Published online Feb 7, 2016. doi: 10.3748/wjg.v22.i5.1919
Peer-review started: July 13, 2015
First decision: August 26, 2015
Revised: September 11, 2015
Accepted: November 9, 2015
Article in press: November 9, 2015
Published online: February 7, 2016
Processing time: 195 Days and 0.3 Hours
Acute liver failure (ALF) is a reversible disorder that is associated with an abrupt loss of hepatic mass, rapidly progressive encephalopathy and devastating complications. Despite its high mortality, an emergency liver transplantation nowadays forms an integral part in ALF management and has substantially improved the outcomes of ALF. Here, we report the case of a 32-year-old female patient who was admitted with grade IV hepatic encephalopathy (coma) following drug-induced ALF. We performed an emergency auxiliary partial orthotopic liver transplantation with a “high risk” graft (liver macrovesicular steatosis approximately 40%) from a living donor. The patient was discharged on postoperative day 57 with normal liver function. Weaning from immunosuppression was achieved 9 mo after transplantation. A follow-up using CT scan showed a remarkable increase in native liver volume and gradual loss of the graft. More than 6 years after the transplantation, the female now has a 4-year-old child and has returned to work full-time without any neurological sequelae.
Core tip: The use of a “high risk” organ (i.e., steatosis graft) bears the risk of poor graft and patient survival. It is commonly recommended to use marginal and steatotic grafts in recipients who are in a relatively good clinical condition (i.e., MELD scores < 20) and avoid using them for fulminant or end-stage liver failure. In the presented case of a young female with acute liver failure, the use of a “high risk” graft (partial liver with approximately 40% macrovesicular steatosis) resulted in an excellent short and long term outcome. She survived immunosuppression weaning without any neurological sequelae after the auxiliary partial orthotopic liver transplantation.
- Citation: Duan WD, Wang XT, Wang HG, Ji WB, Li H, Dong JH. Auxiliary partial liver transplantation for acute liver failure using "high risk" grafts: Case report. World J Gastroenterol 2016; 22(5): 1919-1924
- URL: https://www.wjgnet.com/1007-9327/full/v22/i5/1919.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i5.1919
Acute liver failure (ALF), or fulminant hepatic failure, was first described in 1970 as a potentially reversible disorder[1]. It can arise from many causes and in its most severe forms leads to hepatic encephalopathy (HE), coagulopathy and even progressive multiorgan failure. Although it is rare, this life-threatening disease occurs mostly in young adults and is associated with high mortality. ALF is the most frequent indication for emergency liver transplantation in many countries[2].
Auxiliary liver transplantation (ALT) is the implantation of a donor’s partial or whole liver graft in a heterotopic or orthotopic position with the retention of all or part of the native liver[3,4]. As an alternative to an emergency liver transplantation for acute liver failure, an auxiliary partial orthotopic liver transplantation (APOLT) has been reported to have similar patient survivals as an orthotopic liver transplantation (OLT)[5,6]. With the prospect of native liver regeneration and the eventual withdrawal of immunosuppression, an APOLT seems to be the preferred choice for treating ALF in some situations[7].
Because of the risk of primary nonfunction, delayed graft function, increased complications, or poor long-term outcomes following transplantation, steatotic grafts are used very cautiously and implanted mainly in well selected patients[8]. However, with the increasing incidence of obesity and diabetes in the general population, donor graft steatosis is encountered in many potential donors[9,10]. Here we report a case of a successful APOLT in a young female suffering from acute liver failure with a high Model for End Stage Liver Disease (MELD) score (MELD score = 36.6), using a marginal graft that was from a fatty liver living donor (liver macrovesicular steatosis approximately 40%).
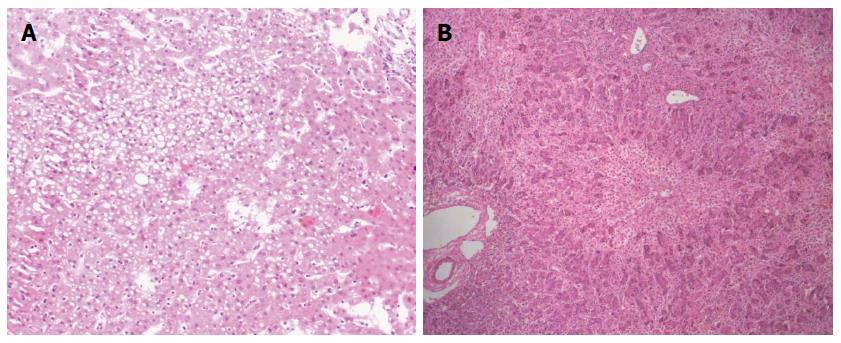
A 32-year-old female with grade IV HE (coma) was transferred to our hospital. Thrity-seven days prior to her current admission, a diagnosis of “suppurative tonsillitis” was made in another hospital. She received antibiotics without improvement. Later, she noted hyperpyrexia, jaundice, and a laboratory test revealed elevated hepatic enzymes. Five days prior to admission, she presented with signs of HE-i.e., the presence of a flapping tremor (hepatic encephalopathy grade II)-and was diagnosed with “subacute liver failure”. She was subsequently treated conservatively without improvement. Her HE deteriorated to a constant coma (grade IV HE). She was then admitted to our hospital for consideration of an emergency living donor liver transplantation (LDLT) with the diagnosis “acute liver failure, suspicion of drug-induced liver injury”. On the day of admission, the laboratory test results showed the following: pH 7.46 (range, 7.35-7.45), serum ALT 304.4 IU/L (range, 0-40 IU/L), AST 222.7 IU/L (range, 0-40 IU/L), total bilirubin 433.6 μmol/L (range, 0-21 μmol/L), platelet count 96 × 109/L (range, 100-300 × 109/L), prothrombin time 62.0 s (range, 12-16 s), INR 6.9 (range, 0.95-1.5), Cr 60.1 μmol/L (range, 30-110 μmol/L), and ammonia 82.7 μg/dL (range, 0-75 μg/dL). While serological markers of hepatitis virus, herpes simplex virus and Epstein-Barr virus infection were negative, that of cytomegalovirus showed positive. The patient remained in a hepatic coma. However, a computed tomography (CT) scan of her head revealed no signs of focal lesion or cerebral edema. Her only candidate for living donor was her 34-year-old sister, whose ABO blood type was identical to the recipient’s. The results of donor’s liver function tests were normal. According to the CT volumetric result, the graft-to-recipient weight ratio (GRWR) was 1.55% if a right lobe graft was to be used. The remnant liver volume in the donor was calculated as 35.4% to the standard liver volume. However, the donor’s height and body weight were 170 cm and 95 kg. Her body mass index (BMI) was calculated to be 32.9 kg/m2. Given an anticipated high risk of donor steatosis, a laparoscopic liver biopsy was performed before proceeding with LDLT. This revealed approximately 40% macrovesicular steatosis (Figure 1A). Thus, a right hepatectomy was considered too risky for the donor. A left graft was thought to be insufficient to support the recipient’s metabolic demand after the LDLT. Considering the report of recipient’s liver biopsy demonstrated only a 50% hepatocyte loss (Figure 1B), the GRWR was more than 0.8% if the donor’s left lobe was used according to the CT volumetric results. Given absence of cerebral edema on CT and the recipient’s young age, we finally decided to perform the APOLT using the left lobe graft after receiving an informed consent of the family and the approval of our Ethics Committee. Therefore, the recipient’s left liver (segments 2-4 and the left part of segment 1 according to Couinaud’s segmentation) was resected with a gall bladder removal. The donor’s left lobe (segments 2-4 to Couinaud’s segmentation with middle hepatic vein; 440.0 g) was orthotopically implanted. The GRWR was calculated to be 0.85%.
The graft’s left hepatic vein (LHV) and middle hepatic vein (MHV) were reconstructed to be a common orifice by venoplasty, as were the recipient’s LHV and MHV. These two orifices were anastomosed in an end-to-end fashion. The portal vein and hepatic artery of the graft were anastomosed to the corresponding recipient’s structures in an end-to-end fashion. A biliary reconstruction was achieved with a retro-colic Roux-en-Y hepaticojejunostomy. Two tubes for drainage and preventing biliary duct stricture were placed via the native liver’s right hepatic duct and the graft’s left hepatic duct, respectively. A portal flow diversion was not performed in the recipient because notably stiffness of the native liver was observed. We utilized Histidine-Triptopan-Ketoglutarate (HTK) solution to preserve the graft, and the cold ischemia time was less than 90 min. The total operative time was 775 min with a blood loss of 4000 mL. A blood transfusion of 5855 mL, including 1250 mL from an autotransfusion, was performed. The patient was then transferred to the SICU. The immunosuppression regimen consisted of tacrolimus, mycophenolate mofetil and low-dose steroids. Ganciclovir was administrated after transplantation.
On posttransplant day 7, the recipient regained consciousness. However, on posttransplant day 10, a repatching of the hepaticojejunostomy anastomosis was performed due to a bile leakage. On posttransplant day 16, she was transferred out of the SICU. After a series of supportive and rehabilitative therapy, the patient discharged on foot from our hospital on posttransplant day 57 with normal liver function and without any neurological disorders.
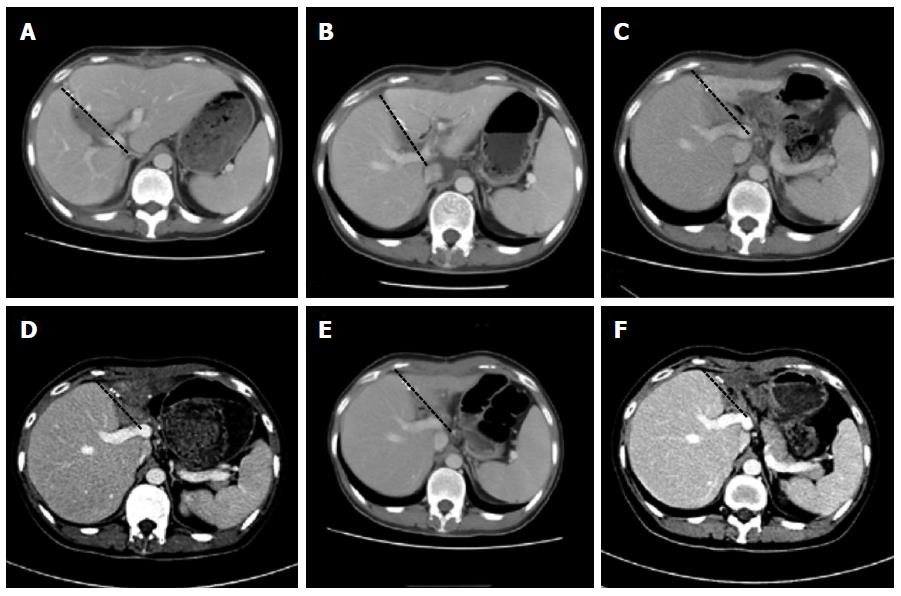
The changes in the native liver and the graft were followed using CT scans and ultrasound-guided percutaneous liver biopsies. The follow-up showed hepatocyte regeneration and a remarkable size increase in the native liver, with a notable decrease in the graft size (Figure 2). Weaning from the immunosuppression was gradually started and eventually accomplished 9 mo after the transplantation. Two years after the transplantation, the young female had a baby. The patient returned to work full-time without any neurological sequelae at review.
ALF, which was previously called fulminant hepatitis or acute hepatic necrosis, is a severe clinical syndrome that is characterized by a rapid deterioration of liver function, subsequent cognitive disturbances and coagulopathy in patients with previously normal liver function[11]. ALF is a potentially fatal condition in which spontaneous recovery is expected in only 10% to 40% of patients. In contrast the short-term survival rate after liver transplantation (LT) is 68% to 84%[12]. In the United States and Europe, patients with ALF are assigned to the highest priority category on the liver transplant waiting list because performing an emergency liver transplantation within 48 to 72 h is crucial for survival[13]. Emergency liver transplantation is now considered an integral part of the management of acute liver failure and the only curative therapy in patients fulfilling poor prognostic criteria[14]. In China, “Organ demand versus supply” is still the greatest obstacle to increasing the frequency of liver transplantation. Thus, it affects treatment decisions for acute liver failure[15]. LDLT is the preferred choice of transplant in ALF patients in counties with a limited cadaveric donor pool[16]. However, in our case, severe right lobe steatosis and a relatively small and fatty left lobe led to a potential therapeutic dilema. Thus, an APOLT with a living donor become an attractive and effective alternative because an APOLT not only solves the problems of donor safety and graft source but also allows immunosuppression to be withdrawn.
In our case, the situation was more challenging. The pretransplant MELD scores, which reflects the severity of liver impairment and has a positive correlation with liver regeneration-associated histopathological parameters (percentage of hepatocyte loss, number of proliferating hepatocytes and numbers of HPCs)[17,18], was as high as 36.6. The King’s College Hospital (KCH) criteria are one of most widespread prognostic systems to identify ALF candidates for liver transplantation. According to the description given by O’Grady[19], the KCH criteria for non-acetaminophen ALF patients are as follows: prothrombin time > 100 s or the presence of any 3 of the following: prothrombin time > 50 s, jaundice to encephalopathy time > 7 d, etiology non-A, non-B hepatitis or drug induced hepatitis, age < 10 years or > 40 years, serum bilirubin > 300 μmol/L. Unfortunately, our young patient fulfilled the criteria (Table 1), which indicated a poor prognosis. In addition, the graft was from a fatty liver living donor with moderate steatosis (approximately 40% macrovesicular steatosis). The use of a “high risk” organ (i.e., steatosis graft) is thought to bring inferior outcomes. The use of marginal and steatotic grafts is commonly recommended for recipients who are in a relatively good clinical condition (i.e., MELD score < 20) but should be avoided in fulminant or end-stage liver failure[20]. However, in our case, the young female survived immunosuppression weaning without any neurological sequelae after the APOLT. The following factors may have contributed to her fully recovery from acute liver failure.
| KCH criteria | Patient’s figures | Positive or negative |
| Prothrombin time > 100 s | 62.0 s | Negative |
| Prothrombin time > 50 s | 62.0 s | Positive |
| Jaundice to encephalopathy time > 7 d | 13 d | Positive |
| Etiology non-A, non-B hepatitis or drug induced hepatitis | Drug induced hepatitis | Positive |
| Age < 10 yr or > 40 yr | 32 yr | Negative |
| Serum bilirubin > 300 μmol/L | 433.6 μmol/L | Positive |
Patient age is an important factor in predicting the regenerative capacity of the native liver. For ALF patients, a recipient’s age strongly affects the outcomes of an emergency transplantation. Although postoperative mortality has more than doubled in those over 50 years of age[21,22], children and recipients aged < 40 years (our patient was 32 years old) appear to have a more favorable outcomes. The volume of viable hepatocytes by histologic examination is also considered to have prognostic value. The likelihood of a histological recovery appears to be minimal in livers with total hepatocyte loss at the time of ALT[23]. The critical mass associated with a good prognosis has been calculated to be between 25 and 40%(the remaining mass of our patient’s liver was calculated to be 50%)[24]. In addition, patients with signs of cerebral edema are unlikely to be suitable for an APOLT (our patient had no signs of cerebral edema)[25]. There are significantly poorer outcomes in patients with cerebral edema. A report by Chan et al[13] demonstrated that moderate to severe cerebral edema (n = 4) and mild cerebral edema (n = 7) were associated with 1-year survival rates of 25% and 44%, respectively[13]. During the transplant procedure in our case, we avoided factors that may have magnified the effect of steatosis, such as a cold ischemic time longer than 10 h, donors without a beating heart, and elderly donors[26]. In addition, the GRWR > 0.8%, portal vein inflow shunting to the native liver mass and delicate venous outflow reconstruction in our case contributed to the decreased risk of graft failure due to portal hyper perfusion[27]. We did not narrow or ligate the native liver’s portal vein because the native liver was markedly stiff. The portal flow was directed to the graft in the early stage because of a high portal pressure in the damaged and stiff native remnant liver due to ALF. Later, once the native liver has recovered and intrahepatic structure has normalized, an opposite effect may occur, which can lead to decreased portal blood supply to the graft[7,28].
The successful experiences from small-for-size graft APOLTs[29-31] may reveal that a newly implanted graft can complement the function of the partially remaining native liver during the early posttransplant period. Then, the remnant native liver expands its function in proportion to its volume growth. The key for the successful treatment of ALF is to ensure that the patient has a sufficiently functioning liver mass to survive the acute illness and allow for native liver recovery. An APOLT is a feasible solution to achieve this goal for ALF under urgent conditions, even with a notably steatotic graft.
A 32-year-old female was diagnosed with “suppurative tonsillitis” one month prior to admission. Later, she noted hyperpyrexia and jaundice, and a laboratory test revealed evaluated hepatic enzymes. Five days prior to admission to our center, she presented with signs of encephalopathy - presence of a flapping tremor (hepatic encephalopathy grade II). The hepatic encephalopathy then deteriorated to a constant coma (hepatic encephalopathy grade IV).
Acute liver failure, suspicion of drug-induced liver injury.
Acetaminophen-induced acute liver failure, liver cirrhosis, virus liver hepatitis, non-virus liver hepatitis.
pH 7.46 (range, 7.35-7.45), serum ALT 304.4 IU/L (range, 0-40 IU/L), AST 222.7 IU/L (range, 0-40 IU/L), total bilirubin 433.6 μmol/L (range, 0-21 μmol/L), platelet count 96 × 109/L (range, 100-300 × 109/L), prothrombin time 62.0 s (range, 12-16 s), INR 6.9 (range, 0.95-1.5), Cr 60.1 μmol/L (range, 30-110 μmol/L), and ammonia 82.7 μg/dL (range, 0-75 μg/dL).
A computed tomography scan of her head revealed no signs of a focal lesion or cerebral edema.
The report of the recipient’s liver biopsy demonstrated only a 50% hepatocyte loss.
An auxiliary partial orthotopic living donor liver transplantation.
Emergency liver transplantation has substantially improved the outcomes of acute liver failure and is an integral part of the management for this condition. Auxiliary partial orthotopic liver transplantation with a living donor is an attractive and effective option because auxiliary partial orthotopic liver transplantation not only solves the problems of donor safety and graft source but also allows immunosuppression to be withdrawn.
Acute liver failure is a reversible disorder associated with an abrupt loss of hepatic mass, rapidly progressive encephalopathy and devastating complications. Auxiliary partial orthotopic liver transplantation is an operation that can be performed with the use of a small-for-size graft because the graft can achieve sufficient regeneration with the support of remnant native liver.
The key for successful treatment of acute liver failure is to ensure that the patient has a sufficient functioning liver mass to survive the acute illness to allow for native liver recovery, and auxiliary partial orthotopic liver transplantation is a feasible solution to achieve this goal for acute liver failure under urgent conditions even with a notably steatotic graft.
This is a very interesting case report of emergency auxiliary living donor liver transplantation in a patient with acute liver failure.
| 1. | Trey C, Davidson CS. The management of fulminant hepatic failure. Prog Liver Dis. 1970;3:282-298. [PubMed] |
| 2. | Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2014;370:1170-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Shaw BW. Auxiliary liver transplantation for acute liver failure. Liver Transpl Surg. 1995;1:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Gubernatis G, Pichlmayr R, Kemnitz J, Gratz K. Auxiliary partial orthotopic liver transplantation (APOLT) for fulminant hepatic failure: first successful case report. World J Surg. 1991;15:660-665; discussion 665-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 125] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | van Hoek B, de Boer J, Boudjema K, Williams R, Corsmit O, Terpstra OT. Auxiliary versus orthotopic liver transplantation for acute liver failure. EURALT Study Group. European Auxiliary Liver Transplant Registry. J Hepatol. 1999;30:699-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 89] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Jaeck D, Boudjema K, Audet M, Chenard-Neu MP, Simeoni U, Meyer C, Nakano H, Wolf P. Auxiliary partial orthotopic liver transplantation (APOLT) in the treatment of acute liver failure. J Gastroenterol. 2002;37 Suppl 13:88-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Jaeck D, Pessaux P, Wolf P. Which types of graft to use in patients with acute liver failure? (A) Auxiliary liver transplant (B) Living donor liver transplantation (C) The whole liver. (A) I prefer auxiliary liver transplant. J Hepatol. 2007;46:570-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Imber CJ, St Peter SD, Lopez I, Guiver L, Friend PJ. Current practice regarding the use of fatty livers: a trans-Atlantic survey. Liver Transpl. 2002;8:545-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Burke A, Lucey MR. Non-alcoholic fatty liver disease, non-alcoholic steatohepatitis and orthotopic liver transplantation. Am J Transplant. 2004;4:686-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Angele MK, Rentsch M, Hartl WH, Wittmann B, Graeb C, Jauch KW, Loehe F. Effect of graft steatosis on liver function and organ survival after liver transplantation. Am J Surg. 2008;195:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Sugawara K, Nakayama N, Mochida S. Acute liver failure in Japan: definition, classification, and prediction of the outcome. J Gastroenterol. 2012;47:849-861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Yamashiki N, Sugawara Y, Tamura S, Nakayama N, Oketani M, Umeshita K, Uemoto S, Mochida S, Tsubouchi H, Kokudo N. Outcomes after living donor liver transplantation for acute liver failure in Japan: results of a nationwide survey. Liver Transpl. 