Published online Feb 7, 2016. doi: 10.3748/wjg.v22.i5.1859
Peer-review started: May 11, 2015
First decision: September 29, 2015
Revised: October 19, 2015
Accepted: November 24, 2015
Article in press: November 24, 2015
Published online: February 7, 2016
Processing time: 261 Days and 0.2 Hours
AIM: To evaluate the clinical value of staging laparoscopy in treatment decision-making for advanced gastric cancer (GC).
METHODS: Clinical data of 582 patients with advanced GC were retrospectively analyzed. All patients underwent staging laparoscopy. The strength of agreement between computed tomography (CT) stage, endoscopic ultrasound (EUS) stage, laparoscopic stage, and final stage were determined by weighted Kappa statistic (Kw). The number of patients with treatment decision-changes was counted. A χ2 test was used to analyze the correlation between peritoneal metastasis or positive cytology and clinical characteristics.
RESULTS: Among the 582 patients, the distributions of pathological T classifications were T2/3 (153, 26.3%), T4a (262, 45.0%), and T4b (167, 28.7%). Treatment plans for 211 (36.3%) patients were changed after staging laparoscopy was performed. Two (10.5%) of 19 patients in M1 regained the opportunity for potential radical resection by staging laparoscopy. Unnecessary laparotomy was avoided in 71 (12.2%) patients. The strength of agreement between preoperative T stage and final T stage was in almost perfect agreement (Kw = 0.838; 95% confidence interval (CI): 0.803-0.872; P < 0.05) for staging laparoscopy; compared with CT and EUS, which was in fair agreement. The strength of agreement between preoperative M stage and final M stage was in almost perfect agreement (Kw = 0.990; 95% CI: 0.977-1.000; P < 0.05) for staging laparoscopy; compared with CT, which was in slight agreement. Multivariate analysis revealed that tumor size (≥ 40 mm), depth of tumor invasion (T4b), and Borrmann type (III or IV) were significantly correlated with either peritoneal metastasis or positive cytology. The best performance in diagnosing P-positive was obtained when two or three risk factors existed.
CONCLUSION: Staging laparoscopy can improve treatment decision-making for advanced GC and decrease unnecessary exploratory laparotomy.
Core tip: Staging laparoscopy plays an important role in advanced gastric cancer (GC) staging, which performs better than computed tomography or endoscopic ultrasound. Staging laparoscopy can improve treatment decision-making for advanced GC and decrease unnecessary exploratory laparotomy. Tumor size (≥ 40 mm), depth of tumor invasion (T4b), and Borrmann type (III or IV) were significantly correlated with either peritoneal metastasis or positive cytology. The best performance in diagnosing P-positive was obtained when two or three risk factors existed.
- Citation: Hu YF, Deng ZW, Liu H, Mou TY, Chen T, Lu X, Wang D, Yu J, Li GX. Staging laparoscopy improves treatment decision-making for advanced gastric cancer. World J Gastroenterol 2016; 22(5): 1859-1868
- URL: https://www.wjgnet.com/1007-9327/full/v22/i5/1859.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i5.1859
It is estimated that 990000 new gastric cancer (GC) cases occur in the world annually[1]. Approximately 42% of these cases occur in China. Due to the absence of a mass screening program, more than 80% of all Chinese cases are discovered at an advanced stage. To improve the survival of these patients, multimodal therapy, including surgery and systemic chemotherapy, are the current recommendations. It is well-known that the specific option of multimodal therapy is based on clinical evaluation of the TNM stage by imaging and exploratory laparotomy[2-7]. Clinically, underestimation of tumor staging by imaging may lead to unnecessary laparotomy, while overestimation of tumor staging by imaging may exclude the opportunity of potential curative surgery as a therapeutic option.
Computed tomography (CT) and endoscopic ultrasound (EUS) are the most widely used conventional modalities for preoperative staging of GC[8]. It was previously reported that CT or EUS has an accuracy of 60%-83% in determining the T stage[9-13]. Approximately 30% of laparotomies were unnecessary and resulted in significant tissue damage due to the low sensitivity of conventional examinations for peritoneal metastasis detection[14-17]. Laparotomy has high accuracy for T staging and high sensitivity for the detection of peritoneal metastasis. However, laparotomy for patients with non-resectable or disseminated disease is unnecessary[18].
To avoid unnecessary conventional exploratory laparotomy, staging laparoscopy as a minimally invasive approach has been recommended prior to surgery planning in order to exclude distant metastases[2,3,19]. For suspected M1 cases, negative findings by staging laparoscopy may help patients regain the potential of radical surgery. In addition to the M stage, the T stage has an important impact in the treatment plan. Neoadjuvant chemotherapy has been reported to increase R0 resection rate in T4a or T4b patients[3,7,20,21]. Since the stage of the tumor is adjusted by staging laparoscopy or laparotomy, parts of the treatment plans should be changed, which may improve patient survival. However, if the treatment plan is changed to neoadjuvant chemotherapy instead of surgery followed by laparotomy, there is no need for a patient to suffer from open-close surgery. Furthermore, damage would be reduced if staging laparoscopy is used instead of laparotomy.
Although staging laparoscopy has been widely described and advocated prior to surgery[2-4], discrepant results have been reported; and results based on Chinese patients have been rarely reported. Our study focuses on the number of treatment plans that changed due to staging laparoscopic findings. The aim of this study is to evaluate the clinical value of staging laparoscopy in treatment decision-making for advanced GC.
A total of 1319 GC patients underwent surgery in Nanfang Hospital from June 1, 2004 to May 1, 2014. A total of 747 patients received staging laparoscopy, in which 112 patients who had early GC and 53 patients who received neoadjuvant chemotherapy were excluded. Thus, 582 patients were enrolled in our study. Preoperative staging entailed physical examinations, fiberoptic gastroscopy, simple chest radiography, and contrast-enhanced CT of the abdomen and pelvis. EUS and lavage cytology examination were performed as needed. All patients enrolled in this study received endoscopy and biopsy, while 150 patients received EUS. Five hundred sixty-three M0 patients were planned to undergo radical surgery, and 19 M1 patients were planned to undergo palliative surgery. Among the enrolled patients, 99 patients underwent supplementary lavage cytology examination. Each patient that underwent gastrectomy had final pathologic staging, while staging for the remaining patients was established by clinical information.
Staging was principally based on the 7th edition of the American Joint Committee on Cancer (AJCC) cancer staging manual[22]. T stages were defined as follows: T2/3, tumor that invades beneath the subserosal connective tissue; T4a, tumor that invades the serosa (visceral peritoneum); and T4b, tumor that invades adjacent structures, such as the spleen, transverse colon, liver, diaphragm, pancreas, abdominal wall, adrenal gland, kidney, small intestine, and retroperitoneum. Metastasis to sites other than regional lymph nodes (distant metastasis) and positive peritoneal cytology were classified as metastatic disease, M1. N stage was not addressed in this study. Modality-specific staging criteria are shown in Table 1[10,12]. The following four situations were considered in order for treatment plans to be changed: the planned operation was radical resection, but the final operation was palliative surgery; the planned operation was palliative surgery, but the final operation was radical resection; the planned operation was gastrectomy alone, but the final operation was combined resection; the planned operation was combined resection, but the final operation was gastrectomy alone.
| Stage | Criteria |
| T stage | CT criteria |
| T2/3 | Neoplasm shows focal or diffuse thickening of gastric wall with transmural involvement, is almost well enhanced, and has smooth outer wall border and clear fat plane around tumor |
| T4a | Transmural tumor with irregular or nodular outer border and/or perigastric fat infiltration |
| T4b | Obliteration of fat plane between gastric tumor and adjacent organ or invasion of adjacent organ |
| M stage | CT criteria |
| M0 | Distant metastasis absent |
| M1 | Distant metastasis present |
| T stage | EUS criteria |
| T2/3 | Tumor extent beyond the muscularis propria up to 4 mm |
| T4a | Tumor extent beyond the muscularis propria greater than 4 mm |
| T4b | Direct extension and invasion of tumor into adjacent organ |
| M stage | EUS criteria |
| M0 | Distant metastasis absent |
| M1 | Distant metastasis present |
| T stage | SL criteria |
| T2/3 | Tumor with clear and smooth outer gastric surface |
| T4a | Tumor with nodular or irregular outer gastric surface |
| T4b | infiltration of adjacent organs |
| M stage | SL criteria |
| M0 | Distant metastasis absent |
| M1 | Distant metastasis present |
CT examinations[9,14,23] were performed using 10 mm slices. All patients underwent contrast-enhanced CT within 4 wk of the operation. Examinations were carried out according to the following protocol: 30 min before CT, after an overnight fast, 100 mL of intravenous sodium iothalamate was delivered by a power injector. Then, all patients were asked to drink 600-800 mL of water to distend the stomach. The procedure was carried out in patients, as previously described by Kim et al[23].
A total of 150 patients were examined by EUS as supplementary staging, and a radial echoendoscope capable of emitting frequencies of 7.5 and 12 MHz was used[24,25]. After an overnight fast, the procedure was carried out in patients, as Power et al[24] described. In addition, all patients were biopsied and pathology confirmed.
The patient was placed in the supine position with legs apart under general anesthesia, the surgeon stood on the left side of the patient, and the camera operator stood between the patient’s legs[19,26]. A first assistant stood at the right side of the patient and held retractors if needed. A 12 mm subumbilical incision was made and a pneumoperitoneum with CO2 under 13-15 mmHg pressure was established through a port. Laparoscopy was performed using a 30° telescope. Two other 5-mm ports were made on the left upper and lower quadrants to allow for the use of grasping and biopsy forceps. If needed, two final 5-mm ports on the right quadrants were made. A thorough abdominal cavity inspection was performed, and biopsies were taken from any tissue suspected to be cancerous. If tumors were positioned in the posterior wall, the lesser sac was inspected. The liver, diaphragm, serosal surfaces, peritoneum, omentum, and pelvic organs were systematically inspected.
Lavage cytology examination was performed in some cases of serosal invasion as a supplementary investigation. Ascitic fluid, if present, was aspirated and sent for cytology examination. If no ascites was present, peritoneal lavage was performed using 300 mL of normal saline instilled into the right and left upper quadrants and pelvis; and washings were collected for cytology examination after the abdomen was gently agitated.
Findings from CT, EUS, and staging laparoscopy were compared with the final stage of GC. Statistically comparable results between the preoperative stage determined by CT, EUS, and staging laparoscopy and the final stage were determined using weighted Kappa statistic (Kw)[27,28]. Weighted Kappa values were defined as follows: values between 0.01 and 0.20 were defined as in slight agreement, values between 0.21 and 0.40 were defined as in fair agreement, values between 0.41 to 0.60 were defined as in moderate agreement, values between 0.61 to 0.80 were defined as in substantial agreement, and values between 0.81 to 1.00 were defined as in almost perfect agreement[29]. Accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. A χ2 test was used to analyze the correlation between peritoneal metastasis or positive cytology and clinical factors, including age, gender, T stage, tumor size, histological type, Borrmann type, tumor location, ECOG score, and lymph node metastasis[27]. Tumor size was sub-classified as < 40 mm or ≥ 40 mm, and type was based on the Borrmann classification. Histological types of GC according to the WHO classification guidelines were categorized into differentiated and undifferentiated types, and tumor locations were graded based on whether the tumor involved each portion of the stomach. P-positive was defined as either peritoneal metastasis or positive cytology. P-negative was defined as neither peritoneal metastasis nor positive cytology. Receiver operating characteristic (ROC) curves were employed to evaluate the effects of several cut-off points on the diagnostic performance of the number of risk factors for P-positive. The summary accuracy measure of Youden’s index (sensitivity + specificity - 1, range: 0-1) was used. By maximal Youden’s index, the best cut-off for diagnosing P-positive was identified. All tests were two-sided, and P values < 0.05 were considered statistically significant. Data analysis was carried out with Statistical Package for Social Sciences (SPSS) version 16.0 (SPSS, Chicago, IL, United States) and Statistical Analysis System (SAS) version 9.2 (SAS, Raleigh, NC, United States).
The strength of agreement between the preoperative T stage and the final T stage was in almost perfect agreement (Kw = 0.838; 95% CI: 0.803-0.872; P < 0.05) for staging laparoscopy, in which 8.4% (49/582) were underestimated and 5.9% (28/582) were overestimated. The strength of agreement between the preoperative T stage and the final T stage was in fair agreement (Kw = 0.287; 95% CI: 0.235-0.339; P < 0.05) for CT; in which 41.2% (240/582) were underestimated, and 7.2% (42/582) were overestimated (Table 2).
| Computed tomography | Staging laparoscopy | |||||||||
| T2/3 | T4a | T4b | T2/3 | T4a | T4b | |||||
| Final stage | ||||||||||
| T2/3 | 115 | 35 | 3 | 126 | 27 | 0 | ||||
| T4a | 82 | 176 | 4 | 49 | 212 | 1 | ||||
| T4b | 22 | 136 | 9 | 0 | 0 | 167 | ||||
| Acc. | Sens. | Spec. | PPV | NPV | Acc. | Sens. | Spec. | PPV | NPV | |
| T2/3 | 76% | 75% | 76% | 53% | 90% | 87% | 82% | 89% | 72% | 93% |
| T4a | 56% | 67% | 47% | 51% | 63% | 87% | 81% | 92% | 89% | 85% |
| T4b | 72% | 5% | 98% | 56% | 72% | 100% | 100% | 100% | 99% | 100% |
Among the 150 patients who underwent EUS, the strength of agreement between the preoperative T stage and the final T stage was in almost perfect agreement (Kw = 0.831; 95% CI: 0.759-0.904; P < 0.05) for staging laparoscopy, in which 6.0% (8/150) were underestimated and 7.3% (10/150) were overestimated. The strength of agreement between preoperative T stage and the final T stage was in fair agreement (Kw = 0.344; 95%CI: 0.239-0.448; P < 0.05) for EUS, in which 10.0% (15/150) were underestimated and 42.0% (63/150) were overestimated (Table 3).
| Endoscopic ultrasound | Staging laparoscopy | |||||||||
| T2/3 | T4a | T4b | T2/3 | T4a | T4b | |||||
| Final stage | ||||||||||
| T2/3 | 28 | 42 | 3 | 64 | 9 | 0 | ||||
| T4a | 4 | 29 | 18 | 8 | 42 | 1 | ||||
| T4b | 0 | 11 | 15 | 0 | 0 | 26 | ||||
| Acc. | Sens. | Spec. | PPV | NPV | Acc. | Sens. | Spec. | PPV | NPV | |
| T2/3 | 67% | 38% | 95% | 88% | 62% | 89% | 88% | 90% | 89% | 88% |
| T4a | 50% | 57% | 46% | 35% | 68% | 88% | 82% | 91% | 82% | 91% |
| T4b | 79% | 58% | 83% | 42% | 90% | 99% | 100% | 99% | 96% | 100% |
The strength of agreement between preoperative M stage and the final M stage was in almost perfect agreement (Kw = 0.990; 95% CI: 0.977-1.000; P < 0.05) for staging laparoscopy; in which 0.3% (2/582) were underestimated, and none was overestimated. The strength of agreement between preoperative M stage and the final M stage was in slight agreement (Kw = 0.169; 95% CI: 0.090-0.243; P < 0.05) for CT; in which 20.8% (121/582) were underestimated, and 0.3% (2/582) were overestimated (Table 4).
| Computed tomography | Staging laparoscopy | ||||||||||
| M0 | M1 | M0 | M1 | ||||||||
| Final stage | M0 | 442 | 2 | 444 | 0 | ||||||
| M1 | 121 | 17 | 2 | 136 | |||||||
| Acc. | Sens. | Spec. | PPV | NPV | Acc. | Sens. | Spec. | PPV | NPV | ||
| M1 | 79% | 89% | 79% | 12% | 100% | 100% | 100% | 100% | 99% | 100% | |
Ninety-nine patients underwent peritoneal lavage cytology examination. Among these 99 patients, seven (7.1%) P0 cases had positive cytology and 16 P1 cases had negative cytology; while 76 patients were in P0CY0, and none was in P1CY1.
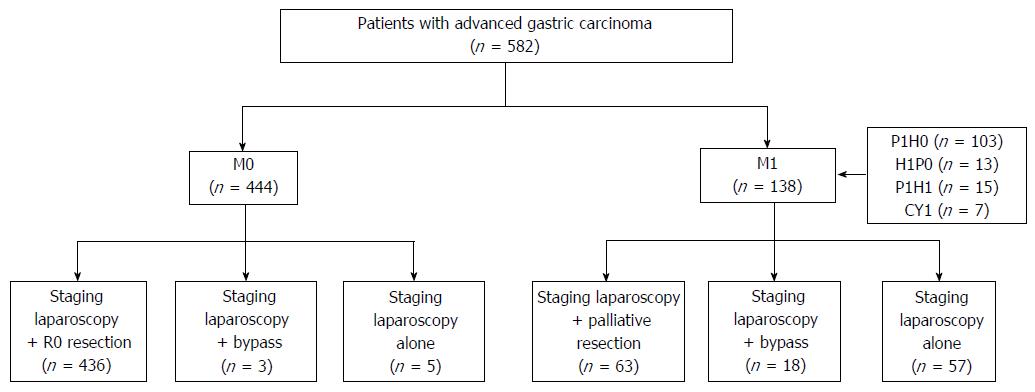
Four hundred forty-four M0 patients and 138 M1 patients were diagnosed by staging laparoscopy. For M0 cases, 436 patients underwent radical gastrectomy and eight patients in T4b received palliative operations (three bypass and five staging laparoscopy alone); and combined resection was considered to be unsuitable for such patients. For M1 cases (103 for peritoneum alone, 13 for liver alone, 15 for liver and peritoneum, and seven positive cytology), 63 patients received palliative resection due to obstruction or bleeding from the tumor (five patients underwent palliative resection as planned), 18 patients underwent bypass (two patients underwent laparoscopic bypass as planned), and 57 patients underwent staging laparoscopy alone (10 patients underwent laparoscopic exploration as planned). In summary, 21 patients underwent laparoscopic gastrojejunostomy, while 62 (10.7%) patients underwent staging laparoscopy alone (Figure 1).
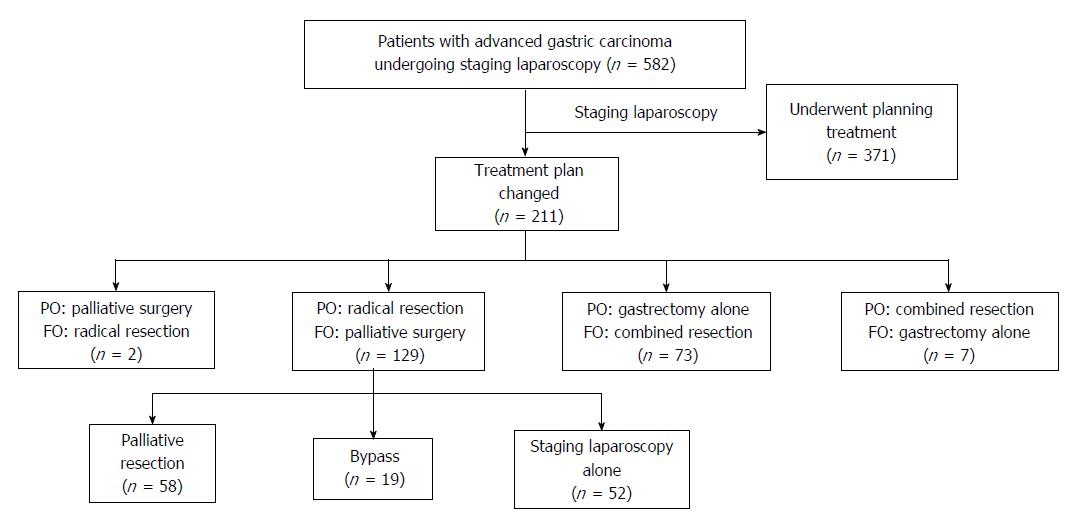
The treatment plans of 211 (36.3%) patients were changed after staging laparoscopy. One hundred twenty-nine (22.2%) patients who were planned to undergo radical gastrectomy were changed to palliative operations (58 patients for palliative gastrectomy, 19 patients for laparoscopic bypass, and 52 patients for staging laparoscopic alone) due to metastases that were not detected by CT. Two patients, who were classified as M1 by CT, underwent radical surgery after being classified as M0 at staging laparoscopy. Seventy-three (12.6%) patients who were planned to undergo gastrectomy alone as non-T4b on CT underwent combined resection. Seven (1.2%) patients who were planned to undergo combined resection as T4b on CT underwent gastrectomy alone (Figure 2).
Sixty-two (10.7%) patients underwent staging laparoscopy alone, including 10 patients who were planned to undergo staging laparoscopy for tumor detection. Among these patients, two patients developed pneumonia; and there was no mortality. Median postoperative hospital stay was 5 d (range, 2-22 d).
Clinical characteristics were compared between P-negative and P-positive patients (Table 5). Gender, tumor size, the middle third involved, depth of tumor invasion, and Borrmann type were found to be significantly correlated with P-positive status by univariate analysis. Among these factors, multivariate analysis indicated that tumor size (≥ 40 mm), depth of tumor invasion (T4b), and Borrmann type (III or IV) were significantly correlated with P-positive status (Table 6).
| Characteristic | P-negative(n = 457) | P-positive(n = 125) | P value |
| Gender | 0.001 | ||
| Male (n = 397) | 328 | 69 | |
| Female (n = 185) | 129 | 56 | |
| Age | 0.573 | ||
| < 65 (n = 441) | 334 | 88 | |
| ≥ 65 (n = 141) | 123 | 37 | |
| ECOG score | 0.751 | ||
| 0 (n = 224) | 175 | 49 | |
| 1 (n = 291) | 227 | 64 | |
| 2 (n = 67) | 55 | 12 | |
| Tumor size (mm) | < 0.001 | ||
| < 40 (n = 238) | 215 | 21 | |
| ≥ 40 (n = 346) | 242 | 104 | |
| Upper third | 0.903 | ||
| Not involved (n = 455) | 358 | 97 | |
| Involved (n = 127) | 99 | 28 | |
| Middle third | < 0.001 | ||
| Not involved (n = 401) | 333 | 68 | |
| Involved (n = 181) | 124 | 57 | |
| Lower third | 0.750 | ||
| Not involved (n = 200) | 159 | 41 | |
| Involved (n = 382) | 298 | 84 | |
| fT stage | < 0.001 | ||
| T2/3 (n = 153) | 145 | 8 | |
| T4a (n = 262) | 232 | 30 | |
| T4b (n = 167) | 80 | 87 | |
| Borrmann type | < 0.001 | ||
| Type I or II (n = 285) | 265 | 20 | |
| Type III (n = 253) | 166 | 87 | |
| Type IV (n = 44) | 26 | 18 | |
| Differentiation | 0.293 | ||
| Differentiated (n = 577) | 454 | 123 | |
| Undifferentiated (n = 5) | 3 | 2 | |
| Lymph node metastasis | 0.305 | ||
| Negative (n = 240) | 183 | 57 | |
| Positive (n = 342) | 274 | 68 |
| Variables | P value | Odd ratio | 95%CI |
| Tumor size (mm) | |||
| < 40 | 1.000 | ||
| ≥ 40 | 0.015 | 2.123 | 1.160-3.887 |
| fT stage | < 0.001 | ||
| T2/3 | 1.000 | ||
| T4a | 0.215 | 1.714 | 0.731-4.020 |
| T4b | < 0.001 | 11.54 | 4.942-26.947 |
| Borrmann type | < 0.001 | ||
| Type I or II | 1.000 | ||
| Type III | < 0.001 | 6.291 | 3.524-11.231 |
| Type IV | < 0.001 | 5.844 | 2.457-13.904 |
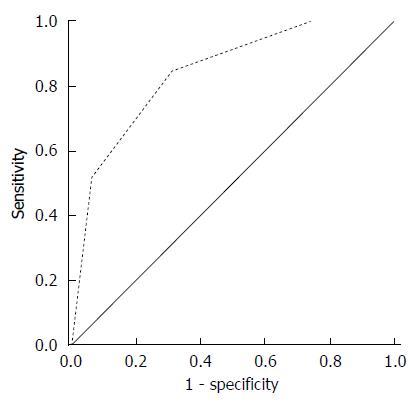
A total of 582 patients were classified according to the number of independent risk factors for P-positive status. Among these patients, 117 had no risk factor (no P-positive), 216 had only one risk factor (19 P-positive), 153 had two risk factors (41 P-positive), and 96 had three risk factors (65 P-positive) (Table 7). Based on the ROC curve (Figure 3) and the number of independent risk factors, patients were classified into two groups. The maximal Youden’s index value (0.535) of the cut-off for diagnosing P-positive patients was obtained when two or three risk factors existed. The summary of the diagnostic accuracy at this cut-off value is shown in Table 7.
| No. of independentrisk factors | P0 CY0 | P1 or CY1 | Total |
| 0, 1 | 314 | 19 | 333 |
| 2, 3 | 143 | 106 | 249 |
| Total | 457 | 125 | 582 |
| Accuracy | 72% | ||
| Sensitivity | 85% | ||
| Specificity | 69% | ||
| PPV | 43% | ||
| NPV | 94% |
Staging laparoscopy is recommended to confirm the absence of peritoneal metastasis prior to surgery in patients with advanced GC[2,3,19]. Staging laparoscopy has a significant advantage in detecting tumors by direct visualization compared with imaging modalities such as CT and EUS. Tissue magnification by laparoscopy allows for the detection of small suspicious peritoneal nodules, which may be undetected by imaging modalities[30,31]. As the entire abdominal cavity could be closely examined by laparoscopy, tiny peritoneal nodules located in the subphrenic space or Douglas pouch can be detected[32]. Staging laparoscopy can be easily performed and causes minimal tissue damage[33].
The use of staging laparoscopy should be decreased if the findings of staging laparoscopy do not change the treatment plan. In the current study, the treatment plans of 211 (36.3%) patients were changed after staging laparoscopy. Two patients classified as M1 by CT regained the opportunity for potential radical resection by staging laparoscopy; and unnecessary laparotomy was avoided in 71 (12.2%) patients, including 19 patients with laparoscopic gastrojejunostomy. Higher rates have been reported by Kakroo et al[14] (14 patients, 28%), de Graaf et al[15] (84 patients, 20.2%), Nakagawa et al[16] (22 patients, 22%), and Muntean et al[17] (17 patients, 37.8%).
Furthermore, staging laparoscopy is an invasive procedure that requires general anesthesia and pneumoperitoneum. It increases hospital cost and anesthesia time and has associated risks[33,34]. However, operation-related complications rarely occur, with rates ranging up to 4.2%[9,17,33,35]. Complications occur more often with exploratory laparotomy[32]. In our study, no perioperative and low postoperative complications (3.2%) were recorded in patients who underwent staging laparoscopy alone. As reported by Karanicolas et al[34], patients who underwent staging laparoscopy alone had a significantly lower rate of in-hospital mortality (5.3% vs 13.1%, P < 0.05) and shorter length of hospitalization (2 d vs 10 d, P < 0.05) than patients who had unnecessary laparotomy.
The major reason some laparotomies are ultimately found to be unnecessary is peritoneal metastasis. Our finding has indicated that tumor size (≥ 40 mm), depth of tumor invasion (T4b), and Borrmann type (III or IV) were independent risk factors. As reported, CA-125, tumor size (> 4 cm tumor size), Borrmann type III or IV, serosa invasion, and positive lymph node metastasis have been reported to be significantly correlated with either peritoneal metastasis or positive cytology[35-37]; which is similar to our finding. Thus, staging laparoscopy should be performed in patients with risk factors, especially with two or three risk factors. Moreover, if lymph nodes are found too close or adherent to the celiac trunk when detected by CT, the relevant area should also be examined to confirm resectability of the disease at staging laparoscopy. These patients may require neoadjuvant chemotherapy to increase R0 resection rate.
Although staging laparoscopy had a good performance in detecting P-positive status, in our study, it failed to find peritoneal disseminations in two cases. Staging laparoscopy using 5-aminolevulinic acid (ALA)-mediated photodynamic diagnosis in advanced GC has been reported to be helpful in detecting peritoneal metastasis[18,38]. Kishi et al[38] reported that the tumor detection rate using 5-ALA photodynamic diagnosis was significantly higher than using white light (72% vs 39%, P < 0.0001) and two peritoneal metastases that were invisible under white light were detected under induced fluorescence. However, this procedure requires special equipment currently not routinely available in clinical practice, like D-LIGHT System.
High-quality staging laparoscopy combined with cytology examination is an important means for improving the clinical evaluation of tumors. The current study on staging laparoscopy procedure included the examination of all abdominal organs, assessment of tumor depth, washing cytology, and biopsy of suspicious peritoneal nodules.
This study has some limitations. Staging laparoscopy has shortcomings compared to CT and EUS. For example, lymph node metastasis is not easily detected by laparoscopy. The standard of cytology examination is not unified, which may result in missed diagnosis of positive cases.
Staging laparoscopy is a valuable technique in staging advanced GC and has an important role in the detection of occult intra-abdominal metastasis not detected by conventional radiological staging. The strengths of the agreement between the staging laparoscopy stage and the final stage were almost perfect. Staging laparoscopy can improve treatment decision-making for advanced GC and decrease unnecessary exploratory laparotomy.
Due to the absence of a mass screening program, more than 80% of all Chinese cases of gastric cancer (GC) are discovered at an advanced stage. To improve patient survival, multimodal therapy including surgery and systemic chemotherapy are the current recommendations. It is well-known that the specific option of multimodal therapy is based on clinical evaluation of the TNM stage by imaging and exploratory laparotomy. The aim of this study is to evaluate the clinical value of staging laparoscopy in treatment decision-making for advanced GC.
Laparoscopy used for staging advanced GC is becoming more common. Few prior reports have analyzed the clinical value of staging laparoscopy in treatment decision-making for advanced GC.
In this study, staging laparoscopy was found to be a useful tool for staging advanced GC. It was more effective especially in detecting peritoneal metastasis. Three clinical characteristics were found to be the risk factors of P-positive advanced GC.
This study suggests that staging laparoscopy has a better performance in staging advanced GC. Undergoing staging laparoscopy prior to open surgery can help to decrease unnecessary exploratory laparotomy.
The weighted Kappa statistic value provides a measure of the agreement between two scorers that classify observations into one of several groups. The range of the weighted Kappa value is from 0 to 1. When the value approaches 1, the agreement is closer to the gold standard. Youden’s index was suggested as a way of summarizing the performance of a diagnostic test. Its value ranges from 0 to 1, in which a zero value means that a diagnostic test gives the same proportion of positive results for groups with and without the disease, such as when the test is useless. A value of one indicates that there are no false positives or false negatives, such as when the test is perfect.
The author of this paper evaluated the clinical value of staging laparoscopy in treatment decision-making for advanced GC. Staging laparoscopy performed better in staging advanced GC. Undergoing staging laparoscopy prior to open surgery can help decrease unnecessary exploratory laparotomy. Tumor size (≥ 40 mm), depth of tumor invasion (T4b), and Borrmann type (III or IV) are significantly correlated with either peritoneal metastasis or positive cytology. The best performance in diagnosing P-positive was obtained when two or three risk factors existed.
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25604] [Article Influence: 1706.9] [Reference Citation Analysis (10)] |
| 2. | National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology.2013 (ver. 2). Available from: http://www.nccn.org. |
| 3. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1911] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 4. | Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737-3743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1542] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 5. | Son T, Hyung WJ, Lee JH, Kim YM, Noh SH. Minimally invasive surgery for serosa-positive gastric cancer (pT4a) in patients with preoperative diagnosis of cancer without serosal invasion. Surg Endosc. 2014;28:866-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Lee SW, Nomura E, Bouras G, Tokuhara T, Tsunemi S, Tanigawa N. Long-term oncologic outcomes from laparoscopic gastrectomy for gastric cancer: a single-center experience of 601 consecutive resections. J Am Coll Surg. 2010;211:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Yano M, Yasuda T, Fujiwara Y, Takiguchi S, Miyata H, Monden M. Preoperative intraperitoneal chemotherapy for patients with serosa-infiltrating gastric cancer. J Surg Oncol. 2004;88:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Hwang SW, Lee DH. Is endoscopic ultrasonography still the modality of choice in preoperative staging of gastric cancer? World J Gastroenterol. 2014;20:13775-13782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Blackshaw GR, Barry JD, Edwards P, Allison MC, Thomas GV, Lewis WG. Laparoscopy significantly improves the perceived preoperative stage of gastric cancer. Gastric Cancer. 2003;6:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Jürgensen C, Brand J, Nothnagel M, Arlt A, Neser F, Habeck JO, Schreiber S, Stölzel U, Zeitz M, Hampe J. Prognostic relevance of gastric cancer staging by endoscopic ultrasound. Surg Endosc. 2013;27:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Leake PA, Cardoso R, Seevaratnam R, Lourenco L, Helyer L, Mahar A, Law C, Coburn NG. A systematic review of the accuracy and indications for diagnostic laparoscopy prior to curative-intent resection of gastric cancer. Gastric Cancer. 2012;15 Suppl 1:S38-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Seevaratnam R, Cardoso R, McGregor C, Lourenco L, Mahar A, Sutradhar R, Law C, Paszat L, Coburn N. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta-analysis. Gastric Cancer. 2012;15 Suppl 1:S3-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 13. | Otsuji E, Kuriu Y, Ichikawa D, Okamoto K, Hagiwara A, Yamagishi H. Tumor recurrence and its timing following curative resection of early gastric carcinoma. Anticancer Res. 2003;23:3499-3503. [PubMed] |
| 14. | Kakroo SM, Rashid A, Wani AA, Akhtar Z, Chalkoo MA, Laharwal AR. Staging Laparoscopy in Carcinoma of Stomach: A Comparison with CECT Staging. Int J Surg Oncol. 2013;2013:674965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | de Graaf GW, Ayantunde AA, Parsons SL, Duffy JP, Welch NT. The role of staging laparoscopy in oesophagogastric cancers. Eur J Surg Oncol. 2007;33:988-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Nakagawa S, Nashimoto A, Yabusaki H. Role of staging laparoscopy with peritoneal lavage cytology in the treatment of locally advanced gastric cancer. Gastric Cancer. 2007;10:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Muntean V, Mihailov A, Iancu C, Toganel R, Fabian O, Domsa I, Muntean MV. Staging laparoscopy in gastric cancer. Accuracy and impact on therapy. J Gastrointestin Liver Dis. 2009;18:189-195. [PubMed] |
| 18. | Yoon H, Lee DH. New approaches to gastric cancer staging: beyond endoscopic ultrasound, computed tomography and positron emission tomography. World J Gastroenterol. 2014;20:13783-13790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Society of American Gastrointestinal and Endoscopic Surgeons. Guidelines for diagnostic laparoscopy. Available from: http://www.sagescms.org. |
| 20. | Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210-5218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 548] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 21. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4736] [Article Influence: 236.8] [Reference Citation Analysis (7)] |
| 22. | Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. New York: Springer 2010; . |
| 23. | Kim TH, Kim JJ, Kim SH, Kim BS, Song HJ, Na SY, Boo SJ, Kim HU, Maeng YH, Hyun CL. Diagnostic value of clinical T staging assessed by endoscopy and stomach protocol computed tomography in gastric cancer: the experience of a low-volume institute. J Gastric Cancer. 2012;12:223-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Power DG, Schattner MA, Gerdes H, Brenner B, Markowitz AJ, Capanu M, Coit DG, Brennan M, Kelsen DP, Shah MA. Endoscopic ultrasound can improve the selection for laparoscopy in patients with localized gastric cancer. J Am Coll Surg. 2009;208:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Kikuchi D, Iizuka T, Hoteya S, Yamada A, Furuhata T, Yamashita S, Domon K, Nakamura M, Matsui A, Mitani T. Prospective Study about the Utility of Endoscopic Ultrasound for Predicting the Safety of Endoscopic Submucosal Dissection in Early Gastric Cancer (T-HOPE 0801). Gastroenterol Res Pract. 2013;2013:329385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Hu Y, Ying M, Huang C, Wei H, Jiang Z, Peng X, Hu J, Du X, Wang B, Lin F. Oncologic outcomes of laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective cohort study from China. Surg Endosc. 2014;28:2048-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Altman DG. Practical statistics for medical research. London: Chapman & Hall/CRC 1991; . |
| 28. | Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70:213-220. [PubMed] |
| 29. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. [PubMed] |
| 30. | Huang B, Sun Z, Wang Z, Lu C, Xing C, Zhao B, Xu H. Factors associated with peritoneal metastasis in non-serosa-invasive gastric cancer: a retrospective study of a prospectively-collected database. BMC Cancer. 2013;13:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Burbidge S, Mahady K, Naik K. The role of CT and staging laparoscopy in the staging of gastric cancer. Clin Radiol. 2013;68:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | D’Ugo DM, Pende V, Persiani R, Rausei S, Picciocchi A. Laparoscopic staging of gastric cancer: an overview. J Am Coll Surg. 2003;196:965-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Song KY, Kim JJ, Kim SN, Park CH. Staging laparoscopy for advanced gastric cancer: is it also useful for the group which has an aggressive surgical strategy? World J Surg. 2007;31:1228-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Karanicolas PJ, Elkin EB, Jacks LM, Atoria CL, Strong VE, Brennan MF, Coit DG. Staging laparoscopy in the management of gastric cancer: a population-based analysis. J Am Coll Surg. 2011;213:644-651, 651.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Tsuchida K, Yoshikawa T, Tsuburaya A, Cho H, Kobayashi O. Indications for staging laparoscopy in clinical T4M0 gastric cancer. World J Surg. 2011;35:2703-2709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Hur H, Lee HH, Jung H, Song KY, Jeon HM, Park CH. Predicting factors of unexpected peritoneal seeding in locally advanced gastric cancer: indications for staging laparoscopy. J Surg Oncol. 2010;102:753-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Fujimura T, Kinami S, Ninomiya I, Kitagawa H, Fushida S, Nishimura G, Kayahara M, Shimizu K, Ohta T, Miwa K. Diagnostic laparoscopy, serum CA125, and peritoneal metastasis in gastric cancer. Endoscopy. 2002;34:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Kishi K, Fujiwara Y, Yano M, Inoue M, Miyashiro I, Motoori M, Shingai T, Gotoh K, Takahashi H, Noura S. Staging laparoscopy using ALA-mediated photodynamic diagnosis improves the detection of peritoneal metastases in advanced gastric cancer. J Surg Oncol. 2012;106:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Namikawa T S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Wang CH