischemia reperfusion injury in rats. Acta Labor Animalis Scientia Sinica. 2013;21:47-52. |
| 17. | Liu YJ, Du JL, Cao LP, Jia R, Shen YJ, Zhao CY, Xu P, Yin GJ. Anti-inflammatory and hepatoprotective effects of Ganoderma lucidum polysaccharides on carbon tetrachloride-induced hepatocyte damage in common carp (Cyprinus carpio L.). Int Immunopharmacol. 2015;25:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Lu CH, Cao JS, Fan H, Xu Q. Mechanisms for improving the liver injury induced by delayed-type hypersensitivity by the Aqueous Extract from Cortex Dictamni. Zhongguo Yaoke Daxue Xuebao. 1999;30:212-215. |
| 19. | Wu XX, Wu LM, Fan JJ, Qin Y, Chen G, Wu XF, Shen Y, Sun Y, Xu Q. Cortex Dictamni extract induces apoptosis of activated hepatic stellate cells via STAT1 and attenuates liver fibrosis in mice. J Ethnopharmacol. 2011;135:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Wang L, Li Z, Li L, Li Y, Yu M, Zhou Y, Lv X, Arai H, Xu Y. Acute and sub-chronic oral toxicity profiles of the aqueous extract of Cortex Dictamni in mice and rats. J Ethnopharmacol. 2014;158 Pt A:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Kulkarni YA, Garud MS. Bauhinia variegata (Caesalpiniaceae) leaf extract: An effective treatment option in type I and type II diabetes. Biomed Pharmacother. 2016;83:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Ghlissi Z, Sayari N, Kallel R, Bougatef A, Sahnoun Z. Antioxidant, antibacterial, anti-inflammatory and wound healing effects of Artemisia campestris aqueous extract in rat. Biomed Pharmacother. 2016;84:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Chen D, Xiong Y, Jiang C, Lv B, Liu F, Wang L, Lin Y. Effects of ginsenosides on rat jejunal contractility. Pharm Biol. 2014;52:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | He J, Huang B, Ban X, Tian J, Zhu L, Wang Y. In vitro and in vivo antioxidant activity of the ethanolic extract from Meconopsis quintuplinervia. J Ethnopharmacol. 2012;141:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Roy N, Laskar RA, Sk I, Kumari D, Ghosh T, Begum NA. A detailed study on the antioxidant activity of the stem bark of Dalbergia sissoo Roxb., an Indian medicinal plant. Food Chem. 2011;126:1115-1121. [RCA] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Amendola D, De Faveri DM, Egües I, Serrano L, Labidi J, Spigno G. Autohydrolysis and organosolv process for recovery of hemicelluloses, phenolic compounds and lignin from grape stalks. Bioresour Technol. 2012;107:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Gong Y, Liu X, He WH, Xu HG, Yuan F, Gao YX. Investigation into the antioxidant activity and chemical composition of alcoholic extracts from defatted marigold (Tagetes erecta L.) residue. Fitoterapia. 2012;83:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Zhou G, Chen Y, Liu S, Yao X, Wang Y. In vitro and in vivo hepatoprotective and antioxidant activity of ethanolic extract from Meconopsis integrifolia (Maxim.) Franch. J Ethnopharmacol. 2013;148:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Wen XB, Miao F, Zhou L, Zhang M, He QL. In vitro antioxidant activity of Parnassia wightiana W. extracts. Zhonguo Ziran Yixue Zazhi. 2012;10:190-195. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Nilsson J, Stegmark R, Åkesson B. Total antioxidant capacity in different pea (Pisum sativum) varieties after blanching and freezing. Food Chem. 2004;86:501-507. [RCA] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Osawa T, Namaki M. A novel type antioxidant isolated from leaf wax of Eucalyptus leaves. Agricul Biol Chem. 1981;45:735-739. [RCA] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 130] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Kikuzaki H, Nakatani N. Antioxidant effects of some ginger constituents. J Food Sci. 1993;58:1407-1410. [RCA] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 323] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 33. | Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. Beijing: China Medical Science Press 2015; . |
| 34. | Guo J, Zhang Y. The clinical application of Cortex Dictamni complex prescription. Zhonghua Zhongyiyao Xuekan. 2005;23:513-514. |
| 35. | Celep E, Aydın A, Yesilada E. A comparative study on the in vitro antioxidant potentials of three edible fruits: cornelian cherry, Japanese persimmon and cherry laurel. Food Chem Toxicol. 2012;50:3329-3335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Mugford CA, Kedderis GL. Sex-dependent metabolism of xenobiotics. Drug Metab Rev. 1998;30:441-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 167] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76:215-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 576] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 38. | Jiang W, Guo MH, Hai X. Hepatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World J Gastroenterol. 2016;22:10180-10188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 87] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 39. | Singh D, Arya PV, Sharma A, Dobhal MP, Gupta RS. Modulatory potential of α-amyrin against hepatic oxidative stress through antioxidant status in Wistar albino rats. J Ethnopharmacol. 2015;161:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Al-Dabbas MM, Al-Ismail K, Kitahara K, Chishaki N, Hashinaga F, Suganuma T, Tadera K. The effects of different inorganic salts, buffer systems, and desalting of Varthemia crude water extract on DPPH radical scavenging activity. Food Chem. 2007;104:734-739. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Ćavar S, Kovač F, Maksimović M. Synthesis and antioxidant activity of selected 4-methylcoumarins. Food Chem. 2009;117:135-142. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Fu M, Feng HJ, Chen Y, Wang DB, Yang GZ. Antioxidant activity of Garcinia xanthochymus leaf, root and fruit extracts in vitro. Zhongguo Ziran Yixue Zazhi. 2012;10:129-134. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Xie Y, Hao H, Wang H, Guo C, Kang A, Wang G. Reversing effects of lignans on CCl4-induced hepatic CYP450 down regulation by attenuating oxidative stress. J Ethnopharmacol. 2014;155:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Prasad A, Pospíšil P. Linoleic acid-induced ultra-weak photon emission from Chlamydomonas reinhardtii as a tool for monitoring of lipid peroxidation in the cell membranes. PLoS One. 2011;6:e22345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Liu Q, Yao H. Antioxidant activities of barley seeds extracts. Food Chem. 2007;102:732-737. [RCA] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 46. | Guillén-Sans R, Guzmán-Chozas M. The thiobarbituric acid (TBA) reaction in foods: a review. Crit Rev Food Sci Nutr. 1998;38:315-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 165] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Pareek A, Godavarthi A, Issarani R, Nagori BP. Antioxidant and hepatoprotective activity of Fagonia schweinfurthii (Hadidi) Hadidi extract in carbon tetrachloride induced hepatotoxicity in HepG2 cell line and rats. J Ethnopharmacol. 2013;150:973-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Colares JR, Schemitt EG, Hartmann RM, Licks F, Soares MD, Bosco AD, Marroni NP. Antioxidant and anti-inflammatory action of melatonin in an experimental model of secondary biliary cirrhosis induced by bile duct ligation. World J Gastroenterol. 2016;22:8918-8928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (2)] |
| 49. | Tian MX, Liu WL, You HJ, Zhao QZ, Ouyang LD, Gao B, Zhang X, Chen NC. Protective effect of Yiguangjian decoction against DNA damage on concanavalin A-induced liver injury mice model. J Tradit Chin Med. 2016;36:471-478. |
| 50. | Lozano-Sepulveda SA, Bautista-Osorio E, Merino-Mascorro JA, Varela-Rey M, Muñoz-Espinosa LE, Cordero-Perez P, Martinez-Chantar ML, Rivas-Estilla AM. S-adenosyl-L-methionine modifies antioxidant-enzymes, glutathione-biosynthesis and methionine adenosyltransferases-1/2 in hepatitis C virus-expressing cells. World J Gastroenterol. 2016;22:3746-3757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | van der Oost R, Beyer J, Vermeulen NP. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol. 2003;13:57-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 2916] [Article Influence: 126.8] [Reference Citation Analysis (0)] |
| 52. | Skrzydlewska E, Farbiszewski R. Antioxidant status of liver, erythrocytes, and blood serum of rats in acute methanol intoxication. Alcohol. 1997;14:431-437. [PubMed] |
| 53. | Cherubini A, Ruggiero C, Polidori MC, Mecocci P. Potential markers of oxidative stress in stroke. Free Radic Biol Med. 2005;39:841-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 286] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 54. | Kim TW, Lee DR, Choi BK, Kang HK, Jung JY, Lim SW, Yang SH, Suh JW. Hepatoprotective effects of polymethoxyflavones against acute and chronic carbon tetrachloride intoxication. Food Chem Toxicol. 2016;91:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Lu YK, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44:723-738. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 611] [Cited by in RCA: 614] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 56. | Liu LG, Yan H, Yao P, Zhang W, Zou LJ, Song FF, Li K, Sun XF. CYP2E1-dependent hepatotoxicity and oxidative damage after ethanol administration in human primary hepatocytes. World J Gastroenterol. 2005;11:4530-4535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 57. | Eggler AL, Gay KA, Mesecar AD. Molecular mechanisms of natural products in chemoprevention: induction of cytoprotective enzymes by Nrf2. Mol Nutr Food Res. 2008;52 Suppl 1:S84-S94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 58. | Siegel D, Gustafson DL, Dehn DL, Han JY, Boonchoong P, Berliner LJ, Ross D. NAD(P)H: quinone oxidoreductase 1: role as a superoxide scavenger. Mol Pharmacol. 2004;65:1238-1247. [PubMed] |
| 59. | Marí M, Cederbaum AI. CYP2E1 overexpression in HepG2 cells induces glutathione synthesis by transcriptional activation of gamma-glutamylcysteine synthetase. J Biol Chem. 2000;275:15563-15571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 60. | Hayes JD, Ebisine K, Sharma RS, Chowdhry S, Dinkova-Kostova AT, Sutherland C. Regulation of the CNC-bZIP transcription factor Nrf2 by Keap1 and the axis between GSK-3 and β-TrCP. Curr Opin Toxicol. 2016;1:92-103. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Niu ZS, Niu XJ, Wang WH. Genetic alterations in hepatocellular carcinoma: An update. World J Gastroenterol. 2016;22:9069-9095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 123] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (3)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Beijing
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Berg T, Novo E S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH