Published online Feb 21, 2016. doi: 10.3748/wjg.v22.i7.2195
Peer-review started: May 5, 2015
First decision: October 14, 2015
Revised: November 11, 2015
Accepted: December 8, 2015
Article in press: December 8, 2015
Published online: February 21, 2016
Processing time: 277 Days and 17.8 Hours
Regulatory T (Treg) cells play key roles in various immune responses. For example, Treg cells contribute to the complex pathogenesis of inflammatory bowel disease (IBD), which includes Crohn’s disease and ulcerative colitis during onset or development of that disease. Many animal models of IBD have been used to investigate factors such as pathogenic cytokines, pathogenic bacteria, and T-cell functions, including those of Treg cells. In addition, analyses of patients with IBD facilitate our understanding of the precise mechanism of IBD. This review article focuses on the role of Treg cells and outlines the pathogenesis and therapeutic strategies of IBD based on previous reports.
Core tip: We review the types and functions of regulatory CD4+ T cells (Treg cells) and describe their roles in the pathologies of the inflammatory bowel diseases, i.e., Crohn’s disease and ulcerative colitis. We have paid particular attention to the use of animal models and human studies to elucidate the mechanisms by which Treg cells influence these diseases and have provided an overview of the potential uses of these cells in therapeutic strategies.
- Citation: Yamada A, Arakaki R, Saito M, Tsunematsu T, Kudo Y, Ishimaru N. Role of regulatory T cell in the pathogenesis of inflammatory bowel disease. World J Gastroenterol 2016; 22(7): 2195-2205
- URL: https://www.wjgnet.com/1007-9327/full/v22/i7/2195.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i7.2195
Inflammatory bowel disease (IBD) is a term that encompasses two major forms of chronic inflammatory intestinal disorders: Crohn’s disease (CD) and ulcerative colitis (UC)[1-3]. Both of these chronic inflammatory diseases feature a typical time of onset during young adulthood and a lifelong course characterized by periods of remission and relapse[4,5]. The pathogenesis of IBD is well known to be complex because of various immunological, environmental, host genetic, and bacterial factors, and the complicated regulatory mechanisms associated with mucosal immunity can also influence the onset or development of IBD[6-8]. Many reports of various animal models of IBD have revealed cellular or molecular mechanisms of IBD and the recent use of anticytokine agents, such as antitumor necrosis factor-α (TNF-α), interleukin (IL)-6R, or IL-13 antibodies, has achieved progress in healing mucosal damage associated with IBD[9,10].
Regulatory T (Treg) cells are known to plays a key role in the pathogenesis of IBD, as well as in other autoimmune disorders or allergies[11-14]. Considerable evidence supports the notion that an altered balance between Foxp3+CD4+ Treg cells and T effector cells in the intestinal microenvironment might contribute to the pathogenesis of IBD[12,15]. The ability to control inflammatory lesions with transferred Treg cells has been demonstrated in several IBD models[16,17]. On the other hand, patients with IBD have been found to harbor significantly reduced numbers of peripheral Treg cells or increased serum level of soluble IL-2Rα[18,19]. Therefore, it is possible that a novel therapy involving Treg cells might effectively treat IBD.
This review will focus on the functions of Treg cells in controlling peripheral immune tolerance within the context of mucosal immunity and will explain the molecular pathogenesis of IBD and possible therapeutic strategies involving Treg cells.
The functions of mucosal immune system depend on the presence of intestinal flora[20]. In the intestinal epithelium, four cell types are found, including columnar cells, goblet cells, endocrine cells, and leukocytes to rest on a continuous basement lamina[21]. In addition, the immune cells in the lamina propria (LP), the leukocytes also involve various immune cells, such as the unique immune cell types that exist in gut- or mucosa-associated lymphoid tissues such as Peyer’s patches, mesenteric lymph nodes, and isolated lymphoid follicles. Balanced mucosal immunity in the gut is important for both immune homeostasis and defense[22-24]. Intestinal immunity is maintained by the proportions of immune cells such as dendritic cells (DCs), macrophages, effector CD4+ or CD8+ T cells, and Treg cells.
Previously reported genome-wide association studies have identified more than 100 distinct loci that confer risk or protection from IBD development although a substantial proportion of these loci are common to both diseases[6]. Many IBD-associated pathways are known to exert heterogeneous effects upon activation in different cell types, and the combination of these cellular outcomes might affect disease manifestation[8,25]. IBD-associated loci can be broadly categorized into several critical pathways such as the innate immune response, intestinal barrier, microbial defense, reactive oxygen, and antimicrobial activity[26]. For example, in humans, nucleotide-binding oligomerization domain-containing protein 2 (NOD2), which is encoded by the NOD2 gene on chromosome 16 plays an important role in the intestinal immune system where it recognizes bacterial peptides and stimulates immune reactions[27]. Mutations in NOD2 gene have been associated with CD[27]. Furthermore, NOD2-/- mice are susceptible to and demonstrate excessive intestinal inflammation when compared with control mice[28]. Treatment with NOD2 ligands, including peptide or muramyl dipeptide has been shown to ameliorate 2,4,6-trinitrobenzenesulfonic acid (TNBS)- or dextran sulfate sodium (DSS)-driven colitis in normal mice[29,30]. Treatment with Lactobacillus peptidoglycan was shown to increase the number of CD103+DCs and Foxp3+ Treg cell in mesenteric lymph nodes and IL-10 expression in the colonic mucosa in a TNBS-driven model of colitis, suggesting that NOD2 activity in the intestinal mucosa potentiates a tolerogenic environment[30].
Homing or migration-associated receptors such as CD62L, C-C chemokine receptor (CCR)7, αEβ7 integrin, α4β7 integrin, CCR4, CCR5, and CCR9 also contribute to the pathogenesis of IBD[31-37]. The expression of these receptors on Treg cells plays a key role in the intestinal immunological homeostasis and defective expression of these receptors has been shown to induce IBD as a result of the deficient migration of Treg cells into the intestine. For example, a loss of CCR7 was found to block Treg cell function in an experimental model of colitis[32].
