Published online Mar 7, 2016. doi: 10.3748/wjg.v22.i9.2799
Peer-review started: September 20, 2015
First decision: October 14, 2015
Revised: October 28, 2015
Accepted: November 19, 2015
Article in press: November 19, 2015
Published online: March 7, 2016
Processing time: 165 Days and 0.2 Hours
AIM: To establish a novel mouse constipation model.
METHODS: Animals were randomly divided into three groups, and intragastrically administered 0-4 °C saline (ice-cold group) or 15-20 °C saline (saline control group) daily for 14 d, or were left untreated (blank control group). Stools were collected 3-24 h after treatment to record the wet and dry weights and the stool form. Intestinal propulsion experiments were carried out and defecation time was measured for six days continuously after suspending treatments. The expressions of PGP9.5 were detected by immunohistochemistry.
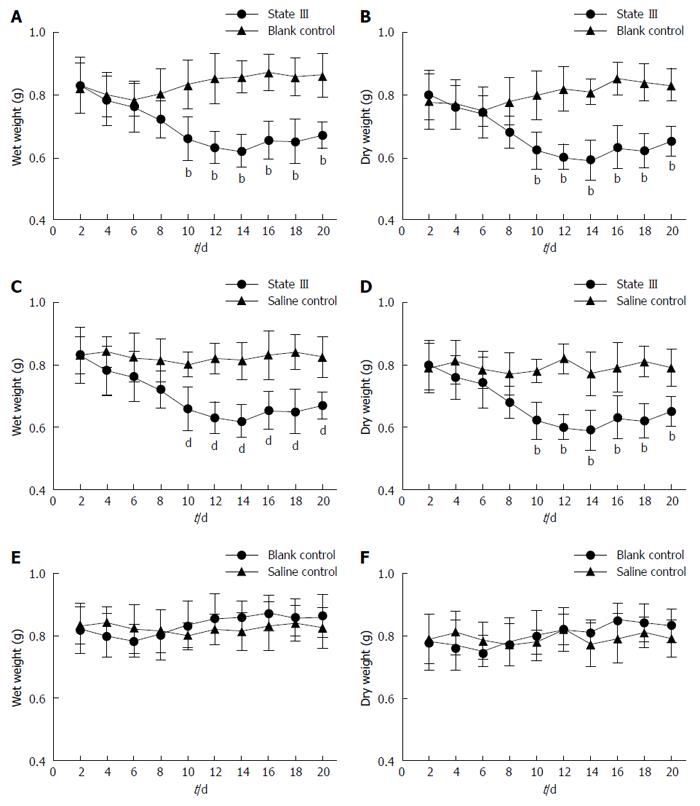
RESULTS: Based on the percentage of stool weight changes compared with baseline (before irritation) in 9-14 d, stool weight changes were classified into three levels. Each level shows a different body state, which is state I (no change: plus or minus 5%), state II (slightly decreased: 5%-15%) and state III (decreased: 15%-25%). In state III, between day 9-14, the stool weights decreased by 15%-25% compared with the baseline, and changed at a rate > 10% compared with blank control values, and the stools became small and dry. Additionally, intestinal functions degenerated in these animals, and PGP9.5-positive expression markedly decreased in jejunum, ileum and proximal colon myenteric plexus.
CONCLUSION: Irritation with ice-cold saline is a stable, repeatable method in building constipation model in mice for exploring the pathogenesis and treatment options of constipation, and the change of stool weight and size may serve as a useful tool to judge a constipation model success or not.
Core tip: Establishing stable animal models is very important for studying disease pathogenesis to develop strategies for prevention and treatment. Evidence from previous researches has shown recurrent and chronic cold water irritation to stomach can inhibit gastrointestinal motility. In this study, we concluded that irritation with ice-cold saline to mice is a stable, repeatable method in building mice constipation model.
- Citation: Liang C, Wang KY, Yu Z, Xu B. Development of a novel mouse constipation model. World J Gastroenterol 2016; 22(9): 2799-2810
- URL: https://www.wjgnet.com/1007-9327/full/v22/i9/2799.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i9.2799
Constipation, which accompanies various diseases, is a symptom of underlying defects in either transit of faecal mass through the gut or defecation, a commonly diagnosed functional gastrointestinal disorder. It usually associates with gastrointestinal motility disorders, which is characterized by a series of complex gastrointestinal symptoms in absence of mechanical obstruction of the gastrointestinal tract. It is generally accepted that impaired gastrointestinal motility and visceral paresthesia form the major pathological and physiological basis for gastrointestinal dysfunction[1-3]. Given the paucity of available clinical data, animal models are required to study the pathogeny of constipation and develop strategies for prevention and treatment, and there is a need to develop optimized models as well[4].
In the past few years, many approaches have been used to produce constipation in experimental animals in vivo. However, most of the work has been carried out in rats. Compared with rats, mice have a lower tolerance and cannot be administered long-term irritative drugs. Research in mice offers multiple advantages, including economic efficiency and availability of a higher number of purebred or genetically manipulated (transgenic and knockout) strains. Moreover, the murine anatomical features and embryonic development are similar to those of humans, and the genomes also show a high degree of homology[5]. Mouse models offer greater versatility for the study of human gene functions and disease pathogenesis than rat models. Hence, in this study, we aimed to produce a stable constipation model in mice, study the pathogenesis of constipation and explore better treatment options.
C57BL/6J mice (SPF-grade, 3-wk-old male, 20-25 g) were purchased from Model Animal Research Center of Nanjing University (Nanjing, China, license number: SCXK 2013-0005). Food and water were available ad libitum, and the animals were housed under controlled environmental conditions. All experimental manipulations were undertaken in accordance with the Principles of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals, published by the National Science Council, China.
The mice were randomly divided into three groups: a 0-4 °C saline-treated group (ice-cold group, n = 30), a normal feeding group (blank control, n = 10), and a 15-20 °C saline-treated group (saline control, n = 10), randomly numbered, and raised in single cages that allowed normal access to food and water. Wire netting was used to facilitate the separation and collection of stools. To eliminate the influence of the biological rhythms, intragastric administrations were conducted at 8:00 am once daily for 14 d. Mice in ice-cold and saline control groups were initially administered either ice-cold or room-temperature saline, respectively, at a dose of 0.2 mL/mouse, and then the dose increased by 0.05 mL/mouse every 5 d. Blank control group mice were raised normally without intragastric administration. Animals were normally raised and monitored for an additional 6 d after the cessation of the intragastric administrations.
During the 3-24 h period after intragastric administration, stool was collected with ceramic cups. After collection, the changes in stool form (pellet size) were observed and recorded before weighing. Then, each ceramic cup was weighed to determine wet weight using an electronic balance. After drying in a microwave oven (medium heat) for 6 min, the cup was weighed again to determine the dry weight.
After the completion of treatment, the differences between the three groups were evaluated at 15 d and 20 d. Mice in each group were randomly assigned to a 15-d subgroup and a 20-d subgroup. The 15 d subgroup was used in the intestinal propulsion experiments at 15 d while the 20 d subgroup was raised normally and used in the same experiments at 20 d. After 12 h of fasting, mice were intragastrically administrated a suspension of black carbon (0.3 mL) and killed 10 min later via cervical dislocation. Then, the section extending from the pylorus to the ileocecal valve was removed. The full length of the intestinal tract as well as the propulsive distance of black carbon in the tract was measured under a tension-free state, and the ratio of the propulsive distance to the length of the intestinal tract was measured for all groups. Additionally, in the next experiment at the 15 d and 20 d time points, we also determined the defecation time after similarly dividing the mice into the 15 d and 20 d subgroups. After 12 h of fasting, the red carbon suspension (0.3 mL) was administered, and the time required to defecate the first stool pellet containing the red indicator was recorded. Animals administered prokinetic agents were pretreated with domperidone suspensions (0.3 mL) for 4 h.
When 14 d intervention finished, the small intestine (jejunum and ileum) and proximal colon tissue specimens (approximately 1cm in length) were removed after 12 h of fasting. Each segment was opened along the mesenteric border and washed twice in saline. When mesenteric tissues and the contents of gut were removed, segments were immediately fixed by immersion in 4% Para formaldehyde for 24 h. Then, they were processed for paraffin embedding in vacuum and cut at a thickness of 10 μm.
Sections were deparaffinized in xylene and hydrated in a graded solution of ethanol. Activity of endogenous peroxidase was blocked with 3% hydrogen peroxide. After three rinses in 0.01 mol/L phosphate-buffered saline (PBS; pH 7.2), non-specific binding was blocked with 5% bovine serum albumin (BSA) for 30 min at 37 °C. The primary antibodies for PGP9.5 (1:1000) were applied to the sections and each specimen was incubated in a moist chamber overnight at 4 °C. The slides were washed for three times in PBS, and incubated with horseradishperoxidase (HRP)-Polymer anti-Mouse/Rabbit lgG for 90 min at 37 °C. After washing in PBS for three times, the localization of target protein was visualized by incubating the sections for 5-10 min in freshly prepared 3,3-diaminobenzidine (DAB) solution. The slides were washed again, counterstained in hematoxylin, and dehydrated. Specificity of the antibody was confirmed by negative control in the absence of primary antibody treatment. Two observers evaluated the slides using an Olympus FV500 optical microscope (Olympus, Tokyo, Japan). Positive immunostaining was evaluated at 5 random visual fields at a magnification of 400. The mean density of positive expression was assessed using image analysis software.
Materials used in this study included saline (sodium chloride injection; Nanjing Chemical Reagent Co, Jiangsu, China), domperidone tablets (Xi’an Janssen Pharmaceutical Ltd, Xi’an, China), wire netting (to facilitate the separation and collection of stools), a precision electronic balance (Sartorius Co ,Beijing, China), acacia gum (gum arabic powder; BASF Chemical Co, Tianjin, China), black and red carbon powder (color toner; Sanheng Information Technology Co, Guangzhou, China), and ceramic cups (high temperature-resistant, radius: 2 cm, height: 3.5 cm). Primary antibodies for immunohistochemistry: PGP9.5 (Abcam, Cambridge, United Kingdom), Secondary antibodies:HRP-Polymer anti-Mouse/Rabbit (Boster Biotech, Wuhan, China)
Red (black) carbon suspensions were prepared as follows: acacia gum (100 g) was added to 800 mL of water and boiled until transparent. Then the above solution was mixed with red (black) carbon powder (50 g) and boiled three times. After cooling down, each solution was diluted with water to 1000 mL and stored at 4 °C. The solutions were agitated prior to use.
Data were expressed as mean ± SD. Statistical analysis was conducted using SPSS 17.0 software with one-factor analysis of variance. Data were compared using the Student Newman Keuls (SNK)-Q test. Differences where P < 0.05 were considered to be statistically significant.
Initially, during the experiments, modestly abnormal defecation occurred as the mice responded to the environmental changes in single cages, but the conditions stabilized after about one week (7-8 d) of adaption. Accordingly, the stool weights at d 0 (before irritation) was used as the baseline to examine changes in stool weight in the d 9-14 period. Based on the percentage of stool weight (wet and dry) changes and stool forms changes compared with baseline in d 9-14, three levels were used: state I (no change: plus or minus 5%, n = 10), state II (slightly decreased: 5%-15%, n = 9) and state III (decreased: 15%-25%, n = 11) compared with baseline (Table 1).
| Groups | Treatments | Performance (after treatment) |
| Ice-cold group | 0-4 °C saline | State I, state II and state III |
| Saline control | 15-20 °C saline | No types of states |
| Blank control | Normal feeding | No types of states |
During consecutive batch experiments, we could observe three different body states (I, II, and III) in the ice-cold group mice, while no such changes were observed in the mice in groups C. In state I, the wet weights and dry weights recorded between d 9-14 changed by -5% to 5% compared with the baseline, and changed at a rate < 5% compared with the corresponding blank control group values, no symptoms of constipation were observed. In state II, the stool wet and dry weights at d 9-14 decreased by 5%-15% compared with the baseline, and changed at a rate (absolute value) < 5% compared with the corresponding blank control group values. Overall, the slight decrease in stool weight in this state compared with blank control group indicated a tendency to constipation. In state III, the stool wet and dry weights at d 9-14 decreased by 15%-25% compared with the baseline, and changed at a rate (absolute value) > 10% compared with the corresponding blank control group values. In this state, the differences in stool weights between ice-cold group and blank control group mice were statistically significant.
After irritation with ice-cold saline, we could observe three different states (I, II, and III), and the constipation symptoms were seen in state III. Compared with blank control group (Figure 1A and B) and saline control group values (Figure 1C and D), the stool weights in state III ice-cold group mice decreased during the experiments , and normalcy was achieved 6 d after the termination of irritation. In comparison, after a similar treatment with room temperature saline (saline control group), such changes were not observed, and the stool weights of the saline control group mice did not significantly differ from the corresponding blank control group values (Figure 1E and F).
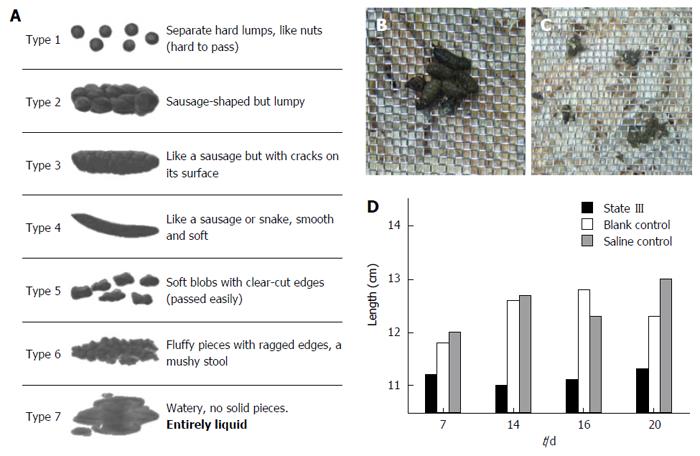
Consistency of the stool is usually described using the Bristol Stool Chart (Figure 2A), where type 1 and 2 indicate constipation, 3 and 4 are normal stools and 5 to 7 are associated with diarrhea and urgency. We utilized 100-150:1 ratio to evaluate human and mouse stools form based on Bristol Stool Chart (according to human and mouse fecal weight ratio). In comparison, as proportions based on the Bristol Scale, under normal conditions, the mouse stools could mainly be classified as (or similar to) type 3 and 4, and rarely as type 1. The stool forms observed in three states changed in a slightly different manner: In state I, stools partially stick together like type 5 or 6 (Figure 2B). In the early intervention, some even adhere to the wire netting similar to type 7 (Figure 2C). In state II, a small amount of the stools could be classified as type 1. However, no significant difference was found compared to blank control group. In state III, most stools became small pellets. We estimated the length of a specific number of stool pellets (n = 25) and termed the value “LS25”. The LS25 value for the state III was lower than the corresponding blank control group or saline control group (Figure 2D). The majority of the state III mice defecated small pellets stools, indicating type 1 or 2, and the proportion of type 3 or 4 stools (Figure 3A and B, first row in each group) decreases after persistent irritation with ice-cold saline. During the recovery stage (15-20 d), the proportion of type 3 or 4 stools in ice-cold group (the first row) increases slowly, but most of the stools were still small pellets, unlike that in the other groups (Figure 3C and D).
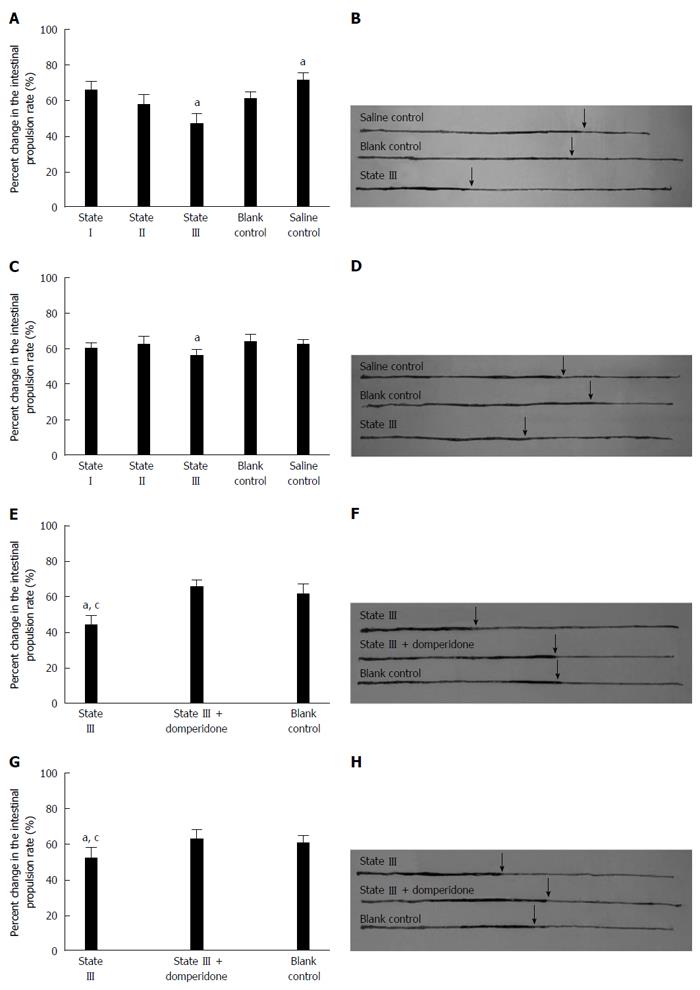
Carbon intestinal propulsion experiments showed that the distances by which black carbon was propelled, significantly decreased in state III mice compared to blank control group at both d 15 (47.03% ± 6.18% vs 61.94% ± 4.80%, P = 0.0211, Figure 4A and B) and d 20 (56.11% ± 4.23% vs 63.73% ± 4.68%, P = 0.0379, Figure 4C and D) and there was an increase in the carbon propulsion rates in saline control group at d 15 compared to blank control group (71.18% ± 4.81% vs 61.94% ± 6.20%, P = 0.0169). In the subsequent experiments, state III mice using domperidone suspensions pretreatment, the carbon propulsion rates were increased compared to those state III mice without using domperidone at both d 15 (65.22% ± 5.43% vs 44.52% ± 8.11%, P = 0.0051, Figure 4E and F) and d 20 (63.14% ± 6.73% vs 52.12% ± 4.05%, P = 0.0196, Figure 4E and F), and did not significantly differ from blank control group values.
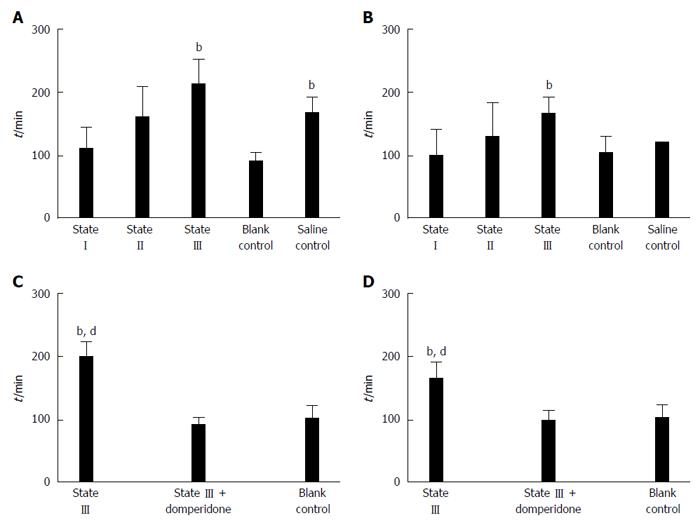
The results showed that, the defecation time was significantly prolonged in state III mice compared to blank control group at d 15 (212.21 ± 40.86 vs 90.33 ± 14.80, P < 0.01, Figure 5A) and d 20 (156.11 ± 26.96 vs 100.67 ± 27.47, P < 0.01, Figure 5B). Defecation at d 15 in saline control group was also delayed compared to that in blank control group (167.11 ± 24.49 vs 90.33 ± 14.80, P < 0.01, Figure 5A). Moreover, after state III mice were pretreated with domperidone suspensions, defecation time was significantly descended compared to state III at the d 15 (90.83 ± 12.53 vs 200.11 ± 24.16, P < 0.01, Figure 5C) and d 20 (98.53 ± 14.13 vs 165.21 ± 27.53, P < 0.01, Figure 5D) , and no difference was observed from blank control group.
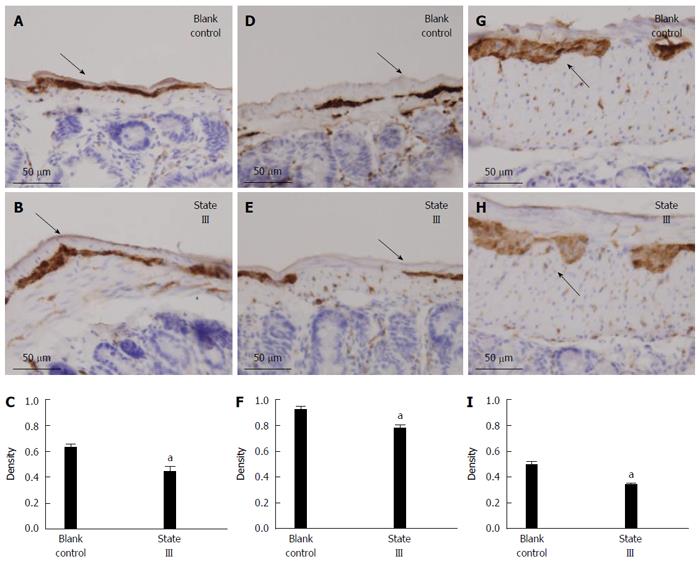
The PGP9.5-positive neuron cells were easily found in the jejunum (Figure 6A), ileum (Figure 6D) and proximal colon (Figure 6G) myenteric plexus in blank control group. Statistical analysis showed, after intragastric administration of ice-cold saline treatment, that the mean density of PGP9.5-positive expression markedly decreased in jejunum (0.6185 ± 0.07 vs 0.4343 ± 0.09, P = 0.0092, Figure 6B and C), ileum (0.9095 ± 0.07 vs 0.7685 ± 0.08, P = 0.0166, Figure 6E and F) and proximal colon (0.4925 ± 0.07 vs 0.3381 ± 0.04, P = 0.0019, Figure 6H and I) myenteric plexus compared with blank control group.
The present study showed that irritation with ice-cold (0-4 °C) saline to mice could induce gastrointestinal dysfunction. During consecutive experiments, mice displayed three different body states (I, II and III) in ice-cold group. For state I, there was no significant change in digestive function. State II mice had slightly decline in intestinal function, showing a trend of constipation, but we do not think they are a stable constipation model, it could be a kind of sub-healthy state between normal and constipation. In state III, the weight of stools decreased, the intestinal digestion and transmission functions degenerated, and continued for some time after the termination of irritation. Besides, the stools became dry and small. Under normal conditions, the mouse stools could mainly be classified as (or similar to) type 3 and 4. State III mice defecated small pellets stools, indicating type 1 or 2, and the proportion of type 3 or 4 stools was decreased. Additionally, the LS25 values for the state III were significantly lower than the values in the blank control group and saline control group. Thus, persistent irritation with ice-cold saline induces digestive tract dysfunction and slows the digestive transmission, which also leads to the formation of smaller-sized stools.
In a morphological study, immunohistochemistry results showed that irritation with ice-cold saline caused changes of enteric nervous system (ENS) in jejunum, ileum and proximal colonic myenteric plexus. The ENS is one component of the neural control system of the digestive tract, which works in concert with the central nervous system[6]. It is found in two layers: myenteric plexus and submucosal plexus. The myenteric plexus is a layer of neurons that runs the length of the gut, from esophagus to anus, between the circular smooth muscle layer and the longitudinal smooth muscle layer. This layer of neurones controls movement along the gut. PGP9.5 is a neuron-specific protein, can accurately locate enteric neurons, the study of its morphology and protein levels has an important role to indicate ENS function[7]. Hence, we used PGP9.5 as an indicator to detect the intestinal tissue expression of ENS. Results showed that PGP9.5 expression in jejunum, ileum and proximal colonic myenteric plexus significantly decreased staining in state III mice compared to blank control group. This suggests that the local regulation of ENS may have changed by irritation with ice-cold saline, which is close to symptoms of constipation.
Furthermore, in saline control group, after administration of saline at room temperature (15-20 °C), the gastrointestinal function also appeared mildly affected, but this treatment appeared to produce different effects at different sites. The specific manifestations included the sthenia of small intestinal digestive function and decrease in the duration of the large intestine transmission process. However, the changes did not last too long. These findings indicate that an effective constipation model cannot be established by the intragastric administration of room-temperature saline, and persistent irritation with ice-cold saline can lead to intestinal dysfunction and finally to constipation.
Previous researches have shown chronic and recurrent cold water irritation to stomach might cause long-term effects on bowel movements, which resulted in the abnormal gastrointestinal motility, such as inhibited jejunal and colonic motility[8,9]. In rats, by gastric instillation 0-4 °C water daily for 14 d, it is found that the weight and water content of the feces were significantly less than the control groups, which was concordant with the symptoms of constipation. Cold water intake can increase nitric oxide (NO) which is a type of gut inhibitory neurotransmitter in the ENS in the myenteric plexus, and up-regulate expression of the 5-HT7 receptor which mediates intestinal smooth muscle relaxation, which might reduce the intestinal motility, besides, visceral sensitivity is lowered in constipation rats[10,11]. In this study, irritation with ice-cold saline to mice induced similar changes. However, different from the rats, mice showed three body states. In our view, differences in individual tolerance changed the gastrointestinal motility and visceral sensitivity in different degrees, which was the main cause of three states. It was reported that[12], the difference in the initial threshold of visceral perception and defecation may cause different performance after cold water intake. Thus, in our opinion, irritation with ice-cold saline induced three states in mice, such changes could be more likely to reflect the performance of the human gastrointestinal tract after stimulation. Recurrent cold water intake is a risk factor for constipation, not the decisive factor. This may be the main reason why only the animals in state III showed symptoms of constipation.
Currently, there are many methods (drug-based and non-drug-based) to reproduce constipation in experimental animals in vivo. However, the existing methods of constipation modeling mostly apply to rats. The available drug-based methods involve both direct and indirect effects. For example, loperamide or clonidine, can inhibit the contraction of intestinal smooth muscles, slow down peristalsis, and prolong the intestinal transmission process[13-15], commonly in studies aiming to identify the different effects of potent laxatives[16-18]. Constipation is also a major gastrointestinal side effect following morphine administration[19]. Morphine can delay gastric emptying, inhibit intestinal nerve excitability, and reduce the fluidity of the intestinal contents, leading to difficulties in defecation[20-22]. Diphenoxylate, an anti-motility drug, also acts on the intestinal tract to affect intestinal smooth muscle contraction, thus reducing defecation[23]. Long-term intragastric administration of rhubarb (a Chinese traditional drug) results in a reduced number of colon smooth muscles and shrinkage of smooth muscle cells, and thereby, in reduced defecation and intestinal transmission function[24]. While there are some non-drug-based methods, these have the disadvantage of being too time-consuming. Such as low fiber diet induce constipation required at least 5 wk[25]. A short-term withholding of water without restricting food intake also reduces the intestinal water content and induces dryness of stools and difficult defecation[26]. While this method appears practical, adverse reactions occur frequently after water withholding. Additionally, this practice also violates ethical guidelines and is not suitable for long-term use.
In general, treatments or strategies for prevention are developed, which can test in the respective animal models, if shown to be safe and valid, are tested in human to evaluate the ability to affect human body state. Some evidence suggest that human biological markers, such as intestinal transit including whole gastrointestinal tract or regional colonic transit, and visceral sensitivity changes which are thought to underlie specific symptoms, serve as a basis for the development of those animal models[4,27]. In this study, many methods were established for evaluation of intestinal function in vivo. The results showed an ability to mime human symptom-based state, which in turn serve as a symbol to estimate a successful model or not.
Notablely, the change of stool weight and size is an important index to distinguish the different state. Because stool weight is 0.5-0.9 g and rate of water content in calculation is not stable, we do not recommend using water content as an evaluation index. We found that ice-cold group start to show different state at 9-10 d. Therefore, we suggest, in 9-14d, according to changes of the stool size combined with weights can filter out the mice in state III, i.e., small-sized stool greatly increased, proportion of pellets longer than 3 mm decreased, and the stool weights decreased by 15%-25% compared with the baseline, and change at a rate (absolute value) > 10% compared with blank control group value.
Mice, which show a lower drug tolerance, cannot be treated long-term with irritative drugs. However, the increased availability of mouse gastrointestinal gene knockout and transgenic models offer a greater scope to probe into the pathogenesis and treatment of constipation. Hence, we explored the establishment of a mouse constipation model and preliminarily built a simple repeatable mouse constipation model. In this drug-free model, we used irritation with ice-cold saline to induce constipation. It is notable that we found that 3-4 wk old mice are most appropriate, thus intervention should be accomplished before adulthood, which means this model does not apply to the correlation study of senile constipation. This model only required a short intervention cycle (14 d) and normalcy was achieved 6 d after the termination of irritation, which is convenient and practical, and avoids side effects due to too strong drug irritation, and thus presents an attractive model to study the pathogeny and pathogenesis underlying constipation, and explore treatment options.
Constipation is a symptom of an underlying condition affecting gut motility. Given the paucity of available clinical data, animal models are required and there is a need to develop optimized models. In existing constipation models, most of them are carried out in rats. Hence, the authors explored the establishment of a mouse constipation model and preliminarily built a simple repeatable mouse constipation model.
Persistent cold water irritation to stomach can inhibited gastrointestinal motility in rats, suggesting that cold water intake is a risk factor for constipation. However, little effort has been tried to establish a mice constipation model with chronic and recurrent cold water irritation.
Irritation with ice-cold saline to mice could induce gastrointestinal dysfunction. Many methods were established for evaluation of intestinal function, which show an ability to mime human symptom-based state. This method is convenient and practical.
It presents an attractive model, and helps to study the pathogeny and pathogenesis underlying constipation, and explore treatment options.
This study covers an interesting topic. It is a sufficiently novel and adds to our canon of knowledge about the pathophysiology and therapeutic options of constipation.
| 1. | Hertig VL, Cain KC, Jarrett ME, Burr RL, Heitkemper MM. Daily stress and gastrointestinal symptoms in women with irritable bowel syndrome. Nurs Res. 2007;56:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Park HJ, Jarrett M, Cain K, Heitkemper M. Psychological distress and GI symptoms are related to severity of bloating in women with irritable bowel syndrome. Res Nurs Health. 2008;31:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Choung RS, Locke GR, Zinsmeister AR, Schleck CD, Talley NJ. Psychosocial distress and somatic symptoms in community subjects with irritable bowel syndrome: a psychological component is the rule. Am J Gastroenterol. 2009;104:1772-1779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Mayer EA, Bradesi S, Chang L, Spiegel BM, Bueller JA, Naliboff BD. Functional GI disorders: from animal models to drug development. Gut. 2008;57:384-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 5. | Mouse Genome Sequencing Consortium, Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty KD, Deri J, Dermitzakis ET, Dewey C, Dickens NJ, Diekhans M, Dodge S, Dubchak I, Dunn DM, Eddy SR, Elnitski L, Emes RD, Eswara P, Eyras E, Felsenfeld A, Fewell GA, Flicek P, Foley K, Frankel WN, Fulton LA, Fulton RS, Furey TS, Gage D, Gibbs RA, Glusman G, Gnerre S, Goldman N, Goodstadt L, Grafham D, Graves TA, Green ED, Gregory S, Guigó R, Guyer M, Hardison RC, Haussler D, Hayashizaki Y, Hillier LW, Hinrichs A, Hlavina W, Holzer T, Hsu F, Hua A, Hubbard T, Hunt A, Jackson I, Jaffe DB, Johnson LS, Jones M, Jones TA, Joy A, Kamal M, Karlsson EK, Karolchik D, Kasprzyk A, Kawai J, Keibler E, Kells C, Kent WJ, Kirby A, Kolbe DL, Korf I, Kucherlapati RS, Kulbokas EJ, Kulp D, Landers T, Leger JP, Leonard S, Letunic I, Levine R, Li J, Li M, Lloyd C, Lucas S, Ma B, Maglott DR, Mardis ER, Matthews L, Mauceli E, Mayer JH, McCarthy M, McCombie WR, McLaren S, McLay K, McPherson JD, Meldrim J, Meredith B, Mesirov JP, Miller W, Miner TL, Mongin E, Montgomery KT, Morgan M, Mott R, Mullikin JC, Muzny DM, Nash WE, Nelson JO, Nhan MN, Nicol R, Ning Z, Nusbaum C, O’Connor MJ, Okazaki Y, Oliver K, Overton-Larty E, Pachter L, Parra G, Pepin KH, Peterson J, Pevzner P, Plumb R, Pohl CS, Poliakov A, Ponce TC, Ponting CP, Potter S, Quail M, Reymond A, Roe BA, Roskin KM, Rubin EM, Rust AG, Santos R, Sapojnikov V, Schultz B, Schultz J, Schwartz MS, Schwartz S, Scott C, Seaman S, Searle S, Sharpe T, Sheridan A, Shownkeen R, Sims S, Singer JB, Slater G, Smit A, Smith DR, Spencer B, Stabenau A, Stange-Thomann N, Sugnet C, Suyama M, Tesler. Initial sequencing and comprarative analysis of the mouse genome. Nature. 2002;420:520-562. [PubMed] |
| 6. | Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 1148] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 7. | Du F, Wang L, Qian W, Liu S. Loss of enteric neurons accompanied by decreased expression of GDNF and PI3K/Akt pathway in diabetic rats. Neurogastroenterol Motil. 2009;21:1229-e114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Chen DP, Xiong YJ, Tang ZY, Yao QY, Ye DM, Liu SS, Lin Y. Characteristics of deslanoside-induced modulation on jejunal contractility. World J Gastroenterol. 2012;18:5889-5896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Yang X, Xi TF, Li YX, Wang HH, Qin Y, Zhang JP, Cai WT, Huang MT, Shen JQ, Fan XM. Oxytocin decreases colonic motility of cold water stressed rats via oxytocin receptors. World J Gastroenterol. 2014;20:10886-10894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Zou BC, Dong L, Wang Y, Wang SH, Cao MB. Expression and role of 5-HT7 receptor in brain and intestine in rats with irritable bowel syndrome. Chin Med J (Engl). 2007;120:2069-2074. [PubMed] |
| 11. | Xu JR, Luo JY, Shang L, Kong WM. Effect of change in an inhibitory neurotransmitter of the myenteric plexus on the pathogenetic mechanism of irritable bowel syndrome subgroups in rat models. Chin J Dig Dis. 2006;7:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Zuo XL, Li YQ, Shi L, Lv GP, Kuang RG, Lu XF, Li JM, Desmond PV. Visceral hypersensitivity following cold water intake in subjects with irritable bowel syndrome. J Gastroenterol. 2006;41:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Bustos D, Ogawa K, Pons S, Soriano E, Bandi JC, Bustos Fernández L. Effect of loperamide and bisacodyl on intestinal transit time, fecal weight and short chain fatty acid excretion in the rat. Acta Gastroenterol Latinoam. 1991;21:3-9. [PubMed] |
| 14. | Shimotoyodome A, Meguro S, Hase T, Tokimitsu I, Sakata T. Sulfated polysaccharides, but not cellulose, increase colonic mucus in rats with loperamide-induced constipation. Dig Dis Sci. 2001;46:1482-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Chen W, Chung HH, Cheng JT. Opiate-induced constipation related to activation of small intestine opioid μ2-receptors. World J Gastroenterol. 2012;18:1391-1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (2)] |
| 16. | Kojima R, Doihara H, Nozawa K, Kawabata-Shoda E, Yokoyama T, Ito H. Characterization of two models of drug-induced constipation in mice and evaluation of mustard oil in these models. Pharmacology. 2009;84:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Kakino M, Izuta H, Tsuruma K, Araki Y, Shimazawa M, Ichihara K, Hara H. Laxative effects and mechanism of action of Brazilian green propolis. BMC Complement Altern Med. 2012;12:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Zhou M, Jia P, Chen J, Xiu A, Zhao Y, Zhan Y, Chen P, Zhang J. Laxative effects of Salecan on normal and two models of experimental constipated mice. BMC Gastroenterol. 2013;13:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Mori T, Shibasaki Y, Matsumoto K, Shibasaki M, Hasegawa M, Wang E, Masukawa D, Yoshizawa K, Horie S, Suzuki T. Mechanisms that underlie μ-opioid receptor agonist-induced constipation: differential involvement of μ-opioid receptor sites and responsible regions. J Pharmacol Exp Ther. 2013;347:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Minnis JG, Patierno S, Kohlmeier SE, Brecha NC, Tonini M, Sternini C. Ligand-induced mu opioid receptor endocytosis and recycling in enteric neurons. Neuroscience. 2003;119:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Niwa T, Nakao M, Hoshi S, Yamada K, Inagaki K, Nishida M, Nabeshima T. Effect of dietary fiber on morphine-induced constipation in rats. Biosci Biotechnol Biochem. 2002;66:1233-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Sato Y. [Improvement effect of Daikenchuto on morphine-induced constipation through gastrointestinal peptides]. Nihon Yakurigaku Zasshi. 2014;143:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Wang J, Huang JH, Cheng YF, Yang GM. Banana resistant starch and its effects on constipation model mice. J Med Food. 2014;17:902-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Fan YH, Xu GP, Feng W. Effects of zhizhu tongbian decoction on the colon ink propelling rate, GDNF, and NOS mRNA expression in rats with slow transit constipation. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32:486-489. [PubMed] |
| 25. | Kakino M, Tazawa S, Maruyama H, Tsuruma K, Araki Y, Shimazawa M, Hara H. Laxative effects of agarwood on low-fiber diet-induced constipation in rats. BMC Complement Altern Med. 2010;10:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Shan JJ, Zhang Y, Diao YL, Qu WS, Zhao XN. Effect of an antidiabetic polysaccharide from Inula japonica on constipation in normal and two models of experimental constipated mice. Phytother Res. 2010;24:1734-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Fioramonti J, Gebhart GF. In vivo and transgenic animal models used to study visceral hypersensitivity. Neurogastroenterol Motil. 2007;19:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: De Ponti F, Jadallah KA, Pasalar M S- Editor: Qi Y L- Editor: Ma JY E- Editor: Wang CH