Published online Apr 14, 2017. doi: 10.3748/wjg.v23.i14.2601
Peer-review started: November 30, 2016
First decision: December 28, 2016
Revised: February 1, 2017
Accepted: March 2, 2017
Article in press: March 2, 2017
Published online: April 14, 2017
Processing time: 135 Days and 19.8 Hours
To investigate the expression and clinical pathological significance of ROR2 and WNT5a in gallbladder squamous/adenosquamous carcinoma (SC/ASC) and adenocarcinoma (AC).
EnVision immunohistochemistry was used to stain for ROR2 and WNT5a in 46 SC/ASC patients and 80 AC patients.
Poorly differentiated AC among AC patients aged > 45 years were significantly more frequent compared with SC/ASC patients, while tumors with a maximal diameter > 3 cm in the SC/ASC group were significantly more frequent compared with the AC group. Positive ROR2 and WNT5a expression was significantly lower in SC/ASC or AC with a maximal mass diameter ≤ 3 cm, a TNM stage of I + II, no lymph node metastasis, no surrounding invasion, and radical resection than in patients with a maximal mass diameter > 3 cm, TNM stage IV, lymph node metastasis, surrounding invasion, and no resection. Positive ROR2 expression in patients with highly differentiated SC/ASC was significantly lower than in patients with poorly differentiated SC/ASC. Positive ROR2 and WNT5a expression levels in highly differentiated AC were significantly lower than in poorly differentiated AC. Kaplan-Meier survival analysis showed that differentiation degree, maximal mass diameter, TNM stage, lymph node metastasis, surrounding invasion, surgical procedure and the ROR2 and WNT5a expression levels were closely related to average survival of SC/ASC or AC. The survival of SC/ASC or AC patients with positive expression of ROR2 and WNT5a was significantly shorter than that of patients with negative expression results. Cox multivariate analysis revealed that poor differentiation, a maximal diameter of the mass ≥ 3 cm, TNM stage III or IV, lymph node metastasis, surrounding invasion, unresected surgery and positive ROR2 or WNT5a expression in the SC/ASC or AC patients were negatively correlated with the postoperative survival rate and positively correlated with mortality, which are risk factors and independent prognostic predictors.
SC/ASC or AC patients with positive ROR2 or WNT5a expression generally have a poor prognosis.
Core tip: Gallbladder carcinoma (GBC) is a highly aggressive malignancy of the biliary tract. However, biological markers for the diagnosis, prognosis, and targeted therapy of GBCs are still not clear. In this study, we investigated the clinicopathological significance of ROR2 and WNT5a in squamous/adenosquamous carcinoma (SC/ASC) and adenocarcinoma (AC) of the gallbladder. We found that positive ROR2 or WNT5a expression in both SC/ASC or AC patients were negatively correlated with postoperative survival rate and positively correlated with mortality. Elevated expression levels of ROR2 or WNT5a are associated with a higher risk of GBC and are independent prognostic predictors.
- Citation: Wu ZC, Xiong L, Wang LX, Miao XY, Liu ZR, Li DQ, Zou Q, Liu KJ, Zhao H, Yang ZL. Comparative study of ROR2 and WNT5a expression in squamous/adenosquamous carcinoma and adenocarcinoma of the gallbladder. World J Gastroenterol 2017; 23(14): 2601-2612
- URL: https://www.wjgnet.com/1007-9327/full/v23/i14/2601.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i14.2601
Gallbladder carcinoma is rare, accounting for approximately 0.28% of the general surgery diseases treated within a given period of time. Gallbladder carcinomas mainly consist of adenocarcinomas (ACs), which account for > 90% of gallbladder carcinomas. Gallbladder squamous carcinoma (SC) and adenosquamous carcinoma (ASC) are rare pathological subtypes of gallbladder carcinoma, with a combined incidence rate accounting for approximately 1.4%-10.4% of all gallbladder carcinomas[1]. Because of this low incidence, the clinicopathological characteristics and biological behavior of SC/ASC remain unclear.
Few studies on this topic are available in the literature, and most of them consist of case reports or clinical case analyses[1-6]. In recent years, some studies have identified the following characteristics of SC/ASC[1]. No special symptoms are shown in the early stage, with the most common clinical manifestations being right upper abdominal pain and discomfort. Most cases are in the late stage at diagnosis, and the efficacy of surgery is therefore poor. Early diagnosis and radical surgery contributes to improvement of the prognosis[2].
The disease is likely to be initiated with squamous epithelium metaplasia in the gallbladder mucosa, followed by carcinogenesis on this basis, though some researchers believe that it occurs in the pluripotent basal cells in the gallbladder mucosa; more in-depth study is awaited[3]. Carcinogenic factors may mainly be related to the long-term chronic irritation of inflammation and cholelithiasis. Some authors have also proposed that carcinogenesis may be related to the long-term effects of bacteria and chemical reactions in bile, forming carcinogens such as anthracene and methylcholanthrene[4].
A number of studies has found that compared with AC, SC/ASC shows stronger proliferation, higher malignancy and directly invades the surrounding tissues and organs much more readily, whereas it shows less frequent regional metastasis in the lymph nodes and other distant organs compared with AC. However, this finding is still controversial, with different perspectives being discussed[5]. Surgery is still the preferred treatment for SC/ASC, but the efficacy of surgery is largely dependent on clinical staging and the applied surgical methods. In addition, postoperative radiotherapy may have some effect[6]. Few or no fundamental systematic studies on the pathogenesis of SC/ASC have been reported in the literature, and most studies are focused on markers of pathological diagnosis, due to the extremely low incidence of SC/ASC and the difficulty of collecting a given number of cases.
Receptor tyrosine kinase-like orphan receptor 2 (ROR2) belongs to the receptor tyrosine kinase (RTK) family. Many members of this kinase family play an important role in the process of morphogenesis and differentiation in mammals. ROR2 can act as a receptor for Wnt5a, leading to signal transduction through the JAK-STAT3 and Wnt/JNK signaling pathways[7,8]. Recent studies have found that the expression of ROR2 is closely related to the occurrence, progression, biological behavior and prognosis of a variety of malignant tumors, such as gastric cancer[9], colorectal cancer[10], liver cancer[11], breast cancer[12], esophageal squamous carcinoma[12] and medulloblastoma[13]. Further studies demonstrated that malignancies with a high expression level of ROR2 are typically poorly differentiated, of a high clinical stage, and prone to metastasis with strong invasiveness. ROR2 is considered to be an important biological indicator for assessing the prognosis of patients with malignant tumors, and a high expression level of ROR2 may suggest a poor prognosis.
Wnt5a is a member of the Wnt family that has received major interest in recent years. The Wnt5a protein is involved in many physiological and pathological processes, such as embryonic development, inflammation and tumor development. It plays an important role in polarity, orientation, deformation of the cell cytoskeleton and a variety of malignant processes in tumor cells. Abnormal expression of the Wnt5a protein can be observed in a variety of epithelial and mesenchymal tumors. Different experimental studies have arrived at opposite conclusions regarding whether Wnt5a is a carcinogen or a suppressor of tumor development. Wnt5a is down-regulated and plays a suppressor role in colorectal cancer[14], neuroblastoma[15] and leukemia[16]. Down-regulation of Wnt5a is positively correlated with the stage of a tumor and is an independent prognostic factor in the various tumor subtypes. In cutaneous melanoma[17], breast cancer cells[18], gastric cancer[19], non-small cell lung cancer[20] and prostate cancer[21], Wnt5a is over-expressed and affects the migration and invasiveness of the tumor, showing the characteristics of an oncogene.
No study addressing the expression levels of ROR2 and Wnt5a in gallbladder SC/ASC and AC has been reported in the literature to date. In the present study, using the EnVision immunohistochemistry method, the expression levels of ROR2 and Wnt5a and their clinical pathological significance were investigated in tissues from 46 cases of gallbladder SC/ASC and 80 cases of gallbladder AC. Additionally, the clinicopathological characteristics of SC/ASC and AC, and the differences in the expression of ROR2 and Wnt5a were compared.
Surgical resection specimens from a total of 46 cases of gallbladder SC/ASC were collected from January 1995 to December 2009, accounting for 4.34% of all gallbladder cancer cases recorded in the same period of time (46/1060). The specimen sources included 16 patients from the Second Xiangya Hospital of Central South University (16/370, 4.32%), 14 from Xiangya Hospital (18/325, 4.31%), 5 from the Third Xiangya Hospital (5/110, 4.55%), 4 from the Hunan Provincial Tumor Hospital (4/100, 4.00%), 5 from the Hunan Provincial People’s Hospital (5/105, 4.76%), and 1 from each of the Central Hospital of Changde and the Central Hospital of Loudi (2/50, 4.00%). Among the 46 cases of SC/ASC, 19 patients were males (41.3%) and 27 patients were females (58.7%). These subjects showed an age range of 35-82 years, with a mean of 55.8 ± 9.6 years, including 3 patients ≤ 45 years of age (6.5%) and 43 patients > 45 years of age (93.5%).
The pathological types included 26 cases of SC (56.5%) and 20 cases of ASC (43.5%). The differentiation among the subjects (judged by the differentiation of SC) included 16 cases of highly differentiated SC (34.8%), 24 cases of moderately differentiated SC (52.2%), and 6 cases of poorly differentiated SC (13.0%). The maximal diameter of the mass was ≤ 3 cm in 20 cases (43.5%) and > 3 cm in 26 cases (56.5%). Gallbladder stones were found in 28 cases (60.9%); regional lymph node metastasis was confirmed through intraoperative and (or) pathologic examination in 29 cases (63.0%) and tumor invasion of the surrounding tissues and organs outside the gallbladder was intraoperatively observed in 30 cases (65.2%). The TNM staging included 5 cases in stage I (10.9%), 7 in stage II (15.2%), 17 in stage III (43.5%) and 14 in stage IV (30.4%). The applied surgical procedures comprised 14 cases of radical resection (30.4%), 18 cases of palliative resection (39.1%) and 14 cases of biopsy alone with no tumor excision (30.4%).
Additionally, surgical specimens from 80 cases of gallbladder AC were collected from the Second Xiangya Hospital and the Central Hospital of Loudi from January 2000 to December 2009 for a comparative analysis. Among the 80 cases of AC, 26 patients were males (32.5%) and 54 patients were females (67.5%). The ages of the subjects ranged from 33-80 years, with a mean of 53.8 ± 9.9 years, including 16 subjects of ≤ 45 years (20.0%) and 64 subjects of > 45 years (80.0%).
The differentiation among this group included 27 cases of highly differentiated carcinoma (33.8%), 25 cases of moderately differentiated carcinoma (33.1%), and 28 cases of poorly differentiated carcinoma (35.0%). The maximal diameter of the mass was ≤ 3 cm in 50 cases (62.5%) and > 3 cm in 30 cases (37.5%). Gallbladder stones were found in 38 cases (47.5%); regional lymph node metastasis was confirmed through intraoperative and (or) pathologic examination in 50 cases (62.5%); tumor invasion of surrounding tissues and organs outside the gallbladder was intraoperatively observed in 49 cases (61.3%). The TNM staging included 8 cases in stage I (10%), 13 in stage II (16.3%), 38 in stage III (47.5%) and 21 in stage IV (26.3%). The applied surgical procedures comprised 26 cases of radical resection (32.5%), 28 cases of palliative resection (35.0%) and 26 cases of biopsy only with no tumor excision (32.5%).
Through mail or telephone interviews, follow-up information was obtained for the 46 cases of SC/ASC and the 80 cases of AC patients, over a follow-up period of 2 years. Among the 46 SC/ASC patients, postoperative survival was ≥ 1 year in 13 cases (4 patients survived over 2 years) and < 1 year in 33 cases, with an average survival time of 10.07 ± 0.78 mo. Among the 80 AC patients, postoperative survival was ≥ 1 year in 23 cases (9 patients survived over 2 years) and < 1 year in 57 cases, with an average survival time of 10.34 ± 0.63 mo. The above gallbladder SC/ASC and AC surgical resection specimens were fixed in 4% formalin for 24 h to prepare conventional paraffin-embedded sections, at a slice thickness of 4 μm.
Rabbit anti-human ROR2 and WNT5a polyclonal antibodies were purchased from Abgent Company (San Diego, CA, United States). The EnVisionTM Detection Kit was obtained from Dao Laboratories (Carpinteria, CA, United States).
The EnVision immunohistochemical method was applied for ROR2 and WNT5a staining, in strict accordance with the operation manual for the reagents. The main procedures were as follows: the slices were dewaxed and washed → treated with 3% H2O2 in methanol for 10 min → treated with trypsin for 15 min → treated with the primary antibody added dropwise, with incubation at 37 °C for 60 min → treated with solution A added dropwise, with incubation at 37 °C for 30 min → developed with the chromogenic regent for 15 min → lightly stained with hematoxylin for 1 min → dehydrated and transparentized, followed by mounting with neutral resin. Brown particles in the cytoplasm indicated ROR2- and WNT5a-positive cells. The rate of positive cells was observed by examining 400 tumor cells in 10 random fields of a section under a microscope at a high magnification. A patient showing an average rate of positive cells ≥ 25% was considered a positive case, while an average rate < 25% was considered a negative case[22-24]. A positive section provided by Beijing Zhongshan Biotechnology Corp (Beijing, China) was used as the positive control, while replacement of the primary antibody with 5% fetal bovine serum was employed as the negative control.
All of the experimental data were input into the SPSS13.0 statistical software package (IBM Corp, Armonk, NY, United States). The relationships between the expression of ROR2 and WNT5a and the histological and clinical factors were investigated with the χ2 test or Fisher’s exact test. The Kaplan-Meier method was applied for univariate survival analysis and log-rank testing. The Cox proportional risk model was utilized to perform multivariate analysis and to determine the 95%CI with the normal approximation test (Wald’s test). A probability level of P < 0.05 was considered significant.
As shown in Table 1, the proportion of poorly differentiated adenocarcinomas among the AC patients aged > 45 years was significantly higher than among the SC/ASC patients (P < 0.05), while the proportion of tumors with a maximal diameter > 3 cm among the patients in the SC/ASC group was significantly higher than in the AC group (P < 0.05). The sex, existence of gallstones, TNM stage, occurrence of lymph node metastasis, invasion of the surrounding tissues and organs, applied surgical procedure, and the average survival of the SC/ASC patients showed no significant difference compared with the AC patients (P > 0.05).
| Clinicopathologic characteristic | SC/ASC,n = 46 | AC,n = 80 | χ2 | P value |
| Sex | ||||
| Male | 19 (41.3) | 26 (32.5) | 0.986 | 0.352 |
| Female | 27 (58.7) | 54 (67.5) | ||
| Age, yr | ||||
| ≤ 45 | 3 (6.5) | 16 (20.0) | 4.143 | 0.042 |
| > 45 | 43 (93.5) | 64 (80.0) | ||
| Degree of differentiation | ||||
| High | 16 (34.8) | 27 (33.8) | 8.515 | 0.014 |
| Moderate | 24 (52.2) | 25 (31.3) | ||
| Poor | 6 (13.0) | 28 (35.0) | ||
| Maximal diameter of the mass, cm | ||||
| ≤ 3 | 20 (43.5) | 50 (62.5) | 4.280 | 0.039 |
| > 3 | 26 (56.5) | 30 (37.5) | ||
| Gallstones | ||||
| No | 18 (39.1) | 42 (52.5) | 2.093 | 0.148 |
| Yes | 28 (60.9) | 38 (47.5) | ||
| TNM stage | ||||
| I + II | 12 (26.1) | 21 (26.3) | 0.287 | 0.866 |
| III | 20 (33.5) | 38 (47.5) | ||
| IV | 14 (30.4) | 21 (26.3) | ||
| Lymph node metastasis | ||||
| No | 17 (37.0) | 30 (37.5) | 0.004 | 0.952 |
| Yes | 29 (63.0) | 50 (62.5) | ||
| Invasion of the surrounding tissue | ||||
| No | 16 (34.8) | 31 (38.8) | 0.197 | 0.658 |
| Yes | 30 (62.5) | 49 (61.3) | ||
| Surgical procedure | ||||
| Radical | 14 (30.4) | 26 (32.5) | 0.215 | 0.898 |
| Palliative | 18 (39.1) | 28 (35.0) | ||
| Unresected | 14 (30.4) | 26 (32.5) | ||
| Average survival time | 10.07 (4-25) | 10.34 (3-27) | 0.014 | 0.906 |
| ROR2 | ||||
| - | 20 (43.5) | 29 (36.2) | 0.642 | 0.386 |
| + | 26 (56.5) | 51 (63.8) | ||
| WNT5a | ||||
| - | 17 (37.0) | 31 (38.7) | 0.040 | 0.858 |
| + | 29 (63.0) | 49 (61.3) |
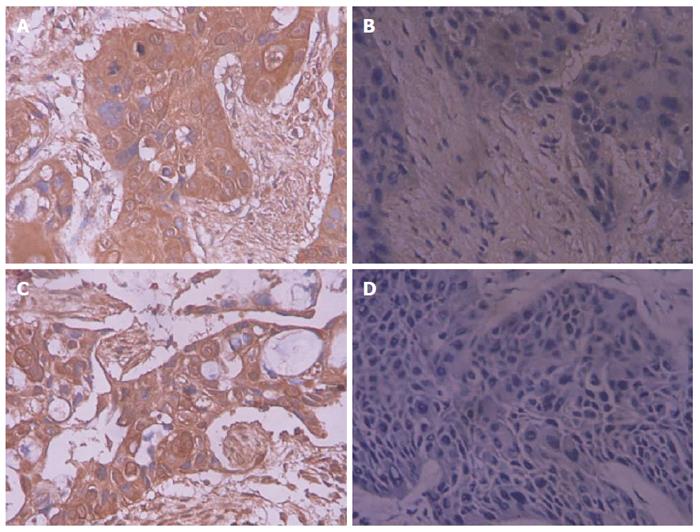
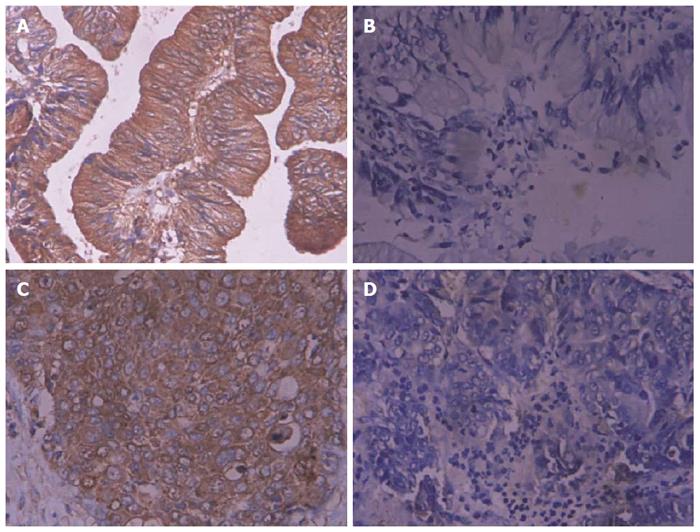
The Brain-derived neurotrophic factor and bone morphogenetic protein receptor type 1A immunohistochemical reaction products were mainly located in the cytoplasm, with occasional nuclear staining observed (Figures 1 and 2). Among the 46 cases of SC/ASC, ROR2 and WNT5a were positively expressed in 26 cases (56.5%) and 29 cases (63.0%), respectively (judged by positive expression of SC, while a case showing positive expression of AC and negative expression of SC was considered to exhibit negative expression). Among the 80 AC cases, ROR2 and WNT5a were positively expressed in 51 (63.8%) and 49 (61.3%) cases. The positive expression rates of ROR2 and WNT5a among the SC/ASC patients showed no significant difference compared with the AC patients (P > 0.05).
The positive expression rates of ROR2 and WNT5a in the patients showing a maximal diameter of the mass ≤ 3 cm, a TNM stage of I + II, no lymph node metastasis, no invasion in the surrounding tissues and organs, and radical resection were significantly lower than in the patients with a maximal diameter of the mass > 3 cm, a TNM stage of IV, lymph node metastasis, invasion in the surrounding tissues and organs, and no resection (P < 0.05 or P < 0.01). The positive expression rate of WNT5a in the patients with highly differentiated SC/ASC was significantly lower than in the patients with poorly differentiated SC/ASC (P < 0.05). The expression of ROR2 and WNT5a was not significantly related to the sex, age, pathological type, or existence of gallstones among the patients (P > 0.05). The detailed data are shown in Table 2.
| Pathologic characteristic | Number of cases | ROR2 | WNT5a | ||||
| Positive cases | χ2 | P value | Positive cases | χ2 | P value | ||
| Pathological type | |||||||
| Squamous carcinoma | 26 | 16 (61.5) | 0.612 | 0.434 | 17 (65.4) | 0.141 | 0.708 |
| Adenosquamous carcinoma | 20 | 10 (50.0) | 12 (60.0) | ||||
| Differentiation | |||||||
| High | 16 | 5 (31.3) | 9.123 | 0.010 | 8 (50.0) | 1.827 | 0.401 |
| Moderate | 24 | 15 (62.5) | 17 (70.8) | ||||
| Poor | 6 | 6 (100.0) | 4 (66.7) | ||||
| Maximal diameter of the mass in cm | |||||||
| ≤ 3 | 20 | 7 (35.0) | 6.669 | 0.010 | 9 (45.0) | 4.945 | 0.026 |
| ≥ 3 | 26 | 19 (73.1) | 20 (76.9) | ||||
| Gallstones | |||||||
| No | 18 | 11 (61.1) | 0.253 | 0.615 | 12 (66.7) | 0.167 | 0.683 |
| Yes | 28 | 15 (53.6) | 17 (60.7) | ||||
| TNM stage | |||||||
| I + II | 12 | 4 (33.3) | 4 (33.3) | ||||
| III | 20 | 10 (50.0) | 7.824 | 0.023 | 14 (70.0) | 6.411 | 0.041 |
| IV | 14 | 12 (85.7) | 11 (78.6) | ||||
| Lymph node metastasis | |||||||
| No | 17 | 5 (29.4) | 8.065 | 0.005 | 7 (41.2) | 6.720 | 0.010 |
| Yes | 29 | 21 (72.4) | 22 (75.9) | ||||
| Invasion of the surrounding tissue | |||||||
| No | 16 | 5 (31.3) | 6.376 | 0.011 | 7 (43.8) | 3.920 | 0.048 |
| Yes | 30 | 21 (70.0) | 22 (73.3) | ||||
| Surgical procedure | |||||||
| Radical | 14 | 4 (28.6) | 7.374 | 0.022 | 5 (35.7) | 77.677 | 0.019 |
| Palliative | 18 | 11 (61.1) | 12 (66.7) | ||||
| Unresected | 14 | 11 (78.6) | 12 (85.7) | ||||
The positive expression rates of ROR2 and WNT5a in the patients with high differentiation, a maximal diameter of the mass ≤ 3 cm, a TNM stage of I + II, no lymph node metastasis, no invasion in the surrounding tissues and organs, and radical resection were significantly lower than in the patients with low differentiation, a maximal diameter of the mass > 3 cm, a TNM stage of IV, lymph node metastasis, invasion in the surrounding tissues and organs, and no resection (P < 0.05 or P < 0.01). The expression of ROR2 and WNT5a was not significantly related to the sex, age, or existence of gallstones in the AC patients (P > 0.05). The detailed data are shown in Table 3.
| Pathologic characteristic | Number of cases | ROR2 | WNT5a | ||||
| Positive cases | χ2 | P value | Positive cases | χ2 | P value | ||
| Differentiation | |||||||
| High | 27 | 12 (44.0) | 10.352 | 0.002 | 11 (40.7) | 8.404 | 0.015 |
| Moderate | 25 | 15 (60.0) | 16 (64.0) | ||||
| Poor | 28 | 24 (85.7) | 22 (78.6) | ||||
| Maximal diameter of the mass in cm | |||||||
| ≤ 3 | 50 | 26 (52.0) | 7.966 | 0.004 | 26 (52.0) | 4.807 | 0.029 |
| > 3 | 30 | 25 (83.3) | 23 (76.7) | ||||
| Gallstones | |||||||
| No | 42 | 26 (61.9) | 0.13 | 0.718 | 26 (61.9) | 0.016 | 0.899 |
| Yes | 38 | 25 (65.8) | 23 (60.5) | ||||
| TNM stage | |||||||
| I + II | 21 | 7 (33.3) | 7 (33.3) | ||||
| III | 38 | 25 (65.8) | 14.968 | 0 | 24 (63.2) | 12.248 | 0.002 |
| IV | 21 | 19 (90.5) | 18 (85.7) | ||||
| Lymph node metastasis | |||||||
| No | 30 | 12 (40.0) | 11.716 | 0.001 | 12 (40.0) | 9.132 | 0.003 |
| Yes | 50 | 39 (78.0) | 37 (74.0) | ||||
| Invasion of the surrounding tissue | |||||||
| No | 31 | 13 (41.9) | 10.422 | 0.002 | 12 (38.7) | 10.834 | 0.001 |
| Yes | 49 | 38 (77.6) | 37 (75.5) | ||||
| Surgical procedure | |||||||
| Radical | 26 | 10 (38.5) | 12.296 | 0.002 | 10 (38.5) | 11.68 | 0.003 |
| Palliative | 28 | 19 (67.9) | 17 (60.7) | ||||
| Unresected | 26 | 22 (84.6) | 22 (84.6) | ||||
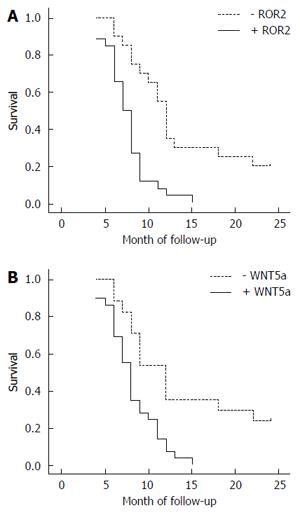
Through mail or telephone interviews, follow-up information was obtained for 46 of the SC/ASC patients, over a follow-up period of 2 years. The 2-year survivors were included in the statistical analysis as censored cases. Among the 46 SC/ASC patients, postoperative survival was ≥ 1 year in 13 cases (4 patients survived for over 2 years) and < 1 year in 33 cases, with an average survival time of 10.07 ± 0.78 mo. The results of the Kaplan-Meier survival analysis showed that the degree of differentiation, maximal diameter of the mass, TNM stage, lymph node metastasis, invasion of the surrounding tissues, and applied surgical procedure were closely related to the average survival of the patients with gallbladder SC/ASC (P < 0.05 or P < 0.01). The survival of the patients with positive expression of ROR2 and WNT5a was significantly shorter compared with the patients with negative expression (P = 0.000, P = 0.001), as shown in Table 4 and in the survival curves in Figure 3.
| Grouping | Number of cases, n | Average survival in mo | χ2 | P value |
| Sex | ||||
| Male | 19 | 10.74 (6-24) | 0.767 | 0.381 |
| Female | 27 | 9.85 (4-24) | ||
| Age, yr | ||||
| ≤ 45 | 3 | 15.67 (8-24) | 2.023 | 0.155 |
| > 45 | 43 | 9.84 (4-25) | ||
| Pathological type | ||||
| Squamous carcinoma | 26 | 10.19 (4-24) | 0.223 | 0.637 |
| Adenosquamous carcinoma | 20 | 10.25 (4-24) | ||
| Degree of differentiation | ||||
| High | 16 | 13.81 (5-24) | 19.125 | 0.000 |
| Moderate | 24 | 8.92 (4-18) | ||
| Poor | 6 | 5.83 (4-9) | ||
| Maximal diameter of the mass, cm | ||||
| ≤ 3 | 20 | 14.35 (7-24) | 31.337 | 0.000 |
| > 3 | 26 | 7.04 (4-11) | ||
| Gallstones | ||||
| No | 18 | 8.22 (4-12) | 3.730 | 0.053 |
| Yes | 28 | 11.50 (4-24) | ||
| TNM stage | ||||
| I + II | 12 | 17.00 (9-24) | 51.139 | 0.000 |
| III | 20 | 9.20 (7-15) | ||
| IV | 14 | 5.86 (4-8) | ||
| Lymph node metastasis | ||||
| No | 17 | 14.24 (4-24) | 16.219 | 0.000 |
| Yes | 29 | 7.86 (4-15) | ||
| Invasion of the surrounding tissue | ||||
| No | 16 | 15.75 (9-24) | 32.271 | 0.000 |
| Yes | 30 | 7.27 (4-12) | ||
| Surgical procedure | ||||
| Radical | 14 | 16.64 (10-24) | 50.165 | 0.000 |
| Palliative | 18 | 8.50 (6-12) | ||
| Unresected | 14 | 6.00 (4-8) | ||
| ROR2 | ||||
| - | 20 | 13.65 (6-24) | 16.502 | 0.000 |
| + | 26 | 7.58 (4-15) | ||
| WNT5a | ||||
| - | 17 | 13.77 (6-24) | 10.844 | 0.001 |
| + | 29 | 8.14 (4-15) | ||
The Cox multivariate analysis showed that poor differentiation, a maximal diameter of the mass ≥ 3 cm, a TNM stage of III or IV, the occurrence of lymph node metastasis, invasion of the surrounding tissues and organs, and unresected surgery were negatively correlated with the postoperative survival rate and positively correlated with mortality, which are risk factors and independent prognostic predictors. Positive expression of ROR2 or WNT5a was negatively correlated with the postoperative survival rate and positively correlated with mortality, which are the risk factors and independent prognostic predictors (Table 5).
| Grouping | Factor | B | SE | Wald | P value | RR | 95%CI | |
| Lower | Upper | |||||||
| Pathological type | SC/ASC | 0.094 | 0.346 | 0.074 | 0.786 | 1.099 | 0.558 | 2.164 |
| Degree of differentiation | High/Moderate/Poor | 0.833 | 0.369 | 5.096 | 0.024 | 2.300 | 1.116 | 4.741 |
| Maximal diameter of the mass, cm | ≤ 3/> 3 | 2.374 | 0.743 | 10.209 | 0.001 | 10.740 | 2.504 | 46.075 |
| Gallstones | No/Yes | 0.641 | 0.420 | 2.329 | 0.127 | 1.898 | 0.833 | 4.324 |
| TNM stage | I + II/III/IV | 1.362 | 0.476 | 8.187 | 0.004 | 3.904 | 1.536 | 9.924 |
| Lymph node metastasis | No/Yes | 1.792 | 0.564 | 10.095 | 0.001 | 6.011 | 1.987 | 18.128 |
| Invasion of the surrounding tissue | No/Yes | 2.648 | 0.782 | 11.466 | 0.001 | 14.126 | 3.050 | 65.413 |
| Surgical procedure | Radical/Palliative/Unresected | 1.104 | 0.481 | 5.268 | 0.022 | 3.016 | 1.175 | 7.743 |
| ROR2 | -/+ | 1.623 | 0.674 | 5.799 | 0.016 | 5.068 | 1.353 | 18.992 |
| WNT5a | -/+ | 1.231 | 0.489 | 6.337 | 0.012 | 3.425 | 1.313 | 8.930 |
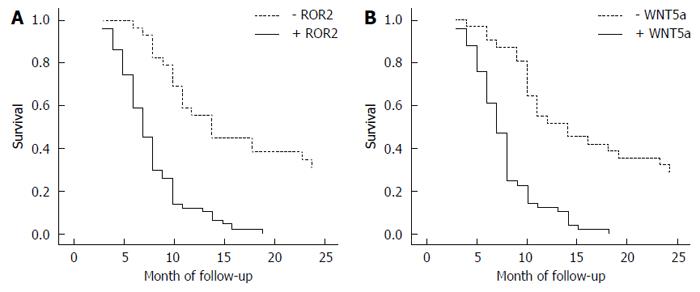
Through mail or telephone interviews, follow-up information was obtained for 80 of the SC/ASC patients, over a follow-up period of 2 years. The 2-year survivors were included in the statistical analysis as censored cases. Among the 80 AC patients, postoperative survival was ≥ 1 year in 23 cases (9 patients survived for over 2 years) and < 1 year in 57 cases, with an average survival time of 10.34 ± 0.63 mo. The results of the Kaplan-Meier survival analysis showed that the degree of differentiation, maximal diameter of the mass, TNM stage, lymph node metastasis, invasion of the surrounding tissues, and applied surgical procedure were closely related to the average survival of the patients with gallbladder AC (P = 0.000). Survival of the patients with positive expression of ROR2 and WNT5a was significantly shorter compared with the patients with negative expression (P = 0.000), as shown in Table 6 and in the survival curves in Figure 4.
| Grouping | Number of cases, n | Average survival in mo | χ2 | P value |
| Sex | ||||
| Male | 26 | 9.58 (3-24) | 2.567 | 0.109 |
| Female | 54 | 11.30 (3-24) | ||
| Age, yr | ||||
| ≤ 45 | 16 | 10.81 (4-24) | 0.003 | 0.956 |
| > 45 | 64 | 10.72 (3-24) | ||
| Degree of differentiation | ||||
| High | 27 | 15.07 (5-24) | 32.501 | 0.000 |
| Moderate | 25 | 10.60 (4-24) | ||
| Poor | 28 | 6.68 (3-14) | ||
| Maximal diameter of the mass, cm | ||||
| ≤ 3 | 50 | 13.70 (6-24) | 68.283 | 0.000 |
| > 3 | 30 | 5.80 (3-10) | ||
| Gallstones | ||||
| No | 42 | 10.19 (3-24) | 0.246 | 0.620 |
| Yes | 38 | 11.34 (4-24) | ||
| TNM stage | ||||
| I + II | 21 | 18.96 (5-24) | 105.825 | 0.000 |
| III | 38 | 9.29 (6-15) | ||
| IV | 21 | 5.14 (3-7) | ||
| Lymph node metastasis | ||||
| No | 30 | 16.27 (4-24) | 42.372 | 0.000 |
| Yes | 50 | 7.42 (3-14) | ||
| Invasion of the surrounding tissue | ||||
| No | 31 | 16.68 (7-24) | 55.535 | 0.000 |
| Yes | 49 | 6.98 (3-11) | ||
| Surgical procedure | ||||
| Radical | 26 | 18.31 (10-24) | 113.141 | 0.000 |
| Palliative | 28 | 8.64 (6-11) | ||
| Unresected | 26 | 5.42 (3-9) | ||
| ROR2 | ||||
| - | 29 | 15.93 (6-24) | 32.994 | 0.000 |
| + | 51 | 7.78 (3-19) | ||
| WNT5a | ||||
| - | 31 | 15.48 (4-24) | 31.654 | 0.002 |
| + | 49 | 7.74 (3-18) | ||
The Cox multivariate analysis showed that poor differentiation, a maximal diameter of the mass ≥ 3 cm, a TNM stage of III or IV, the occurrence of lymph node metastasis, the invasion of the surrounding tissues and organs, and unresected surgery were negatively correlated with the postoperative survival rate and positively correlated with mortality, which are risk factors and independent prognostic predictors. The positive expression of ROR2 or WNT5a was negatively correlated with the postoperative survival rate and positively correlated with mortality, which are risk factors and independent prognostic predictors (Table 7).
| Grouping | Factor | B | SE | Wald | P value | RR | 95%CI | |
| Lower | Upper | |||||||
| Degree of differentiation | High/Moderate/Poor | 1.097 | 0.496 | 4.982 | 0.027 | 2.995 | 1.133 | 7.918 |
| Maximal diameter of the mass, cm | ≤ 3/> 3 | 0.985 | 0.463 | 4.526 | 0.033 | 2.678 | 1.081 | 6.636 |
| Gallstones | No/Yes | 0.412 | 0.381 | 1.169 | 0.280 | 1.510 | 0.716 | 3.186 |
| TNM stage | I + II/III/IV | 1.396 | 0.459 | 9.250 | 0.002 | 4.039 | 1.643 | 9.931 |
| Lymph node metastasis | No/Yes | 1.351 | 0.465 | 8.441 | 0.004 | 3.861 | 1.552 | 9.606 |
| Invasion of the surrounding tissue | No/Yes | 1.644 | 0.583 | 7.952 | 0.005 | 5.176 | 1.651 | 16.227 |
| Surgical procedure | Radical/Palliative/ Unresected | 1.737 | 0.531 | 10.701 | 0.001 | 5.680 | 2.006 | 16.083 |
| ROR2 | -/+ | 2.023 | 0.674 | 9.009 | 0.003 | 7.561 | 2.018 | 28.333 |
| WNT5a | -/+ | 1.931 | 0.649 | 8.853 | 0.003 | 6.896 | 1.933 | 24.607 |
Gallbladder cancer is not common in the digestive system, and AC accounts for > 85% of all gallbladder cancers. Most ACs are highly or moderately differentiated, with few poorly differentiated cases being observed[22]. SC and ASC are rare subtypes of gallbladder cancer, showing a combined incidence rate of 1.4%-10.4% among all gallbladder cancers. Of the 1060 cases of gallbladder cancer examined in this study, 46 (4.34%) were SC/ASC, which is consistent with the data reported in most of the literature[1,2].
SC/ASC shares similar clinical manifestations with AC, including an insidious onset and lack of specific clinical manifestations in the early stage, or only symptoms of chronic cholecystitis; hence, early diagnosis is very difficult. If a patient experiences persistent upper abdominal pain, mass and jaundice, the disease has progressed to the late stage, with abnormal results being obtained in various examinations[1-6,22]. The data evaluated in this study showed that 73.8% the SC/ASC cases were in TNM stage III or IV, similar to the percentage for AC (73.8%). According to most of the available literature, the proliferation of SC is stronger than that of AC, associated with high malignancy. The prognosis of SC/ASC is poor compared with AC, but its metastasis potential is low. Therefore, most gallbladder SCs manifest as a giant lump and are prone to direct invasion of the surrounding organs, with little metastasis being observed in the lymph nodes and distant organs[1-6,25].
The data from this study showed that the proportion of SC/ASC cases displaying a maximal diameter of the mass > 3 cm (56.5%) was significantly higher than for the AC cases (37.5%), while the incidence of lymph node metastasis and invasion of the surrounding tissues and organs was not significantly different between these groups. The prognosis of gallbladder SC/ASC and AC was very poor. All of the patients of TNM stage of III or above died within 2.5 years after surgery, with 5-year survival rates of less than 4% being observed, while the 5-year survival of the patients presenting TNM stage I was > 60%. Therefore, early diagnosis is particularly important.
The data from this study showed that the average postoperative survival of the SC/ASC patients was 10.07 ± 0.78 mo, similar to that of the AC patients (10.34 ± 0.63 mo). Only 4 SC/ASC patients and 9 AC patients in TNM stage I + II survived for over 2 years, demonstrating the poor prognosis of both SC/ASC and AC. Similar to AC, the main treatment for SC/ASC is surgery, but the rate of radical resection is low[1-6,25]. In this study, the radical resection rate among the 46 SC/ASC cases was 30.4%, similar to that for AC (32.5%). The survival time of the patients in the radical resection group was significantly longer than in the palliative resection group and the no resection group; therefore, extended radical surgery may improve the prognosis of the patients.
SC/ASC and AC are not sensitive to chemotherapy, but radiation therapy may have an effect on SC/ASC patients, as reported in the literature, though determination of the specific outcome awaits further observations and the accumulation of relevant data[1-6]. In summary, the results of this study suggest that the clinical manifestations, biological behavior, treatment and postoperative prognosis of SC/ASC are similar to those of AC, with no obvious differences being observed.
The ROR family of receptors belongs to a class of orphan receptors among the receptor tyrosine kinases, which are highly evolutionarily conserved. In mammals, the ROR family consists of two structurally related proteins, ROR1 and ROR2. ROR2 plays an important role in the development of the nervous system and limbs. It has been reported that ROR2 can bind to Wnt5a, CKI and other factors involved in the regulation of canonical and non-canonical Wnt signaling pathways, but the relationship between ROR2 and tumor cell migration remains unclear[24].
The structure of the ROR2 protein includes three important domains: a cytoplasmic domain, a transmembrane domain and an extracellular domain[25]; it has also been shown that ROR2 exhibits a Wnt receptor domain-like structure[26]. Binding of ROR2 with Wnt5a can trigger a signaling pathway mediated by ROR2, and the Wnt5a protein may have an antagonistic effect on the canonical Wnt signaling pathway via this pathway. However, when the receptors of Frizzled4 and LRPS are expressed on the cell surface, the effect of Wnt5a and ROR2 binding is significantly reduced or lost, and the difference in the degree of binding can reflect the activation of the canonical Wnt signal transduction pathway.
The functions of ROR2 and Wnt5a suggest that Wnt5a may play different roles in different tumors. To date, only a few studies examining ROR2 in relation to the occurrence and development of human tumors have been reported. As mentioned above, expression of ROR2 is closely associated with the biological behavior as well as the clinical manifestations of various malignant tumors, including digestive tract cancer[9-11,12], breast cancer[11], and medulloblastoma[13]. Further studies suggested that high expression of ROR2 is often founded in poorly differentiated malignancies in a late clinical stage with strong invasiveness, which is consistent with our results. Therefore, ROR2 is a significant potential biological marker for evaluating the prognosis of the patients with malignant tumors, and high expression of ROR2 may suggest a poor prognosis.
The Wnt5a gene is located on chromosome 3p14.2-p21.1 and encodes a growth factor rich in cysteine, which is involved in signaling transduction between cells during the growth and differentiation of cells[27,28]. Wnt5a can activate both the canonical Wnt/-catenin pathway and the non-canonical Wnt/Ca2+ pathway. Additionally, the Wnt/Ca2+ pathway can interact with the canonical Wnt pathway to play roles in cell differentiation, maturation and tumor development[22,23]. The main biological function of Wnt5a is related to the development and maturation of normal tissues and organs.
Wnt5a is closely associated with a variety of malignant tumors, but the existing reports indicate that the biological effects of Wnt5a are not consistent in different malignancies. Thus, there is significant disagreement in the understanding of the roles of Wnt5a, and some studies have suggested that Wnt5a presents the characteristics of an oncogene. The existing evidence indicates that sustained expression or over-expression of Wnt5a plays an important role in the onset of cancer, through affecting the proliferation, differentiation, invasion and metastasis of tumor cells. For example, Wnt5a is over-expressed and influences the migration and invasion of tumors in cutaneous melanoma[17], breast cancer cells[18], gastric cancer[19], non-small cell lung cancer[20] and prostate cancer[21], exhibiting the characteristics of an oncogene. Further studies revealed that malignant tumors exhibiting high expression of Wnt5a are often poorly differentiated, in a late clinical stage and prone to metastasis with strong invasiveness. Wnt5a is considered to be an important biological indicator for assessing the prognosis of patients with malignant tumors, and high expression of Wnt5a suggests a poor prognosis.
Wnt5a may display the characteristics of a tumor suppressor gene. It can act as a tumor suppressor to partially reduce or delay the occurrence and metastasis of a malignant tumor. Iozzo et al[24] found that Wnt5a was up-regulated in most malignant tumors but was down-regulated in pancreatic cancer, indicating that the roles of Wnt5a in tumors are not fully consistent. Liang et al[25] observed that a lack of Wnt5a readily led to the occurrence of human hematopoietic malignancies and that Wnt5a could act as a tumor suppressor to inhibit the proliferation of B cells and tumorigenesis; therefore, Wnt5a is a potential therapeutic target for human acute lymphoblastic leukemia and myeloid leukemia. In addition, Wnt5a plays the role of suppressor in colorectal cancer[14], neuroblastoma[15] and leukemia[16], where its expression is down-regulated. Thus, considerable controversy remains regarding the biological role of Wnt5a in different tumors.
No study addressing the expression levels of ROR2 and Wnt5a in gallbladder SC/ASC and AC tissues has previously been reported in the literature. The results of the present study showed that the positive expression rates of ROR2 and WNT5a were not significantly different between SC/ASC and AC tissues (P > 0.05). While the positive expression rates of Wnt were not significantly different between highly differentiated SC/AS and poorly differentiated SC/AS (P > 0.05), the positive expression rates of ROR2 and WNT5a in the SC/ASC or AC patients with high differentiation, a maximal diameter of the mass ≤ 3 cm, a TNM stage of I + II, no lymph node metastasis, no invasion in the surrounding tissues and organs, and radical resection were significantly lower than in the patients with a maximal diameter of the mass > 3 cm, a TNM stage of IV, lymph node metastasis, invasion in the surrounding tissues and organs, and no resection (P < 0.05 or P < 0.01).
The results of the Kaplan-Meier survival analysis showed that the degree of differentiation, maximal diameter of the mass, TNM stage, lymph node metastasis, invasion of the surrounding tissues, and applied surgical procedure were closely related to the average survival of the patients with gallbladder SC/ASC or AC (P < 0.05 or P < 0.01). Survival of the patients presenting positive expression of ROR2 and WNT5a was significantly shorter compared with the patients presenting negative expression results (P < 0.01). Cox multivariate analysis revealed that poor differentiation, a maximal diameter of the mass ≥ 3 cm, a TNM stage of III or IV, the occurrence of lymph node metastasis, invasion of the surrounding tissues and organs, and unresected surgery were negatively correlated with the postoperative survival rate of the patients and positively correlated with mortality, which are risk factors and independent prognostic predictors.
Our experimental results are consistent with reported findings regarding the expression levels of ROR2 and Wnt5a in other epithelial malignancies from researchers from other countries, suggesting that ROR2 and Wnt5a play an important role in the occurrence, progression, biological behavior and prognosis of gallbladder SC/ASC and AC. Gallbladder SC/ASC and AC cases showing high expression of ROR2 and Wnt5a are highly malignant, exhibit rapid progression, and are prone to regional lymph node metastasis with strong invasiveness. Thus, ROR2 and Wnt5a are both important biological markers reflecting the prognosis of patients with gallbladder SC/ASC and AC. Considering the findings presented in the relevant literature, detection of the expression levels of ROR2 and (or) Wnt5a in benign gallbladder lesions may have important clinical pathological significance in the prevention and early diagnosis of gallbladder cancer. Further research on this topic is awaited.
Gallbladder carcinoma (GBC) is the most common and aggressive type of carcinoma among the biliary tree cancers. Early diagnosis and radical surgery contribute improved prognosis of GBCs. The diagnosis of GBC mainly depends on non-invasive auxiliary imaging and invasive examination, such as laparoscopy and biopsy. However, ideal biological markers for the diagnosis, prognosis and targeted therapy of GBCs have not been established. In this study, we detected the expression levels of ROR2 and WNT5a in surgical resection specimens from a total of 46 cases of gallbladder squamous/adenosquamous carcinoma (SC/ASC) and 80 cases of gallbladder adenocarcinomas (AC). The results showed that ROR2 and WNT5a expression are negatively correlated with postoperative survival rate and positively correlated with mortality.
Tumor markers have had an increasing significance in the diagnosis and evaluation of GBC. Previous studies found that CA242, CA15-3, CA19-9 and CA125 are fairly good markers for discriminating patients of carcinoma of the gallbladder from cholelithiasis. Combined CA242 and CA125 detection achieved the best sensitivity and specificity. Serum markers seem to be less sensitive when used individually in carcinomas of the gallbladder but may prove useful in combination. However, the ideal biological markers for the diagnosis, prognosis and targeted therapy of GBCs have not been established.
This is the first report investigating ROR2 and WNT5a expression in clinical samples from two different types of gallbladder cancer (SC/ASC and AC) using the EnVision immunohistochemical staining technique, and reveals their correlation with clinicopathologic characteristics in both types of gallbladder cancer.
The expression and clinicopathological significance of ROR2 and Wnt5a could be applied to the prevention and early diagnosis of gallbladder cancer in benign gallbladder lesions. The combined detection of ROR2 and WNT5a as biological markers might increase sensitivity for the diagnosis and prognosis of GBC.
SC/ASC and AC are two major types of gallbladder cancer with slightly different clinicopathologic characteristics. Squamous carcinoma shows slight differences from adenocarcinoma gallbladder cancer with an advanced T stage but less common nodal involvement and distant metastasis (Kalayarasan et al, Am J Surg 2013; 206: 380-385).
The paper is significant hence it studies a rare type of tumor.
| 1. | Kim WS, Jang KT, Choi DW, Choi SH, Heo JS, You DD, Lee HG. Clinicopathologic analysis of adenosquamous/squamous cell carcinoma of the gallbladder. J Surg Oncol. 2011;103:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Chan KM, Yu MC, Lee WC, Jan YY, Chen MF. Adenosquamous/squamous cell carcinoma of the gallbladder. J Surg Oncol. 2007;95:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Mingoli A, Brachini G, Petroni R, Antoniozzi A, Cavaliere F, Simonelli L, Chirletti P, Modini C. Squamous and adenosquamous cell carcinomas of the gallbladder. J Exp Clin Cancer Res. 2005;24:143-150. [PubMed] |
| 4. | Kondo M, Dono K, Sakon M, Shimizu J, Nagano H, Nakamori S, Umeshita K, Wakasa K, Monden M. Adenosquamous carcinoma of the gallbladder. Hepatogastroenterology. 2002;49:1230-1234. [PubMed] |
| 5. | Oohashi Y, Shirai Y, Wakai T, Nagakura S, Watanabe H, Hatakeyama K. Adenosquamous carcinoma of the gallbladder warrants resection only if curative resection is feasible. Cancer. 2002;94:3000-3005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Nishihara K, Nagai E, Izumi Y, Yamaguchi K, Tsuneyoshi M. Adenosquamous carcinoma of the gallbladder: a clinicopathological, immunohistochemical and flow-cytometric study of twenty cases. Jpn J Cancer Res. 1994;85:389-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Garcea G, Neal CP, Pattenden CJ, Steward WP, Berry DP. Molecular prognostic markers in pancreatic cancer: a systematic review. Eur J Cancer. 2005;41:2213-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Miller JR. The Wnts. Genome Biol. 2001;3:3001. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 302] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 9. | Kubo T, Kuroda Y, Shimizu H, Kokubu A, Okada N, Hosoda F, Arai Y, Nakamura Y, Taniguchi H, Yanagihara K. Resequencing and copy number analysis of the human tyrosine kinase gene family in poorly differentiated gastric cancer. Carcinogenesis. 2009;30:1857-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Lara E, Calvanese V, Huidobro C, Fernández AF, Moncada-Pazos A, Obaya AJ, Aguilera O, González-Sancho JM, Sánchez L, Astudillo A. Epigenetic repression of ROR2 has a Wnt-mediated, pro-tumourigenic role in colon cancer. Mol Cancer. 2010;9:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Liu S, Gong J, Morishita A, Nomura T, Miyoshi H, Tani J, Kato K, Yoneyama H, Deguchi A, Mori H. Use of protein array technology to investigate receptor tyrosine kinases activated in hepatocellular carcinoma. Exp Ther Med. 2011;2:399-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Li L, Ying J, Tong X, Zhong L, Su X, Xiang T, Shu X, Rong R, Xiong L, Li H. Epigenetic identification of receptor tyrosine kinase-like orphan receptor 2 as a functional tumor suppressor inhibiting β-catenin and AKT signaling but frequently methylated in common carcinomas. Cell Mol Life Sci. 2014;71:2179-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Lee SE, Lim SD, Kang SY, Suh SB, Suh YL. Prognostic significance of Ror2 and Wnt5a expression in medulloblastoma. Brain Pathol. 2013;23:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Ying J, Li H, Yu J, Ng KM, Poon FF, Wong SC, Chan AT, Sung JJ, Tao Q. WNT5A exhibits tumor-suppressive activity through antagonizing the Wnt/beta-catenin signaling, and is frequently methylated in colorectal cancer. Clin Cancer Res. 2008;14:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | Blanc E, Roux GL, Bénard J, Raguénez G. Low expression of Wnt-5a gene is associated with high-risk neuroblastoma. Oncogene. 2005;24:1277-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Roman-Gomez J, Jimenez-Velasco A, Cordeu L, Vilas-Zornoza A, San Jose-Eneriz E, Garate L, Castillejo JA, Martin V, Prosper F, Heiniger A. WNT5A, a putative tumour suppressor of lymphoid malignancies, is inactivated by aberrant methylation in acute lymphoblastic leukaemia. Eur J Cancer. 2007;43:2736-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Dissanayake SK, Wade M, Johnson CE, O’Connell MP, Leotlela PD, French AD, Shah KV, Hewitt KJ, Rosenthal DT, Indig FE. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem. 2007;282:17259-17271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 285] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 18. | Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S, Trümper L, Binder C. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci USA. 2006;103:5454-5459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 279] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 19. | Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66:10439-10448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 352] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 20. | Huang CL, Liu D, Nakano J, Ishikawa S, Kontani K, Yokomise H, Ueno M. Wnt5a expression is associated with the tumor proliferation and the stromal vascular endothelial growth factor--an expression in non-small-cell lung cancer. J Clin Oncol. 2005;23:8765-8773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Yamamoto H, Oue N, Sato A, Hasegawa Y, Yamamoto H, Matsubara A, Yasui W, Kikuchi A. Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene. 2010;29:2036-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 189] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 22. | Kühl M. The WNT/calcium pathway: biochemical mediators, tools and future requirements. Front Biosci. 2004;9:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Prieve MG, Moon RT. Stromelysin-1 and mesothelin are differentially regulated by Wnt-5a and Wnt-1 in C57mg mouse mammary epithelial cells. BMC Dev Biol. 2003;3:2. [PubMed] |
| 24. | Iozzo RV, Eichstetter I, Danielson KG. Aberrant expression of the growth factor Wnt-5A in human malignancy. Cancer Res. 1995;55:3495-3499. [PubMed] |
| 25. | Liang H, Chen Q, Coles AH, Anderson SJ, Pihan G, Bradley A, Gerstein R, Jurecic R, Jones SN. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell. 2003;4:349-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 257] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 26. | Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 585] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 27. | Clark CC, Cohen I, Eichstetter I, Cannizzaro LA, McPherson JD, Wasmuth JJ, Iozzo RV. Molecular cloning of the human proto-oncogene Wnt-5A and mapping of the gene (WNT5A) to chromosome 3p14-p21. Genomics. 1993;18:249-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 941] [Cited by in RCA: 1012] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Jurcic P, Ozkan Z, Rungsakulkij N S- Editor: Qi Y L- Editor: Filipodia E- Editor: Wang CH