Many animal models of IBD have been developed based on the various aspects in the pathogenesis or mechanisms of IBD[16,38]. Mice have been widely used to examine the contribution of bacteria and specific bacterial factors to the pathogenesis of IBD[9]. Studies conducted in germ-free, specific pathogen-free, and gnotobiotic mice have suggested that defined or specific microbial flora plays a fundamental role in the initiation and development of IBD[39-41]. Additional experimental colitis mouse models have been induced using chemical drugs such as DSS, TNBS, oxazolone, or acetic acid[9,42-46]. In addition, adoptive transfer models in which a T-cell deficient mouse strain is reconstituted with Treg cell-depleted naïve T cells from a congenic donor mouse have been widely used[16,38,47]. Additional genetic models, including gene knockout, transgenic, or mutant animals, have been utilized to understand the genetic and molecular pathogenesis of IBD[9,16,38]. As spontaneous models of IBD, several animal models mice and rats are known to understand the pathogenesis of IBD or investigate new therapeutic strategy for IBD[9,38].
Studies conducted in mouse models of IBD have indicated that the Treg cell compartment is particularly sensitive to microbiotal changes[48]. Treg cells from germ-free mice are generally less suppressive and express lower level of Foxp3 than do Treg cells from normal mice[49-51]. The colons of germ-free mice harbor reduced number of Treg cells, although this population was shown to increase in response to a decrease in bacterial load following vancomycin treatment[52,53]. Citrobactor rodentium-infected mouse models have been the preferred infection models in acute intestinal inflammation[54]. Helicobactor-infected mice have been also used to be a bacterial infection model in combination with the other model[47]. In addition, toll-like receptor 9 (TLR9)-deficient mice harbor increased numbers of Treg cells in the small intestine, suggesting that inflammatory signals from bacterial DNA play a role in inhibiting induced Treg cell differentiation or Treg cell proliferation[12,55].
IL-2-/-, IL-2R-/-, and IL-10-/- mice are known IBD models in which reduced Treg cell number or dysfunction of Treg cells are observed[41,19,56-60]. In addition, innate or mucosal immunity-related gene-deficient mice, such as NOD2-/-, myeloid differentiation primary response 88 (MYD88)-/-, nuclear factor-κB (NF-κB)-/-, cytokine-deficiency induced colitis susceptibility 1 (CDCS1)-/-, multidrug resistance gene 1a (MDR1a)-/-, and TLR5-/- mice, also reveal inflammatory lesions in the colon[27,61-70]. T-cell receptor (TCR)-/- mice are also known to be one of IBD models[60]. Overexpression of TNF-α and signal transducer and activator of transcription4 (STAT4) gene in mice results in the development of IBD-like lesions[16,71]. Moreover, there are various gene-manipulated models, in which epithelial barrier- and immune regulation-associated genes are manipulated by knockdown, knockin, conditional knockout, or transgenic mice (Table 1)[9,16,38,39,72-82]. Among them, a reduction of Treg cell number or impaired Treg cell function is observed in several models (Table 1)[19,59,60,68,72,77,78].
| Group | Model | Association | Ref. |
| Chemical drug-induced model | DSS | TNF-α, IL-17, reduced Treg cells | [43] |
| TNBS | IL-12, IL-17, reduced Treg cells | [44] | |
| Oxazolone | IL-4 | [45] | |
| Acetic acid (rat) | Myeloperoxidase | [46] | |
| Adoptive transfer model | Naïve T cell→Rag-/-/SCID | Recovery by Treg cell transfer | [16] |
| Bacteria-infected model | C. rodentium | Acute infection, IL-17 | [54] |
| Helicobacter, etc. | Combined with the other models | [47] | |
| Spontaneous model | SAMP/Yit/Fc | Dysfunction of Treg cell | [83] |
| C3H/HeJBir | Increased susceptibility to bacteria | [84] | |
| Cotton-top Tamarin (monkey) | C.Jejuni infection | [85] | |
| LEC (rat) | Reduced Treg cells | [86] | |
| Gene-manipulated model | IL-2-/- | Reduced Treg cells | [59] |
| IL-2R-/- | Reduced Treg cells | [19] | |
| IL-10-/- | Reduced Treg cells | [60] | |
| NOD2-/- | Impaired innate immunity | [61] | |
| Myd88-/- | Impaired TLR signal | [65] | |
| NF-κB-/- | Impaired pro-inflammatory signal | [62] | |
| Cdcs1-/- | Impaired pro-inflammatory signal | [67] | |
| Mdr1a-/- | Reduced iTreg cell differentiation | [68] | |
| TCR-/- | Defective adaptive immunity | [60] | |
| TGFβ-/- | Reduced Treg cell | [72] | |
| JAK3-/- | Balance of Th1 and Th2 | [73] | |
| Muc2-/- | ER stress | [74] | |
| A20-/- | Impaired Myd88 signal | [75] | |
| TCR-/-SOCS1-/- | Increased IFN-γ and IL-4 | [76] | |
| CD4/TGFβ-/- | Impaired Treg cell function | [77] | |
| CD4/PDK1-/- | Reduced Treg cell | [78] | |
| CD4/Blimp1-/- | Increased IL-17 | [79] | |
| TNF∆ARETG | Increased CD8 function | [71] | |
| STAT4TG | Increased TNF-α | [80] | |
| T/CD40LTG | Thymic dysfunction | [81] | |
| B/CD40LTG | Increased IFN-γ | [82] |
As one of spontaneous IBD models based on the pathogenic factor by Treg cells, senescence-accelerated mouse protein (SAMP)1/YitFc (SAMP) mice are well known[83]. A functional abnormality in Treg cells in SAMP mice is observed[83]. C3H/HeJBir mice with high susceptibility to bacteria have been also used to be a model of IBD[84]. In addition, a monkey model, cotton-top tamarin, infected with Campylobactor Jejuni is known to be a spontaneous model of IBD[85]. Moreover, long-evans cinnamon (LEC) rat is a spontaneous IBD model based on Treg cell-associated pathogenicity[86]. The LEC rat was first described as a naturally occurring mutant with a defect in thymocyte development: specifically, T-cell differentiation is arrested at the transition from CD4+CD8+ double positive to CD4+CD8- single positive (SP), but not to CD4-CD8+ SP, thymocytes[87]. Accordingly, in LEC rats, peripheral CD4+ T cells did not function as Th cells in terms of antibody production against T cell-dependent antigens, as well as in IL-2 production[87]. In addition, significantly reduced numbers of thymic and peripheral Foxp3+Treg cell and a defect in the suppressive activity of Treg cells were observed in these rats[86]. Interestingly, the proportion of Treg cells in the LP is significantly decreased in LEC rats when compared with control rats[86]. These findings suggest that the dysfunction in the Treg cell-controlled regulatory system may play a crucial role in the development of IBD.
Forkhead box P3 (Foxp3)-expressing Treg cells are a suppressive subset of CD4+ T cells that control autoimmunity, allergy, infection, and tumors[11,88]. Natural Treg (nTreg) cells arise as a discrete and largely stable lineage in the thymus[89,90]. The nTreg subset exhibits a TCR repertoire that is distinct from those of Foxp3-conventional T cells and induced (iTreg) cells[91-94].
nTreg cells require IL-2 for the development and maintenance and were initially identified by their elevated expression of the high-affinity IL-2 receptor (CD25)[11]. Mice that lack the ability to conduct IL-2-mediated signaling following the injection of anti-IL-2 mAb or as a result of IL-2 or IL-2R gene knockout exhibit defects in the number and function of nTreg cells[95-98]. The most important finding in mice with deficient IL-2 signaling is the development of spontaneous autoimmune lesions, including IBD[96-98].
nTreg cells share phenotypic features with naïve and memory conventional T cells[89]. Most Treg cells in circulation and the lymphoid organs express CCR7 and the adhesion receptor CD62L, which direct their recirculation through lymphoid tissues; effector or activated Treg cells comprise a minor fraction of these[88,99]. Similar to activated conventional T cells, this Treg cell population exhibits phenotypes such as CD62LloCCR7loCD44hi killer cell lectin-like receptor subfamily G member 1 (KLRG1)+CD103+ or CD45RAloCD25hi is thought to have encountered antigens[33,100-102]. These cells exhibit enhanced migration through nonlymphoid tissues.
Tissue-resident Treg cells can be found in nonlymphoid tissues, even under noninflammatory conditions[89,103]. Various tissues such as the skin, lungs, liver, salivary gland, lacrimal gland, intestinal mucosa, adipose tissue, and placenta are known to harbor substantial numbers of Treg cells in both humans and mice[14,104]. For example, CCR7+Foxp3+ Treg cells were found to reside in the salivary glands of healthy humans and mice whereas the number of CCR7+Foxp3+ Treg cells was extremely decreased in the tissues from patients and model mice with Sjögren’s syndrome, an autoimmune disease that affects the exocrine glands such as the salivary and lacrimal glands[104,105].
iTreg cells are generated from peripheral naïve conventional CD4+ T cells during the course of an immune response[12]. In healthy mice, most peripheral Treg cells in the spleen and lymph nodes are thymus-derived nTreg cells. By contrast, the iTreg population resides in the intestinal LP and gut-associated lymphoid tissue (GALT)[48,106]. iTreg cell generation involves naïve T-cell activation in the presence of transforming growth factor-β (TGF-β): in this setting, antigen exposure induces iTreg cell differentiation[107]. iTreg cells are particularly prevalent at mucosal surfaces where tolerance is induced against various antigens within the colonizing microbiota[52,108]. In addition, ingested food contains a plethora of antigens: in particular, food antigen feeding experiments identified a TGF-β1-producing CD4+ T cell population in the LP and GALT[109]. Immune responses to these dietary molecules can be actively suppressed by inducing oral tolerance[110]. Experiments with germ-free mice have suggested that bacterial commensals are essential for the development of normal numbers of colonic Treg cells[109]. In the human microbiota, 17 clostridial strains that induce Treg cell gut-homing and expansion by producing large amounts of short-chain fatty acids (SCFAs), which are bacterial breakdown products of plant-derived fibers were identified[111,112]. In particular, the SCFAs propionate, butyrate, and acetate can restore clonal Treg cell numbers in germ-free or antibiotic-treated mice[112].
Although the gastrointestinal tract harbors the largest reservoir of tissue-resident Treg cells in the body, recent studies have demonstrated that Treg cells are also highly enriched in the visceral adipose tissue (VAT)[113]. Interestingly, more than 50% Treg cells of CD4+ T cells in the VAT of 20-week-old mice are found[113]. Differences in the expressions of IL-10, CCR1, CCR2, CCR4, GATA binding protein 3 (GATA3), and peroxisome proliferator-activated receptor γ (PPARγ) have been observed between VAT Treg cells and effector Treg cells in lymphoid tissues[113,114]. Moreover, mice with a Treg cell-specific PPARγ deletion that were fed a high-fat diet exhibited impaired restoration of insulin sensitivity and glucose tolerance following pioglitazone treatment[113,114]. These findings suggest that VAT Treg cells might be critical mediators of the effects of treatment for lipid metabolism disorders.
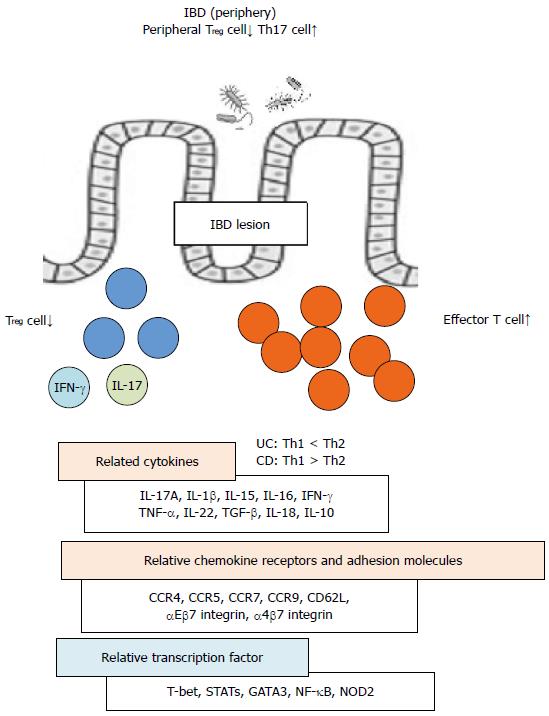
The phenotype and function of Treg cells in the inflamed mucosa or periphery of patients with IBD or animal models have been described as considerably different from those in peripheral lymphoid organs of healthy controls[12,115] (Figure 1). For example, patients with IBD exhibit reduced numbers of peripheral Treg cells and increased numbers peripheral Th17 cells[116]. By contrast, the mRNA expression levels of Foxp3 are elevated in the mucosa of patients with IBD along with elevated levels of IL-17A, IL-1β, and IL-6 mRNA[116]. In IBD models, dysfunctional suppressive Treg cell function plays a crucial role in the development of IBD lesions via the upregulation of T-cell specific T-box transcription factor (T-bet), STAT-1, and nuclear factor-κB (NF-κB)-mediated peripheral Treg cell activation[86]. Intestinal immune system activation in response to bacterial antigens and pathologic cytokine production by intestinal T cells is induced through various transcription factors or signal molecules, including T-bet, GATA-3, and STATs and plays a critical role in the development of IBD[72,117-119]. CD is associated with the production of Th1 cytokines such as IFN-γ and TNF-α and in an IBD model naïve T cells transferred into T cell-deficient mice can be controlled by a coinjection of Treg cells which suppress Th1 effector cell functions such as IFN-γ production[120,121]. Moreover, UC development in humans is associated with Th2 cytokines such as IL-5[122].
Recent studies have demonstrated that Foxp3+ Treg cells express retinoic acid receptor-related orphan receptor gamma t and are thus able to differentiate into Th17 cells, a process that is associated with a decreased suppressive Treg cell function in patients with IBD. Treg cells were found to suppress colonic inflammation by downregulating Th17 responsiveness via TGF-β in an adoptive transfer mouse model of colitis[123,124]. Additionally, CCR7 was shown to regulate the intestinal Th1/Th17/Treg cell balance during CD-like murine ileitis[125]. Furthermore, IFN-γ+IL-17+ coproducing CD4+ T cells which express high levels of T-bet, CD26, and IL-22 resemble the pathogenic Th17 cells that contribute to intestinal inflammation in IBD[126]. Epithelial-derived IL-18 also regulates both colonic Th17 cell differentiation and Treg cell function in the context of IBD-associated intestinal inflammation[124].
A recent study described Clostridium bacteria as potent inducers of Foxp3+ Treg cells in the colonic mucosa[111]. Additionally, CD4+CD8+αα colonic Treg cells were reported to produce IL-10 in response to Faecalibacterium prausnitzii and the frequencies of CD4+CD8+αα lymphocytes in the LP lymphocytes and peripheral blood were found to be significantly lower in patients with IBD than in healthy controls[127]. These findings suggest that colonic bacteria-induced CD4+CD8+αα Treg cells may control or prevent IBD.
New and effective therapies involving anti-TNF antibodies have yielded remarkable progress in the field of IBD therapy[10]. In addition, clinical trial applications are underway for target molecules such as IL-6/IL-6R, IL-12/23, IL-17A/F, IL-13, interferon-gamma-inducible protein 10 (IP10), CCR9, Janus kinase 3 (JAK3), similar to mothers against decapentaplegic (Smad)7/TGF, α4β7/β7, and mucosal vascular addressin cell adhesion molecule (MAdCAM)[4,5]. Target molecules such as IL-17A/F, IL-13, IP10, JAK3, α4β7/β7, or MAdCAM also represent promising therapeutic strategies for UC[4,5,15,22,24].
The Foxp3+Treg cell frequency was found to be significantly lower in active patients with IBD than in healthy controls[18,116]. On day 14 after an initial dose of anti-TNFα infusion therapy, patients with IBD exhibited a significant increase in the frequency of circulating Treg cells along with two- to three-fold increase in Foxp3 expression, which paralleled a reduction of IBD[128]. Furthermore, another report demonstrated that anti-TNFα mAb [infliximab (IFX)] therapy yielded a significant and sustained relative increase in peripheral blood Treg cells; a change in C-reactive protein levels and durable clinical response was associated with this sustained increase in circulating Foxp3+Treg cells[129]. IFX therapy was also shown to downregulate the mucosal expression of Foxp3 mRNA and protein in patients with UC and CD[129].
In patients with active IBD, anti-TNF-α treatment rapidly enhances the frequency of functional Foxp3+Treg cells in the blood and potentiates their suppressive function[128]. In addition, in these patients increased apoptosis of local Foxp3+Treg cells is observed in the inflamed mucosa when compared with noninflamed control colon tissues, along with a reduced frequency and increased apoptosis of peripheral blood Treg cells and elevated caspase activity in the serum[130]. During anti-TNF-α antibody treatment, a decrease in the apoptosis of Treg cells was found to correlate closely with an increase in peripheral Treg cell numbers and a decrease of caspase and disease activity[130].
CD4+CD25+CD127loCD45RA+ Treg cells appear to be a promising population from which to expand Treg cells for autologous Treg cell transfer therapy in patients with CD[131]. Expanded CD45RA+ Treg cells carry an epigenetically stable FOXP3 locus and do not convert to a Th17 phenotype in in vitro culture and CD45RA+ Treg cells from patients with CD were found to home to the human small intestine in a C.B-17 severe combined immune deficiency xenotransplant model[131]. There remains an unmet need for the development of novel therapies for IBD, as current drug therapies frequently fail to maintain long-term remission and may be complicated by significant side effects. Although cellular therapies are emerging as a potentially attractive many hindrances to successful clinical therapy remain in terms of sufficient cell numbers, cell maintenance, and cell sources.
