Published online Feb 14, 2016. doi: 10.3748/wjg.v22.i6.2111
Peer-review started: May 20, 2015
First decision: July 19, 2015
Revised: August 18, 2015
Accepted: September 28, 2015
Article in press: September 30, 2015
Published online: February 14, 2016
Processing time: 249 Days and 13.6 Hours
AIM: To investigate the specific carbohydrate diet (SCD) as nutritional therapy for maintenance of remission in pediatric Crohn’s disease (CD).
METHODS: Retrospective chart review was conducted in 11 pediatric patients with CD who initiated the SCD as therapy at time of diagnosis or flare. Two groups defined as SCD simple (diet alone, antibiotics or 5-ASA) or SCD with immunomodulators (corticosteroids and/or stable thiopurine dosing) were followed for one year and compared on disease characteristics, laboratory values and anthropometrics.
RESULTS: The mean age at start of the SCD was 11.8 ± 3.0 years (range 6.6-17.6 years) with five patients starting the SCD within 5 wk of diagnosis. Three patients maintained a strict SCD diet for the study period and the mean time for liberalization was 7.7 ± 4.0 mo (range 1-12) for the remaining patients. In both groups, hematocrit, albumin and ESR values improved while on strict SCD and appeared stable after liberalization (P-value 0.006, 0.002, 0.002 respectively). The majority of children gained in weight and height percentile while on strict SCD, with small loss in weight percentile documented with liberalization.
CONCLUSION: Disease control may be attainable with the SCD in pediatric CD. Further studies are needed to assess adherence, impact on mucosal healing and growth.
Core tip: Enteral nutrition is effective for both induction and maintenance therapy for pediatric Crohn’s disease (CD), but adherence to a formula-based diet can be challenging. The specific carbohydrate diet (SCD) may offer a real-food nutritional therapy. Mild liberalization after response to a strict diet has not been described and may improve adherence while maintaining therapeutic effect. Laboratory parameters improved when following a strict SCD and were stable after liberalization. Despite this restrictive diet, growth was supported. The SCD may offer an alternative or adjunct to traditional medication therapy for pediatric CD.
- Citation: Burgis JC, Nguyen K, Park K, Cox K. Response to strict and liberalized specific carbohydrate diet in pediatric Crohn’s disease. World J Gastroenterol 2016; 22(6): 2111-2117
- URL: https://www.wjgnet.com/1007-9327/full/v22/i6/2111.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i6.2111
The western lifestyle and a diet high in refined sugars and processed foods have been implicated to induce an abnormal immunological response in the digestive tract and potentially contribute to the increased incidence of inflammatory bowel disease (IBD) over the last 60 years[1-3]. Diets high in fruits, vegetables and fiber have shown in some studies to be protective against the risk of developing IBD[4,5]. Proposed mechanisms of how nutrition impacts intestinal inflammation include modifying the intestinal microbiota, generating an immune response to dietary antigen exposure and alteration in inflammatory cytokine profiles[6-8].
Manipulation of nutrition creates a therapeutic opportunity in IBD. Enteral nutrition using formula as therapy has clear efficacy for inducing remission in pediatric Crohn’s disease (CD)[9-11]. However, formula-based diets carry a risk of taste fatigue, may require a nasogastric tube or change the social dynamic around meals, and long term adherence may be difficult[12]. Diets with limited ingredients such as the specific carbohydrate diet (SCD) may be more accepted and easier to follow for patients and their families.
The SCD was introduced by Haas in the 1920s as a therapy for celiac disease and was popularized by Gottschall, a biochemist and mother of a patient with ulcerative colitis in the late 1980s as a therapy for various intestinal disorders[13,14]. The basic premise of SCD is restriction to simple carbohydrates found in fruits, honey, yogurt, vegetables and nuts. No grains or starches including wheat, rice, corn or potatoes are permitted. The diet postulates that undigested complex carbohydrates are malabsorbed and then fermented in the colon, producing acid and inflammatory byproducts, which worsen diarrhea and lead to bacterial overgrowth. The SCD allows proteins such as meat, poultry, fish and lactose free natural cheeses. Processed meats and other dairy products are not permitted. Fresh fruits and vegetables and some legumes, such as specific soaked beans and lentils, are encouraged. There is a focus on food quality and elimination of processed foods, specifically sucrose. The multi-factorial challenges of adhering to and remaining “legal” on the SCD can not be understated, and include limited availability of legal ready-to-eat foods and the demands of home food preparation. Given these challenges, concerns regarding patient adherence have been raised, which parallel questions about long term enteral nutrition use[15,16].
A recent case series of seven children with CD on the SCD without immunosuppressive medications demonstrated symptom improvement via pediatric CD activity index after three months and significant improvement of serum albumin, CRP, hematocrit (HCT) and stool calprotectin over a long follow-up period (average 14.6 +/- 10.8 mo)[17]. A second prospective report of nine pediatric patients with active CD on the SCD demonstrated significantly improved disease index scores over the initial 12 wk. Improvement was sustained in 7 children who completed 52 wk on the diet, including mucosal healing documented in two patients by capsule endoscopy. No changes or addition of medications occurred during the study period[18]. There are limited case reports of other diets with varied carbohydrate restrictions in patients with CD with positive outcomes[19-21]. Other diets restricting refined sugar and modified fiber intake have shown mixed results[22,23].
Of all these restricted carbohydrate diets, the SCD is the most restrictive and thus, difficult to maintain long term even when a distinct positive response is observed while following the diet strictly. Studies have shown that partial enteral nutrition at greater than 50% of caloric intake may be effective maintenance therapy in CD[11]. There is rationale that over time allowing a small amount of illegal foods or ingredients on the SCD may not alter the potential therapeutic effect. Liberalization represents a real-world application of the SCD to facilitate longer adherence and possibly broader use.
No studies to date have evaluated the impact of mild liberalization from a strict SCD on disease control and growth parameters. We completed a retrospective chart review of pediatric patients with CD on the SCD to help evaluate clinical response while on a strict and on a liberalized SCD.
Retrospective chart review was conducted of pediatric patients with CD followed in the pediatric gastroenterology practice at Lucile Packard Children’s Hospital between 2003 and 2012. These patients were diagnosed with CD based on standard criteria including symptomatology, laboratory values, radiologic studies and endoscopy with pathologic confirmation. These patients chose to follow the SCD as primary therapy on their own accord. The SCD was started at the time of diagnosis or at the time of a disease flare. Some patients added the SCD to their medication therapy.
Data including age at diagnosis, disease location, medication use, dietary adherence, anthropometrics, and laboratory and imaging findings were extracted from the medical record (Table 1). Patients were stratified into two groups defined by overall treatment approach for induction and maintenance therapy including: SCD alone or in combination with only 5ASA or antibiotics vs SCD in combination with immunomodulating medications including corticosteroids or thiopurines. Corticosteroids were tapered over a maximum 6 wk time period and thiopurine dose was stable.
| Gender | Age at diagnosis (yr) | Prior treatment | Disease length (mo) | Concurrent treatment | Time liberalized (mo) | Follow-up (mo) | Added food |
| SCD Simple | |||||||
| F | 11.9 | None | 0 | None | 5.2 | 8.2 | Wheat daily, then corn, yeast, potatoes |
| M | 11 | None | 1.2 | None | 9.5 | 15.1 | Illegal meal on major holidays |
| M | 6.5 | None | 1.2 | None | Strict | 9.0 | Strict |
| M | 17 | Antibiotic | 7.2 | 5ASA1 | Strict | 14.2 | Strict |
| 5ASA | |||||||
| F | 7.7 | Antibiotic | 6.6 | None2 | 10 | 12.4 | Illegal meal per week |
| SCD with Immunomodulators | |||||||
| M | 11.8 | None | 0 | Prednisone × 6 wk | 2.5 | 8.8 | Rice daily, "rare" corn + Illegal meal per month |
| M | 14.4 | None | 1.2 | Prednisone × 4 wk | 12 | 13.7 | Illegal meal per week + rare illegal snacks |
| M | 12.2 | Budesonide | 3.0 | Budesonide × 4 wk | 14 | 17.5 | Illegal snacks daily then daily potatoes |
| F | 12.2 | 6MP | 21.6 | Prednisone × 4 wk, 6MP | 5 | 9.9 | Daily rice + Illegal meal per month |
| M | 10.5 | 5ASA, 6MP | 15.6 | 5ASA, 6MP | 3 | 9.5 | Illegal meal every 2 wk |
| M | 6.3 | 5ASA, 6MP | 39.6 | 5ASA, 6MP | Strict | 12.1 | Strict |
Patients followed the diet strictly for various time periods, and the SCD was liberalized by patients based on personal preference. Time at liberalization was defined as any significant variance from the SCD including one illegal meal more than once every month or the addition of an illegal ingredient on a regular basis. End of follow-up was defined as clinic visit or laboratory testing at approximately 12 mo while still using the SCD as primary therapy, whether strict or liberalized.
Descriptive statistics including mean, SD and range are reported for patient characteristics. Wilcoxon rank sum tests were used to compare laboratory values. Visual representation of median, quartiles and 95%CI are given in Figures 1-3. Test of statistical inference was deferred for weight and height due to the nature of covariance with patient age. The statistical methods of this study were reviewed by KT Park, MD from Stanford University. Technical appendix, statistical code, and dataset are available from the corresponding author. This study was reviewed and approved by the Stanford University Human Subjects Research Institutional Review Board. As a retrospective review, a waiver of consent was granted for all study participants. Technical appendix, statistical code, and dataset are available from the corresponding author at jburgis@stanford.edu. A waiver of consent was granted for this study; the presented data are anonymized and the risk of identification is low. This retrospective chart review served as a pilot study to NCT01749813 which is registered as a clinical trial. There are no conflicts of interest to disclose. No animal studies were conducted. Funding provided by private donation from George Serrurier through the Lucile Packard Foundation for Children’s Health.
There were 11 patients who met eligibility criteria and underwent detailed medical record review including eight males and three females. One patient had isolated colonic disease and the remainder had small bowel and colonic disease at diagnosis. Including pathologic descriptions of gastritis, five patients had upper gastrointestinal tract involvement at diagnosis. Three patients had history of perianal disease; one with rectal fissures, one with fistula with seton in place, and another with history of perirectal abscess. The mean age at diagnosis of CD was 11.0 ± 3.2 years (range 6.3-17 years) and mean age at start of the SCD 11.8 ± 3.0 years (range 6.6-17.6 years). Mean duration of disease until initiation of the SCD 8.8 ± 11.8 mo (range 0-44.4 mo) with five patients starting the SCD within 5 wk of diagnosis (Table 1).
Of five patients in the simple SCD group, three used the SCD alone without other medications; one patient completed an 8 wk vancomycin taper due to concurrent Clostridium difficile infection; one patient continued a 5-aminosalicylic acid and antibiotics for a fistula with seton in place (Ciprofloxacin for 4 wk and vancomycin for 12 wk). Of the six patients in the SCD with immunomodulator group, three patients started the SCD with a brief (4-6 wk course of corticosteroids) and one patient weaned off budesonide after 4 wk. Three patients in this group were on a stable dose of 6-mercaptopurine and continued 5-aminosalicylic acid. No patients were on biologics. No new medications were started during the study period (Table 1).
Three patients maintained a strict SCD diet for mean 11.8 ± 2.1 mo (range 9-14.2). The remainder of patients liberalized their diet at various times. The mean time for liberalization was 7.7 ± 4.0 mo (range 1-12). End of follow-up for all patients occurred at a mean 11.9 ± 3.0 mo (range 8.2-17.5) after starting SCD. For patients who liberalized, mean follow-up time on a liberalized diet was 4.2 ± 1.7 mo (range 1.7-6.5). Half of the patients added an illegal ingredient to their daily diet and the other patients added illegal meals or snacks at varying frequency, some as often as daily.
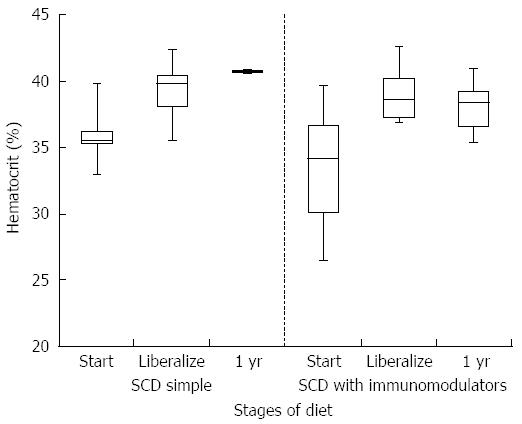
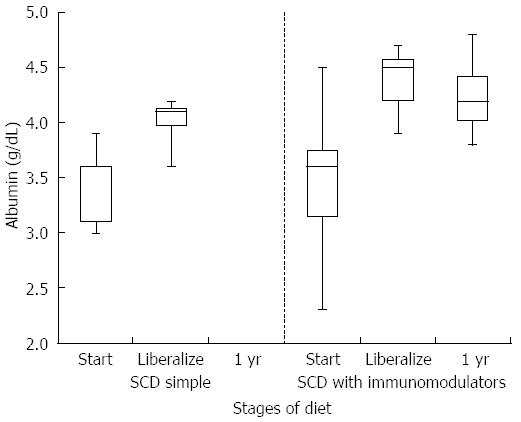
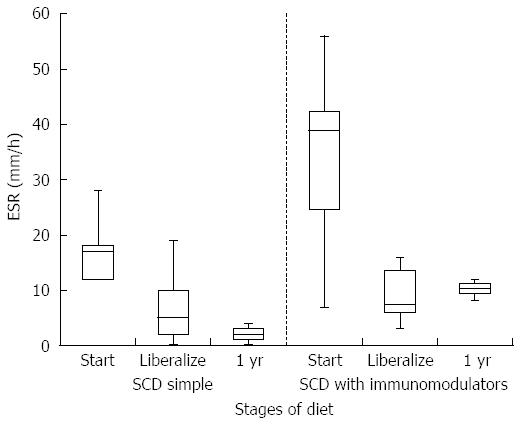
HCT values improved significantly while on a strict SCD for all patients (mean at start of SCD 35.6% vs mean at liberalization 39.7%, P-value 0.006). Improvement was similar between the simple SCD group and the SCD with immunomodulator group (P-value 0.855). After liberalization, HCT values appear to remain stable (Figure 1). The box plots for all figures are defined by 1st and 3rd quartile with median as middle line and error bars defined by minimum and maximum values. Significant improvement in albumin levels also occurred between start of SCD and time of liberalization (3.4 g/dL vs 4.2 g/dL respectively, P-value 0.002). There was a larger change in albumin in the SCD with immumodulator group compared to the SCD simple group (0.9 g/dL vs 0.6 g/dL, respectively, P-value < 0.001). One patient in the SCD simple group had an albumin level of 3.8 g/dL after liberalization, which was stable. Albumin appeared to show a small drop in SCD with immunomodulator group after liberalization (Figure 2). Inflammation as measured by erythrocyte sedimentation rate (ESR) significantly decreased in all patients while on strict SCD (mean at start of SCD 26.5 mm/h vs mean at liberalization 8.27 mm/h, P-value 0.002). There was no significant difference in the change in ESR between the SCD simple and SCD with immunomodulator groups (P-value 0.14). ESR appeared relatively stable after liberalization for both groups (Figure 3).
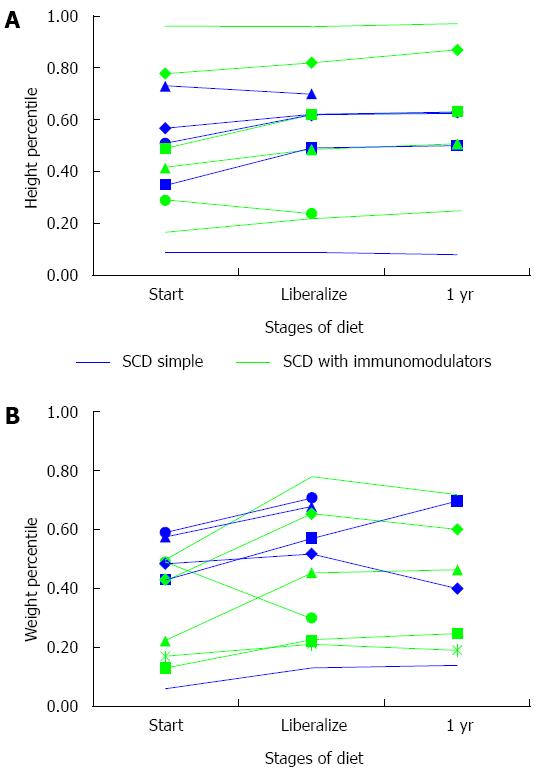
Ten (90%) children gained in weight percentile while following a strict SCD diet (Figure 4A Height percentile-for-age and 4B Weight percentile-for-age). Nine (82%) patients had stable or increased height percentiles on a strict SCD. In the eight children who liberalized, weight loss was seen in 50% with a mean decrease of less than 1 percentile. Height percentiles during liberalization increased by 1 percentile or more in all by one patient. Among all patients, only one had weight and height below the 10th percentile while on SCD, and she was using the SCD alone without other medications.
Despite preliminary data from two case series on the effectiveness of the SCD in pediatric patients with CD, it is currently unknown whether mild liberalization after initial response to a strict SCD could be detrimental. Building on the benefit of enteral nutrition in pediatric patients with CD, the SCD - even with mild liberalization - may offer a more sustainable real food therapeutic intervention. With the prospect of life-long medication therapy with intimidating side effect profiles, this nutritional therapy - even if difficult and restrictive - fills a need for patients and families willing to embrace the challenge.
Our retrospective study demonstrates improvements in anemia, albumin and inflammatory markers for patients in both cohorts when following a strict SCD. The majority of patients also demonstrated gains in both weight and height percentiles while on strict diet. After mild liberalization, most of these clinical laboratory changes were maintained for the follow-up period. Over time with liberalization, growth was relatively stable with a small mean decline in weight percentile and increased height percentile for most patients. A more thorough assessment of remission on the SCD, whether strict or liberalized, by disease index scores or measures of mucosal healing was not possible in this series due to the retrospective nature and varied provider practice. Evaluation at specific time points appeared to be limited by patients in this cohort as many had infrequent clinic visits and wanted to minimize surveillance, even blood draws.
Appropriate nutritional surveillance is paramount when dietary therapy is undertaken for CD, and adequate provision of calories is an obvious concern for a disease process inherent with inflammation, malabsorption and growth failure[24]. As a retrospective review, we were unable to directly assess calorie provision, but an improved or stable weight and height percentile for most patients is a highlight of our study. Longer term follow-up would be needed to assess improvements in weight and height velocity over time. Close follow-up and detailed dietary logs reviewed by a registered dietician would be necessary to monitor caloric intake and macro and micronutrient needs for patients on dietary therapy in a more comprehensive prospective study.
Anecdotally, our patients are attracted to the SCD vs enteral nutrition as it offers an opportunity to eat conventional palatable foods as their main caloric source, avoidance of tube feedings and a less disruptive social dynamic around meals. Adolescents face additional challenges as they gain independence in preparing meals, encounter peer pressure and consume more meals outside the home. Self-management, patient activation and adherence to therapy for IBD will continue to be a goal[25]. We have seen improved acceptance among children whose parents have proficient cooking skills and dedicated time in the kitchen. Success has also been tied to social networks and connections with other families on the SCD for additional support and resources. Practical concerns have been raised in the literature about the SCD such as financial burdens on the family, long term adherence, physician knowledge of and comfort with patients and families who want to avoid standard medication therapy[16,26]. These again are similar barriers and issues reported by pediatric gastroenterologists with the use of enteral nutrition as primary therapy for CD[15]. Use of complementary therapies in children with IBD is frequent practice, and nutritional supplements and vitamins are commonly used[27-29]. Many patients and families facing this challenging diagnosis are motivated to find an alternative approach, and dietary therapies are frequently sought out. Provider awareness is paramount to maintain a therapeutic alliance with the patient and offer appropriate clinical monitoring.
Overall, limited research exists on the use of the SCD as monotherapy or adjuvant to enteral or medication therapy. Our retrospective review provides additional support for the SCD to control inflammation as demonstrated by improvements in anemia, albumin and inflammatory markers and supporting stable growth parameters. To help understand its’ possible therapeutic mechanism, we are currently completing a prospective pilot study of pediatric patients with CD on the SCD investigating the impact on disease activity, inflammatory markers including fecal calprotectin, cytokine profiles and intestinal microbiota populations.
