Published online Apr 21, 2017. doi: 10.3748/wjg.v23.i15.2795
Peer-review started: January 5, 2017
First decision: February 9, 2017
Revised: February 13, 2017
Accepted: March 15, 2017
Article in press: March 15, 2017
Published online: April 21, 2017
Processing time: 105 Days and 5.3 Hours
To report on a more accurate diagnostic possibility offered by endoscopic ultrasound-guided cutting of holes and deep biopsy (EUS-CHDB) for pathologic diagnosis of gastric infiltrative tumors and gastrointestinal submucosal tumors.
Ten consecutive patients who were suspected of having gastric invasive tumors or gastrointestinal submucosal tumors underwent EUS-CHDB with a novel vertical diathermic loop. We reviewed their medical data and analysed the effectiveness and safety of this new method. The final diagnosis was based on the surgical pathology or clinical/imaging follow-up.
EUS-CHDB was performed successfully in all the ten patients. Neither severe haemorrhage nor perforation occurred in any patient. Among the ten patients, there were three cases of gastric linitis plastica, one case of gastric lymphoma, five cases of gastrointestinal stromal tumors (GISTs), and only one case of chronic non-atrophic gastritis. That is, nine (90%) of the cases treated by EUS-CHDB showed positive findings.
EUS-CHDB may be a technically feasible and safe option for patients with gastric infiltrative tumors or gastrointestinal submucosal tumors. EUS-CHDB may be used as a remedial or even preferred biopsy method for submucosal lesions.
Core tip: This was a prospective clinical diagnostic trial seeking to evaluate the efficacy of endoscopic ultrasound-guided cutting of holes and deep biopsy for the diagnosis of gastric infiltrative tumors and gastrointestinal submucosal tumors using a novel vertical diathermic loop. This new technique was proved to be a safe, technically feasible and more accurate option for patients with gastric infiltrative tumors or gastrointestinal submucosal tumors.
- Citation: Liu YM, Yang XJ. Endoscopic ultrasound-guided cutting of holes and deep biopsy for diagnosis of gastric infiltrative tumors and gastrointestinal submucosal tumors using a novel vertical diathermic loop. World J Gastroenterol 2017; 23(15): 2795-2801
- URL: https://www.wjgnet.com/1007-9327/full/v23/i15/2795.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i15.2795
Gastric infiltrative tumors, commonly referred to as gastric linitis plastica (GLP) and gastric lymphoma, along with gastrointestinal submucosal tumors often have more than one negative pathological finding on ordinary endoscopic biopsies. Such troublesome phenomena are associated with the biopsy technique itself and the deep location of the tumors. GLP, which is also known as Borrmann type IV or diffuse infiltrating-type gastric cancer or scirrhous carcinoma, is an invasive and diffuse carcinoma that is characterized by a thickened, tough and rigid gastric wall and a narrow cavity[1]. In general, GLP infiltrates the submucosal layer without destroying the superficial structure of the gastric wall; thus, pathological findings in the mucosal layer are not available for making a diagnosis via common biopsy[2]. Therefore, making an early diagnosis is difficult, and peritoneal metastasis is common at the time of detection, which results in a poor prognosis[3,4]. Primary gastric lymphomas, which are the major malignant tumors of the stomach secondary to gastric carcinoma, mostly originate in the submucosa, and their diagnosis via endoscopic forceps biopsy is often difficult, with a positive rate of only 29% in the initial detection[5,6]. For patients with primary gastric lymphoma, in contrast to those with GLP, effective chemotherapy produces a good outcome, and unnecessary surgery is avoided. Therefore, the distinction between GLP and gastric lymphoma is also significant for treatment. Similarly, for gastrointestinal stromal tumors (GISTs), which generally originate from the muscularis propria, a common endoscopic biopsy cannot acquire the correct tumor tissue that is usually resected during surgery or endoscopic submucosal dissection (ESD) for immunohistochemistry analysis.