Although the precise molecular pathogenesis of IBD remains unclear, recent therapeutic strategies involving antibodies against pathogenic cytokines or chemokines have provided treatment opportunities for many more patients suffering from IBD. Additionally, Treg cells may provide a more effective therapy for IBD; therefore the unknown mechanisms with respect to Treg cell differentiation, function, and maintenance should be clarified.
| 1. | Blumberg RS, Saubermann LJ, Strober W. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr Opin Immunol. 1999;11:648-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 328] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 2. | Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1487] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 3. | Fiocchi C. Inflammatory bowel disease pathogenesis: therapeutic implications. Chin J Dig Dis. 2005;6:6-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 949] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 5. | Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1347] [Cited by in RCA: 1577] [Article Influence: 112.6] [Reference Citation Analysis (0)] |
| 6. | Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Buning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, International IBDGC, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3979] [Cited by in RCA: 3709] [Article Influence: 264.9] [Reference Citation Analysis (1)] |
| 7. | Knights D, Lassen KG, Xavier RJ. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut. 2013;62:1505-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 353] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 8. | Huttenhower C, Kostic AD, Xavier RJ. Inflammatory bowel disease as a model for translating the microbiome. Immunity. 2014;40:843-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 252] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 9. | Nell S, Suerbaum S, Josenhans C. The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat Rev Microbiol. 2010;8:564-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 288] [Article Influence: 18.0] [Reference Citation Analysis (1)] |
| 10. | Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7:6-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 11. | Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1981] [Cited by in RCA: 2055] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 12. | Mayne CG, Williams CB. Induced and natural regulatory T cells in the development of inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1772-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 13. | Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 511] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 14. | Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol. 2013;14:1007-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 283] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 15. | Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 291] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 16. | Mizoguchi A. Animal models of inflammatory bowel disease. Prog Mol Biol Transl Sci. 2012;105:263-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 194] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 17. | Martin B, Banz A, Bienvenu B, Cordier C, Dautigny N, Bécourt C, Lucas B. Suppression of CD4+ T lymphocyte effector functions by CD4+CD25+ cells in vivo. J Immunol. 2004;172:3391-3398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Niederau C, Backmerhoff F, Schumacher B, Niederau C. Inflammatory mediators and acute phase proteins in patients with Crohn’s disease and ulcerative colitis. Hepatogastroenterology. 1997;44:90-107. [PubMed] |
| 19. | Nielsen OH, Ciardelli T, Wu Z, Langholz E, Kirman I. Circulating soluble interleukin-2 receptor alpha and beta chain in inflammatory bowel disease. Am J Gastroenterol. 1995;90:1301-1306. [PubMed] |
| 20. | Colombo BM, Scalvenzi T, Benlamara S, Pollet N. Microbiota and mucosal immunity in amphibians. Front Immunol. 2015;6:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | McAvoy JW, Dixon KE. Cell specialization in the small intestinal epithelium of adult Xenopus laevis: structural aspects. J Anat. 1978;125:155-169. [PubMed] |
| 22. | Krishnan K, Arnone B, Buchman A. Intestinal growth factors: potential use in the treatment of inflammatory bowel disease and their role in mucosal healing. Inflamm Bowel Dis. 2011;17:410-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Paclik D, Lohse K, Wiedenmann B, Dignass AU, Sturm A. Galectin-2 and -4, but not galectin-1, promote intestinal epithelial wound healing in vitro through a TGF-beta-independent mechanism. Inflamm Bowel Dis. 2008;14:1366-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol. 2008;14:348-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 237] [Cited by in RCA: 267] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 25. | Lassen KG, Kuballa P, Conway KL, Patel KK, Becker CE, Peloquin JM, Villablanca EJ, Norman JM, Liu TC, Heath RJ. Atg16L1 T300A variant decreases selective autophagy resulting in altered cytokine signaling and decreased antibacterial defense. Proc Natl Acad Sci USA. 2014;111:7741-7746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 301] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 26. | Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2019] [Cited by in RCA: 1979] [Article Influence: 131.9] [Reference Citation Analysis (2)] |
| 27. | Rubino SJ, Selvanantham T, Girardin SE, Philpott DJ. Nod-like receptors in the control of intestinal inflammation. Curr Opin Immunol. 2012;24:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Barreau F, Meinzer U, Chareyre F, Berrebi D, Niwa-Kawakita M, Dussaillant M, Foligne B, Ollendorff V, Heyman M, Bonacorsi S. CARD15/NOD2 is required for Peyer’s patches homeostasis in mice. PLoS One. 2007;2:e523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Watanabe T, Asano N, Murray PJ, Ozato K, Tailor P, Fuss IJ, Kitani A, Strober W. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest. 2008;118:545-559. [PubMed] |
| 30. | Macho Fernandez E, Valenti V, Rockel C, Hermann C, Pot B, Boneca IG, Grangette C. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut. 2011;60:1050-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 279] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 31. | Venturi GM, Conway RM, Steeber DA, Tedder TF. CD25+CD4+ regulatory T cell migration requires L-selectin expression: L-selectin transcriptional regulation balances constitutive receptor turnover. J Immunol. 2007;178:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Schneider MA, Meingassner JG, Lipp M, Moore HD, Rot A. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J Exp Med. 2007;204:735-745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 33. | Suffia I, Reckling SK, Salay G, Belkaid Y. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J Immunol. 2005;174:5444-5455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 262] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 34. | Denning TL, Kim G, Kronenberg M. Cutting edge: CD4+CD25+ regulatory T cells impaired for intestinal homing can prevent colitis. J Immunol. 2005;174:7487-7491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Yuan Q, Bromley SK, Means TK, Jones KJ, Hayashi F, Bhan AK, Luster AD. CCR4-dependent regulatory T cell function in inflammatory bowel disease. J Exp Med. 2007;204:1327-1334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Kang SG, Piniecki RJ, Hogenesch H, Lim HW, Wiebke E, Braun SE, Matsumoto S, Kim CH. Identification of a chemokine network that recruits FoxP3(+) regulatory T cells into chronically inflamed intestine. Gastroenterology. 2007;132:966-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Guo Z, Jang MH, Otani K, Bai Z, Umemoto E, Matsumoto M, Nishiyama M, Yamasaki M, Ueha S, Matsushima K. CD4+CD25+ regulatory T cells in the small intestinal lamina propria show an effector/memory phenotype. Int Immunol. 2008;20:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Corridoni D, Arseneau KO, Cominelli F. Inflammatory bowel disease. Immunol Lett. 2014;161:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 39. | Eckmann L. Animal models of inflammatory bowel disease: lessons from enteric infections. Ann N Y Acad Sci. 2006;1072:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Uhlig HH, Powrie F. Mouse models of intestinal inflammation as tools to understand the pathogenesis of inflammatory bowel disease. Eur J Immunol. 2009;39:2021-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 379] [Article Influence: 18.0] [Reference Citation Analysis (1)] |
| 42. | Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 376] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 43. | Boehm F, Martin M, Kesselring R, Schiechl G, Geissler EK, Schlitt HJ, Fichtner-Feigl S. Deletion of Foxp3+ regulatory T cells in genetically targeted mice supports development of intestinal inflammation. BMC Gastroenterol. 2012;12:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Reardon C, Wang A, McKay DM. Transient local depletion of Foxp3+ regulatory T cells during recovery from colitis via Fas/Fas ligand-induced death. J Immunol. 2008;180:8316-8326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis: A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998;188:1929-1939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 421] [Cited by in RCA: 402] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 46. | Singh VP, Patil CS, Jain NK, Singh A, Kulkarni SK. Effect of nimesulide on acetic acid- and leukotriene-induced inflammatory bowel disease in rats. Prostaglandins Other Lipid Mediat. 2003;71:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Cahill RJ, Foltz CJ, Fox JG, Dangler CA, Powrie F, Schauer DB. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun. 1997;65:3126-3131. [PubMed] |
| 48. | Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 283] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 49. | Ostman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. Eur J Immunol. 2006;36:2336-2346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 50. | Singh B, Read S, Asseman C, Malmström V, Mottet C, Stephens LA, Stepankova R, Tlaskalova H, Powrie F. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 377] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 51. | Strauch UG, Obermeier F, Grunwald N, Gürster S, Dunger N, Schultz M, Griese DP, Mähler M, Schölmerich J, Rath HC. Influence of intestinal bacteria on induction of regulatory T cells: lessons from a transfer model of colitis. Gut. 2005;54:1546-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 52. | Tanoue T, Honda K. Induction of Treg cells in the mouse colonic mucosa: a central mechanism to maintain host-microbiota homeostasis. Semin Immunol. 2012;24:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1480] [Cited by in RCA: 1426] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 54. | Spahn TW, Ross M, von Eiff C, Maaser C, Spieker T, Kannengiesser K, Domschke W, Kucharzik T. CD4+ T cells transfer resistance against Citrobacter rodentium-induced infectious colitis by induction of Th 1 immunity. Scand J Immunol. 2008;67:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 398] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 56. | Poussier P, Ning T, Chen J, Banerjee D, Julius M. Intestinal inflammation observed in IL-2R/IL-2 mutant mice is associated with impaired intestinal T lymphopoiesis. Gastroenterology. 2000;118:880-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Sohn KJ, Shah SA, Reid S, Choi M, Carrier J, Comiskey M, Terhorst C, Kim YI. Molecular genetics of ulcerative colitis-associated colon cancer in the interleukin 2- and beta(2)-microglobulin-deficient mouse. Cancer Res. 2001;61:6912-6917. [PubMed] |
| 58. | Davidson NJ, Leach MW, Fort MM, Thompson-Snipes L, Kuhn R, Muller W, Berg DJ, Rennick DM. T helper cell 1-type CD4 T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med. 1996;184:241-251. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 306] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 59. | Waidmann M, Bechtold O, Frick JS, Lehr HA, Schubert S, Dobrindt U, Loeffler J, Bohn E, Autenrieth IB. Bacteroides vulgatus protects against Escherichia coli-induced colitis in gnotobiotic interleukin-2-deficient mice. Gastroenterology. 2003;125:162-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 60. | Burich A, Hershberg R, Waggie K, Zeng W, Brabb T, Westrich G, Viney JL, Maggio-Price L. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001;281:G764-G778. [PubMed] |
| 61. | Watanabe T, Kitani A, Murray PJ, Wakatsuki Y, Fuss IJ, Strober W. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity. 2006;25:473-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 171] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 62. | Fox JG, Rogers AB, Whary MT, Ge Z, Taylor NS, Xu S, Horwitz BH, Erdman SE. Gastroenteritis in NF-kappaB-deficient mice is produced with wild-type Camplyobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect Immun. 2004;72:1116-1125. [RCA] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 63. | Dennis A, Kudo T, Kruidenier L, Girard F, Crepin VF, MacDonald TT, Frankel G, Wiles S. The p50 subunit of NF-kappaB is critical for in vivo clearance of the noninvasive enteric pathogen Citrobacter rodentium. Infect Immun. 2008;76:4978-4988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Gibson DL, Ma C, Bergstrom KS, Huang JT, Man C, Vallance BA. MyD88 signalling plays a critical role in host defence by controlling pathogen burden and promoting epithelial cell homeostasis during Citrobacter rodentium-induced colitis. Cell Microbiol. 2008;10:618-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 168] [Article Influence: 8.8] [Reference Citation Analysis (4)] |
| 65. | Hapfelmeier S, Stecher B, Barthel M, Kremer M, Müller AJ, Heikenwalder M, Stallmach T, Hensel M, Pfeffer K, Akira S. The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J Immunol. 2005;174:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 326] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 66. | Maggio-Price L, Shows D, Waggie K, Burich A, Zeng W, Escobar S, Morrissey P, Viney JL. Helicobacter bilis infection accelerates and H. hepaticus infection delays the development of colitis in multiple drug resistance-deficient (mdr1a-/-) mice. Am J Pathol. 2002;160:739-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 67. | Beckwith J, Cong Y, Sundberg JP, Elson CO, Leiter EH. Cdcs1, a major colitogenic locus in mice, regulates innate and adaptive immune response to enteric bacterial antigens. Gastroenterology. 2005;129:1473-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 68. | Tanner SM, Staley EM, Lorenz RG. Altered generation of induced regulatory T cells in the FVB.mdr1a-/- mouse model of colitis. Mucosal Immunol. 2013;6:309-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Maggio-Price L, Bielefeldt-Ohmann H, Treuting P, Iritani BM, Zeng W, Nicks A, Tsang M, Shows D, Morrissey P, Viney JL. Dual infection with Helicobacter bilis and Helicobacter hepaticus in p-glycoprotein-deficient mdr1a-/- mice results in colitis that progresses to dysplasia. Am J Pathol. 2005;166:1793-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 70. | Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 355] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 71. | Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1070] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 72. | Neurath MF, Weigmann B, Finotto S, Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E, Mudter J, Galle PR. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J Exp Med. 2002;195:1129-1143. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 495] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 73. | Murata Y, Yamashita A, Saito T, Sugamura K, Hamuro J. The conversion of redox status of peritoneal macrophages during pathological progression of spontaneous inflammatory bowel disease in Janus family tyrosine kinase 3(-/-) and IL-2 receptor gamma(-/-) mice. Int Immunol. 2002;14:627-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 74. | Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, Thornton DJ, Png CW, Crockford TL, Cornall RJ. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 521] [Cited by in RCA: 584] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 75. | Hammer GE, Turer EE, Taylor KE, Fang CJ, Advincula R, Oshima S, Barrera J, Huang EJ, Hou B, Malynn BA. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat Immunol. 2011;12:1184-1193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 76. | Chinen T, Kobayashi T, Ogata H, Takaesu G, Takaki H, Hashimoto M, Yagita H, Nawata H, Yoshimura A. Suppressor of cytokine signaling-1 regulates inflammatory bowel disease in which both IFNgamma and IL-4 are involved. Gastroenterology. 2006;130:373-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 77. | Sledzińska A, Hemmers S, Mair F, Gorka O, Ruland J, Fairbairn L, Nissler A, Müller W, Waisman A, Becher B. TGF-β signalling is required for CD4+ T cell homeostasis but dispensable for regulatory T cell function. PLoS Biol. 2013;11:e1001674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 78. | Park SG, Mathur R, Long M, Hosh N, Hao L, Hayden MS, Ghosh S. T regulatory cells maintain intestinal homeostasis by suppressing γδ T cells. Immunity. 2010;33:791-803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 79. | Salehi S, Bankoti R, Benevides L, Willen J, Couse M, Silva JS, Dhall D, Meffre E, Targan S, Martins GA. B lymphocyte-induced maturation protein-1 contributes to intestinal mucosa homeostasis by limiting the number of IL-17-producing CD4+ T cells. J Immunol. 2012;189:5682-5693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 80. | O’Malley JT, Eri RD, Stritesky GL, Mathur AN, Chang HC, Hogenesch H, Srinivasan M, Kaplan MH. STAT4 isoforms differentially regulate Th1 cytokine production and the severity of inflammatory bowel disease. J Immunol. 2008;181:5062-5070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 81. | Clegg CH, Rulffes JT, Haugen HS, Hoggatt IH, Aruffo A, Durham SK, Farr AG, Hollenbaugh D. Thymus dysfunction and chronic inflammatory disease in gp39 transgenic mice. Int Immunol. 1997;9:1111-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 82. | Kawamura T, Kanai T, Dohi T, Uraushihara K, Totsuka T, Iiyama R, Taneda C, Yamazaki M, Nakamura T, Higuchi T. Ectopic CD40 ligand expression on B cells triggers intestinal inflammation. J Immunol. 2004;172:6388-6397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Ishikawa D, Okazawa A, Corridoni D, Jia LG, Wang XM, Guanzon M, Xin W, Arseneau KO, Pizarro TT, Cominelli F. Tregs are dysfunctional in vivo in a spontaneous murine model of Crohn’s disease. Mucosal Immunol. 2013;6:267-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 84. | Elson CO, Cong Y, Sundberg J. The C3H/HeJBir mouse model: a high susceptibility phenotype for colitis. Int Rev Immunol. 2000;19:63-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 85. | Johnson LD, Ausman LM, Rolland RM, Chalifoux LV, Russell RG. Campylobacter-induced enteritis and diarrhea in captive cotton-top tamarins (Saguinus oedipus) during the first year of life. Comp Med. 2001;51:257-261. [PubMed] |
| 86. | Ishimaru N, Yamada A, Kohashi M, Arakaki R, Takahashi T, Izumi K, Hayashi Y. Development of inflammatory bowel disease in Long-Evans Cinnamon rats based on CD4+CD25+Foxp3+ regulatory T cell dysfunction. J Immunol. 2008;180:6997-7008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 87. | Agui T, Oka M, Yamada T, Sakai T, Izumi K, Ishida Y, Himeno K, Matsumoto K. Maturational arrest from CD4+8+ to CD4+8- thymocytes in a mutant strain (LEC) of rat. J Exp Med. 1990;172:1615-1624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 628] [Cited by in RCA: 624] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 89. | Liston A, Gray DH. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol. 2014;14:154-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 344] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 90. | Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5576] [Cited by in RCA: 5945] [Article Influence: 258.5] [Reference Citation Analysis (0)] |
| 91. | Feuerer M, Hill JA, Kretschmer K, von Boehmer H, Mathis D, Benoist C. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc Natl Acad Sci USA. 2010;107:5919-5924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 92. | Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, Ziegelbauer J, Yassai M, Li SH, Relland LM. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 342] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 93. | Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 453] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 94. | Relland LM, Williams JB, Relland GN, Haribhai D, Ziegelbauer J, Yassai M, Gorski J, Williams CB. The TCR repertoires of regulatory and conventional T cells specific for the same foreign antigen are distinct. J Immunol. 2012;189:3566-3574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 95. | Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169:4850-4860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 378] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 96. | Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 622] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 97. | Papiernik M, de Moraes ML, Pontoux C, Vasseur F, Pénit C. Regulatory CD4 T cells: expression of IL-2R alpha chain, resistance to clonal deletion and IL-2 dependency. Int Immunol. 1998;10:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 319] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 98. | Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 900] [Cited by in RCA: 988] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 99. | Lee JH, Kang SG, Kim CH. FoxP3+ T cells undergo conventional first switch to lymphoid tissue homing receptors in thymus but accelerated second switch to nonlymphoid tissue homing receptors in secondary lymphoid tissues. J Immunol. 2007;178:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 100. | Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rötzschke O, Falk K. CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood. 2005;105:2877-2886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 240] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 101. | Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 502] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 102. | Stephens GL, Andersson J, Shevach EM. Distinct subsets of FoxP3+ regulatory T cells participate in the control of immune responses. J Immunol. 2007;178:6901-6911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 103. | Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 334] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 104. | Ishimaru N, Nitta T, Arakaki R, Yamada A, Lipp M, Takahama Y, Hayashi Y. In situ patrolling of regulatory T cells is essential for protecting autoimmune exocrinopathy. PLoS One. 2010;5:e8588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 105. | Ishimaru N, Yamada A, Nitta T, Arakaki R, Lipp M, Takahama Y, Hayashi Y. CCR7 with S1P1 signaling through AP-1 for migration of Foxp3+ regulatory T-cells controls autoimmune exocrinopathy. Am J Pathol. 2012;180:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 106. | Bilate AB, Lafaille JJ. It takes two to tango. Immunity. 2011;35:6-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 107. | Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875-1886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3466] [Cited by in RCA: 3831] [Article Influence: 174.1] [Reference Citation Analysis (7)] |
| 108. | Atarashi K, Umesaki Y, Honda K. Microbiotal influence on T cell subset development. Semin Immunol. 2011;23:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 109. | Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 576] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 110. | Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241:241-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 488] [Cited by in RCA: 463] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 111. | Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2568] [Cited by in RCA: 2969] [Article Influence: 185.6] [Reference Citation Analysis (0)] |
| 112. | Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2937] [Cited by in RCA: 4162] [Article Influence: 320.2] [Reference Citation Analysis (1)] |
| 113. | Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1750] [Cited by in RCA: 1715] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 114. | Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549-553. [PubMed] |
| 115. | Kullberg MC, Hay V, Cheever AW, Mamura M, Sher A, Letterio JJ, Shevach EM, Piccirillo CA. TGF-beta1 production by CD4+ CD25+ regulatory T cells is not essential for suppression of intestinal inflammation. Eur J Immunol. 2005;35:2886-2895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 116. | Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010;30:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 330] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 117. | Rengarajan J, Szabo SJ, Glimcher LH. Transcriptional regulation of Th1/Th2 polarization. Immunol Today. 2000;21:479-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 319] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 118. | Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1765] [Cited by in RCA: 1823] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 119. | Asnagli H, Murphy KM. Stability and commitment in T helper cell development. Curr Opin Immunol. 2001;13:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 120. | Plevy SE, Landers CJ, Prehn J, Carramanzana NM, Deem RL, Shealy D, Targan SR. A role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn’s disease. J Immunol. 1997;159:6276-6282. [PubMed] |
| 121. | Iijima H, Neurath MF, Nagaishi T, Glickman JN, Nieuwenhuis EE, Nakajima A, Chen D, Fuss IJ, Utku N, Lewicki DN. Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1. J Exp Med. 2004;199:471-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 122. | Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261-1270. [PubMed] |
| 123. | Ueno A, Jijon H, Chan R, Ford K, Hirota C, Kaplan GG, Beck PL, Iacucci M, Fort Gasia M, Barkema HW. Increased prevalence of circulating novel IL-17 secreting Foxp3 expressing CD4+ T cells and defective suppressive function of circulating Foxp3+ regulatory cells support plasticity between Th17 and regulatory T cells in inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19:2522-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 124. | Harrison OJ, Srinivasan N, Pott J, Schiering C, Krausgruber T, Ilott NE, Maloy KJ. Epithelial-derived IL-18 regulates Th17 cell differentiation and Foxp3+ Treg cell function in the intestine. Mucosal Immunol. 2015;8:1226-1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 195] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 125. | McNamee EN, Masterson JC, Veny M, Collins CB, Jedlicka P, Byrne FR, Ng GY, Rivera-Nieves J. Chemokine receptor CCR7 regulates the intestinal TH1/TH17/Treg balance during Crohn’s-like murine ileitis. J Leukoc Biol. 2015;97:1011-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 126. | Globig AM, Hennecke N, Martin B, Seidl M, Ruf G, Hasselblatt P, Thimme R, Bengsch B. Comprehensive intestinal T helper cell profiling reveals specific accumulation of IFN-γ+IL-17+coproducing CD4+ T cells in active inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:2321-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 127. | Sarrabayrouse G, Bossard C, Chauvin JM, Jarry A, Meurette G, Quévrain E, Bridonneau C, Preisser L, Asehnoune K, Labarrière N. CD4CD8αα lymphocytes, a novel human regulatory T cell subset induced by colonic bacteria and deficient in patients with inflammatory bowel disease. PLoS Biol. 2014;12:e1001833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 128. | Boschetti G, Nancey S, Sardi F, Roblin X, Flourié B, Kaiserlian D. Therapy with anti-TNFα antibody enhances number and function of Foxp3(+) regulatory T cells in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:160-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 129. | Li Z, Arijs I, De Hertogh G, Vermeire S, Noman M, Bullens D, Coorevits L, Sagaert X, Schuit F, Rutgeerts P. Reciprocal changes of Foxp3 expression in blood and intestinal mucosa in IBD patients responding to infliximab. Inflamm Bowel Dis. 2010;16:1299-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 130. | Veltkamp C, Anstaett M, Wahl K, Möller S, Gangl S, Bachmann O, Hardtke-Wolenski M, Länger F, Stremmel W, Manns MP. Apoptosis of regulatory T lymphocytes is increased in chronic inflammatory bowel disease and reversed by anti-TNFα treatment. Gut. 2011;60:1345-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 131. | Canavan JB, Scottà C, Vossenkämper A, Goldberg R, Elder MJ, Shoval I, Marks E, Stolarczyk E, Lo JW, Powell N. Developing in vitro expanded CD45RA+ regulatory T cells as an adoptive cell therapy for Crohn’s disease. Gut. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 170] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Lakatos PL, Schicho R, Wlodarczyk M S- Editor: Qi Y L- Editor: A E- Editor: Liu XM