2012;18:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Chan G, Taqi A, Marotta P, Levstik M, McAlister V, Wall W, Quan D. Long-term outcomes of emergency liver transplantation for acute liver failure. Liver Transpl. 2009;15:1696-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | O’Grady J. Timing and benefit of liver transplantation in acute liver failure. J Hepatol. 2014;60:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Huang J. Ethical and legislative perspectives on liver transplantation in the People’s Republic of China. Liver Transpl. 2007;13:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Ikegami T, Taketomi A, Soejima Y, Yoshizumi T, Sanefuji K, Kayashima H, Shimada M, Maehara Y. Living donor liver transplantation for acute liver failure: a 10-year experience in a single center. J Am Coll Surg. 2008;206:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Katoonizadeh A, Decaestecker J, Wilmer A, Aerts R, Verslype C, Vansteenbergen W, Yap P, Fevery J, Roskams T, Pirenne J. MELD score to predict outcome in adult patients with non-acetaminophen-induced acute liver failure. Liver Int. 2007;27:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 18. | Katoonizadeh A, Nevens F, Verslype C, Pirenne J, Roskams T. Liver regeneration in acute severe liver impairment: a clinicopathological correlation study. Liver Int. 2006;26:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439-445. [PubMed] |
| 20. | McCormack L, Petrowsky H, Jochum W, Mullhaupt B, Weber M, Clavien PA. Use of severely steatotic grafts in liver transplantation: a matched case-control study. Ann Surg. 2007;246:940-96; discussion 940-96;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 187] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 21. | Bernal W, Cross TJ, Auzinger G, Sizer E, Heneghan MA, Bowles M, Muiesan P, Rela M, Heaton N, Wendon J. Outcome after wait-listing for emergency liver transplantation in acute liver failure: a single centre experience. J Hepatol. 2009;50:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Wigg AJ, Gunson BK, Mutimer DJ. Outcomes following liver transplantation for seronegative acute liver failure: experience during a 12-year period with more than 100 patients. Liver Transpl. 2005;11:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Quaglia A, Portmann BC, Knisely AS, Srinivasan P, Muiesan P, Wendon J, Heneghan MA, O’Grady JG, Samyn M, Hadzic D. Auxiliary transplantation for acute liver failure: Histopathological study of native liver regeneration. Liver Transpl. 2008;14:1437-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | O’Grady J. Liver transplantation for acute liver failure. Best Pract Res Clin Gastroenterol. 2012;26:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Ciria R, Davila D, Heaton N. Auxiliary liver transplantation in children. Curr Opin Organ Transplant. 2011;16:489-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Salizzoni M, Franchello A, Zamboni F, Ricchiuti A, Cocchis D, Fop F, Brunati A, Cerutti E. Marginal grafts: finding the correct treatment for fatty livers. Transpl Int. 2003;16:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Heaton N. Small-for-size liver syndrome after auxiliary and split liver transplantation: donor selection. Liver Transpl. 2003;9:S26-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Faraj W, Dar F, Bartlett A, Melendez HV, Marangoni G, Mukherji D, Vergani GM, Dhawan A, Heaton N, Rela M. Auxiliary liver transplantation for acute liver failure in children. Ann Surg. 2010;251:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Kasahara M, Takada Y, Egawa H, Fujimoto Y, Ogura Y, Ogawa K, Kozaki K, Haga H, Ueda M, Tanaka K. Auxiliary partial orthotopic living donor liver transplantation: Kyoto University experience. Am J Transplant. 2005;5:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Kobayashi T, Sato Y, Yamamoto S, Oya H, Hara Y, Watanabe T, Kokai H, Hatakeyama K. Feasibility of auxiliary partial living donor liver transplantation for fulminant hepatic failure as an aid for small-for-size graft: single center experience. Transplant Proc. 2009;41:262-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Ohno Y, Mita A, Ikegami T, Masuda Y, Urata K, Nakazawa Y, Kobayashi A, Terada M, Ikeda S, Miyagawa S. Temporary auxiliary partial orthotopic liver transplantation using a small graft for familial amyloid polyneuropathy. Am J Transplant. 2012;12:2211-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Auzinger G S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH