Published online Feb 21, 2016. doi: 10.3748/wjg.v22.i7.2357
Peer-review started: May 25, 2015
First decision: June 25, 2015
Revised: September 5, 2015
Accepted: November 30, 2015
Article in press: November 30, 2015
Published online: February 21, 2016
Processing time: 251 Days and 13.4 Hours
AIM: To explore the changes of X-box binding protein 1 splicing (XBP1s) and inflammatory cytokine expression in patients with ulcerative colitis (UC) in response to endoplasmic reticulum stress (ERS).
METHODS: Reverse transcription polymerase chain reaction and quantitative polymerase chain reaction were performed to detect the forms of XBP1s and the expression of interleukin (IL)-2, interferon (IFN)-γ, and IL-17α. Differences between patients with UC and normal subjects were then determined.
RESULTS: Mononuclear cells of the peripheral blood of normal subjects and UC patients with were stimulated with no drugs (control), phytohemagglutinin (PHA), thapsigargin (TG), or both PHA and TG. XBP1s in patients with UC exhibited splicing, which was greater with co-stimulation than single stimulation. Co-stimulation increased the expression level of IL-2, IFN-γ, and IL-17α.
CONCLUSION: The T lymphocytes of both normal subjects and patients with UC responded to ERS by activating the XBP1s-mediated signalling pathway, upregulating the expression of inflammatory cytokines, and increasing the occurrence of inflammation. The mononuclear cells in the peripheral blood of patients with UC were more sensitive to ERS than those in the peripheral blood of normal subjects.
Core tip: Endoplasmic reticulum stress (ERS) can repair stress-induced cell damage and restore normal cell function by the inositol-requiring enzyme 1/X-box binding protein 1 splicing (IRE1/XBP1) signalling pathway. However, the link to ulcerative colitis (UC) remains unclear. In the present study, we report that T lymphocytes respond to ERS by activating the IRE1/XBP1 signalling pathway, upregulating the expression of inflammatory cytokines, and increasing the occurrence of inflammation. In addition, mononuclear cells in the peripheral blood of patients with UC were more sensitive to ERS than normal subjects.
- Citation: Li N, Wang XM, Jiang LJ, Zhang M, Li N, Wei ZZ, Zheng N, Zhao YJ. Effects of endoplasmic reticulum stress on the expression of inflammatory cytokines in patients with ulcerative colitis. World J Gastroenterol 2016; 22(7): 2357-2365
- URL: https://www.wjgnet.com/1007-9327/full/v22/i7/2357.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i7.2357
The endoplasmic reticulum regulates protein synthesis and folding and promotes the synthesis of various lipids, such as cholesterol and steroids. Various physiological or pathological states, such as an increase in unfolded proteins, synthesis of misfolded proteins, dysregulation of intracellular calcium ions, and changes in intracellular redox state, can cause the occurrence of endoplasmic reticulum stress (ERS)[1]. ERS can repair stress-induced cell damage and restore normal cell functions through an unfolded protein response. Inositol-requiring enzyme 1(IRE1)/X-box binding protein 1 splicing (XBP1) is an important signalling pathway in ERS; IREI/XBPI can reduce protein synthesis, promote protein degradation, increase chaperone generation, slow down ERS occurrence, and return cellular functions to normal. Under ERS, the IREI/XBPI pathway can activate c-Jun N-terminal kinase (JNK)[2,3] related signalling pathways. As a result, release of cytokines and bio-chemotactic factors is promoted, and the body’s natural immune system is activated. Researchers have investigated changes in Toll-like receptors[4] and cytokines as well as the immune system to explain the pathogenesis of inflammatory bowel disease and ulcerative colitis (UC). The IREI/XBPI signalling pathway can conserve the integrity of the intestinal mucosal barrier, maintain the number of intestinal Paneth cells, and promote the secretion of cytokines inside the intestine. In this manner, the intestinal mucosa is protected, and the occurrence of intestinal inflammation is reduced[5-7]. However, studies have yet to describe the effects of ERS on T lymphocytes and the corresponding changes in patients in response to ERS. Hence, we determined whether XBPI influences the incidence of UC by adjusting the acquired immune response.
