INTRODUCTION
Intestinal ischemia-reperfusion (IR) is a frequently observed phenomenon in health and disease[1,2]. It occurs as part of normal physiology, for example in exercising healthy individuals[3,4], in whom redistribution of blood flow to the vital organs and muscles leads to significant intestinal hypoperfusion with up to 80% reduced mesenteric blood flow[3,5]. Moreover, intestinal IR occurs in a variety of pathophysiological situations. It is observed in patients with acute mesenteric ischemia due to mesenteric arterial embolism or venous thrombosis. In addition, intestinal IR occurs in situations with severe blood loss and/or hypovolemia, leading to redistribution of blood flow to the most important organs, including heart and brains. This form of intestinal IR is frequently observed in patients undergoing major surgery or in patients with trauma, shock or sepsis[1,6]. Lastly, intestinal ischemia is a well-known player in the development and perpetuation of intestinal inflammation, including in patients with inflammatory bowel disease[7,8].
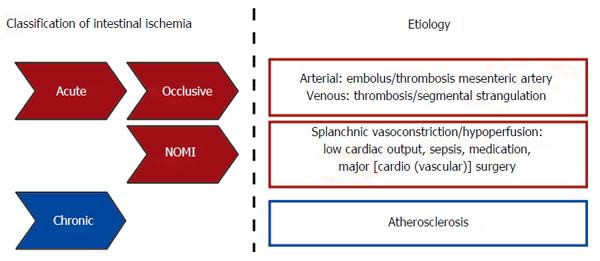
Based on etiological background, intestinal ischemia is divided into a chronic and an acute form (Figure 1). Chronic gastrointestinal ischemia (CGI) is most commonly due to atherosclerotic disease and has been reviewed elsewhere[9,10]. In contrast to CGI, acute intestinal ischemia is a consequence of a rapid reduction in intestinal blood flow, caused by occlusion (occlusive disease) or severe hypoperfusion [non-occlusive mesenteric ischemia (NOMI)] of mesenteric vessels (Figure 1)[1,11,12]. Acute intestinal ischemia is a potentially fatal clinical emergency with an overall mortality of 60% to 80%[1,2,11]. Mortality rates are especially high when ischemia progresses towards bowel necrosis, leading to a severe inflammatory response, sepsis and shock[11,12].
Figure 1 Schematic overview of intestinal ischemia based on etiological background.
NOMI: Nonocclusive mesenteric ischemia.
It is remarkable that although medical practice has evolved considerably, the mortality rates of intestinal ischemia did not improve over the past 70 years[1,13]. This is partly a consequence of the continued difficulty to recognize acute mesenteric ischemia at an early stage, which is the key to reducing high morbidity and mortality[14]. The second major reason for intestinal IR-related high morbidity and mortality rates is the paucity in preventive and/or therapeutic options. Obviously, rapid reperfusion after intestinal ischemia is of major importance, but reperfusion can paradoxically also contribute to tissue injury and severe inflammation[11,15,16]. Improved insight into the mechanisms underlying intestinal IR-related complications in man are imperative to work towards novel therapeutic strategies. This review provides an overview of the current knowledge on the pathophysiology of human intestinal ischemia-reperfusion. Development of unique human IR models allowed detailed investigation of the consequences of human intestinal IR on several important players of the intestinal barrier function.
THE HUMAN INTESTINAL EPITHELIAL BARRIER
The primary functions of the human gastrointestinal tract are digestion of food, absorption of nutrients and water, and electrolyte exchange. In addition, the gut provides a barrier between the internal milieu and the potentially toxic intraluminal content, including commensal and pathogenic microorganisms and their products[17,18]. For this purpose, the intestinal tract is equipped with several barriers. The first anatomical site at which the host encounters the gut microbiota is the mucus layer[19,20]. This layer serves as a physical and chemical barrier, preventing adherence of microbiota to the epithelium and impedes translocation of potential pathogens and their toxins to the internal milieu[19-21]. The secreted mucins are continuously produced by specialized goblet cells that are found in the small and large intestinal epithelium. Secretory mucins are stored in bulky apical granules of the goblet cells[21]. The mucin-containing granules are secreted from the apical surface both constitutively and in response to a variety of external stimuli[19,21]. The second line of defence is formed by the intestinal epithelium[22] which consists of a cohesive monolayer of columnar epithelial cells[23], firmly connected by tight junction complexes that seal the paracellular pathway[18,24,25]. Next to providing a physical barrier, enterocytes actively participate in innate immunity, by acting as immune sensors of microbial pathogens and commensal organisms[17]. The signaling loop that mediates the epithelial response to microorganisms is based on sensing of structural motifs, known as pathogen-associated molecular patterns (PAMPs) by pattern-recognition receptors (PRRs) on epithelial cells[26]. PAMPs are expressed by both commensal and pathogenic microorganisms, and include lipopolysaccharide (LPS), lipoprotein and peptidoglycans[17].
A third line of defense is formed by Paneth cells, highly specialized epithelial cells residing in the crypts of the small intestine in between the stem cells[27-30]. Paneth cells are the main source of antimicrobial proteins, including α-defensins and lysozyme, in the small intestine[28,31,32]. These proteins are stored in granules and released into the crypt lumen both constitutively and in response to bacterial threats[32] to prevent microbial invasion[33,34]. In addition, the antimicrobial proteins disseminate into the mucus layer in order to reinforce the mucosal barrier function[33-35]. Lastly, Paneth cells provide critical stem cell niche factors, including EGF, Wnt3 and Notch[36].
From studies in a variety of animal models we have learned that intestinal IR can severely damage the intestinal epithelial barrier. Our current knowledge on (patho)physiological mechanisms of IR-induced intestinal injury in a variety of animal models has recently been excellently reviewed elsewhere[37]. So far, translation to the human setting was only possible in post-mortem studies or in patients undergoing surgery with resection of necrotic intestinal segments after exposure to extensive periods of ischemia, which only allowed investigation of the end-stage of the disease. To overcome these problems, we have developed in recent years novel human experimental intestinal IR models. These models allowed us to investigate the pathophysiology and underlying mechanisms of human small intestinal IR and colonic IR.
THE HUMAN SMALL INTESTINAL IR MODEL
Development of a standardized experimental human model for small intestinal IR was considered to be of significant advantage in addressing clinical questions. Important issues during development of an in vivo human model were first, that it should be ethically justified, safe, and would allow only minimally invasive experimental procedures. Second, the model should enable investigators to expose a healthy intestinal segment to various periods of ischemia and reperfusion. Third, it should allow collection of both tissue and blood during the experimental protocol at set time points, yielding reproducible and consistent results. This led to the inclusion of patients undergoing abdominal surgery with a Roux-Y or similar small intestinal reconstruction (e.g., during pylorus-preserving pancreatico-duodenectomy, pancreatico-jejunostomy, hepatico-jejunostomy, or Frey procedure). In these patients, a variable length of healthy jejunum is usually resected in continuity with the specimen as part of the standard surgical procedure. Approximately 6 cm of this healthy part of jejunum was used for the experiments; therefore no additional tissue had to be resected for study purposes.
In short, after identification and localization of the major anatomical structures and tumor during surgery, a healthy jejunal segment with a central mesenteric arteriole and venule was isolated by transsection at both ends with a linear cutting stapler. This segment was subjected to various periods of ischemia by placing 2 atraumatic vascular clamps across the mesentery. Meanwhile, surgery proceeded as planned. Removal of the clamps led to reperfusion as confirmed by regaining of normal pink color and restoration of intestinal motility. Tissue was sampled at selective time point using the linear cutting stapler, and blood was sampled by direct puncture of the venule draining the isolated jejunal segment. Arterial blood was sampled from the radial artery line, present in all patients as part of routine intraoperative monitoring. This allowed assessing concentration gradients in plasma across the isolated jejunal tissue. At the end of the experiment, 2 cm of jejunum that remained untreated during surgery was resected and served as internal control tissue. This segment underwent similar surgical handling as the isolated part of jejunum while it was not exposed to IR. A more detailed description of the human intestinal IR model, including a diagram of the model, can be found in reference[38].
In this newly developed human experimental model to study small intestinal IR, the maximum time of reperfusion of the isolated ischemic jejunum is dependent on the surgical procedure, i.e., the moment at which the surgeon decides that the pancreatico- or hepatico-jejunal anastomosis has to be created. Using this model, we obtained insight into the pathophysiology of short an prolonged periods of ischemia followed by reperfusion and we elucidated the key molecular events during intestinal IR in man.
SHORT PERIODS OF SMALL INTESTINAL ISCHEMIA WITH REPERFUSION: MECHANISMS TO PREVENT EPITHELIAL LINING DAMAGE
The human intestine is frequently exposed to short periods of ischemia, both in physiological and pathophysiological situations. Maintenance of the intestinal epithelial lining is of major importance, since loss of this barrier facilitates translocation of potentially harmful intestinal luminal contents towards the circulation, causing a severe inflammatory response. Using the human experimental IR model, we obtained insight into the duration of ischemia that the human jejunum can withstand before extensive barrier damage occurs.
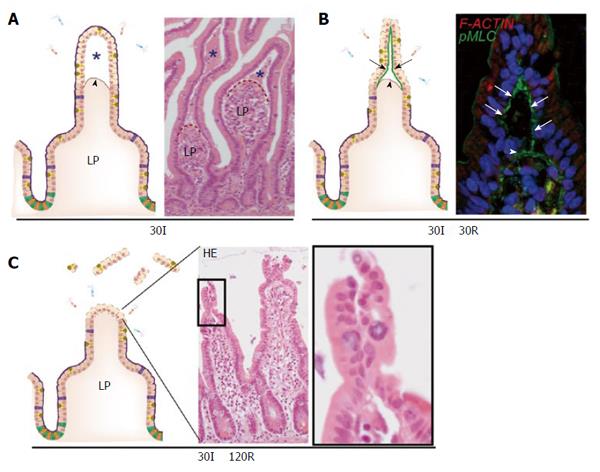
A key feature of short small intestinal ischemia (without reperfusion) is the appearance of subepithelial spaces (Figure 2A). These can be observed as early as 15 min after the onset of ischemia, but are more pronounced at 30 min of ischemia[5,39]. These spaces have been described previously in animal studies and are classically called the spaces of Gruenhagen[40,41]. These subepithelial spaces are the consequence of detachment of the upper, ischemically damaged, epithelial cells from the basal membrane with simultaneous contraction of the basement membrane muscle fibers emanating up to the tips of the villi[6,40]. Detachment of the epithelial cells from their basement membrane was only observed at the villus tips, probably because these cells are more susceptible to ischemia. This is in part due to a countercurrent flow in the villi which causes lower oxygen supply to the upper parts of the villus compared to the crypts[7]. In addition, enterocytes obtain more pro-apoptotic characteristics as they migrate up towards the tip of the villus, where they undergo apoptosis and are shed into the lumen at the end of their life cycle (also called “anoikis”)[42,43]. To our surprise, detachment of epithelial cells at the villus tip did not directly result in a disrupted epithelial lining. Instead, we observed that early after onset of reperfusion, the sheets of loose epithelial cells were pulled together[5,39]. Our studies suggested that this was achieved by accumulation of non-muscle type myosin fibers, phosphorylated myosin light chain (pMLC), at the basal side of the loose epithelial cells leading to active constriction of the IR-damaged cells at the villus tips, resulting in a dramatic reduction in wound size (Figure 2B)[39]. This phenomenon, which we termed zipper-like epithelial constriction, morphologically resembled purse-string contraction in small epithelial wounds, which is similarly characterized by accumulation of actin and myosin at the apical side of leading edge cells followed by active contraction to close small epithelial defects[44-47]. Indeed, accumulation of filamentous actin and pMLC was observed in the epithelial defects at 120 min of reperfusion, indicating that purse string contraction is involved in wound healing of IR-damaged small intestine.
Figure 2 The human small intestine has efficient mechanisms to prevent excessive epithelial lining damage.
A: Cartoon and hematoxylin-eosin staining demonstrating the appearance of subepithelial spaces (asterisks) after ischemia as a result of retraction of the basement membrane (arrowhead); B: Early during reperfusion, loose IR-damaged epithelial sheets are pulled together through active contraction of pMLC at the basal side of epithelial cells (green line, arrows), bringing these cells together; C: Zipper-like constriction of the epithelium is associated with rapid restoration of the epithelial lining and prevents exposure of lamina propria to intraluminal content. LP: Lamina propria; pMLC: Phosphorylated myosin light chain; F-ACTIN: Filamentous actin.
The combined mechanisms of villus retraction, constriction of loose epithelial cells and rapid pMLC-mediated closure of the epithelial defects resulted in a morphologically restored epithelial lining within 120 min of reperfusion of ischemically damaged jejunum (Figure 2C). This mechanism could therefore be involved in limiting bacterial translocation and vigorous inflammation in response to environmental conditions that cause a mild decreased splanchnic perfusion, such as physical exercise and surgery[48].
The fact that IR-induced zipper-like constriction of the epithelium was induced even before epithelial cells were shed into the lumen, emphasizes that it is not only essential to close small epithelial defects but importantly, it is crucial to limit the development of epithelial wounds.
PROLONGED SMALL INTESTINAL ISCHEMIA WITH REPERFUSION: PHYSICAL INTESTINAL BARRIER INTEGRITY LOSS AND OCCURRENCE OF LOCAL INFLAMMATION
In contrast to short periods of ischemia of the small intestine, the epithelial lining starts to disintegrate after more than 45 min of small intestinal ischemia. Damage of villus tips further continues during reperfusion, and apoptosis of enterocytes at the villus tips becomes particularly apparent at 30 min of reperfusion. It seems that restorative mechanisms as described above, preventing exposure of the lamina propria to the intraluminal content, fail after longer periods of small intestinal ischemia[49]. This is consistent with data from animal models, in which it has been shown that with increasing duration of the ischemic periods, progressive cell death occurs from villus to crypt[40,50]. Additionally, restoration of IR-induced damage was not achieved after 120 min of reperfusion in tissue exposed to > 45 min of ischemia. This results in prolonged exposure of lamina propria immune cells to PAMPs and damage-associated molecular patterns (DAMPs) which both elicit inflammation.
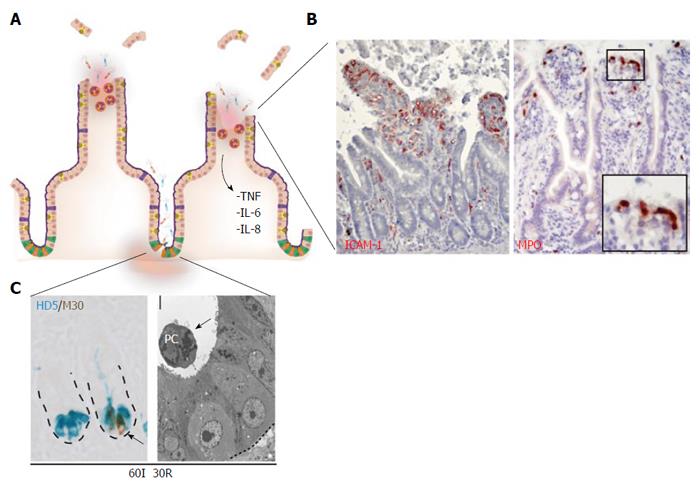
Consequently, and in contrast to the findings presented for short periods of small intestinal ischemia, an inflammatory response develops during the reperfusion phase after prolonged ischemia (Figure 3A). First, enhanced expression of intercellular adhesion molecule-1 (ICAM-1) becomes apparent on the endothelium early during reperfusion, which is accompanied by sequestration and influx of neutrophils into the damaged villus tips (Figure 3B)[49]. Neutrophils accumulated just beneath the damaged epithelial lining, which has been described to play a role in sterilizing the wound (Figure 3B, right panel)[6,51]. However, it should be taken into account that activated neutrophils can also augment to reperfusion-induced damage, since local release of their antimicrobial proteins including myeloperoxidase results in formation of reactive oxygen species (ROS)[52,53]. Second, the inflammatory response was accompanied by complement activation, represented by the deposition of activated C3, a downstream target in the complement activation cascade, in IR-damaged epithelial cells in the lumen[49].
Figure 3 Prolonged small intestinal ischemia-reperfusion results in physical and immunological barrier function loss and inflammation.
A: Prolonged IR leads to disruption of the epithelial lining (physical barrier integrity loss) and Paneth cell loss (immunological barrier integrity loss); B: Inflammatory responses are characterized by increased endothelial expression of ICAM-1 (left panel) with sequestration of MPO-positive neutrophils into the villus tips (right panel), and increased expression and release of inflammatory cytokines including IL-6, IL-8 and TNF; C: Left panel: Prolonged IR leads to Paneth cell apoptosis, as shown by the co-localization of M30 (brown: apoptosis) and human defensin 5 (blue: Paneth cells). Right panel: EM picture of apoptotic Paneth cell, shed into the crypt lumen. ICAM-1: Intercellular adhesion molecule-1; MPO: Myeloperoxidase; HD5: Human defensin-5.
Complement activation has been identified as a key mediator of IR-induced inflammation in mainly murine IR models[54], with involvement of the classical[55], the alternative[56,57] as well as the lectin[58] activation pathway. Complement activation fragments have chemoattractant properties and are capable of inducing production of chemokines and cytokines[59]. In line with this, increased mRNA expression of pro-inflammatory cytokines interleukin (IL)-6, IL-8 and tumor necrosis factor (TNF)-α was observed as early as 30 min after induction of reperfusion, which was accompanied by increased arteriovenous concentration differences of IL-6 and IL-8 across the small intestine[49].
PROLONGED SMALL INTESTINAL IR: IMMUNOLOGICAL BARRIER FUNCTION LOSS AND INCREASED SYSTEMIC INFLAMMATION
Apart from the physical barrier, mainly comprised of a mucus layer and an epithelial lining, the intestine is also dependent on its immunological barrier function to prevent bacterial translocation. An important player in the immunological defense is the Paneth cell, a pyramidal shaped cell located in the crypts of Lieberkühn in the small intestine[36], and continuously produce and secrete antimicrobial proteins into the lumen, thereby sterilizing the crypt[27,31]. In addition, they actively sense the presence of microbiota and directly respond to bacterial threats by releasing their antimicrobials, and are therefore crucial in limiting bacterial translocation[33]. The highly secretory nature of Paneth cells makes these cells particularly susceptible to endoplasmic reticulum (ER) stress[60]. The ER is an important cellular organelle involved in processing and folding of proteins. Environmental conditions that impose stress on the ER, such as hypoxia and oxidative stress, can result in the accumulation of unfolded or misfolded proteins in the ER lumen, resulting in disruption of ER homeostasis[61]. In response to ER stress, a number of highly specific signaling pathways, collectively called unfolded protein response (UPR), are activated. The UPR is directed at restoring ER (and cellular) homeostasis and one of the strategies involved is induction of a translational block, which is mediated by PERK-dependent phosphorylation of eukaryotic translation-initiation factor 2α (eIF2α)[62,63]. A second important pathway is the highly evolutionary conserved IRE1 branch of the UPR. Activation of this pathway induces alternative splicing of X-box binding protein 1 (XBP1) mRNA, resulting in the production of functional XBP1 protein, which plays a crucial role in cellular survival and adaptation following ER stress in the intestine[60,61]. However, if ER stress is extensive or prolonged, signaling typically switches from pro-survival to pro-apoptotic. The transcription factor C/EBP homologous protein (CHOP), which functions downstream of the PERK and ATF6 pathway, can promote apoptotic cell death upon sustained ER stress[64].
Interestingly, we observed in our human intestinal IR studies that the UPR was among the most highly upregulated pathways, particularly after prolonged reperfusion. Hallmarks of UPR activation, including XBP1 splicing and increased expression of UPR-related genes including GADD34 and CHOP, were observed at 30 min of reperfusion of ischemically damaged intestine[34]. Although IR was previously linked to ER stress in a variety of other organs[65-67], this was the first report of ER stress in the pathophysiology of human intestinal IR.
Interestingly, we observed that prolonged periods of ischemia (> 45 min) followed by reperfusion, resulted in increasingly evident ER stress in the human intestine and particularly in Paneth cells, which was consistent with the pioneering work from Kaser et al[60], who linked genetically - induced intestinal ER stress to hypomorphic Paneth cells. In line, human intestinal IR-induced ER stress was accompanied by Paneth cell apoptosis (Figure 3C), which correlated strongly with the level of ER stress. Apoptotic Paneth cells were shed into the crypt lumen (Figure 3C, right panel) and this significantly lowered their numbers, which was particularly true for intestinal tissue exposed to 60 min of ischemia followed by reperfusion[34]. In animal studies, Paneth cell loss in the otherwise normal intestine has been shown to increase bacterial load in mesenteric lymph nodes (MLN) and spleen, as a measure of increased bacterial translocation in the Paneth cell deficient intestine[33]. Moreover, Paneth cells were recently identified as the central players in the development of intestinal inflammation, since Paneth cell specific deletion of Xbp1 was sufficient to induce severe enteritis with a Crohn’s disease-like phenotype[68].
In line with these studies, we showed in rats that loss of the intestinal epithelial barrier as observed in human intestinal IR, in combination with Dithizone-induced loss of Paneth cells, led to an increase in bacterial translocation to MLN, liver and spleen, as well as increased systemic inflammation. This demonstrated the crucial function of Paneth cells in limiting bacterial translocation[34], and suggested that Paneth cell loss is a new phenomenon contributing to human intestinal IR-induced inflammatory complications and IR-related morbidity and mortality.
Since Paneth cells have been shown to be crucial for stem cell survival by providing critical growth factors[36], and stem cells are of major importance for regeneration of IR-damaged intestinal epithelium, future studies are directed at elucidating whether IR-induced Paneth cell loss also contributes to the development of transmural damage, a highly lethal complication associated with intestinal ischemic disease[1,11,14]. Improved knowledge on these mechanisms might lead to novel strategies to improve and/or restore stem cell function following intestinal IR and could have great potential in reducing the high morbidity and mortality of intestinal ischemia.
FIRST INSIGHT INTO THE PATHOPHYSIOLOGY OF HUMAN COLONIC IR
Colon IR is the most common form of intestinal ischemia, and is observed in a variety of situations including infections and vasculitis, as well as in patients undergoing aortic surgery or cardiac bypass surgery[1,69-71]. To study the pathophysiology of human colonic IR we developed a human experimental colon IR model[72,73], analogous to the model for human small intestinal IR as described above[34,38,49]. The first interesting observation was that human colon tissue was far more resistant to ischemia than human jejunum[73]. The epithelial lining of the human colon remained intact even after 60 min of ischemia, with or without reperfusion. In addition, apoptosis of colonocytes was limited to only few cells in the surface epithelium, whereas in the human small intestine IR was associated with massive apoptosis of epithelial cells in the villus tips and crypts[34,49,74].
Apart from the epithelial lining, a crucial layer of antimicrobial defense, particularly in the colon, is the mucus layer. This layer creates a physical and chemical barrier that prevents adherence of microbiota to the epithelium (Figure 4A)[19-21]. To study the consequences of IR on the mucus layer in more detail, implementation of a rat colon IR model was imperative to overcome problems with preservation of the mucus layer and staining for bacteria.
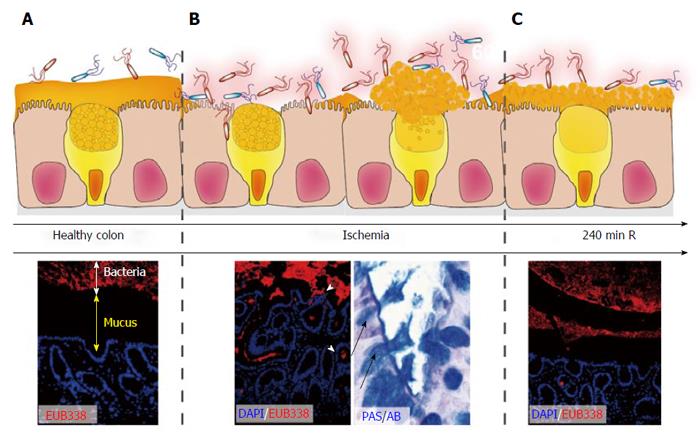
Figure 4 Rapid restoration of colon ischemia-reperfusion-induced mucus barrier loss by goblet cell compound exocytosis.
In healthy colon, bacteria are separated from the epithelium by a thick mucus layer (A; DAPI, Blue: nuclei. EUB338, Red: bacteria); During ischemia and early reperfusion, the mucus barrier is damaged leading to penetration of bacteria deep into the colonic crypts (B). Goblet cells respond by massive release of their granules into the crypt lumen (so called compound exocytosis), which led to a recoverd mucus layer at 240 min of reperfusion in the rat intestine (C). Note that bacterial clearance is accompanied by depletion of goblet cell contents.
A newly observed and likely important pathophysiological consequence of 60 min of colonic ischemia was disruption of this mucus layer, which was accompanied by intrusion of bacteria into the colonic epithelium (Figure 4B). Intriguingly, goblet cells react rapidly in response to these bacterial threats with expulsion of their mucus - containing granules (Figure 4B, right panel), a phenomenon known as goblet cell “compound exocytosis”[21,75]. Compound exocytosis is characterized by expansion of mucin volume and rapid replacement of degraded mucus barriers, and has been described as a mechanism to reinforce the barrier and exclude pathogens from the normally sterile crypts[21]. Indeed, the mucus layer covering the epithelium was fully restored within 4 h of reperfusion of ischemically damaged colon (Figure 4C) and a time-dependent clearance of bacteria from the colonic crypts was observed. This was accompanied by only mild inflammatory responses, particularly if compared to small intestinal IR[73].
Therefore, compound exocytosis seemed an efficient mechanism to prevent prolonged exposure of microbiota to the epithelium thereby preventing intestinal inflammation. This might explain why patients with colonic ischemia tend to have a milder course of the disease as compared to small intestinal IR[71,76-78]. In addition, mucus organization could explain why ischemia of the right-sided colon is often more severe than left-sided colonic ischemia, since thickness of the inner mucus layer increases from the proximal to distal colon[71].
TRANSLATION TO CLINICAL PRACTICE
Our improved understanding of the pathophysiological mechanisms of small intestinal IR paves a way to develop and experimentally test new therapies that can limit ischemic damage as well as reperfusion-induced intestinal tissue damage. This is particularly relevant for patients undergoing aortic surgery with temporary abrogation of the mesenteric blood flow, patients with severe mesenteric hypoperfusion due to sepsis or shock, or patients revascularized after an ischemic insult (i.e., after embolectomy). In addition, improved knowledge on the sequelae of human intestinal IR is important for the field of intestinal transplantation.
However, probably the most important factor that determines the outcome of patients with acute intestinal ischemia is our ability to recognize intestinal ischemia at an early stage[14]. There is often a considerable diagnostic delay due to the nonspecific clinical presentation in combination with the lack of early, non-invasive diagnostic markers for intestinal ischemia. A promising marker for intestinal ischemia is intestinal fatty acid binding protein (I-FABP), a small (14-kD) cytosolic protein specifically present in mature enterocytes at the tip of the villus that is released into the circulation upon enterocyte damage[5,79-82]. Using the human intestinal IR model, the characteristics of I-FABP were studied in more detail. We have shown that I-FABP is a very sensitive marker of intestinal villus tip damage, as arteriovenous concentration differences were already increased 15 min after onset of ischemia, whereas morphological changes were hardly noticeable at this time point[83]. Longer ischemia resulted in massive increase of arteriovenous I-FABP concentration differences, and I-FABP levels correlated with the degree of histological epithelial damage.
More importantly however, systemic I-FABP levels were increased in patients that were included in the experimental IR protocol compared to control patients that were exposed to similar surgical handling without being exposed to intestinal IR using the experimental protocol[83]. This demonstrated that IR of a 6 centimeter small intestinal segment can be detected using plasma I-FABP measurement, which emphasizes the potential value of plasma I-FABP in the early diagnosis of intestinal ischemia. Prospective trials now need to show whether I-FABP levels can be used for decision making in patients with suspected intestinal ischemia.
CONCLUSION
Over the past years, the human experimental intestinal IR models have led to a more in depth knowledge on the pathophysiology of human small intestinal IR and colon IR. We have been able to identify the duration of ischemia that the human small intestine can undergo before extensive physical and immunological barrier damage and inflammation occur. Our studies show that the human small intestine is adapted to withstand short periods of ischemia by ingenious mechanisms involved in prevention and rapid repair of epithelial lining damage, thereby preventing severe inflammation. This explains why humans can endure short periods of intestinal IR as a consequence of strenuous exercise (physiological), or during major (cardiovascular) surgery with cross clamping of the aorta or extensive blood loss. In contrast, longer periods of ischemia result in extended physical barrier damage and immunological barrier compromise, typically observed in the small intestine.
The colonic epithelial lining appeared more resistant to ischemia and reperfusion, and goblet cells reacted rapidly to IR-induced mucus barrier loss by expulsion of their contents, which resulted in rapid restoration of the protective mucus barrier.
With increasing knowledge on the pathophysiology of human intestinal IR, future studies will be directed towards implementation of this knowledge on intestinal IR, to work towards better detection, prevention and therapy for intestinal ischemia/reperfusion in man.