Endoscopic ultrasound (EUS) is reliable in the diagnosis and staging of gastrointestinal malignancies and has become an indispensable evaluation in patients who are suspected of having submucosal tumors of the upper gastrointestinal tract[7,8]. Specimens obtained with a standard endoscopic biopsy rarely provide a confirmative diagnosis because lesions in the submucosa are difficult to reach directly with forceps. The excavating biopsy, snaring biopsy and endoscopic mucosal resection (EMR), derived from the traditional biopsy methods, are considered unsafe for frequent complications such as haemorrhage and perforation[9,10]. EUS enables the detection of the thickness of the wall and invasive depth of lesions. Therefore, it may reduce the operational risks and complications to finish a deep biopsy under the guidance of EUS in real time, which makes up for the deficiency of routine endoscopy. In addition, given the deficiencies of the currently available biopsy methods, such as a large wound, an inadequate depth and a rigid gastric wall, we invented a novel assistant tool, a reform of the diathermic snare. It contains a control handle, an insulated sheath and an electric mental wire whose tail end wields a small loop vertically. This vertical diathermic loop connected with an electrosurgical unit can be used to cut holes by fulguration into the tumor through the biopsy channel first, and the biopsy forceps can be poked into the holes to acquire deep tissue samples. Here, we retrospectively investigated the safety and efficacy of this novel process, called EUS-guided cutting of holes and deep biopsy (EUS-CHDB), for diagnosing gastric infiltrative tumors and gastrointestinal submucosal tumors.
From March 2014 to June 2016, ten consecutive patients (including five men) at our department who were suspected of having gastric invasive tumors or GISTs underwent EUS-CHDB with the novel electric ring. All patients had undergone ordinary endoscopic biopsies one to four times, and their pathology showed negative results. We extracted and analyzed their medical data. The study was approved by the Institutional Review Board of Fudan University Shanghai Cancer Center, and informed consent for the procedure was obtained.
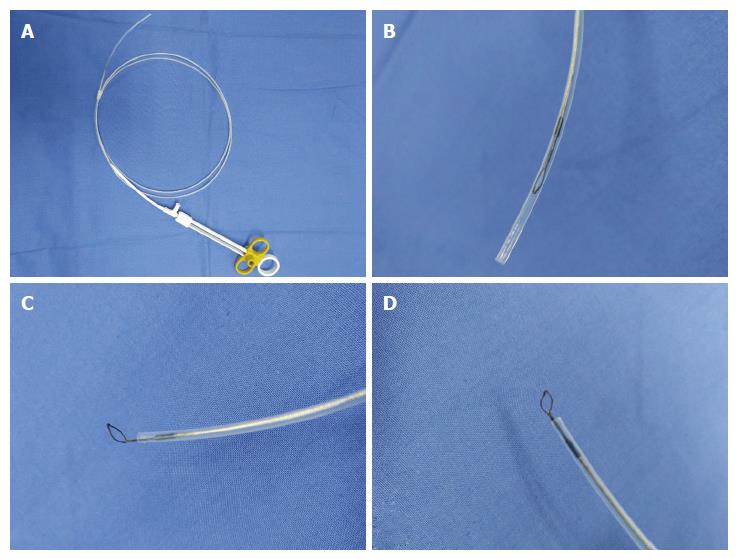
EUS equipment in our endoscopy centre is composed of an ultrasound mainframe (Hitachi Preirus; Hitachi, Tokyo, Japan) and linear echoendoscopes (PANTAX EG-3870UTK and EG-3270UK; PANTAX, Tokyo, Japan). Biopsy instruments consisted of the following: a vertical diathermic loop, composed of a control handle, an insulated sheath and an electric mental wire whose end vertically wields a small loop with a diameter of 3-5 mm. The available length of this device was 155 cm. The small ring could be pushed out with the largest length at 1.5 cm or pulled into the sheath with a diameter of approximately 2 mm (Figure 1). An electrosurgical unit was connected to the handle of the loop, and energy delivery was controlled by a foot switch. A small conventional biopsy forceps with a diameter of 1.8 mm was attached.
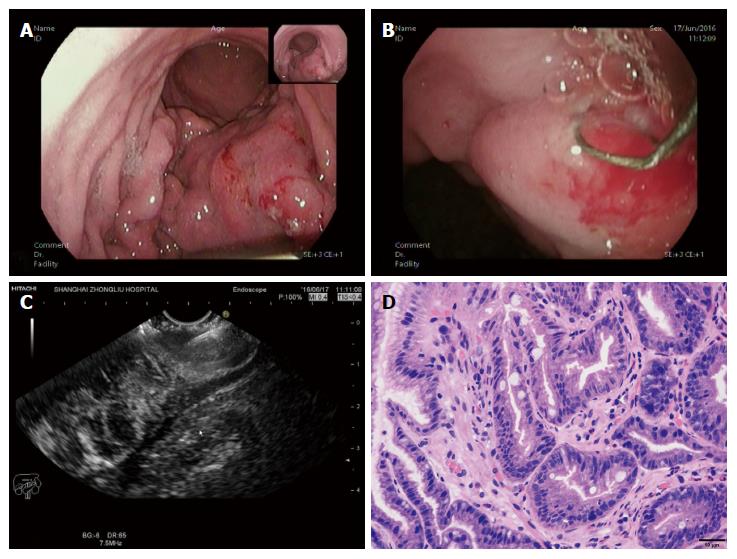
All patients experienced conventional EUS examination first to determine the targeted focus characterized by the obviously thick and rigid wall on the tumor. Subsequently, the vertical diathermic loop connected to an electrosurgical unit could be used to cut cylindrical holes under the guidance of EUS by fulguration into the targeted focus through the biopsy channel, and then a conventional biopsy forceps was poked into the holes to acquire the deep tissue samples. The diameters of the holes were approximately 3-5 mm, and the depth to the location was equivalent to the muscularis propria. Every tumor required cutting 2-3 holes to acquire more than seven blocks of tissue specimens. When the biopsy was finished, if the incision was too broad or still bleeding after spraying thrombin locally, an endoclip was selectively used to close it (Figure 2).
EUS-CHDB was performed successfully in all the 10 patients. The median age of the patients was 52.4 years (range: 41-75 years), and the male to female ratio was 5:5. Characteristics of the patients and tumors and the final diagnoses are shown in Table 1.
| Age, median (range), yr | 52.4 (41-75) | ||
| Male, n (%) | 5 (50) | ||
| Tumour thickness, median (range), mm | 25.34 (10.6-45.2) | ||
| Tumour location, n | |||
| Gastric fundus | 2 | ||
| Gastric body | 5 | ||
| Gastric fundus and body | 1 | ||
| Gastric body and antrum | 1 | ||
| Descending duodenum | 1 | ||
| Final diagnosis, n | |||
| Pathology obtained by EUS-CHDB | Surgical resection or clinical follow-up | ||
| Histological type | Gastric adenocarcinoma | GIST | Gastric lymphoma |
| Gastric adenocarcinoma | 3 | 0 | 0 |
| GIST | 0 | 5 | 0 |
| Gastric lymphoma | 0 | 0 | 1 |
| Chronic non-atrophic gastritis | 1 | 0 | 0 |
| The overall accuracy rate | 90% | ||
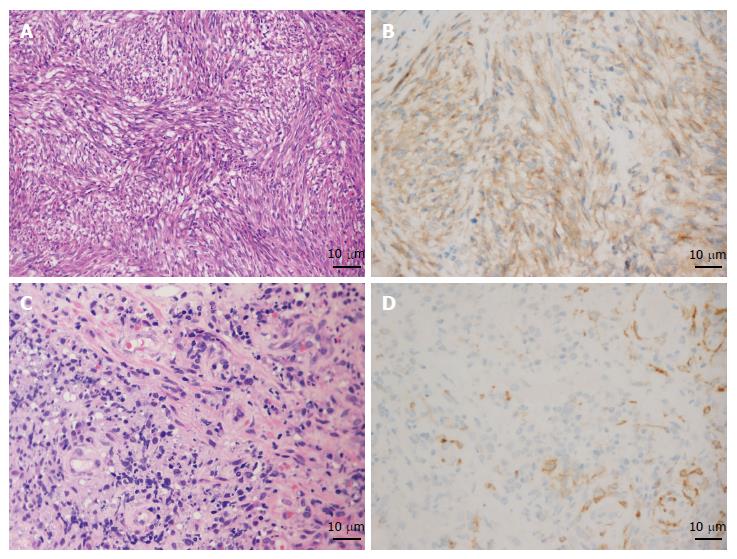
The average numbers of cut holes and tissue specimens were 2.29 (range: 2-3) and 8.57 (range: 7-12), respectively. There were two cases still haemorrhaging after spraying thrombin locally and three cases showing a large wound, so endoclips were used to close the incision. Neither severe haemorrhage nor perforation occurred in any patient. According to the pathology results from the samples obtained by EUS-CHDB, among the ten patients, there were three GLP cases, one gastric lymphoma case, five GIST cases, and only one case showing chronic non-atrophic gastritis (Figure 3). Three of the five patients diagnosed with GISTs underwent surgical treatment, and the surgical pathology findings were consistent with the EUS-CHDB findings. The patient showing chronic non-atrophic gastritis was diagnosed with GLP by surgical resection. According to the clinical/imaging follow-up, the diagnosis of patients without surgical resection was consistent with the EUS-CHDB findings. That is, nine (90%) of the cases treated by EUS-CHDB had positive findings.
Primary gastric lymphoma (PGL) accounts for 2% to 8% of all malignant gastric tumors, which spread mainly in a longitudinal growth style. Suekane et al[11] divided the patients into four groups according to the endoscopic ultrasonography findings: superficially spreading type, diffusely infiltrating type, mass-forming type, and mixed type. Among the four groups, the superficially spreading type had the highest frequency with EUS imaging features of diffused gastric wall thickening and replacing of the second and third layers with a hypoecho mass. Based on the morphological characteristics of the gastric tumor visualized by common endoscopy or EUS, a differential diagnosis of GLP and gastric lymphoma is difficult in most cases. In our outpatient department of endoscopy, a 34-year-old female patient who was clinically suspected of having gastric lymphoma for more than 3 years but treated as chronic gastritis subsequently suffered from a huge abdominal mass suspected of being the product of metastasis. We suggest that the correct histopathologic findings are closely tied to the prognosis of GLP; the sooner the pathology, the better the treatment. However, a study showed that only eight cases were correctly diagnosed by preoperative biopsy among 45 cases of GPL[12]. Thus, finding an effective biopsy method may be the key point.
A meta-analysis[4] showed that compared with Borrmann type "others" , B-4 had a higher incidence of poorly differentiated carcinoma (P < 0.01), lymph node metastasis (P < 0.01), peritoneal metastasis (P < 0.01), and serosal invasion (P < 0.01). Kim et al[2] showed that the missed rate of ordinary biopsies in diagnosing Borrmann type IV gastric cancer was as high as 55.9%. Lesions showing thickening of the gastric mucosa generally originate from the submucosa, whose tissue samples obtained by large-capacity biopsy forceps can improve the diagnostic rate, but only to 17%[13]. For patients with gastric infiltrative tumors whose initial biopsy is negative for malignancy, the following two to four biopsies enable an increase in the positive rate, but limited, and repeated endoscopy examinations and biopsies may bring more suffering, cost burdens and even delayed treatment.
Along with the advancement of endoscopic diagnostic and therapeutic technology, several novel technologies, such as EUS-guided fine needle aspiration (EUS-FNA), excavating biopsy, snaring biopsy and EMR, have emerged. EUS-FNA for gastric infiltrative tumors can be used to acquire cytology findings, but for large tissue specimens for histopathology analysis, with the disadvantages of difficult operations, long time and high expenses, the positive finding rate is still undesirable[14-16]. Al-Haddad et al[17] reported that the sensitivity, specificity and accuracy rates of EUS-FNA in the diagnosis of lymphoma were 80%-87%, 92%-93%, and 83%-89%, respectively. Recently, Zhou et al[18] confirmed that endoscopic ultrasound-guided deep and large biopsy technique provided a definitive and conclusive diagnosis in 29 (80.6%) of the 36 patients suspected of having gastric infiltrating tumors. Excavating biopsy, snaring biopsy and endoscopic mucosal resection (EMR), which generally cannot obtain the correct deep tissue samples, are considered unsafe due to frequent complications such as haemorrhage and perforation[18].