Main instruments: The following instruments were used in this study: low-temperature high-speed centrifuge (Eppendorf, Hamburg, Germany); dry thermostat (Hangzhou ORSUS biotechnology Co, Hangzhou, China); DNA diffusion meter (PE, United States); real-time polymerase chain reaction (PCR) system (Eppendorf); polyacrylamide gel electrophoresis and electrotransferring instrument (Bio-Rad Co., Hercules, CA, United States); inverted fluorescence microscope (Nikon, Tokyo, Japan); CO2 incubator (Thermo Scientific, Boston, MA, United States); high-speed desktop centrifuge (Eppendorf); low-temperature high-speed desktop centrifuge (Eppendorf); vortex turbulencer (Beijing Tongzheng Biotechnology Co., Ltd., Beijing, China); cryogenic tank (Haier Electric Co., Qingdao, China); chemiluminescence analyser (DLR, USA); temperature controller module (Eppendorf; pure water (Millipore, Billerica, MA, United States); Ultraviolet (UV) spectrometer (Pharmacia, Piscataway, NJ, United States); and agarose gel electrophoresis tank (Beijing Liuyi Instrument plant, Beijing, China).
Reagents: The following reagents were used in this study: chloroform, isopropanol, and ethanol (Beijing Chemical Plant); concanavalin A (ConA) (Sigma, St Louis, MO, Unites States); phytohemagglutinin (PHA) (Sigma); thapsigargin (TG) (Sigma); RPMI-1640 medium (Beijing Neuron biotech Co.); FBS (Gibco, Carlsbad, CA, United States); RNAiso Plus (Takara, Tokyo, Japan); RNA reverse transcription kit (Takara, Japan); real-time kit (Takara, Japan); SYBR (Kangwei Reagent Company, Xinzhuang, China); agarose (GENE Co., Chai Wan, China); Goodview (SBSbBio Genentech, Shanghai, China); and lymphocyte stratification fluid (Sigma).
Normal control (Ctrl) subjects and patients with UC: The normal Ctrl group comprised 18 healthy volunteers (13 males and five females) aged 25–55 years (with a mean age of 40 years). These volunteers were selected from May 2012 to March 2013 from the Department of Gastroenterology, Hospital of General Staff Headquarters and excluded from the UC diagnostic criteria. All of the normal subjects were screened for high blood pressure, coronary heart disease, cerebrovascular accidents and other cardiovascular diseases, diabetes, thyroid dysfunction, and other endocrine, gastroenterological, and metabolic diseases, liver-kidney-pancreatic diseases, hypoxia-related diseases, inflammation, cancer, and stress response. A total of 21 UC patients aged 23–54 years (with a mean age of 39 years) were selected from May 2012 to March 2013 from the Department of Gastroenterology, Hospital of General Staff Headquarters, Xiyuan Hospital and West division of Chaoyang Hospital. Of the 21 patients, 16 were males and five were females. These patients were diagnosed with moderate to severe UC in accordance with the “consensus of diagnosis and treatment of UC”, enacted in Jinan in 2007. The patient data are shown in Table 1.
| No. | Gender | Age (yr) | Enteroscopic diagnosis | Pathological appearance |
| 1 | M | 25 | Severe, in acute period | Acute and chronic inflammation |
| 2 | M | 52 | Severe, in acute period | Acute and chronic inflammation |
| 3 | F | 48 | Moderate-severe, in acute period | Chronic and chronic inflammation |
| 4 | M | 43 | Moderate-severe, in remission period | Chronic inflammation |
| 5 | F | 57 | Moderate, in acute period | Chronic inflammation |
| 6 | M | 50 | Moderate, in acute period | Chronic inflammation |
| 7 | M | 42 | Moderate, in remission period | Chronic inflammation |
| 8 | M | 23 | Moderate, in acute period | Acute and chronic inflammation |
| 9 | M | 41 | Moderate -severe, in acute period | Acute and chronic inflammation |
| 10 | M | 23 | Moderate, in acute period | Acute inflammation |
| 11 | M | 45 | Moderate-severe, in acute period | Acute and chronic inflammation |
| 12 | M | 35 | Severe, in remission period | Acute and chronic inflammation |
| 13 | M | 47 | Severe, in acute period | Acute and chronic inflammation |
| 14 | M | 33 | Moderate -severe, in remission period | Chronic inflammation |
| 15 | F | 56 | Moderate, in remission period | Chronic inflammation |
| 16 | M | 51 | Moderate, in acute period | Chronic inflammation |
| 17 | M | 40 | Moderate, in remission period | Acute inflammation |
| 18 | M | 22 | Moderate, in acute period | Acute and chronic inflammation |
| 19 | M | 39 | Moderate-severe, in remission period | Chronic inflammation |
| 20 | M | 27 | Moderate, in acute period | Acute inflammation |
| 21 | M | 43 | Moderate-severe, in remission period | Acute inflammation |
Cell isolation and primary culture were performed in accordance with the principles and methods of American Type Culture Collection (ATCC).