Crohn’s disease (CD) is thought to be triggered by autoimmunity, an exaggerated inflammatory cascade and interactions with gut microbiota. Standard therapy involves immunosuppressive medications but enteral nutrition (EN) can also modify the inflammatory response and alter the intestinal flora, providing an alternative therapeutic modality. Although EN has been shown effective to improve patient symptoms and control inflammation in pediatric CD, adherence to this formula-based diet can be challenging. The specific carbohydrate diet (SCD), which excludes complex carbohydrates such as refined sugar, gluten, starches and lactose, may provide a more palatable and flexible real-food alternative. In this study we evaluated the efficacy of a strict and liberalized SCD to improve markers of inflammation and growth parameters in pediatric CD.
Patient driven interest in nutritional therapies is on the rise as a more holistic approach to avoid potential side effects of immunosuppressive medications. The risk for malnutrition and growth failure characteristic of pediatric CD makes nutritional decisions critical. Limited studies have been conducted on the effectiveness of various dietary modifications for disease control in pediatric CD. This study provides additional experience and expertise to help guide patient treatment decisions.
In this study, a strict SCD was effective to improve laboratory values and maintain growth parameters in pediatric patients with CD. These results are similar to limited prior studies. Our study also demonstrated the feasibility of strict adherence to the SCD for a mean of 7.7 ± 4.0 mo, much longer than standard EN regimens of 8-12 wk. In addition, patient laboratory values and growth parameters were stable despite mild diet liberalization.
This study demonstrates that the SCD offers therapeutic benefit as a nutritional modification to control inflammation in pediatric CD. Mild liberalization of the SCD, after a period of strict adherence, may provide an option for maintenance therapy which can promote long term adherence. More research is needed to help understand how restricted carbohydrate content can impact disease activity.
CD is a chronic inflammatory condition of the gastrointestinal tract with a relapsing and remitting course. EN: Enteral nutrition refers to a diet restricted to a nutrient rich liquid formula. SCD: SCD avoids complex carbohydrates, excluding grains and most dairy products, focusing on meats, fruits, fresh fruits, vegetables, oils, nuts and honey.
The authors aim to investigate the SCD as nutritional therapy for maintenance of remission in pediatric CD. This is a quite non-complicated study using retrospective chart review. The authors studied 11 pediatric patients with CD who initiated the SCD as therapy at time of diagnosis or flare. Two groups defined as SCD simple (diet alone, antibiotics or 5-ASA) or SCD with immunomodulators (corticosteroids and/or stable thiopurine dosing) were followed for one year and compared on disease characteristics, laboratory values and anthropometrics. Conclusions of the authors are that disease control may be attainable with the SCD in pediatric CD. Enteral nutrition is indeed effective for both induction and maintenance therapy for pediatric CD.
| 1. | Asakura H, Suzuki K, Kitahora T, Morizane T. Is there a link between food and intestinal microbes and the occurrence of Crohn’s disease and ulcerative colitis? J Gastroenterol Hepatol. 2008;23:1794-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 718] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 3. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3598] [Article Influence: 257.0] [Reference Citation Analysis (6)] |
| 4. | Amre DK, D’Souza S, Morgan K, Seidman G, Lambrette P, Grimard G, Israel D, Mack D, Ghadirian P, Deslandres C. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn’s disease in children. Am J Gastroenterol. 2007;102:2016-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JR, Fuchs CS, Willett WC, Richter JM, Chan AT. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2013;145:970-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 544] [Cited by in RCA: 471] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 6. | Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1339] [Cited by in RCA: 1395] [Article Influence: 77.5] [Reference Citation Analysis (2)] |
| 7. | Fell JM. Control of systemic and local inflammation with transforming growth factor beta containing formulas. JPEN J Parenter Enteral Nutr. 2005;29:S126-S128; discussion S129-133, S184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Lionetti P, Callegari ML, Ferrari S, Cavicchi MC, Pozzi E, de Martino M, Morelli L. Enteral nutrition and microflora in pediatric Crohn’s disease. JPEN J Parenter Enteral Nutr. 2005;29:S173-S175; discussion S175-178, S184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 9. | Zachos M, Tondeur M, Griffiths AM. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2007;CD000542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Akobeng AK, Thomas AG. Enteral nutrition for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2007;CD005984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Yamamoto T, Nakahigashi M, Umegae S, Matsumoto K. Enteral nutrition for the maintenance of remission in Crohn’s disease: a systematic review. Eur J Gastroenterol Hepatol. 2010;22:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Gupta K, Noble A, Kachelries KE, Albenberg L, Kelsen JR, Grossman AB, Baldassano RN. A novel enteral nutrition protocol for the treatment of pediatric Crohn’s disease. Inflamm Bowel Dis. 2013;19:1374-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Haas SV, Haas MP. The treatment of celiac disease with the specific carbohydrate diet; report on 191 additional cases. Am J Gastroenterol. 1955;23:344-360. [PubMed] |
| 14. | Gottschall E. Breaking the vicious cycle- Intestinal health through diet. Baltimore: The Kirkton Press 1994; . |
| 15. | Stewart M, Day AS, Otley A. Physician attitudes and practices of enteral nutrition as primary treatment of paediatric Crohn disease in North America. J Pediatr Gastroenterol Nutr. 2011;52:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 16. | Hou JK, Lee D, Lewis J. Diet and inflammatory bowel disease: review of patient-targeted recommendations. Clin Gastroenterol Hepatol. 2014;12:1592-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 17. | Suskind DL, Wahbeh G, Gregory N, Vendettuoli H, Christie D. Nutritional therapy in pediatric Crohn disease: the specific carbohydrate diet. J Pediatr Gastroenterol Nutr. 2014;58:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Cohen SA, Gold BD, Oliva S, Lewis J, Stallworth A, Koch B, Eshee L, Mason D. Clinical and mucosal improvement with specific carbohydrate diet in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2014;59:516-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 19. | Heaton KW, Thornton JR, Emmett PM. Treatment of Crohn’s disease with an unrefined-carbohydrate, fibre-rich diet. Br Med J. 1979;2:764-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 90] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Chiba M, Abe T, Tsuda H, Sugawara T, Tsuda S, Tozawa H, Fujiwara K, Imai H. Lifestyle-related disease in Crohn’s disease: relapse prevention by a semi-vegetarian diet. World J Gastroenterol. 2010;16:2484-2495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 142] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (4)] |
| 21. | Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report. Nutr J. 2014;13:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (1)] |
| 22. | Ritchie JK, Wadsworth J, Lennard-Jones JE, Rogers E. Controlled multicentre therapeutic trial of an unrefined carbohydrate, fibre rich diet in Crohn’s disease. Br Med J (Clin Res Ed). 1987;295:517-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Brandes JW, Körst HA, Littman KP. [Sugar-free diet as long-term or interval treatment in the remission phase of Crohn disease--a prospective study]. Leber Magen Darm. 1982;12:225-228. [PubMed] |
| 24. | Critch J, Day AS, Otley A, King-Moore C, Teitelbaum JE, Shashidhar H. Use of enteral nutrition for the control of intestinal inflammation in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2012;54:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 25. | Hommel KA, Greenley RN, Maddux MH, Gray WN, Mackner LM. Self-management in pediatric inflammatory bowel disease: A clinical report of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2013;57:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Hwang C, Ross V, Mahadevan U. Popular exclusionary diets for inflammatory bowel disease: the search for a dietary culprit. Inflamm Bowel Dis. 2014;20:732-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Wong AP, Clark AL, Garnett EA, Acree M, Cohen SA, Ferry GD, Heyman MB. Use of complementary medicine in pediatric patients with inflammatory bowel disease: results from a multicenter survey. J Pediatr Gastroenterol Nutr. 2009;48:55-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Heuschkel R, Afzal N, Wuerth A, Zurakowski D, Leichtner A, Kemper K, Tolia V, Bousvaros A. Complementary medicine use in children and young adults with inflammatory bowel disease. Am J Gastroenterol. 2002;97:382-388. [PubMed] [DOI] [Full Text] |
| 29. | Day AS, Whitten KE, Bohane TD. Use of complementary and alternative medicines by children and adolescents with inflammatory bowel disease. J Paediatr Child Health. 2004;40:681-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Sergi CM S- Editor: Ma YJ L- Editor: A E- Editor: Ma S