GISTs, demonstrated to stem from gain-of-function mutations of the platelet-derived growth factor receptor alpha gene and/or c-kit gene[19,20], account for 1% of primary gastrointestinal tumors. Although surgical resection has been the main modality of treatment[21], many cases are locally advanced and unresectable at present. In addition, after curative resections, approximately 40% of cases will develop recurrences in the form of local or distant metastasis[22,23]. Imatinib mesylate (IM), a tyrosine kinase targeted therapy, is now the standard adjuvant therapy before or after surgical resection of non-metastatic GISTs with a significant risk of recurrence[24,25]. In locally advanced or unresectable GISTs, use of neoadjuvant IM helps decrease the extent of resection and surgical morbidity by diminishing the tumor and reducing the intraoperative spillage of tumor cells[26-28]. Obtaining the gene mutation status from the biopsy specimens before neoadjuvant IM therapy makes the process more accurate, which is in accordance with the latest edition of the NCCN clinical practice guidelines (2016) for gastrointestinal stromal tumors. Decisions to improve the outcomes in the management of GISTs are based on the risk of progression, pathologic characteristics, and tumor location[29]. Limited by a fine needle, specimens obtained by EUS-FNA are often not sufficient to allow immunohistochemistry and genotype analysis, which could occur with EUS-CHDB with careful operations to reduce the intraoperative spillage of tumor cells[30].
Using the vertical diathermic loop, gastric infiltrative tumors were open to the biopsy forceps safely by electric coagulation, which resulted in a positive rate of 90% in this study. In particular, for a rigid and tough wall, the diathermic loop, similar to a knife, makes the tumor incised and revealed. The vertical angle makes cutting holes in tumors easier than with other electroexcision tools or biopsy forceps. Under the guidance of EUS, we can determine the most characterized targeted focus, guarantee electroexcision without risk, and avoid areas with abundant irregular branch blood flow signals. In this way, EUS-CHDB was preliminarily proved to be quite safe because neither severe haemorrhaging nor perforation occurred in any patient. In contrast to EUS-FNA, EUS-CHDB is less costly, easier to carry out and, most significantly, allows more histopathology findings. Meanwhile, EUS-CHDB can obtain a larger tissue specimen and may thus increase the rate of positive diagnostic findings compared with conventional biopsies. For the only negative case who was suspected of having GLP but suffered a negative pathology, the rational speculation was focused on an inadequate depth, insufficient biopsy samples or invalid biopsy on the tissue that received electrocoagulation. For negative cases, if necessary, EUS-FNA combined with EUS-CHDB may offer a satisfactory solution.
As a limitation, this study was performed at a single centre with a small number of patients and without a control group. Further large studies on this method are needed to clarify the indications and clinical outcomes.
In conclusion, under the guidance of high-definition longitudinal axis EUS, the vertical diathermic loop can safely and efficiently break up the shield of a thick and rigid wall of the gastric or mucous layer above the tumor. EUS-CHDB may be a technically feasible and safe option for patients with gastric infiltrative tumors or gastrointestinal submucosal tumors. EUS-CHDB could be recommended as a remedial or even preferred biopsy method for some submucosal lesions.
Gastric infiltrative tumors, such as gastric linitis plastica (GLP) and gastric lymphoma, and gastrointestinal submucosal tumors often have more than one negative pathological finding on ordinary endoscopic biopsies. The authors invented a novel vertical diathermic loop to perform EUS-guided cutting of holes and deep biopsy (EUS-CHDB).
For patients suspected with gastric infiltrative tumors or gastrointestinal submucosal tumors, diagnosis via endoscopic forceps biopsy is often difficult, with a low positive rate in the initial detection. Several novel technologies, such as EUS-guided fine needle aspiration, excavating biopsy, snaring biopsy and endoscopic mucosal resection, have emerged. However, the positive finding rate of the above techniques is still undesirable.
This study reported on a more accurate diagnostic possibility offered by EUS-CHDB for pathologic diagnosis of gastric infiltrative tumors and gastrointestinal submucosal tumors. This new technique was proved to be a safe, technically feasible and more accurate option for patients with gastric infiltrative tumors or gastrointestinal submucosal tumors.