Peripheral blood mononuclear cell suspensions were obtained from normal volunteers and patients with UC; these suspensions were then seeded in 12-well plates with 1 mL of the cell suspension in each well. The cell density was adjusted to 5 × 108 cells/L.
The experiment was divided into two groups, namely, the peripheral blood mononuclear cells from normal volunteers and those from patients with UC. Each group was further divided into four subgroups, namely, the Ctrl group, the PHA stimulation group, the TG stimulation group, and the PHA + TG co-stimulation group. Each group was set in triplicate wells, and the experiment was repeated thrice.
Drugs were not added to the Ctrl group. A 20 μL PHA solution was added to the PHA stimulation group at a final concentration of 5 μg/mL. Thirty microliters TG solution was added to the TG stimulation group at a final concentration of 300 nmol/L. Twenty microliters PHA solution and 0 μL TG solution were added to the PHA + TG co-stimulation group. The groups were cultured at 37 °C and 5% CO2 for 12 h; afterwards, the cells were collected to extract RNA for subsequent use.
Extraction of total RNA: The total RNA was extracted using the TRIzol method and stored at -70 °C for reverse transcription.
Analysis of experimental results: After the reaction, the amplification curve and the melting curve of real-time PCR were confirmed. Meanwhile, the PCR quantitation standard curve was performed. The primer sequences are shown in Table 2.
| Human cytokines | Primer name | Sequence |
| IL-2 | Forward | 5’-AAGTTTTACATGCCCAAGAAGG-3’ |
| Reverse | 5’-AAGTGAAAGTTTTTGCTTTGAGCTA-3’ | |
| IFN-γ | Forward | 5’-AGGGAAGCGAAAAAGGAGTCA-3’ |
| Reverse | 5’-GGACAACCATTACTGGGATGCT-3’ | |
| IL-17α | Forward | 5’-GAGCCCCAAAAGCAAGAGGAA-3’ |
| Reverse | 5’-TGCGGGCATACGGTTTCATC-3’ | |
| IL-4 | Forward | 5’-GCCAAGACCCCTTCGAGAAAT-3’ |
| Reverse | 5’-CCGTCCCTGTTATCTGCCTCC-3’ | |
| GAPDH | Forward | 5’-TGTGGGCATCAATGGATTTGG-3’ |
| Reverse | 5’-ACACCATGTTATTCCGGGTCAAT-3’ |
PCR products were then added onto the agarose gel tank for electrophoresis to observe the splicing of XBP1 among the primer sequences of the groups (Table 3).
| XBP1 | Primer name | Sequence |
| Human XBP1 | Forward | 5’-A AAC AG A GTA GCA GCT CAG ACT GC-3’ |
| Reverse | 5’-TC CTT CTG GGT AGA CCT CTG GGA G-3’ | |
| Mouse XBP1 | Forward | 5’-A AAC AG A GTA GCA GCG CAG ACT GC-3’ |
| Reverse | 5’-TC CTT CTG GGT AGA CCT CTG GG |
The experimental data were expressed as mean ± SD, and the statistical software Graphpad Prism 5.0 (La Jolla, CA, United States) was used to plot the data. T tests were performed to determine significant differences between groups; where P < 0.05 was considered statistically different and P < 0.01 was considered significantly and statistically different.
Human T lymphocytes were stimulated and activated, and, from the perspective of ERS effects, they produced effects towards the endoplasmic reticulum through XBPI. Under the ERS state, T lymphocytes could stimulate the adaptive immune system through XBPI, thus promoting the expression of inflammatory cytokines.
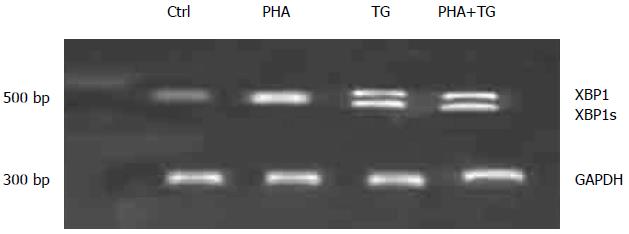
The peripheral blood mononuclear cells of UC patients were extracted, stimulated, experimentally grouped, and then divided into the Ctrl group and the PHA stimulation group (with a stimulation concentration of 5 μg/mL). The cells of the TG group were collected 12 h after the stimulation to detect XBPIs. The results are shown in Figure 1. The expression of XBPIs in the PHA-TG co-stimulation group was significantly increased relative to that in the Ctrl group.
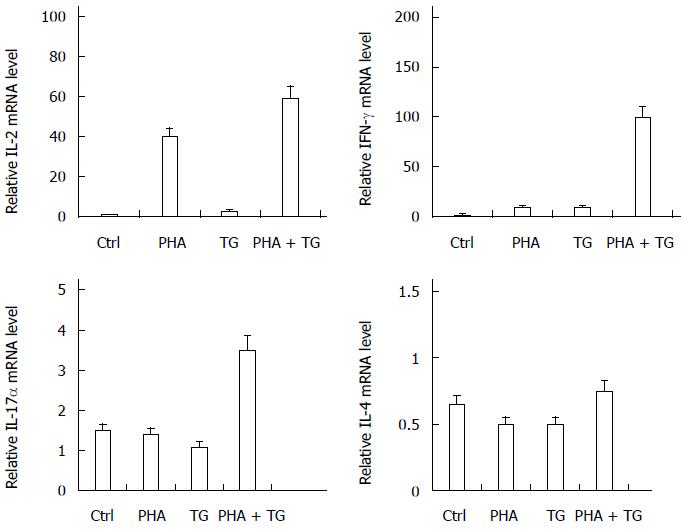
The cells were collected from the four groups after 12 h of stimulation, and the expression of interleukin (IL)-2, interferon (IFN)-γ, IL-17α, IL-4, and other cytokines were detected. The results are shown in Figure 2. After TG-PHA co-stimulation, the expression of IL-2, IFN-γ, and IL-17α was significantly increased relative to that in the Ctrl group.
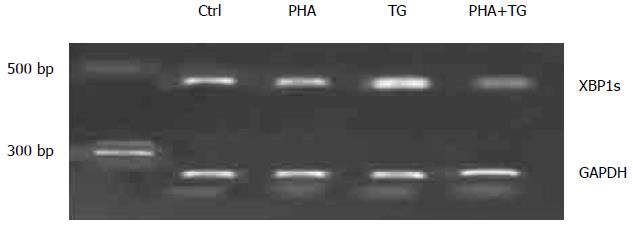
To study the changes of XBP1s and the differences in the expression of inflammatory factors in the peripheral blood mononuclear cells of the healthy Ctrl group and the UC group, additional peripheral blood was extracted from normal volunteers. From this blood, mononuclear cells were extracted, and these cells were stimulated PHA, TG, or both. The experiment was divided into four groups, namely, the Control group, the PHA stimulation group (with a concentration of 5 μg/mL), the TG stimulation group (with a final concentration of 300 nmol/L) and the PHA + TG co-stimulation group. The cells were harvested after 12 h of stimulation to detect the status of XBPIs. The results are shown in Figure 3. XBPIs showed no significant change after PHA-TG co-stimulation.
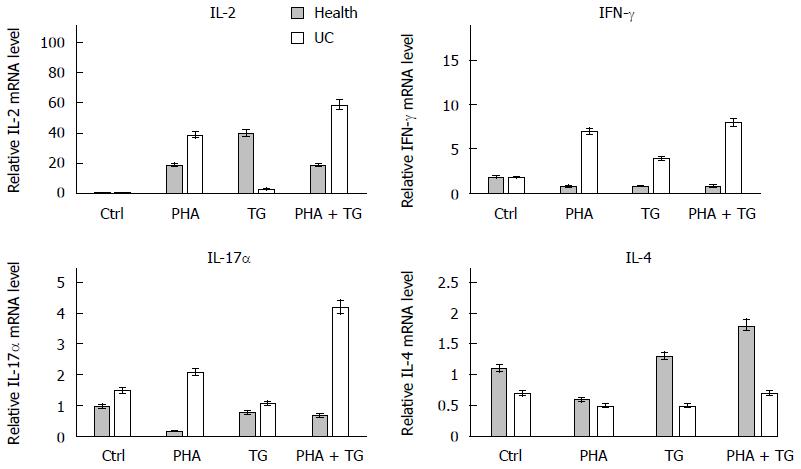
To study the difference in the expression of inflammatory cytokines in the peripheral blood mononuclear cells of the healthy Ctrl group and the UC group under ERS, we conducted the following experiment. The subjects were divided into two groups: the normal group and the UC patient group. Each group was then further subdivided into four subgroups, namely, the Ctrl group, the PHA stimulation group (with a final concentration of 5 μg/mL), the TG stimulation group (with a final concentration of 300 nmol/L), and the PHA+TG co-stimulation group. The cells were harvested after 12 h of stimulation to detect the expression of 1L-2, IFN-γ, 1L-17α, and other cytokines. The results are shown in Figure 4. The expression levels of IL-2, IFN-γ, and IL-17α in the PHA-TG co-stimulation group were significantly increased compared with the levels found in normal volunteers.
PHA stimulation activated T lymphocytes in the peripheral blood mononuclear cells of normal volunteers and UC patients. When TG stimulation was performed under the ERS status, PHA-TG co-stimulation on the peripheral blood mononuclear cells of UC patients caused significant enhancement of XBP1s compared with the Ctrl group, the PHA stimulation group, and the TG stimulation group. No significant difference in XBP1s was found in the normal population. Meanwhile, PHA-TG co-stimulation of peripheral blood mononuclear cells in normal subjects did not significantly change the expression of IL-2, IFN-γ, and IL-17α. However, the expression of the above cytokines in UC patients was increased with co-stimulation, and the differences were statistically significant, indicating that under the same ERS status, the T lymphocytes in UC patients were much more sensitive towards ERS than normal subjects. UC patients may express more pro-inflammatory cytokines, thus increasing the inflammation response.
Inflammatory intestinal diseases (including UC and Crohn’s disease) are chronic non-specific diseases that occur inside the intestine[8]. Currently, the causes are still unclear, although one study has shown that some environmental factors and dietary factors can modulate the clinical course of inflammatory bowel disease (IBD)[9]. Most scholars have classified UC and Crohn’ disease as autoimmune diseases[10]. Because the cause of these diseases is unknown, the diagnosis rate is low, and the treatment effects and prognoses are poor. However, the use of biological drugs has opened up new horizons in the management of inflammatory bowel diseases, but the long prognosis remains uncertain[11]. Thus, the problem of diagnosis and treatment continues to be perpetuated. As an organelle of the body, the endoplasmic reticulum plays an important role in the synthesis, maturation, and transport of proteins as well as in the maintenance of calcium homeostasis[12], which affects the folding, quality control, and transport regulation of proteins[13]. Any changes of in vitro and in vivo conditions affect the functions of the endoplasmic reticulum, thus blocking protein processing and causing a large number of folded proteins to accumulate inside the endoplasmic reticulum, leading to endoplasmic reticulum dysfunction[14]. To ease the pressure of the endoplasmic reticulum, the chaperone binding protein (BiP) dissociates from three important signalling molecules PKR-like endoplasmic reticulum kinase (PERK), activating transcription factor 6 (ATF6), and inositol requiring 1 (IRE1) inside the ERS pathway[13,15-17]. Normally, BiP directly acts on the folding protein to promote protein folding and input the synthesised proteins into the endoplasmic reticulum. BiP also releases the pressure of the endoplasmic reticulum, thereby returning endoplasmic reticulum functions back to normal. If the folded proteins of the endoplasmic reticulum were continuously generated at an enormous rate, the pressure would be sustained and the loading would be increased. The PERK, ATF6, and IRE1 pathways would be activated and would reduce the synthesis of proteins, promote the degradation of proteins, and increase the generation of BiP, thus slowing the occurrence of ERS. However, if the pressure load inside the endoplasmic reticulum is not lifted, it would eventually lead to apoptosis[18]. During the occurrence of ERS, the immune system, the channels associated with inflammation and pressure, and the oxidative stress pathways are affected; hence, a number of chronic metabolic diseases and autoimmune diseases, such as type 2 diabetes, fatty liver, neuropathic lesions and various tumours and inflammatory intestinal diseases, would be induced[19]. Recent studies have linked ER stress and the UPR to IBD[20].
Experiments have shown that under ERS, the IRE1/XBP1 pathway could activate the body’s natural immune system through TLR (Toll-like receptors)[4]. On the one hand, the activation of XBP1 within macrophages could promote the induction of NOX2 NADPH oxidase by TLR[21]. On the other hand, it could activate the ROS system[22], thus causing the activation of XBP1. This activation would ultimately activate NF-KB, JNK, and other signalling pathways[2,23,24], promote the secretion of IL-6, TNFα, and other cytokines, and act in the early stage of immune response[22]. Under ERS, the expression of XBP1s significantly increased after activation of human T lymphocytes[25]; the expression of IL-2, IFT-γ, and IL-17α increased, and inflammation increased. Compared with inactivated T lymphocytes, the activated T lymphocytes exhibited a significant enhancement of XBP1s after TG stimulation. XBP1s is the active form of XBP1. When the IRE1/XBP1 signalling pathway was activated, XBP1s was significantly enhanced[26]. In comparison with T lymphocytes activated by single stimuli, the mRNA expression levels of IL-2, IFN-γ, and IL-17α was significantly increased after the co-stimulation of the activator and TG[27-29]. Collectively activated T lymphocytes affect ERS by XBP1, namely; under ERS, XBP1 could not only activate the innate immune response[30] but also activate the body’s acquired immune response and release cytokines. Acquired immune response is one of the most important parts of the immune system, playing a key role in the body’s processing of the immune response. Acquired immunity participates in and affects the occurrence and development of various autoimmune diseases, and influences their prognosis. Previous studies found that the incidence of inflammatory intestinal diseases was most closely related to the autoimmune system. The TLR family was shown to regulate T cells through 15 natural immunity and CD4+CD25+[31] and to play important regulatory roles in the pathogenesis of IBD by adaptive immunity. Some cytokines, such as TNFα, IL-17α, IL-23, and TGF-β, play important roles in the pathogenesis of inflammatory intestinal diseases, though the inflammatory intestinal diseases are still not clearly understood. In this study, key molecules in the three channels of endoplasmic reticulum were detected. The expressions of IRE1/XBP1 and ATF6 in the intestinal epithelial mucosa of patients with severe UC were much higher than expressions in the normal volunteers, while the expression of PERK was reduced[32]. ERS occurred in patients with inflammatory intestinal disease, while the specific mechanism was still unclear.
Our experiment was designed and conducted based on ERS activation of the acquired immune system in UC patients, and it role in the occurrence and development of UC. We sampled the peripheral blood of UC patients, separated the mononuclear cells, and then stimulated them with PHA to activate the T lymphocytes. Meanwhile, the TG stimulation was also performed to detect the conditions of XBP1s. Under ERS, UC patients exhibited obvious XBP1s inside the activated T lymphocytes, which was significantly enhanced compared with the unstimulated group and the PHA or TG single-stimulation group. We also detected the cytokines of each group after treatment and found that the mRNA expression of IL-2, IFN-γ, and IL-17α in the peripheral blood mononuclear cells of UC patients was significantly increased after co-stimulation by PHA and TG. We also isolated the peripheral blood mononuclear cells from healthy subjects and stimulated them with PHA, TG, or a combination of both. The results showed that after co-stimulation, the expression of IL-2, IFN-γ, and IL-17α, as well as XBP1s was increased. However, compared with the UC patients, the expression of cytokines and XBP1s in the other groups was significantly reduced. Thus, we believe that the peripheral blood mononuclear cells of UC patients were much more sensitive to ERS. Under ERS, the expression of XBP1s in the peripheral blood mononuclear cells of activated UC patients was much more obvious and could activate the body to achieve the acquired immune response to promote the expression of cytokines, such as IL-2, IFN-γ and IL-17α, aggravating the occurrence of UC.
The activation of T lymphocytes and peripheral blood mononuclear cells in UC patients by TG stimulation significantly increased the expression of IL-2, IFN-γ, and IL-17α. The expression of IL-14, however, was not significantly changed. T helper (Th) cells inside the body’s T cells were divided into two subtypes (Th1 and Th2), and IL-2 and IFN-γ were expressed by Th1-type cells, while IL-4 was expressed by Th2 cells. Under normal circumstances, Th1yu and TH2 exist in dynamic equilibrium. The expression of IL-2, IFN-γ, and IL-17α increased, while IL-4 was not increased, indicating that under ERS, lymphocytes drifted towards Th1. The Th1 cells mainly mediate the cellular immune response, and are thus involved in inducing the occurrence of organ-specific autoimmune diseases. This observation further indicated that the body was more likely to develop autoimmune diseases under ERS, which might be an important mechanism for the occurrence of UC. Under ERS, the expression of IL-2, IFN-γ, and IL-17α in the lymphocytes of UC patients were increased, while the expression of IL-4 showed no significant change, indicating that the lymphocytes in UC patients drifted towards TH1; no such phenomenon occurred in healthy people, which is consistent with literature[33].
IL-17α is a cytokine secreted and released by the TH17 subgroup. As a pro-inflammation cytokine, IL-17α often plays roles in autoimmune and infectious diseases[34]. The expression of IL-17α was significantly increased during the process of UC[35]. We used CT to stimulate the peripheral blood mononuclear cells of normal subjects and activated UC patients and found that compared with normal people, the expression of IL-17α was increased in the lymphocytes of UC patients. In addition, the expression of IL-17α increased by the PHA–CG co-stimulation was significantly higher than PHA stimulation alone. We used TG to stimulate the activated human T lymphocytes, and the expression of IL-17α was significantly increased, indicating that ERS could activate T lymphocytes to express IL-17α, thus aggravating inflammation. We used TG to stimulate the activated lymphocytes in UC patients, and the expression level was significantly higher than that in PHA or TG stimulation alone, indicating that UC patients were much more sensitive to ERS.
In summary, under ERS, activated human T lymphocytes could affect the endoplasmic reticulum and promote the expression of inflammatory cytokines through XBP1s, and the lymphocytes drifted towards Th1. Therefore, we considered that under ERS, the acquired immune response could be activated, thus promoting the expressions of inflammatory cytokines and aggravating the occurrence of autoimmune diseases. UC is considered an autoimmune disease. Compared with normal healthy individuals, the peripheral blood lymphocytes of UC patients were much more sensitive to ERS. The lymphocytes could respond to ERS through XBP1s, thus activating the body to achieve the acquired immune response, promoting the expressions of IL-2, IL-17α, and IFN-γ. This response might contribute to the pathogenesis of UC; blocking this pathway might be one way to treat UC, although it still needs to be confirmed by further experiments.
In conclusion, this study found that human T lymphocytes responded to ERS through XBP1s and promoted the secretions of IFN-γ, IL-17α, and IL-2. In addition, we also found that the peripheral blood mononuclear cells of UC patients were much more sensitive to ERS. The T lymphocytes of the patient could respond to ERS through the IRE1/XBP1 pathway, activate the body to obtain the acquired immune response, and promote the expression of inflammatory cytokines. Hence, T lymphocytes play important roles towards the occurrence and development of inflammation.
Various physiological or pathological states can cause the occurrence of endoplasmic reticulum stress (ERS); ERS can repair stress-induced cell damage and restore normal cell functions through an unfolded protein response. Inositol-requiring enzyme 1(IRE1)/X-box binding protein 1 splicing (XBP1) is an important signalling pathway in ERS. Although the IREI/XBPI signalling pathway can conserve the integrity of the intestinal mucosal barrier, maintain the number of intestinal Paneth cells, and promote the secretion of cytokines inside the intestine, no studies have yet described the effects of ERS on T lymphocytes and the corresponding changes in patients in response to ERS. Hence, the authors determined whether XBPI influences the incidence of UC by adjusting the acquired immune response.
Experiments showed that under ERS, the expression of XBP1s is significantly increased after human T lymphocytes were activated and that the IRE1/XBP1 pathway could activate the body’s natural immune system through TLR. This activation would ultimately activate NF-κB, JNK, and other signalling pathways, promote the secretion of IL-6, TNFα, and other cytokines, and act in the early stage of immune response.
This study provided direct evidence that T lymphocytes of patients with UC respond similarly to those of normal subjects. The response was through the XBP1s-mediated signalling pathway and was associated with activation of the expression of inflammatory cytokines and increased occurrence of inflammation. In contrast, the mononuclear cells of peripheral blood of UC patients were more sensitive to ERS than the normal subjects.
Mononuclear cells of peripheral blood may be a possible target in the treatment of UC.
ERS, the stress status of the endoplasmic reticulum, repairs stress-induced cell damage and restores normal cell function. IRE1/XBP1 are two kinds of proteins that promote the pathway of ERS repair.
In this interesting study, Nan et al explored changes in XBP1s forms and the expression of inflammatory cytokines in patients with UC towards ERS. The results of this study are good.
| 1. | Moore KA, Plant JJ, Gaddam D, Craft J, Hollien J. Regulation of sumo mRNA during endoplasmic reticulum stress. PLoS One. 2013;8:e75723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2367] [Article Influence: 91.0] [Reference Citation Analysis (1)] |
| 3. | Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1045] [Cited by in RCA: 1101] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 4. | Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1641] [Cited by in RCA: 1616] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 5. | Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1177] [Cited by in RCA: 1169] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 6. | Keshav S, Lawson L, Chung LP, Stein M, Perry VH, Gordon S. Tumor necrosis factor mRNA localized to Paneth cells of normal murine intestinal epithelium by in situ hybridization. J Exp Med. 1990;171:327-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1256] [Cited by in RCA: 1275] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 8. | Morís G. Inflammatory bowel disease: an increased risk factor for neurologic complications. World J Gastroenterol. 2014;20:1228-1237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Cabré E, Domènech E. Impact of environmental and dietary factors on the course of inflammatory bowel disease. World J Gastroenterol. 2012;18:3814-3822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Schmidt C, Stallmach A. Etiology and pathogenesis of inflammatory bowel disease. Minerva Gastroenterol Dietol. 2005;51:127-145. [PubMed] |
| 11. | Rencz F, Péntek M, Bortlik M, Zagorowicz E, Hlavaty T, Śliwczyński A, Diculescu MM, Kupcinskas L, Gecse KB, Gulácsi L. Biological therapy in inflammatory bowel diseases: access in Central and Eastern Europe. World J Gastroenterol. 2015;21:1728-1737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Arbabian A, Brouland JP, Gélébart P, Kovàcs T, Bobe R, Enouf J, Papp B. Endoplasmic reticulum calcium pumps and cancer. Biofactors. 2011;37:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Adolph TE, Niederreiter L, Blumberg RS, Kaser A. Endoplasmic reticulum stress and inflammation. Dig Dis. 2012;30:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1032] [Cited by in RCA: 1114] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 15. | Scharl M, Rogler G. Inflammatory bowel disease pathogenesis: what is new? Curr Opin Gastroenterol. 2012;28:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Dai MX, Zheng XH, Yu J, Yin T, Ma MJ, Zhang L, Liu M, Ma Y, Liu LW, Gao X. The impact of intermittent and repetitive cold stress exposure on endoplasmic reticulum stress and instability of atherosclerotic plaques. Cell Physiol Biochem. 2014;34:393-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2364] [Cited by in RCA: 3187] [Article Influence: 227.6] [Reference Citation Analysis (0)] |
| 18. | Yoo SA, You S, Yoon HJ, Kim DH, Kim HS, Lee K, Ahn JH, Hwang D, Lee AS, Kim KJ. A novel pathogenic role of the ER chaperone GRP78/BiP in rheumatoid arthritis. J Exp Med. 2012;209:871-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2298] [Cited by in RCA: 2255] [Article Influence: 140.9] [Reference Citation Analysis (0)] |
| 20. | Luo K, Cao SS. Endoplasmic reticulum stress in intestinal epithelial cell function and inflammatory bowel disease. Gastroenterol Res Pract. 2015;2015:328791. [PubMed] |
| 21. | Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 843] [Cited by in RCA: 843] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 22. | Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277-2293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1149] [Cited by in RCA: 1270] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 23. | Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24-32. [PubMed] |
| 24. | Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071-3084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 615] [Article Influence: 30.8] [Reference Citation Analysis (1)] |
| 25. | Kaser A, Blumberg RS. Endoplasmic reticulum stress in the intestinal epithelium and inflammatory bowel disease. Semin Immunol. 2009;21:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Bae J, Prabhala R, Voskertchian A, Brown A, Maguire C, Richardson P, Dranoff G, Anderson KC, Munshi NC. A multiepitope of XBP1, CD138 and CS1 peptides induces myeloma-specific cytotoxic T lymphocytes in T cells of smoldering myeloma patients. Leukemia. 2015;29:218-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Staege MS, Hansen G, Baersch G, Burdach S. Functional and molecular characterization of interleukin-2 transgenic Ewing tumor cells for in vivo immunotherapy. Pediatr Blood Cancer. 2004;43:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Rice J, Dossett ML, Ohlén C, Buchan SL, Kendall TJ, Dunn SN, Stevenson FK, Greenberg PD. DNA fusion gene vaccination mobilizes effective anti-leukemic cytotoxic T lymphocytes from a tolerized repertoire. Eur J Immunol. 2008;38:2118-2130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. 2015;116:1022-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 563] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 30. | Siddique I, Khan I. Mechanism of regulation of Na-H exchanger in inflammatory bowel disease: role of TLR-4 signaling mechanism. Dig Dis Sci. 2011;56:1656-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Kanai T, Kawamura T, Dohi T, Makita S, Nemoto Y, Totsuka T, Watanabe M. TH1/TH2-mediated colitis induced by adoptive transfer of CD4+CD45RBhigh T lymphocytes into nude mice. Inflamm Bowel Dis. 2006;12:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Tréton X, Pédruzzi E, Cazals-Hatem D, Grodet A, Panis Y, Groyer A, Moreau R, Bouhnik Y, Daniel F, Ogier-Denis E. Altered endoplasmic reticulum stress affects translation in inactive colon tissue from patients with ulcerative colitis. Gastroenterology. 2011;141:1024-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Olsen T, Rismo R, Cui G, Goll R, Christiansen I, Florholmen J. TH1 and TH17 interactions in untreated inflamed mucosa of inflammatory bowel disease, and their potential to mediate the inflammation. Cytokine. 2011;56:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 34. | Dong C. Differentiation and function of pro-inflammatory Th17 cells. Microbes Infect. 2009;11:584-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Zhang X, Yu P, Wang Y, Jiang W, Shen F, Wang Y, Tu H, Yang X, Shi R, Zhang H. Genetic polymorphisms of interleukin 17A and interleukin 17F and their association with inflammatory bowel disease in a Chinese Han population. Inflamm Res. 2013;62:743-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Orbell JH, Toth E S- Editor: Yu J L- Editor: Filipodia E- Editor: Liu XM