Published online Feb 21, 2016. doi: 10.3748/wjg.v22.i7.2294
Peer-review started: August 3, 2015
First decision: September 9, 2013
Revised: October 8, 2015
Accepted: December 19, 2015
Article in press: December 19, 2015
Published online: February 21, 2016
Processing time: 181 Days and 23.3 Hours
Hepatitis B virus (HBV) infection has received increasing public attention. HBV is the prototypical member of hepadnaviruses, which naturally infect only humans and great apes and induce the acute and persistent chronic infection of hepatocytes. A large body of evidence has demonstrated that dysfunction of the host anti-viral immune response is responsible for persistent HBV replication, unresolved inflammation and disease progression. Many regulatory factors are involved in immune dysfunction. Among these, T cell immunoglobulin domain and mucin domain-3 (Tim-3), one of the immune checkpoint proteins, has attracted increasing attention due to its critical role in regulating both adaptive and innate immune cells. In chronic HBV infection, Tim-3 expression is elevated in many types of immune cells, such as T helper cells, cytotoxic T lymphocytes, dendritic cells, macrophages and natural killer cells. Tim-3 over-expression is often accompanied by impaired function of the above-mentioned immunocytes, and Tim-3 inhibition can at least partially rescue impaired immune function and thus promote viral clearance. A better understanding of the regulatory role of Tim-3 in host immunity during HBV infection will shed new light on the mechanisms of HBV-related liver disease and suggest new therapeutic methods for intervention.
Core tip: Here, we discuss the current knowledge of the interaction between hepatitis B virus (HBV) and host immunity, addressing the important role of T cell immunoglobulin domain and mucin domain-3 (Tim-3) in HBV infection. Tim-3 expression on both adaptive and innate immune cells is elevated in HBV infection. Increasing Tim-3 expression inhibits, and blocking Tim-3 expression rescues, the anti-viral immune response, indicating that Tim-3 is a potential target for controlling HBV infection. Finally, we describe remaining unsolved problems in this field and analyze the potential of Tim-3 as a novel drug target in the treatment of HBV-related liver diseases.
- Citation: Liu Y, Gao LF, Liang XH, Ma CH. Role of Tim-3 in hepatitis B virus infection: An overview. World J Gastroenterol 2016; 22(7): 2294-2303
- URL: https://www.wjgnet.com/1007-9327/full/v22/i7/2294.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i7.2294
Hepatitis B virus (HBV) infection is a well-known and increasingly severe public health problem worldwide. According to epidemiological data, the number of people with resolved or present HBV infection has reached an alarming 2 billion[1,2]. For most patients with chronic HBV infection, the present drug treatment is incapable of thoroughly eliminating the virus, owing to HBV DNA integration into the host genome and the formation of covalently closed circular DNA structures[3]. Furthermore, many patients are threatened by a lifetime 15%-40% risk of developing HBV-related cirrhosis, liver failure and hepatocellular carcinoma (HCC)[1,2]. The HBV genome contains 3200 bp and forms a relaxed circular, partially double-stranded structure[4]. The HBV genome contains 4 compact overlapping open reading frames encoding different viral proteins: preS/S, preCore/Core, polymerase (pol) and X. Because of multiple alternative start codons, surface proteins exist in 3 forms, termed small, medium and large surface proteins, which are needed for virion assembly. The core protein forms the viral nucleocapsid and has a secreted counterpart termed e antigen. The polymerase is a multi-functional enzyme that serves as a DNA-dependent DNA polymerase, reverse transcriptase and RNase H. X is the smallest gene of HBV; this gene is composed of 452 nucleotides and encodes a 17-kDa protein[5]. A large body of evidence has demonstrated that HBV can cooperate with other etiological factors and then trigger tumorigenesis and the development of HCC. Thus, suppression of HBV DNA replication and the clearance of viral products are the main goals of HBV treatment.
Considerable evidence has shown that host immunity is responsible for the control of HBV infection and is the primary determinant of HBV disease progression. Impaired function of adaptive immunocytes, particularly HBV-specific CD8+ T cells, is considered to be the primary cause of widespread viral infection. HBV tends to stimulate an immunosuppressive environment that is beneficial for its survival. For example, HBV infection increases the number of regulatory T cells (Tregs), which repress effector T cell activity[6]. However, impairments in the adaptive immune response cannot explain all events that occur during HBV infection, because various components of the innate immune system also participate in disease progression. Indeed, the activation of dendritic cells (DCs), natural killer cells (NKs) and macrophages during acute infection leads to a bona fide clinical outcome, whereas persistent HBV infection at least partly results from dysregulation of the innate immune response at early stages of infection[7]. Therefore, studying the interaction between HBV and host immunity and uncovering the reason why the immune response is dysregulated in HBV infection are critical.
Innate and adaptive immunocyte activation is regulated by a set of inhibitory surface receptor-ligand pairs, or immune checkpoints. Among these pairs, T cell immunoglobulin domain and mucin domain-3 (Tim-3) and its matched ligand are currently attracting increasing attention because of their demonstrated potential as a target for immunotherapy for infectious diseases and cancers. Although Tim-3 was first identified as a surface molecule specifically expressed on CD4+ T helper 1 (Th1) and CD8+ type 1 (Tc1) cells[8], further studies have revealed that Tim-3 is also expressed on many other cell types undergoing dynamic changes during infection. In the resting state, Tim-3 is expressed on only a very small percentage of CD4+ or CD8+ T cells, and its over-expression may indicate T cell exhaustion and represent a pathological immune state[9]. However, innate immune cells including monocytes, macrophages and DCs show constitutive and high-level Tim-3 expression that can be further elevated in some diseases. Tim-3 is the prototypical member of the Tim family, which includes 8 members (Tim-1- Tim-8) in mice and 3 members in humans (Tim-1, -3, -4). Tim family members share a similar molecular structure consisting of 4 parts: an N-terminal IgV domain, a mucin domain, a transmembrane domain and a cytoplasmic tail[9]. Galectin-9 (Gal-9), a widely expressed S-type lectin, was the first identified ligand for Tim-3. The interaction of Tim-3 with Gal-9 leads to apoptosis of Th1 cells and inhibition of Th1 and Tc1 cell-mediated immunity[10]. Emerging evidence has shown that additional Tim-3 ligands exist, including phosphatidylserine, carbohydrate moieties and the alarmin high-mobility group box 1[11]. Carcinoembryonic antigen cell adhesion molecule 1 (CEACAM1), another membrane molecule that inhibits T cell activation, is a newly identified ligand for Tim-3. Binding of Tim-3 and CEACAM1 appears to be necessary for the T cell inhibiting function of Tim-3, and this interaction has a crucial role in regulating anti-tumour immunity[12]. Thus, the interactions of Tim-3 with its ligands play important roles in different immune-related diseases by regulating both innate and adaptive immunity. Although Tim-3 has gained public attention as an inhibitory immune regulator, its role in regulating the host immune response is complicated and remains controversial in several fields, particularly in infectious diseases.
This review will briefly describe the function of Tim-3 in regulating immunity, which has become a hot topic of research in recent years. In addition, this review will discuss in detail the important role of Tim-3 in HBV infection, because studies in this field are currently lacking.
T cell exhaustion, which is characterized as low proliferative ability, decreased cytokine production and suppressed cytotoxicity, often occurs in individuals with chronic viral infections including HBV, hepatitis C virus (HCV) and human immunodeficiency virus (HIV). T cell exhaustion has been detected in both animal models and clinical patients. In particular, dysfunction of antigen-specific CD8+ T cells is believed to be one of the most important reasons why viruses such as HBV, HCV and HIV escape from the anti-viral immune response. One of the key characteristics of exhausted T cells is the combined over-expression of several inhibitory surface markers, such as programmed death 1 receptor(PD-1), lymphocyte-activation gene 3 (LAG-3) and Tim-3[13,14]. Although PD-1 has been extensively studied, Tim-3 has attracted increasing attention in recent years. However, the role of Tim-3 in infectious diseases remains unclear.
The identification of Tim-3 as a negative regulator of the anti-viral adaptive immune response was first reported in chronic HIV infection. The numbers of Tim-3+CD8+ T and Tim-3+CD4+ T cells are increased in patients infected with HIV[15,16], and compared with Tim-3- cells, Tim-3+CD4+ and Tim-3+CD8+ T cells show impaired functions. Furthermore, blocking Tim-3 with a neutralizing antibody rescues the anti-viral immune response to a certain extent[15].
In 2009, our laboratory was the first to demonstrate the crucial role of Tim-3 in inhibiting hepatic CD8+ T cells in HBV infection. In a mouse model with hydrodynamic injection of HBV-bearing plasmids, augmented Tim-3 expression was detected on hepatic CD8+ T cells which displayed decreased interferon (IFN)-γ production. Furthermore, Tim-3 silencing enhanced IFN-γ production and even indirectly affected HBV neutralizing antibody production, suggesting the potential role of Tim-3 in the host antiviral immune response against HBV infection[17].
Two years later, Wu et al[18] studied the relationship between Tim-3 expression on peripheral T cell subsets and disease progression in patients with chronic hepatitis B (CHB). They found that Tim-3 expression is increased on CD4+ and CD8+ T cells, and its expression is associated with the severity of CHB. Tim-3 expression may also indicate the severity of liver injury because its expression has been found to be markedly and positively correlated with alanine aminotransferase (ALT), aspartate aminotransferase (AST), the international normalized ratio (INR) and total bilirubin (TB). After control of CHB infection, Tim-3 expression decreases. Moreover, the percentage of Tim-3+ T cells is negatively correlated with plasma IFN-γ and T-bet mRNA levels, indicating that high levels of Tim-3 expression inhibit T cell activity[18].
In 2012, the same group investigated the immunocompetence of Tim-3+CD8+ and Tim-3-CD8+ T cells and the effects of Tim-3 expression on lymphocyte proliferation and cytokine secretary capacity. Compared with Tim-3-CD8+ cells, Tim-3+CD8+ T cells show a lower capacity to proliferate and produce cytokines upon antigen challenge. Interference of the Tim-3 pathway by either anti-Tim-3 antibodies or Tim-3 short hairpin RNAs rescues CD8+ T cell activity, improving their proliferation and enhancing their cytokine secretary capacity, which suggests the potential of Tim-3 to become a new drug target for controlling HBV infection[19].
Similar results have been observed in HCV infection. During chronic HCV infection, Tim-3 expression on CD4+ and CD8+ T cells is elevated, and these Tim-3+ T cells exhibit a CD127lowCD57high and CD45RA-CCRhigh phenotypes, indicating the impaired function of these effector cells. Accordingly, blockade of Tim-3 expression enhances cell proliferation and promotes cytokine production[20]. The level of Tim-3 expression on CD4+CD25+ T cells is negatively correlated with the proliferative capacity of effector T cells, and blocking Tim-3 results in the rapid expansion of these cells[21]. This finding has been further confirmed in studies with HCV vaccination. Upon stimulation with live-attenuated HCV vaccine, effector T cells isolated from patients infected with HCV display a diminished anti-viral response compared to those isolated from healthy individuals. Furthermore, Tim-3 blockade substantially rescues the impaired function of Tim-3+ T cells, indicating that Tim-3 is responsible for the peripheral tolerance during persistent HCV infection. Of note, the recovery of T cell function after Tim-3 blockade may at least partially result from an enhanced antigen presentation ability of DCs because researchers have also found that Tim-3 may inhibit DC maturation during chronic HCV infection[22].
The co-expression of Tim-3 and other inhibitory regulators such as PD-1 in HBV and HCV infections is also important. Studies have shown that PD-1+ Tim-3+ T cells are abundant among the central memory T cell subsets, particularly in the liver, during chronic HCV infection. Moreover, compared to HCV mono-infection, the percentage of PD-1+Tim-3+ T cells is much higher in patients co-infected with HIV and HCV. Co-expression of PD-1 and Tim-3 on HCV-specific T cells may also reflect liver disease progression. Although the function of PD-1 and Tim-3 co-expression remains unclear, researchers have hypothesized that co-expression of various inhibitors may increase the risk of persistent and refractory virus infection[23].
Although Tim-3 has been well recognized as a marker of T cell exhaustion and a negative regulator of adaptive immunity in chronic virus infection, its roles in acute viral infection and bacterial infection are different. Elevated Tim-3 expression is observed on T cells in the early stage of acute hepatitis B (AHB); however, this elevation is transient and quickly reversed at the convalescence stage. Moreover, unlike CHB, the level of Tim-3 expression is not correlated with either different stages of hepatic injury or serum IFN-γ levels in patients with AHB[19]. In active TB infection, Tim-3 is up-regulated on both CD4+ and CD8+ T cells; however, in contrast to chronic virus infection, Tim-3+ effector T cells show more active anti-TB responses. One possible explanation for this finding is that Tim-3 is not only a marker of T cell exhaustion but also a marker of T cell differentiation, because CD127 is also expressed on these cells, indicating the complicated roles of Tim-3 in different microenvironments[24]. Similar results have been observed in mice infected with Listeria monocytogenes. Tim-3 expression is induced in cytotoxic T cells in infected wild-type mice, and the host exhibits a much stronger immune response compared to that in Tim-3-knockout mice[25]. Because other members in the Tim family may substitute for Tim-3 in Tim-3-deficient mice, the hypothesis that Tim-3 may play an important positive role in the adaptive immune response should be considered.
Tregs, defined as CD4+CD25+FOXP3+ regulatory T cells, can repress the functions of other immune cells via cell-to-cell contact and secretion of immunosuppressive cytokines such as transforming growth factor (TGF)-β and interleukin (IL)-10[26]. Th17 cells are a new subtype of CD4+ T cells that secrete the cytokine IL-17[27]. Accumulating data support the negative roles of these 2 cell types in chronic viral infections including HBV. Indeed, the proportion and absolute numbers of both cell types are increased in both peripheral blood mononuclear cells and liver tissues in patients with CHB[28-32]. The number of circulating Th17 cells is also positively associated with the levels of liver injury markers[33]. Moreover, the differentiation pathways of Th17 cells and Tregs are controlled by similar cytokines, and the Treg/Th17 ratio is strongly associated with HBV load. Furthermore, imbalance of the Treg/Th17 ratio is involved in HBV-related diseases and may become a novel drug target in the future[34,35]. HBV has also been reported to enhance the function of Tregs. In particular, co-culture of T cells with HepG2.2.15 cells, a hepatoma cell line stably integrated with the HBV genome, promotes Treg development and induces the expression of Treg-related genes[36].
The Tim-3/Gal-9 interaction is known to be involved in Treg function. Accordingly, blocking the Tim-3-Gal-9 pathway results in an obvious decrease in the suppressive activity of Tregs in vitro[37,38]. In mice, Tim-3 is constitutively expressed on natural Treg cells. In contrast, Tim-3 is not expressed on human Treg cells ex vivo but is up-regulated after activation. Tim-3+ Treg cells also display increased expression of other inhibitory receptors including LAG-3, cytotoxic T-lymphocyte antigen 4 (CTLA-4), glucocorticoid-induced TNF receptor and PD-1[37]. In addition, Tim-3 has been identified as a marker of Tregs in tumors[39].
Tim-3 is over-expressed in Tregs in patients chronically infected with HCV. Tim-3+ Tregs tend to resist apoptotic signals and show a higher capacity to proliferate, leading to Treg accumulation. Moreover, Ji et al[40] have reported that HCV infection leads to elevated Gal-9 and TGF-β production in hepatocytes. Co-culture of HCV-infected hepatocytes and CD4+ T cells induces increased Tim-3 expression on CD4+ T cells, and the Tim-3/Gal-9 interaction enhances TGF-β/IL-10 production by CD4+ T cells, which accelerates the differentiation of CD4+ T cells into Tregs[40]. However, the regulatory effects of Tim-3 on Tregs in HBV infection remain to be clarified.
Tim-3 has also been reported to be involved in regulating Th17 cells. Tim-3 appears to suppress the activation and cytokine secretion of Th17 cells, and Tim-3 expression is impaired in many auto-immune diseases such as Guillain-Barré syndrome and psoriasis[41,42]. However, few studies have focused on the expression pattern of Tim-3 on Th17 cells during persistent HBV or HCV infection, which highlights significant gaps in this research field.
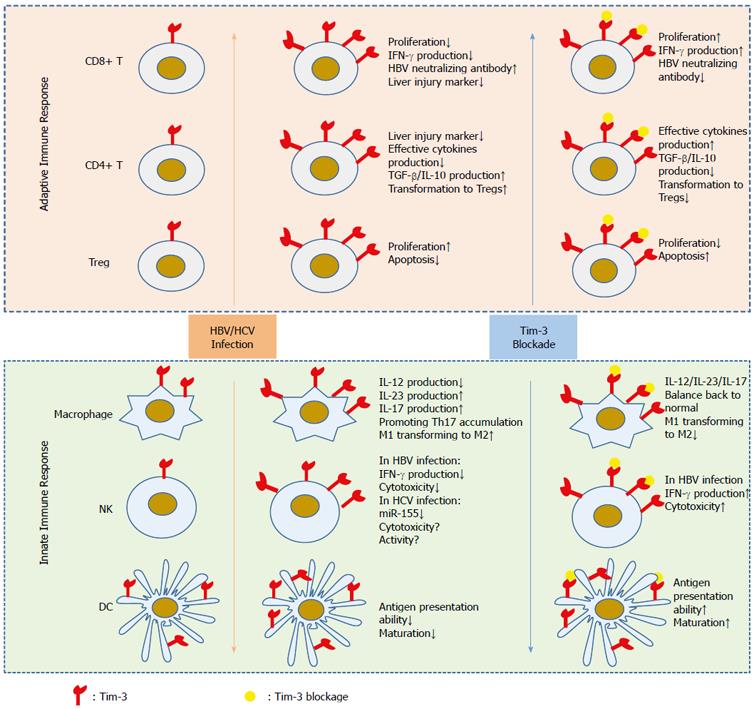
In summary, during chronic HBV infection, Tim-3 is induced in adaptive immune cells and represses host anti-viral immunity. Furthermore, blocking Tim-3 seems to be beneficial for controlling viral activity and may become a future therapeutic approach for treating CHB (Figure 1).
Because of the key role of adaptive immunity, the role of innate immunity in HBV infection has largely been ignored in previous studies. However, increased understanding of pathogen-associated molecular patterns and signaling pathways that control the activation of innate immunity has highlighted the importance of the innate immune system in HBV-related diseases[7]. A large body of evidence has proved that Tim-3 regulates innate immune response. Unlike adaptive T cells, innate immune cells such as monocytes/macrophages, NKs and NK T cells (NKTs) constitutively express Tim-3, which can be further elevated in some diseases including chronic viral infection. Interference of Tim-3 pathway changes the function of innate immune cells[43,44].
Monocytes and macrophages are important components of the innate immune system. Upon stimulation with inflammatory signals, monocytes rapidly infiltrate sites of infection and then differentiate into macrophages that kill pathogens. In HBV infection, macrophages are crucial in modulating chronic liver injury and HBV clearance. Macrophages can be classified into 2 types: M1 and M2. M1 cells contribute to HBV clearance, whereas M2 cells impair the host immune response, promote HBV infection and accelerate tumorigenesis[45,46]. In addition, HBV tends to promote M2 polarization[6,7]. Several studies have reported that pathogens can induce Tim-3 over-expression in macrophages and monocytes, which may regulate the activation and cytokine production of these cells[47]. Moreover, lipopolysaccharide, a Toll-like receptor (TLR) ligand, can repress Tim-3 expression on macrophages and at least partially rescue their function, suggesting that TLRs and their downstream pathways may be involved in the regulation of Tim-3 expression[48]. Of note, the role of Tim-3 in natural immunity is much more complex than its role in adaptive immunity. Recently, our laboratory has found that Tim-3 regulate the polarization of macrophages and accelerate the transformation of M1 macrophages into M2 macrophages, which then suppress the inflammatory response in HCC[44]. Together, these data indicate the complicated role of Tim-3 in regulating innate immune cells.
Tim-3 expression has been reported to be strongly elevated on monocytes in patients with CHB and further elevated in those patients with acute-on-chronic liver failure (ACLF). Tim-3 expression on monocytes is also positively associated with the level of ALT in patients with CHB, indicating its role in disease progression[49]. Concordantly, Tim-3 expression is increased on monocytes from patients with HCV infection and is positively correlated with IL-17 levels in CD4+ T cells, thus promoting Th17 cell accumulation. However, blocking Tim-3 on monocytes restores the balance of IL-12, IL-23 and IL-17 signaling via the STAT3 pathway[50,51].
As the most potent antigen-presenting cells (APCs), DCs play a critical role in the innate immune response and greatly affect CHB progression. In particular, DCs control HBV recognition in vivo. Accumulating evidence has demonstrated that compared to DCs from healthy controls, DCs from patients infected with HBV exhibit impaired function[52,53], yet the reasons for this impairment remain unclear. Researchers have focused on developing a curative DC vaccine that activates the immune response through rescuing the function of DCs to treat HBV.
Constitutive expression of Tim-3 can be observed on DCs and may be positively associated with DC activation. Accordingly, stimulating Tim-3 with Gal-9 promotes TNF-α synthesis and secretion in cultured DCs[54]. Furthermore, using Gal-9 to activate Tim-3 signaling in tumor-bearing animal models can enhance the number of mature DCs and aid in the anti-tumor immune response[55]. However, some researchers have obtained opposing results, suggesting that Tim-3 might also be a negative regulator of DCs[56]. Compared with Tim-3-deficient bone marrow-derived DCs (BMDCs), Tim-3+ BMDCs exhibit an impaired function phenotype. In particular, the latter cells showed a much poorer capacity to produce cytokines such as IFN-β1, IFN-α and IL-6[57]. The regulatory effect of Tim-3 on DCs is intermittent; many other factors, such as different pathogens and different ligands of Tim-3, can modulate the regulatory effect of Tim-3 on DCs.
In 2014, Ma et al[22] first engineered a live-attenuated HCV vaccine to stimulate immune cells. These researchers found that DCs isolated from healthy individuals demonstrated enhanced antigen presentation ability after stimulation, whereas DCs isolated from patients infected with HCV showed diminished responses after stimulation. Furthermore, blocking Tim-3 substantially rescued the function of DCs, indicating that Tim-3 may inhibit DC maturation. The effect of Tim-3 on DCs in HBV infection remains to be clarified; however, these findings observed in HCV infection may provide some clues[22].
Over half of all liver lymphocytes are innate immune lymphocytes, and NKs account for the majority of these cells. Moreover, the percentage of NKs in the liver is approximately five-fold greater than that in spleen or blood, further indicating their important roles in liver diseases. Indeed, accumulating evidence has indicated the critical roles of NK cells in HBV-related diseases. The number and activity of circulating NKs are remarkably decreased in patients with CHB[58-60], and impaired NK function leads to persistent HBV infection and HCC tumorigenesis[61].