This study provides the evidence supporting a new technique about biopsy, EUS-CHDB, which may improve the diagnostic accuracy and even assist in the early diagnosis of gastric infiltrative tumors and gastrointestinal submucosal tumors.
EUS: an endoscopy with an ultrasonic probe in the head end, which can detect and scan lesions of the gastrointestinal tract, pancreas, liver, gallbladder and adjacent organs by virtue of ultrasound. GLP: a special type of diffuse infiltrative gastric carcinoma with tumor growth direction to the submucosa so that the gastric cavity becomes small and gastric full layer is thickening and hardening.
Gastric infiltrative tumors, such as gastric linitis plastica and gastric lymphoma, and gastrointestinal submucosal tumors often have more than one negative pathological finding on ordinary endoscopic biopsies. The authors invented a novel vertical electrode loop to perform EUS-guided cutting of holes and deep biopsy (EUS-CHDB). The authors report on a more accurate diagnostic possibility offered by EUS-CHDB for pathologic diagnosis of gastric infiltrative tumors and gastrointestinal submucosal tumors. The results are interesting.
| 1. | Yokota T, Teshima S, Saito T, Kikuchi S, Kunii Y, Yamauchi H. Borrmann’s type IV gastric cancer: clinicopathologic analysis. Can J Surg. 1999;42:371-376. [PubMed] |
| 2. | Kim JI, Kim YH, Lee KH, Kim SY, Lee YJ, Park YS, Kim N, Lee DH, Kim HH, Park do J. Type-specific diagnosis and evaluation of longitudinal tumor extent of borrmann type IV gastric cancer: CT versus gastroscopy. Korean J Radiol. 2013;14:597-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Yook JH, Oh ST, Kim BS. Clinicopathological analysis of Borrmann type IV gastric cancer. Cancer Res Treat. 2005;37:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Luo Y, Gao P, Song Y, Sun J, Huang X, Zhao J, Ma B, Li Y, Wang Z. Clinicopathologic characteristics and prognosis of Borrmann type IV gastric cancer: a meta-analysis. World J Surg Oncol. 2016;14:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 5. | Psyrri A, Papageorgiou S, Economopoulos T. Primary extranodal lymphomas of stomach: clinical presentation, diagnostic pitfalls and management. Ann Oncol. 2008;19:1992-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Vetro C, Chiarenza A, Romano A, Amico I, Calafiore V, Di Raimondo C, Coppolino F, Di Raimondo F. Prognostic assessment and treatment of primary gastric lymphomas: how endoscopic ultrasonography can help in tailoring patient management. Clin Lymphoma Myeloma Leuk. 2014;14:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Mortensen MB, Edwin B, Hünerbein M, Liedman B, Nielsen HO, Hovendal C. Impact of endoscopic ultrasonography (EUS) on surgical decision-making in upper gastrointestinal tract cancer: an international multicenter study. Surg Endosc. 2007;21:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Han C, Lin R, Shi H, Liu J, Qian W, Ding Z, Hou X. The role of endoscopic ultrasound on the preoperative T staging of gastric cancer: A retrospective study. Medicine (Baltimore). 2016;95:e4580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Cantor MJ, Davila RE, Faigel DO. Yield of tissue sampling for subepithelial lesions evaluated by EUS: a comparison between forceps biopsies and endoscopic submucosal resection. Gastrointest Endosc. 2006;64:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Chiyo T, Kobara H, Mori H, Katsuki N, Haba R, Masaki T. Submucosal Endoscopic Sampling for Indefinite Gastric Linitis Plastica Infiltrating into the Submucosal Layer. J Gastrointestin Liver Dis. 2015;24:375-378. [PubMed] |
| 11. | Suekane H, Iida M, Yao T, Matsumoto T, Masuda Y, Fujishima M. Endoscopic ultrasonography in primary gastric lymphoma: correlation with endoscopic and histologic findings. Gastrointest Endosc. 1993;39:139-145. [PubMed] |
| 12. | Butkeviciene L, Dubinskiene L, Verbiene I. [Diagnosis of malignant lymphoma of the stomach]. Medicina (Kaunas). 2002;38:172-175. [PubMed] |
| 13. | Hunt GC, Smith PP, Faigel DO. Yield of tissue sampling for submucosal lesions evaluated by EUS. Gastrointest Endosc. 2003;57:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Rong L, Kida M, Yamauchi H, Okuwaki K, Miyazawa S, Iwai T, Kikuchi H, Watanabe M, Imaizumi H, Koizumi W. Factors affecting the diagnostic accuracy of endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) for upper gastrointestinal submucosal or extraluminal solid mass lesions. Dig Endosc. 2012;24:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Buscaglia JM, Nagula S, Jayaraman V, Robbins DH, Vadada D, Gross SA, DiMaio CJ, Pais S, Patel K, Sejpal DV. Diagnostic yield and safety of jumbo biopsy forceps in patients with subepithelial lesions of the upper and lower GI tract. Gastrointest Endosc. 2012;75:1147-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Lee JH, Choi KD, Kim MY, Choi KS, Kim DH, Park YS, Kim KC, Song HJ, Lee GH, Jung HY. Clinical impact of EUS-guided Trucut biopsy results on decision making for patients with gastric subepithelial tumors ≥ 2 cm in diameter. Gastrointest Endosc. 2011;74:1010-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Al-Haddad M, Savabi MS, Sherman S, McHenry L, Leblanc J, Cramer H, Emerson R, O’Neil J, Khashab M, Dewitt J. Role of endoscopic ultrasound-guided fine-needle aspiration with flow cytometry to diagnose lymphoma: a single center experience. J Gastroenterol Hepatol. 2009;24:1826-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Zhou XX, Pan HH, Usman A, Ji F, Jin X, Zhong WX, Chen HT. Endoscopic ultrasound-guided deep and large biopsy for diagnosis of gastric infiltrating tumors with negative malignant endoscopy biopsies. World J Gastroenterol. 2015;21:3607-3613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Ahn JB, Ha TK, Lee HR, Kwon SJ. An Insufficient Preoperative Diagnosis of Borrmann Type 4 Gastric Cancer in Spite of EMR. J Gastric Cancer. 2011;11:59-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [PubMed] |
| 21. | Zheng S, Chen LR, Wang HJ, Chen SZ. Analysis of mutation and expression of c-kit and PDGFR-alpha gene in gastrointestinal stromal tumor. Hepatogastroenterology. 2007;54:2285-2290. [PubMed] |
| 22. | Roberts PJ, Eisenberg B. Clinical presentation of gastrointestinal stromal tumors and treatment of operable disease. Eur J Cancer. 2002;38 Suppl 5:S37-S38. [PubMed] |
| 23. | Rutkowski P, Debiec-Rychter M, Ruka W. Gastrointestinal stromal tumors: key to diagnosis and choice of therapy. Mol Diagn Ther. 2008;12:131-143. [PubMed] |
| 24. | Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y, Kitamura Y. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, doubleblind, placebo-controlled trial. Lancet. 2009;373:1097-104. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1075] [Cited by in RCA: 983] [Article Influence: 57.8] [Reference Citation Analysis (1)] |
| 25. | Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 690] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 26. | Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster J, Al-Batran SE. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 703] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 27. | Shrikhande SV, Marda SS, Suradkar K, Arya S, Shetty GS, Bal M, Shukla PJ, Goel M, Mohandas KM. Gastrointestinal stromal tumors: case series of 29 patients defining the role of imatinib prior to surgery. World J Surg. 2012;36:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Koontz MZ, Visser BM, Kunz PL. Neoadjuvant imatinib for borderline resectable GIST. J Natl Compr Canc Netw. 2012;10:1477-1482; quiz 1482. [PubMed] |
| 29. | Tielen R, Verhoef C, van Coevorden F, Gelderblom H, Sleijfer S, Hartgrink HH, Bonenkamp JJ, van der Graaf WT, de Wilt JH. Surgical treatment of locally advanced, non-metastatic, gastrointestinal stromal tumours after treatment with imatinib. Eur J Surg Oncol. 2013;39:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | von Mehren M. Management of Gastrointestinal Stromal Tumors. Surg Clin North Am. 2016;96:1059-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Fernandez JM, Sato H S- Editor: Qi Y L- Editor: A E- Editor: Wang CH