Tim-3 also appears to have conflicting effects on NKs. In some reports, the Tim-3-Gal-9 interaction has been shown to enhance IFN-γ production and Tim-3 has been described as a marker of fully mature NKs[62], whereas in other reports, for example, during HIV infection, Tim-3 has been found to inhibit the function of NKs, weakening the NK cell-mediated anti-viral immune response[63,64].
Our group first revealed the regulatory role of Tim-3 expression on NK cells in HBV infection. Increased Tim-3 expression on NK cells was detected in patients with CHB and in HBV-transgenic mice. In addition, Tim-3 expression on NK cells was positively correlated with the serum ALT levels in patients with CHB. Blockade of the Tim-3/Gal-9 interaction induced increased cytotoxicity and up-regulated IFN-γ production in both NKs from patients with CHB and in NK92 cell lines, strongly suggesting that Tim-3 plays negative roles in NKs during HBV[43]. However, the role of Tim-3 appears to be more complicated in HCV infection. Some studies have demonstrated that Tim-3 expression is elevated in NKs and that Tim-3 over-expression tends to result from down-regulated miR-155 in NKs during HCV infection, similarly to chronic HBV infection. Blocking Tim-3 can rescue the function of NKs, whereas reconstituting miR-155 can down-regulate Tim-3[65]. However, a recent study has reported the opposite results. Golden-Mason et al[66] have analyzed Tim-3 expression on NKs, demonstrating not only elevated expression of Tim-3 on these cells but also a positive correlation between Tim-3 and NK activity. These Tim-3+ NKs also showed a stronger response to IFN-α stimulation and exhibited more intense killing activity[66]. Thus, the role of Tim-3 on NKs requires additional studies.
NKT-like cells, defined as CD3+CD16+CD56+ cells, refer to a small population of T cells that co-express NK markers, for example, NK1.1 and CD56. If activated, these cells produce abundant pro-inflammatory cytokines and anti-inflammatory cytokines, including IFN-γ, MCP-1, and IL-4. Similarly to its expression on monocytes and NKs, Tim-3 over-expression is also observed on NKT-like cells and is positively associated with the level of ALT in patients with CHB. Moreover, ACLF may further elevate Tim-3 expression[49].
Above all, elevated Tim-3 expression is observed in innate immunocytes and exerts a suppressive effect on their function during HBV infection (Figure 1). However, the role of Tim-3 in innate immune cells is complicated and requires further study.
In 2012, Chinese scientists examined polymorphisms of the Tim-3 gene in a population of 712 individuals. Among these individuals, 182 represented healthy controls, and the others were patients with HBV-related liver diseases. The Tim-3-1541C/T, -1516G/T, -882C/T, -574G/T and +4259T/G polymorphisms were examined and analyzed, and the results showed that allele T-containing genotypes (GT+ TT), allele T and the allele T-containing haplotype (CTCGT) of the -1516G/T polymorphism occur more often in patients with CHB. The allele T-containing genotypes and allele T of -1516G/T are also associated with lymph node metastasis and tumor grade of HCC[67]. Other researchers have identified 2 other single nucleotide polymorphisms, rs31223 and rs246871, which correlate with the progression of HBV-induced liver disease. The minor allele “C” in rs31223 represents an increased chance of sero-clearance of HBsAg, whereas the genotype “CC” in rs246871 suggests an increased likelihood of developing HBV-related HCC. Furthermore, the haplotype blocks CGC* and TGC* strongly correlate with serum HBsAg sero-clearance, whereas CAT*, CGT*, TAC* and TGT* tend to be markedly correlated with HBV-induced HCC[68]. In accordance with their containment functions in negatively regulating immunity, polymorphisms of Tim-3 and PD-1 may differentially and interactively predispose individuals to HBV-related liver disease progression. The combined carriage of PD1+8669 AA/TIM3 -1516 GT or TT shows a higher frequency in patients with cirrhosis than in patients without cirrhosis. Patients with HCC also have a higher frequency of this combined carriage than do patients with cirrhosis[69]. Together, these findings suggest that Tim-3 polymorphisms may affect disease susceptibility and HCC traits associated with HBV infection.
The relationship between tumors and Tim-3 has been studied for many years, and Tim-3 may become the next major target in the treatment of cancer. In several cancer models, including breast cancer, colon cancer and melanoma, Tim-3 is induced in tumor-infiltrated lymphocytes and appears to mark exhausted CD8+ T cells because PD-1+Tim-3-CD8+ cells remain able to produce bona fide cytokines such as IFN-γ, IL-2 and TNF-α[70]. Here, we focus on the role of Tim-3 in liver cancer and discuss whether it can be regarded as a novel drug target in the treatment of liver cancer.
Chronic HBV infection is one of the most important risk factors for HCC, accounting for up to 54% of HCC cases worldwide, and this percentage is even higher in China. Accumulating evidence has supported the hypothesis that Tim-3 plays roles in HCC, particularly by modulating the tumor microenvironment. For instance, Tim-3 expression is elevated on CD4+ and CD8+ T cells infiltrating tumor tissues compared to those cells infiltrating the adjacent tissues, and Tim-3+ T cells exhibit a senescence phenotype. Furthermore, the number of Tim-3+ tumor-infiltrating cells is negatively correlated with patient survival, and Tim-3/Gal-9 signaling induces T cell senescence. Kupffer cells (KCs) have the highest Gal-9 expression, and Tim-3+ T cells and Gal-9+ KCs show a co-localization pattern in HCC. Blocking Tim-3/Gal-9 signaling re-activates tumor-infiltrating T cells, which display increased T cell proliferation and enhanced cytokine production. Moreover, in the HCC microenvironment, IFN-γ secreted by tumor-infiltrating T cells stimulates Gal-9 expression on APCs[71].
Tumor-associated macrophages (TAMs) are a major component of the tumor microenvironment and play a critical role in promoting tumor progression. Recently, our laboratory has discovered the important role of Tim-3 in TAM polarization in HCC. Specifically, elevated Tim-3 expression is negatively associated with tumor grade and patient survival. Moreover, ectopic expression of Tim-3 induces altered M2 activation, with a phenotype that promotes tumor development[44]. Our results further emphasize the critical role of Tim-3 as a new component in HCC progression.
In fact, Tim-3 is not expressed only in immune cells; our in vivo and in vitro experiments both demonstrated that Tim-3 is also expressed in HCC cells and that Tim-3 serves as an oncoprotein in these cells (unpublished data). These new data suggest that Tim-3 may have other functions in addition to immune inhibition.
HCC remains refractory to current chemotherapeutic drugs and generally is associated with a poor prognosis. Moreover, many patients with HCC cannot receive local ablative or surgical interventions because HCC is frequently diagnosed at an advanced stage. Recently, cancer immunotherapy has attracted substantial attention, and immune checkpoint blockade has achieved great success in many clinical trials. Although blockade of CTLA-4 and PD-1 has shown objective responses in several cancers, some issues still remain. For example, some patients have been shown to be non-responders to immunotherapies that target CTLA-4 and PD-1 in various clinical trials[72,73]. In this context, the discovery of novel immune checkpoints is urgently required, and Tim-3 is a potential candidate. In 2010, Sakuishi reported that combined inhibition of Tim-3 and PD-1 demonstrated greater inhibition of tumor growth than PD-1 inhibition alone. Specifically, half of all tumor-bearing mice treated with combined blockade of Tim-3 and PD-1 displayed complete tumor regression, suggesting the crucial role of Tim-3 in tumor progression[74]. However, additional studies are required to demonstrate the therapeutic role of Tim-3 blockade in HCC treatment.
During HBV infection, Tim-3 expression is elevated on both adaptive and innate immune cells. This increased Tim-3 expression inhibits the anti-viral immune response, indicating that Tim-3 is a potential target for controlling HBV infection (Figure 1). However, several critical questions remain to be clarified. For example, how does HBV infection induce ectopic Tim-3 expression in different types of immune cells? What are the downstream signaling pathways promoted by Tim-3 in both adaptive and innate immune cells? Moreover, the regulatory role of Tim-3 in the innate immune response remains unclear, and Tim-3 may possess dual functions depending on the specific context. Therefore, we cannot be blindly optimistic to the potential of Tim-3 as a drug target for controlling chronic infection and HCC.
| 1. | Chen CJ, Yang HI. Natural history of chronic hepatitis B REVEALed. J Gastroenterol Hepatol. 2011;26:628-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 230] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 2. | Fattovich G, Olivari N, Pasino M, D’Onofrio M, Martone E, Donato F. Long-term outcome of chronic hepatitis B in Caucasian patients: mortality after 25 years. Gut. 2008;57:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Sung WK, Zheng H, Li S, Chen R, Liu X, Li Y, Lee NP, Lee WH, Ariyaratne PN, Tennakoon C. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat Genet. 2012;44:765-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 745] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 4. | Summers J, Mason WS. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982;29:403-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1118] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 5. | Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64:51-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1094] [Cited by in RCA: 1121] [Article Influence: 43.1] [Reference Citation Analysis (2)] |
| 6. | Loggi E, Gamal N, Bihl F, Bernardi M, Andreone P. Adaptive response in hepatitis B virus infection. J Viral Hepat. 2014;21:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Busca A, Kumar A. Innate immune responses in hepatitis B virus (HBV) infection. Virol J. 2014;11:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Sabatos CA, Chakravarti S, Cha E, Schubart A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4:1102-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 532] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 9. | Zhu C, Anderson AC, Kuchroo VK. TIM-3 and its regulatory role in immune responses. Curr Top Microbiol Immunol. 2011;350:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1334] [Cited by in RCA: 1638] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 11. | Dolina JS, Braciale TJ, Hahn YS. Liver-primed CD8+ T cells suppress antiviral adaptive immunity through galectin-9-independent T-cell immunoglobulin and mucin 3 engagement of high-mobility group box 1 in mice. Hepatology. 2014;59:1351-1365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 574] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 13. | Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 814] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 14. | Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2481] [Cited by in RCA: 3192] [Article Influence: 212.8] [Reference Citation Analysis (1)] |
| 15. | Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763-2779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 545] [Cited by in RCA: 649] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 16. | Kassu A, Marcus RA, D’Souza MB, Kelly-McKnight EA, Golden-Mason L, Akkina R, Fontenot AP, Wilson CC, Palmer BE. Regulation of virus-specific CD4+ T cell function by multiple costimulatory receptors during chronic HIV infection. J Immunol. 2010;185:3007-3018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Ju Y, Hou N, Zhang XN, Zhao D, Liu Y, Wang JJ, Luan F, Shi W, Zhu FL, Sun WS. Blockade of Tim-3 pathway ameliorates interferon-gamma production from hepatic CD8+ T cells in a mouse model of hepatitis B virus infection. Cell Mol Immunol. 2009;6:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Wu W, Shi Y, Li S, Zhang Y, Liu Y, Wu Y, Chen Z. Blockade of Tim-3 signaling restores the virus-specific CD8+ T-cell response in patients with chronic hepatitis B. Eur J Immunol. 2012;42:1180-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Wu W, Shi Y, Li J, Chen F, Chen Z, Zheng M. Tim-3 expression on peripheral T cell subsets correlates with disease progression in hepatitis B infection. Virol J. 2011;8:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122-9130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 381] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 21. | Moorman JP, Wang JM, Zhang Y, Ji XJ, Ma CJ, Wu XY, Jia ZS, Wang KS, Yao ZQ. Tim-3 pathway controls regulatory and effector T cell balance during hepatitis C virus infection. J Immunol. 2012;189:755-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Ma CJ, Ren JP, Li GY, Wu XY, Brockstedt DG, Lauer P, Moorman JP, Yao ZQ. Enhanced virus-specific CD8+ T cell responses by Listeria monocytogenes-infected dendritic cells in the context of Tim-3 blockade. PLoS One. 2014;9:e87821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120:4546-4557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 24. | Qiu Y, Chen J, Liao H, Zhang Y, Wang H, Li S, Luo Y, Fang D, Li G, Zhou B. Tim-3-expressing CD4+ and CD8+ T cells in human tuberculosis (TB) exhibit polarized effector memory phenotypes and stronger anti-TB effector functions. PLoS Pathog. 2012;8:e1002984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Gorman JV, Starbeck-Miller G, Pham NL, Traver GL, Rothman PB, Harty JT, Colgan JD. Tim-3 directly enhances CD8 T cell responses to acute Listeria monocytogenes infection. J Immunol. 2014;192:3133-3142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Barbi J, Pardoll D, Pan F. Treg functional stability and its responsiveness to the microenvironment. Immunol Rev. 2014;259:115-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 27. | Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 438] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 28. | Yan J, Liu XL, Xiao G, Li NL, Deng YN, Han LZ, Yin LC, Ling LJ, Liu LX. Prevalence and clinical relevance of T-helper cells, Th17 and Th1, in hepatitis B virus-related hepatocellular carcinoma. PLoS One. 2014;9:e96080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Wu W, Li J, Chen F, Zhu H, Peng G, Chen Z. Circulating Th17 cells frequency is associated with the disease progression in HBV infected patients. J Gastroenterol Hepatol. 2010;25:750-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Ge J, Wang K, Meng QH, Qi ZX, Meng FL, Fan YC. Implication of Th17 and Th1 cells in patients with chronic active hepatitis B. J Clin Immunol. 2010;30:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 31. | Sun HQ, Zhang JY, Zhang H, Zou ZS, Wang FS, Jia JH. Increased Th17 cells contribute to disease progression in patients with HBV-associated liver cirrhosis. J Viral Hepat. 2012;19:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG, Janssen HL. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005;41:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 412] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 33. | Zhang GL, Xie DY, Ye YN, Lin CS, Zhang XH, Zheng YB, Huang ZL, Peng L, Gao ZL. High level of IL-27 positively correlated with Th17 cells may indicate liver injury in patients infected with HBV. Liver Int. 2014;34:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Chen Y, Fang J, Chen X, Pan C, Liu X, Liu J. Effects of the Treg/Th17 cell balance and their associated cytokines in patients with hepatitis B infection. Exp Ther Med. 2015;9:573-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Yu XP, Guo RY, Su ML, Ming DS, Lin CZ, Deng Y, Lin ZZ, Su ZJ. Dynamic Changes of Treg and Th17 Cells and Related Cytokines Closely Correlate With the Virological and Biochemical Response in Chronic Hepatitis B Patients Undergoing Nucleos(t)ide Analogues Treatment. Hepat Mon. 2013;13:e15332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Zhang HH, Mei MH, Fei R, Liu F, Wang JH, Liao WJ, Qin LL, Wei L, Chen HS. Regulatory T cells in chronic hepatitis B patients affect the immunopathogenesis of hepatocellular carcinoma by suppressing the anti-tumour immune responses. J Viral Hepat. 2010;17 Suppl 1:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 37. | Gautron AS, Dominguez-Villar M, de Marcken M, Hafler DA. Enhanced suppressor function of TIM-3+ FoxP3+ regulatory T cells. Eur J Immunol. 2014;44:2703-2711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 193] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 38. | Ju Y, Shang X, Liu Z, Zhang J, Li Y, Shen Y, Liu Y, Liu C, Liu B, Xu L. The Tim-3/galectin-9 pathway involves in the homeostasis of hepatic Tregs in a mouse model of concanavalin A-induced hepatitis. Mol Immunol. 2014;58:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, Yang Q, Zhang X, Lu B. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS One. 2012;7:e30676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 329] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 40. | Ji XJ, Ma CJ, Wang JM, Wu XY, Niki T, Hirashima M, Moorman JP, Yao ZQ. HCV-infected hepatocytes drive CD4+ CD25+ Foxp3+ regulatory T-cell development through the Tim-3/Gal-9 pathway. Eur J Immunol. 2013;43:458-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Kanai Y, Satoh T, Igawa K, Yokozeki H. Impaired expression of Tim-3 on Th17 and Th1 cells in psoriasis. Acta Derm Venereol. 2012;92:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Liang SL, Wang WZ, Huang S, Wang XK, Zhang S, Wu Y. Th17 helper cell and T-cell immunoglobulin and mucin domain 3 involvement in Guillain-Barré syndrome. Immunopharmacol Immunotoxicol. 2012;34:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao D, Liu Y, Zhu F, Zhang L, Sun W. T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B. J Hepatol. 2010;52:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 44. | Yan W, Liu X, Ma H, Zhang H, Song X, Gao L, Liang X, Ma C. Tim-3 fosters HCC development by enhancing TGF-β-mediated alternative activation of macrophages. Gut. 2015;64:1593-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 260] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 45. | Dai K, Huang L, Sun X, Yang L, Gong Z. Hepatic CD206-positive macrophages express amphiregulin to promote the immunosuppressive activity of regulatory T cells in HBV infection. J Leukoc Biol. 2015;98:1071-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Bility MT, Cheng L, Zhang Z, Luan Y, Li F, Chi L, Zhang L, Tu Z, Gao Y, Fu Y. Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages. PLoS Pathog. 2014;10:e1004032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 47. | Zhang Y, Ma CJ, Wang JM, Ji XJ, Wu XY, Moorman JP, Yao ZQ. Tim-3 regulates pro- and anti-inflammatory cytokine expression in human CD14+ monocytes. J Leukoc Biol. 2012;91:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 48. | Yang X, Jiang X, Chen G, Xiao Y, Geng S, Kang C, Zhou T, Li Y, Guo X, Xiao H. T cell Ig mucin-3 promotes homeostasis of sepsis by negatively regulating the TLR response. J Immunol. 2013;190:2068-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 49. | Rong YH, Wan ZH, Song H, Li YL, Zhu B, Zang H, Zhao Y, Liu HL, Zhang AM, Xiao L. Tim-3 expression on peripheral monocytes and CD3+CD16/CD56+natural killer-like T cells in patients with chronic hepatitis B. Tissue Antigens. 2014;83:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Wang JM, Shi L, Ma CJ, Ji XJ, Ying RS, Wu XY, Wang KS, Li G, Moorman JP, Yao ZQ. Differential regulation of interleukin-12 (IL-12)/IL-23 by Tim-3 drives T(H)17 cell development during hepatitis C virus infection. J Virol. 2013;87:4372-4383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Zhang Y, Ma CJ, Wang JM, Ji XJ, Wu XY, Jia ZS, Moorman JP, Yao ZQ. Tim-3 negatively regulates IL-12 expression by monocytes in HCV infection. PLoS One. 2011;6:e19664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 52. | Op den Brouw ML, Binda RS, van Roosmalen MH, Protzer U, Janssen HL, van der Molen RG, Woltman AM. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology. 2009;126:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 186] [Article Influence: 10.3] [Reference Citation Analysis (2)] |
| 53. | Woltman AM, Op den Brouw ML, Biesta PJ, Shi CC, Janssen HL. Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function. PLoS One. 2011;6:e15324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (2)] |
| 54. | Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 590] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 55. | Nagahara K, Arikawa T, Oomizu S, Kontani K, Nobumoto A, Tateno H, Watanabe K, Niki T, Katoh S, Miyake M. Galectin-9 increases Tim-3+ dendritic cells and CD8+ T cells and enhances antitumor immunity via galectin-9-Tim-3 interactions. J Immunol. 2008;181:7660-7669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 173] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 56. | Patel J, Bozeman EN, Selvaraj P. Taming dendritic cells with TIM-3: another immunosuppressive strategy used by tumors. Immunotherapy. 2012;4:1795-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 57. | Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012;13:832-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 693] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 58. | Peppa D, Micco L, Javaid A, Kennedy PT, Schurich A, Dunn C, Pallant C, Ellis G, Khanna P, Dusheiko G. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathog. 2010;6:e1001227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 217] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 59. | Sun C, Fu B, Gao Y, Liao X, Sun R, Tian Z, Wei H. TGF-β1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog. 2012;8:e1002594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 60. | Li Y, Wang JJ, Gao S, Liu Q, Bai J, Zhao XQ, Hao YH, Ding HH, Zhu F, Yang DL. Decreased peripheral natural killer cells activity in the immune activated stage of chronic hepatitis B. PLoS One. 2014;9:e86927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 61. | Sun C, Sun H, Zhang C, Tian Z. NK cell receptor imbalance and NK cell dysfunction in HBV infection and hepatocellular carcinoma. Cell Mol Immunol. 2015;12:292-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 62. | Gleason MK, Lenvik TR, McCullar V, Felices M, O’Brien MS, Cooley SA, Verneris MR, Cichocki F, Holman CJ, Panoskaltsis-Mortari A. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012;119:3064-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 63. | Ndhlovu LC, Lopez-Vergès S, Barbour JD, Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF, Lanier LL. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119:3734-3743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 416] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 64. | Finney CA, Ayi K, Wasmuth JD, Sheth PM, Kaul R, Loutfy M, Kain KC, Serghides L. HIV infection deregulates Tim-3 expression on innate cells: combination antiretroviral therapy results in partial restoration. J Acquir Immune Defic Syndr. 2013;63:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Cheng YQ, Ren JP, Zhao J, Wang JM, Zhou Y, Li GY, Moorman JP, Yao ZQ. MicroRNA-155 regulates interferon-γ production in natural killer cells via Tim-3 signalling in chronic hepatitis C virus infection. Immunology. 2015;145:485-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 66. | Golden-Mason L, Waasdorp Hurtado CE, Cheng L, Rosen HR. Hepatitis C viral infection is associated with activated cytolytic natural killer cells expressing high levels of T cell immunoglobulin- and mucin-domain-containing molecule-3. Clin Immunol. 2015;158:114-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Li Z, Liu Z, Zhang G, Han Q, Li N, Zhu Q, Lv Y, Chen J, Xing F, Wang Y. TIM3 gene polymorphisms in patients with chronic hepatitis B virus infection: impact on disease susceptibility and hepatocellular carcinoma traits. Tissue Antigens. 2012;80:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Liao J, Zhang Q, Liao Y, Cai B, Chen J, Li L, Wang L. Association of T-cell immunoglobulin and mucin domain-containing molecule 3 (Tim-3) polymorphisms with susceptibility and disease progression of HBV infection. PLoS One. 2014;9:e98280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 69. | Li Z, Li N, Zhu Q, Zhang G, Han Q, Zhang P, Xun M, Wang Y, Zeng X, Yang C. Genetic variations of PD1 and TIM3 are differentially and interactively associated with the development of cirrhosis and HCC in patients with chronic HBV infection. Infect Genet Evol. 2013;14:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 70. | Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175-2186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 890] [Cited by in RCA: 1075] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 71. | Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, Liu J, Shi L, Liu C, Wang G. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 409] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 72. | Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2712] [Cited by in RCA: 2755] [Article Influence: 211.9] [Reference Citation Analysis (3)] |
| 73. | Melero I, Lasarte JJ. Genetic basis for clinical response to CTLA-4 blockade. N Engl J Med. 2015;372:783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 74. | Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187-2194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1279] [Cited by in RCA: 1694] [Article Influence: 105.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Garcia-Roman R, Villinger F S- Editor: Gong ZM L- Editor: A E- Editor: Liu XM