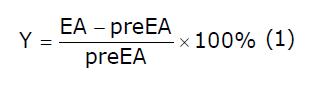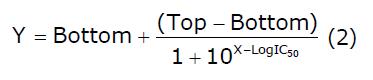Published online Feb 7, 2016. doi: 10.3748/wjg.v22.i5.1834
Peer-review started: May 12, 2015
First decision: July 19, 2015
Revised: August 4, 2015
Accepted: November 9, 2015
Article in press: November 9, 2015
Published online: February 7, 2016
Processing time: 255 Days and 17.9 Hours
AIM: To investigate whether electroacupuncture (EA) at ST25 affects jejunal motility in vivo and if so, whether a sympathetic pathway is involved.
METHODS: Jejunal motility was assessed using a manometric balloon placed in the jejunum approximately about 3-5 cm away from the suspensory ligament of the duodenum in anesthetized animals. The effects of EA at ST25 were measured in male Sprague-Dawley rats, some of which were treated with propranolol or clenbuterol (EA intensities: 1, 3, 5, 7, and 9 mA), and in male transient receptor potential vanilloid-1 (TRPV1) (capsaicin receptor) knockout mice (EA intensities: 1, 2, and 4 mA).
RESULTS: Anesthetized rats exhibited three types of fasting jejunal motor patterns (types A, B, and C), and only type C rats responded to EA stimulation. In type C rats, EA at ST25 significantly suppressed the motor activity of the jejunum in an intensity-dependent manner. The inhibitory effect of EA was weakened by propranolol (β adrenoceptor antagonist) and disappeared with clenbuterol (β adrenoceptor agonist) induced inhibition of motility, suggesting that the effect of EA on motility is mediated via a sympathetic pathway. Compared with wild-type mice, EA at ST25 was less effective in TRPV1 knockout mice, suggesting that this multi-modal sensor channel participates in the mechanism.
CONCLUSION: EA at ST25 was found to inhibit jejunal motility in an intensity-dependent manner, via a mechanism in which sympathetic nerves and TRPV1 receptors play an important role.
Core tip: Evidence from clinical studies has shown that acupuncture on the abdomen was effective for both diarrhea and abdominal pain, suggesting that acupuncture at this point may inhibit gastrointestinal motility. However, its mechanism has not been established. In this work, we concluded that abdominal electroacupuncture (EA) (at ST25) may alter jejunal motility via a somatosympathetic reflex, and that transient receptor potential vanilloid-1 may serve as an EA-responsive channel mediating this reflex.
- Citation: Yu Z, Zhang N, Lu CX, Pang TT, Wang KY, Jiang JF, Zhu B, Xu B. Electroacupuncture at ST25 inhibits jejunal motility: Role of sympathetic pathways and TRPV1. World J Gastroenterol 2016; 22(5): 1834-1843
- URL: https://www.wjgnet.com/1007-9327/full/v22/i5/1834.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i5.1834
Intestinal motility is one of the most critical physiological functions for transportation and absorption of food. Intestinal dysmotility occurs in a range of diseases[1-5], including chronic intestinal pseudo-obstruction, chronic constipation, and irritable bowel syndrome (IBS), and symptoms include abdominal pain, nausea, and constipation. As one of the major pathophysiological features of functional gastrointestinal disorders, intestinal dysmotility is becoming a serious public health problem[6] resulting in decreased quality of life of individuals and an economic burden, however, at present, options for treatment are limited[5].
Acupuncture is a traditional medical treatment that has been practiced in China for thousands of years. It is becoming increasingly accepted and has even been practiced in emergency departments to treat gastrointestinal symptoms[7]. Its therapeutic effects on vomiting and nausea were first formally confirmed in November 1997[8]. A considerable number of studies have shown that electroacupuncture (EA) has therapeutic effects on gastrointestinal disorders[9-15]; examples include restoration of impaired gastric accommodation in vagotomized dogs and amelioration of impaired gastric motility and slow waves induced by rectal distension. The mechanism of such effects has mainly been attributed to modulation of the autonomic nervous system[16,17].
Evidence from clinical studies has shown that acupuncture on the abdomen was effective for both diarrhea and abdominal pain, suggesting that acupuncture at this point may inhibit gastrointestinal motility and/or reduce gastrospasms[10,18,19]. In animal models, it was found that acupuncture at the hind paw increased gastric and duodenal motility via parasympathetic pathways, whereas application of acupuncture at the abdomen showed an inhibitory effect by increasing sympathetic activity[8]. However, little effort has been made to investigate the effect of EA on jejunal motility.
Activation of Aδ- and C-fibers forms the basis of acupuncture’s effects on autonomic nervous function[20]. The capsaicin receptor or transient receptor potential vanilloid-1 (TRPV1) is a member of the TRP-cation-channel superfamily[21], and it is mainly expressed in Aδ- and C-fibers. TRPV1 acts as a sensor for heat, pH, and inflammation[22], and it has also been shown to participate in mechanosensation[23]; therefore, TRPV1 channels could be affected by the physical stimulation associated with acupuncture, thus producing an autonomic response[24].
We hypothesized that abdominal EA (at ST25) may alter jejunal motility via a somatosympathetic reflex, and that TRPV1 may serve as an EA-responsive channel mediating this reflex.
Sprague-Dawley rats (male, 180-230 g) were purchased from the Model Animal Research Center of Nanjing Medical University (Nanjing, China; license number: SCXK 2013-0005).
TRPV1-/- mice (male, 22-28 g, B6.129X1-TRPV1tm1Jul/NJU, J003770) and their wild-type counterparts (WT male, 22-28 g) were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China).
All animals had free access to food and water, and were housed under controlled environmental conditions (22 °C, 40%-60% relative humidity, 12/12 h light/dark cycle). After two weeks of feeding adaptation, animals were used for our experiments.
The drugs used in the experiments were urethane (U2500, Sigma, St. Louis, MO, United States), propranolol hydrochloride (P0084, Sigma), and clenbuterol hydrochloride (C5423, Sigma). The concentration and doses of the drugs were as follows: urethane (20%; 0.8 g/kg), propranolol (0.4%; initial dose, 1.0 mL·kg-1; maintenance dose, 40 μL·min-1·kg-1), and clenbuterol (0.2%; maintenance dose, 80 μL·min-1·kg-1).
The animals were fasted overnight with free access to water and then anesthetized with urethane (ip). The trachea was cannulated to keep the respiratory tract unobstructed. A catheter was inserted into the left jugular vein for drug administration. A small incision was made in the jejunum, about 3-5 cm away from the suspensory ligament of the duodenum. A small balloon made of flexible condom rubber was inserted into the jejunal area via the incision. The pressure in the balloon was measured by a transducer (YP201; Chengdu Instrument Factory, Chengdu, China) and the signal was collected with a physiological signal-acquisition system (RM6240; Chengdu Instrument Factory) for further analysis. The jejunal baseline pressure was kept between 0.8 and 1.0 kPa. During the experiment, the animal was kept on an electric heating board to maintain a temperature of 37 °C ± 0.5 °C.
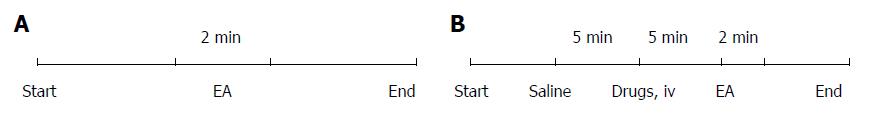
The experimental time course for the EA-only rodent (no drugs) is shown in Figure 1A, while that for rats treated with both EA and drugs is shown in Figure 1B. Different intensities of EA (1, 3, 5, 7, and 9 mA for rats; 1, 2, and 4 mA for mice) were applied at ST25 in an ascending order. The higher intensity stimuli could only be applied when jejunal motility recovered to the baseline.
A pair of needles (diameter, 0.3 mm) were inserted (approximately 5 mm deep) into the unilateral Tianshu point (ST25). ST25 was located bilaterally, 5 mm lateral to the intersection between the upper 2/3 and the lower 1/3, in the line between the xiphoid process and the pubic symphysis upper border[25]. EA was performed at unilateral ST25 for 2 min. The needles were connected to a Han electroacupuncture therapeutic apparatus (LH402A; Beijing Huawei Industrial Development Corporation, Beijing, China). The parameters were set as follows: pulse duration: 1 ms; pulse frequency: 2 Hz and 15 Hz alternate; wave form: square wave.
The average amplitude over two minutes of jejunal motility (contractions; kPa) during EA was compared with the basal amplitude (pre EA, average amplitude over two minutes). If a decrease of over 5% was observed versus the basal amplitude[16], the response was considered to be inhibited. Equation (1) was fitted to the percentage decrease of amplitude.
Math 7
Data were analyzed using SPSS 18.0 software (SPSS, Chicago, United States). Differences between two groups were compared using an independent-sample t test. P < 0.05 was considered statistically significant. All data are expressed as mean ± standard error (SE). The data-curve fitting for different intensities used equation (2):
Math 8
where X is log of intensity, Y is response, decreasing as X increases, Top and Bottom are plateaus in the same unit as Y, and LogIC50 uses the same log unit as X.
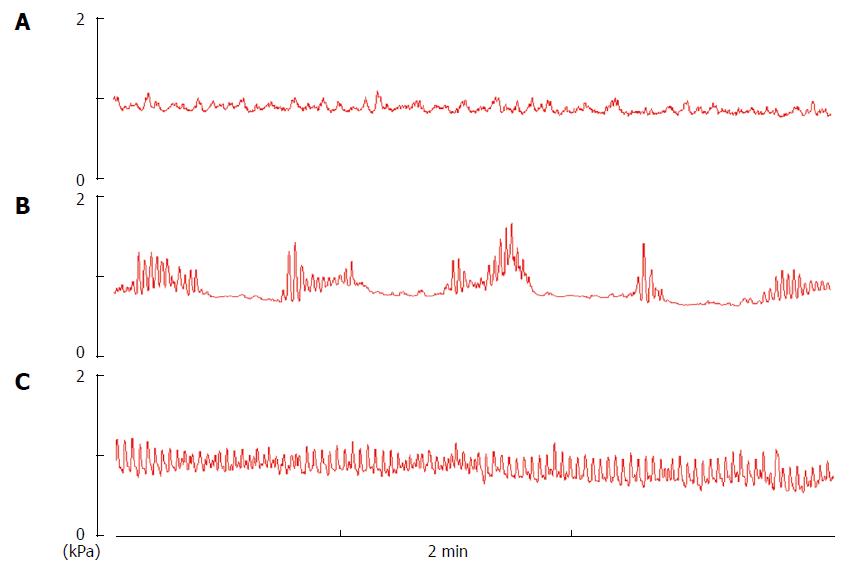
In anesthetized rats, three types of fasting jejunal motor patterns were generally observed under resting conditions (Figures 2 and 3). Type A showed a quiescent period with virtually no contraction when balloon pressure was increased to 0.8-1.0 kPa by expanding the volume of the balloon to 0.1 mL with warm water. Type B showed arrhythmic contractions at a frequency of 1-2/min, and the contractile amplitude was about 0.4-0.8 kPa. Type C showed cyclic contractions at a frequency of 3-8/min, and the contractile amplitude was about 0.5-1.0 kPa.
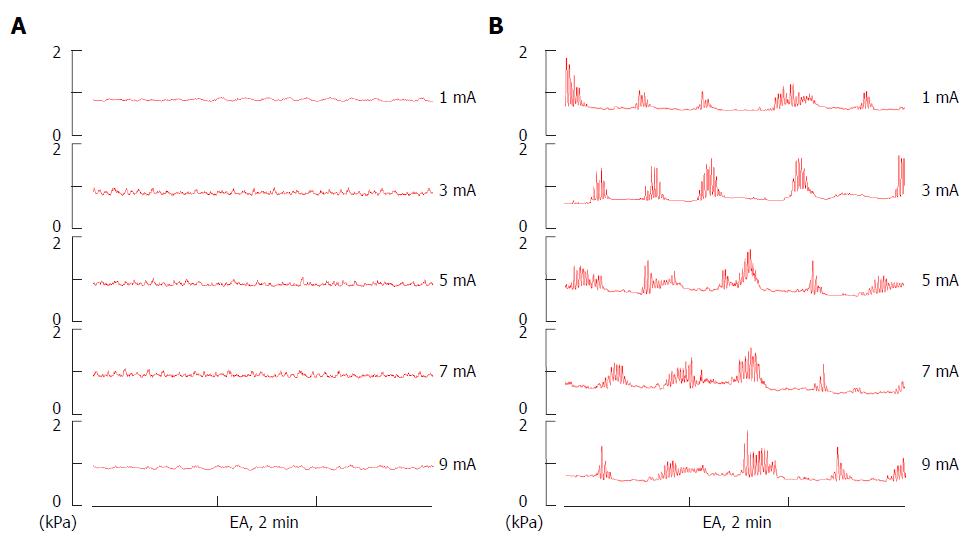
In the resting condition of anesthetized mice, no typical jejunal motor patterns described above but arrhythmic jejunal contractions at a frequency of 12-30/min were observed in the TRPV1-/- and WT mice, and the contractile amplitude was approximately 0.05-0.15 kPa, when balloon pressure was maintained at about 0.1-0.2 kPa by filling the balloon with 0.05 mL of warm water (Figure 4A and B). The jejunal motility observed in TRPV1-/- and WT mice showed no visible difference.
To investigate the effect of EA at ST25 on jejunal motility, different stimulation intensities (1, 3, 5, 7, and 9 mA) were applied to the needles inserted in the rat abdomen. EA stimulation showed no effect on jejunal motility at any intensity in types A (n = 8; Figure 3A) and B rats (n = 8; Figure 3B).
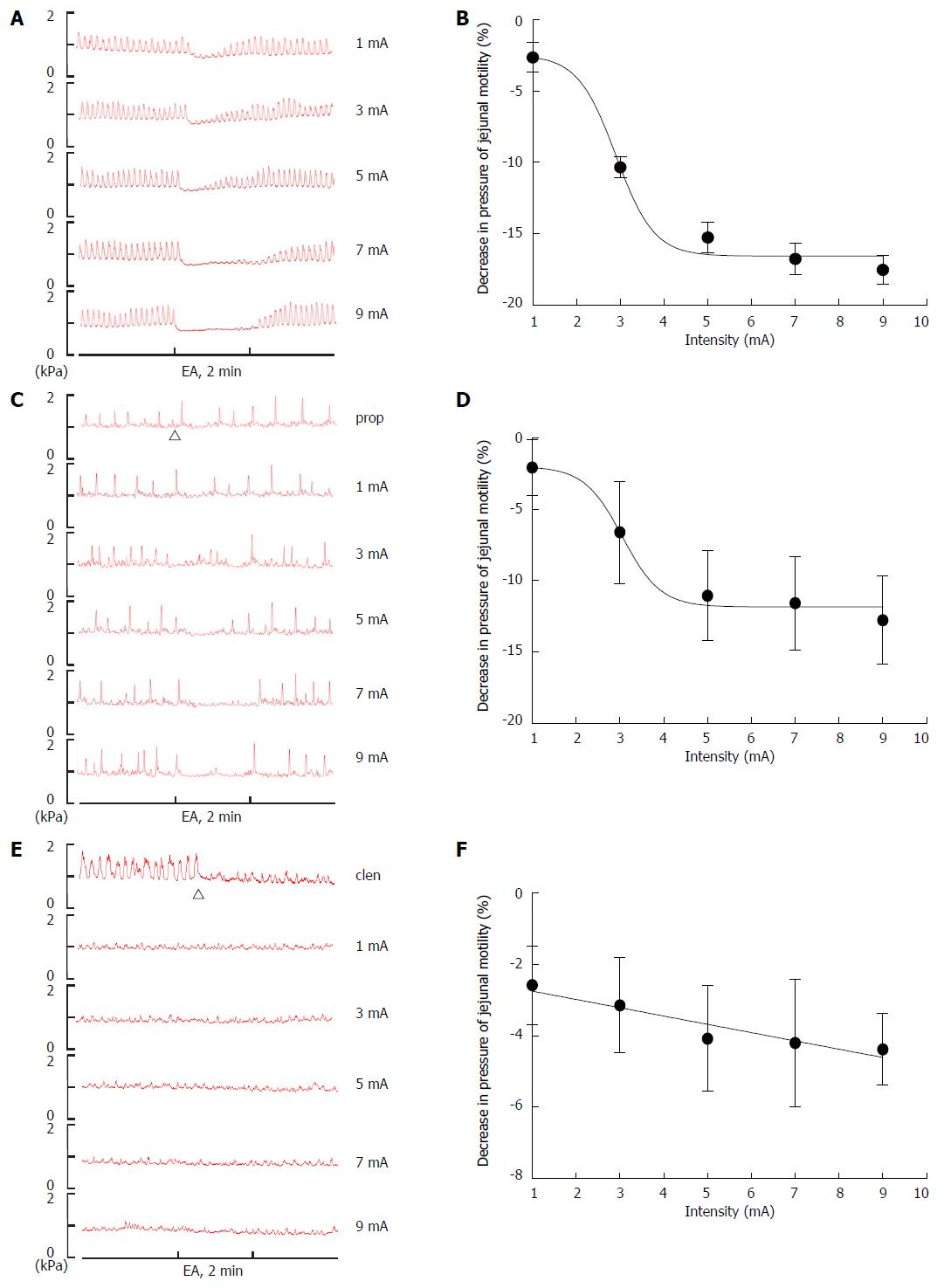
Compared to type C rats (n = 8), EA at ST25 significantly suppressed jejunal motor activity at all intensities except 1 mA (Figure 5A), and the logIC50 value of the stimulation was 2.90 ± 0.15 mA (Figure 5B). An important feature of the EA effect was its dependence on stimulation intensity. Jejunal contraction amplitude decreased with increasing EA intensity, in a sigmoidal manner down to a plateau at intensities ≥ 5 mA.
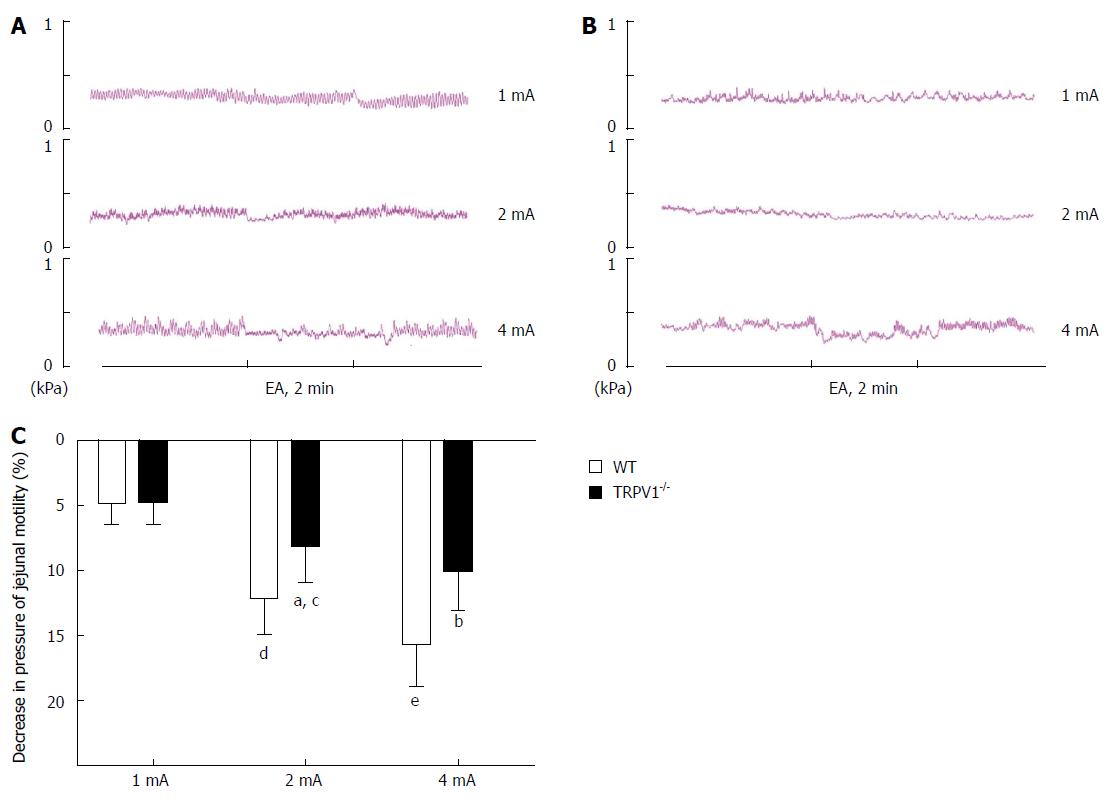
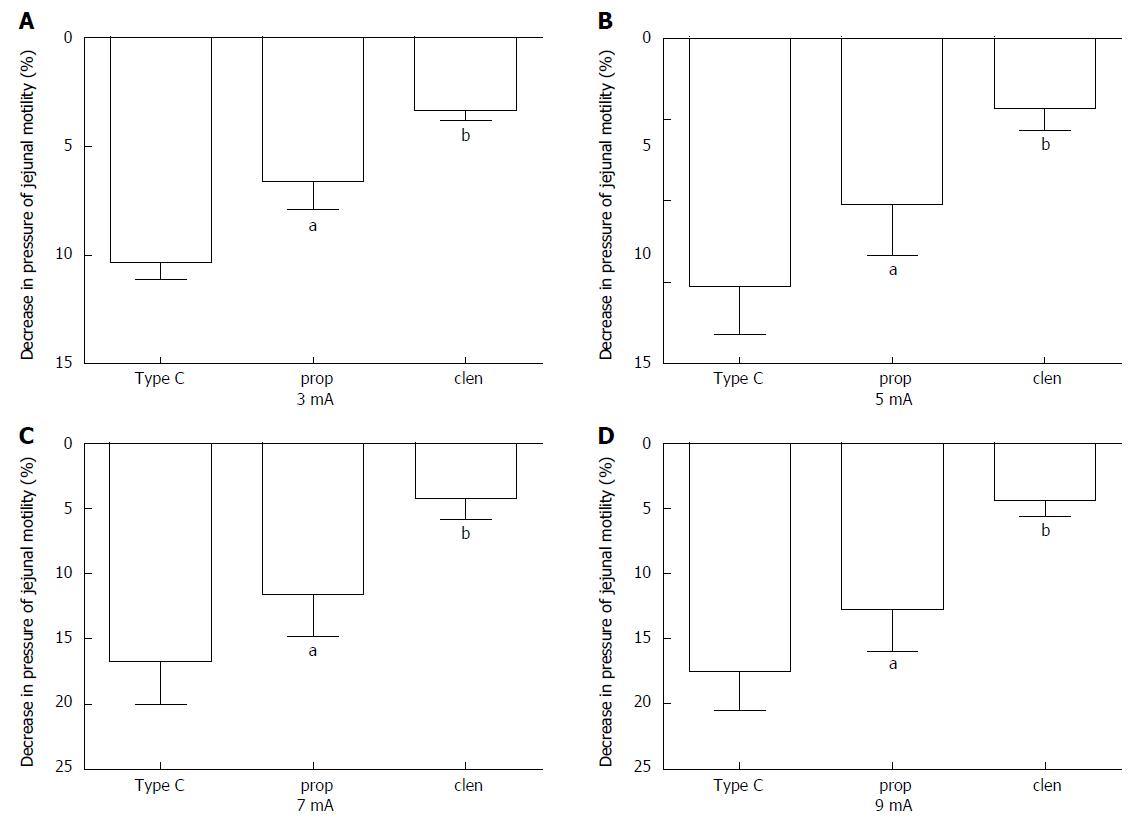
To determine whether sympathetic pathways were involved in the effect of EA at ST25 in regulating jejunal motility, either the β adrenoceptor antagonist, propranolol (n = 8), or the β adrenoceptor agonist, clenbuterol (n = 8), was administered before EA on type C rats. After administration, different EA intensities (1, 3, 5, 7, and 9 mA) were applied to rats in an ascending order. The propranolol results produced a curve with a similar shape to that for the drug-free rats, but the logIC50 value was about 6% higher at 3.06 ± 0.18 mA (Figure 5C and D). Propranolol significantly reduced the EA effect at all intensities above 1 mA (Figure 6A-D). On the other hand, clenbuterol alone almost completely inhibited motility, and EA at ST25 did not modify this effect significantly (Figure 5E, 5F, 6A-D). Taken together, these results suggest that sympathetic pathways may mediate the inhibitory effect of EA.
To explore the afferent pathway underlying the EA stimulation in regulating jejunal motility, TRPV1 knockout mice were used in the study. In WT mice (n = 8), an intensity-dependent decrease in jejunal motility was also observed as the result of EA stimulation at ST25 in the rats. At an intensity of 1 mA, EA produced no significant effect, but at 2 mA, a slight inhibitory effect was observed, and then at 4 mA the effect increased significantly in magnitude. In TRPV1-/- mice (n = 8), the effect of EA at 2 mA and 4 mA was significantly reduced when compared to that for WT mice (Figure 4A-C); what’s more important, it showed no significance between 2 mA and 4 mA, that is, intensity-dependent inhibition did not occur between them, suggesting that TRPV1 receptor may serve as one of the afferent pathways underlying the EA stimulation in regulating jejunal motility.
Two distinct patterns of small intestinal motility were found after fasting and feeding[17]. The typical motor pattern in the interdigestive state is the migrating motor complex (MMC)[26]. The MMC consists of three phases: I, a period of motor quiescence; II, a period of irregular low amplitude contractions; and III, a period of regular high amplitude contractions. Compared to human and dogs, the MMC cycle in rodent is more irregular. It has been shown in rats and mice that the MMC was observed in the small bowel[27,28], but it is rather difficult to distinguish three phases, so they are denoted as phase-I-like contractions, phase-II-like contractions, and phase-III-like contractions in rats, and no phases denoted in mice. In the present study, three relatively fixed types of fasting jejunal motor patterns were generally observed in the rats: A (phase I-like contractions), B (phase II-like contractions), and C (phase III-like contractions). However, in the anesthetized mice, no fixed motor pattern described above but arrhythmic jejunal contractions were observed in the TRPV1-/- and WT mice, which was similar to a previous study[28].
In contrast to this traditional description of gastric waves, Tatewaki et al[29] reported two fixed types of gastric contraction patterns in rats. The difference between the fasting motor patterns observed in types A and B rats may be due to the difference in vagal efferent activity that mediates the phase III gastric contractions. Since the stomach and jejunum are innervated by the same segment nerves[30], it is possible that the difference in the fasting jejunal motor patterns was due to the same nervous activity that produces the gastric contraction patterns mentioned above. Tatewaki’s work suggests that manual acupuncture could shift one contraction pattern to another, whereas in our study, EA stimulation at ST25 did not have this effect, in fact, it had no effect at all on type A or B rats. However, EA did show a significant intensity-dependent inhibition of motility in type C rats.
Acupuncture has been reported to be efficacious in the treatment of gastrointestinal motility disorders in numerous clinical and animal studies[17]. More specifically, acupuncture at ST25 has been shown to be advantageous in these cases[19,31,32], yet the mechanism of its effect on motility remains unclear. A series of studies demonstrated that acupuncture at the abdominal area inhibited gastric and duodenal motility by increasing sympathetic activity[33]; whether the effect of EA at ST25 on jejunal motility shares the same mechanism has not been reported. To determine whether sympathetic pathways are involved in the mechanism of the EA effect, we used propranolol (β adrenoceptor antagonist) and clenbuterol (β adrenoceptor agonist). Our data show that EA at ST25 still inhibited jejunal motility in propranolol-treated type C rats, but that this effect was significantly reduced (Figure 6). Clenbuterol blocked jejunal motility almost completely and EA had no effect in its presence. Taken together, these results suggest that a sympathetic pathway is involved in the effect of EA.
Sato et al[16] and Noguchi et al[33,34] concluded that acupuncture of the abdomen altered gastrointestinal motility and secretion via a somatovisceral sympathetic reflex and its afferent nerve pathway was composed of abdominal cutaneous and muscle afferent nerves. Somatic afferent nerve fibers are composed of Aα- (group I), Aβ- (group II), Aδ- (group III) and C-fibers (group IV). According to a number of studies[35,36], the mean stimulation threshold for induction of firing in rat Aδ- and C-fibers are approximately 2 mA and 5 mA, respectively, whereas in mice they are 2 mA and 3 mA. To determine which somatic afferent nerve fibers participate in the effect of EA at ST25, we used a range of EA stimulation intensities (currents). It was found that EA at ST25 significantly suppressed the jejunal motor activity at all intensities in the drug-free group, except at 1 mA. In all cases, the effect increased sigmoidally with intensity to a plateau.
It has been reported that TRPV1 is mainly coexpressed with Aδ- and C-fibers, rather than with myelinated nociceptors[22]. To confirm the role of Aδ- and C-fibers in the EA effect, we repeated the experiment in TRPV1 knockout mice. Compared with the wild-type mice, EA at ST25 had a significantly reduced effect on jejunal motility. This suggests that TRPV1 in Aδ- and C-fibers is involved in the action of EA at ST25, and these results are in agreement with previous reports[37]. On the other hand, recent studies showed that the vast majority of the sympathetic neurons were TRPV1-positive[38], and sympathetic denervation could reduce significantly the expression of TRPV1, calcitonin gene-related peptide and/or phosphorylated PKC, suggesting that the activation of TRPV1 receptors is modulated sympathetically by the action of ATP released from sympathetic efferents to activate the PKC cascade[39]. Therefore, further studies exploring TRPV1 receptor will provide new insights into the mechanism(s) underlying the acupuncture-induced jejunal motility via a sympathetic pathway.
In conclusion, the present study has found that EA stimulation at ST25 inhibits jejunal motility in an intensity-dependent manner via a sympathetic pathway, and that TRPV1 receptors in Aδ- and C-fibers seem to play an important role in the mechanism.
The authors thank Hong Feng (Nanjing University of Chinese Medicine), Yuqin Li (Nanjing University of Chinese Medicine) and Yidan Wang (Nanjing University of Chinese Medicine) for their help in the experiments of this study.
Intestinal dysmotility is one of the major pathophysiological features of functional gastrointestinal disorders. Although electroacupuncture (EA) at abdominal acupoint, ST25, is widely used in the clinical setting to treat intestinal dysmotility disease, the underlying mechanisms of its action remain unexplained.
In animal models, it was found that acupuncture at the hind paw increased gastric and duodenal motility via parasympathetic pathways, whereas application of acupuncture at the abdomen showed an inhibitory effect by increasing sympathetic activity. However, little effort has been made to investigate the effect of EA on jejunal motility.
In this study, the authors found that anesthetized rats exhibited three types of fasting jejunal motor patterns. Only type C (phase-III like contractions) rats responded to EA stimulation and the effect of EA on motility is mediated via a sympathetic pathway.
An important feature of the inhibitory effect of EA on jejunal motility, its dependence on stimulation intensity, was uncovered, that is, jejunal contraction amplitude decreased with increasing EA intensity, in a sigmoidal manner down to a plateau at intensities ≥ 5 mA. This work may help acupuncture practitioners select stimulation intensities for the treatment of patients with gastrointestinal disorders.
The effects that electroacupunture have on jejunal motility were investigated in healthy rats in the presence or absence of either a beta-adrenoceptor agonist or an antagonist, and in TRPV1 k/o mice relative to wild-type counterparts. These interesting experiments revealed that electroacupunture suppressed jejunal motility, and that beta-adrenoreceptors and TRPV1 mediate components of this effect.
| 1. | Billiauws L, Corcos O, Joly F. Dysmotility disorders: a nutritional approach. Curr Opin Clin Nutr Metab Care. 2014;17:483-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 2. | Gfroerer S, Fiegel H, Ramachandran P, Rolle U, Metzger R. Changes of smooth muscle contractile filaments in small bowel atresia. World J Gastroenterol. 2012;18:3099-3104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Seidl H, Gundling F, Pehl C, Pfeiffer A, Schepp W, Schmidt T. Small bowel motility in functional chronic constipation. Neurogastroenterol Motil. 2009;21:1278-e122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Rychter J, Espín F, Gallego D, Vergara P, Jiménez M, Clavé P. Colonic smooth muscle cells and colonic motility patterns as a target for irritable bowel syndrome therapy: mechanisms of action of otilonium bromide. Therap Adv Gastroenterol. 2014;7:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 5. | Rosa-E-Silva L, Gerson L, Davila M, Triadafilopoulos G. Clinical, radiologic, and manometric characteristics of chronic intestinal dysmotility: the Stanford experience. Clin Gastroenterol Hepatol. 2006;4:866-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Talley NJ. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol Motil. 2008;20 Suppl 1:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 214] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 7. | Zhang AL, Parker SJ, Smit de V, Taylor DM, Xue CC. Acupuncture and standard emergency department care for pain and/or nausea and its impact on emergency care delivery: a feasibility study. Acupunct Med. 2014;32:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Su YS, He W, Wang C, Shi H, Zhao YF, Xin JJ, Wang XY, Shang HY, Hu L, Jing XH. “Intensity-response” effects of electroacupuncture on gastric motility and its underlying peripheral neural mechanism. Evid Based Complement Alternat Med. 2013;2013:535742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Lima FA, Ferreira LE, Pace FH. Acupuncture effectiveness as a complementary therapy in functional dyspepsia patients. Arq Gastroenterol. 2013;50:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Anastasi JK, McMahon DJ, Kim GH. Symptom management for irritable bowel syndrome: a pilot randomized controlled trial of acupuncture/moxibustion. Gastroenterol Nurs. 2009;32:243-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 11. | Zhang X, Jin HF, Fan YH, Lu B, Meng LN, Chen JD. Effects and mechanisms of transcutaneous electroacupuncture on chemotherapy-induced nausea and vomiting. Evid Based Complement Alternat Med. 2014;2014:860631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Glickman-Simon R, Tessier J. Guided imagery for postoperative pain, energy healing for quality of life, probiotics for acute diarrhea in children, acupuncture for postoperative nausea and vomiting, and animal-assisted therapy for mental disorders. Explore (NY). 2014;10:326-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Zheng CH, Huang GY, Xu XH, Wang Y, Zhang MM, Wang W, Jing XH, Zhu B. Electro-acupuncture with different current intensities to treat functional constipation: a study protocol for a randomized controlled trial. Trials. 2013;14:344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Ouyang H, Xing J, Chen J. Electroacupuncture restores impaired gastric accommodation in vagotomized dogs. Dig Dis Sci. 2004;49:1418-1424. [PubMed] |
| 15. | Chen J, Song GQ, Yin J, Koothan T, Chen JD. Electroacupuncture improves impaired gastric motility and slow waves induced by rectal distension in dogs. Am J Physiol Gastrointest Liver Physiol. 2008;295:G614-G620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Sato A, Sato Y, Suzuki A, Uchida S. Neural mechanisms of the reflex inhibition and excitation of gastric motility elicited by acupuncture-like stimulation in anesthetized rats. Neurosci Res. 1993;18:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 170] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Yin J, Chen JD. Gastrointestinal motility disorders and acupuncture. Auton Neurosci. 2010;157:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Huang R, Zhao J, Wu L, Dou C, Liu H, Weng Z, Lu Y, Shi Y, Wang X, Zhou C. Mechanisms underlying the analgesic effect of moxibustion on visceral pain in irritable bowel syndrome: a review. Evid Based Complement Alternat Med. 2014;2014:895914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Zhou S, Zeng F, Liu J, Zheng H, Huang W, Liu T, Chen D, Qin W, Gong Q, Tian J. Influence of acupuncture stimulation on cerebral network in functional diarrhea. Evid Based Complement Alternat Med. 2013;2013:975769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Uchida S, Kagitani F, Suzuki A, Aikawa Y. Effect of acupuncture-like stimulation on cortical cerebral blood flow in anesthetized rats. Jpn J Physiol. 2000;50:495-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Rahmati R. The transient receptor potential vanilloid receptor 1, TRPV1 (VR1) inhibits peristalsis in the mouse jejunum. Arch Iran Med. 2012;15:433-438. [PubMed] |
| 22. | Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2577] [Cited by in RCA: 2724] [Article Influence: 104.8] [Reference Citation Analysis (1)] |
| 23. | Bielefeldt K, Davis BM. Differential effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation in mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G130-G138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Wu SY, Chen WH, Hsieh CL, Lin YW. Abundant expression and functional participation of TRPV1 at Zusanli acupoint (ST36) in mice: mechanosensitive TRPV1 as an “acupuncture-responding channel”. BMC Complement Altern Med. 2014;14:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 25. | Yu Z, Xia Y, Ju C, Shao Q, Mao Z, Gu Y, Xu B. Electroacupuncture regulates glucose-inhibited neurons in treatment of simple obesity. Neural Regen Res. 2013;8:809-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (2)] |
| 26. | Taniguchi H, Ariga H, Zheng J, Ludwig K, Takahashi T. Effects of ghrelin on interdigestive contractions of the rat gastrointestinal tract. World J Gastroenterol. 2008;14:6299-6302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Wang HP, Qin QG, Liu K, Gao XY, Zhu B. [Effects of acupuncture at “Tianshu” (ST 25) on electrical and mechanical motor of jejunum smooth muscles at different phases of the interdigestive migrating motor complex in normal rats]. Zhen Ci Yan Jiu. 2014;39:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Spencer NJ, Sanders KM, Smith TK. Migrating motor complexes do not require electrical slow waves in the mouse small intestine. J Physiol. 2003;553:881-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Tatewaki M, Harris M, Uemura K, Ueno T, Hoshino E, Shiotani A, Pappas TN, Takahashi T. Dual effects of acupuncture on gastric motility in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R862-R872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 30. | Jacobowitz D. Histochemical studies of the autonomic innervation of the gut. J Pharmacol Exp Ther. 1965;149:358-364. [PubMed] |
| 31. | Guo X, Chen J, Lu Y, Wu L, Weng Z, Yang L, Xin Y, Lin X, Liang Y, Fang J. Electroacupuncture at He-Mu points reduces P2X4 receptor expression in visceral hypersensitivity. Neural Regen Res. 2013;8:2069-2077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (1)] |
| 32. | Liu Z, Liu J, Zhao Y, Cai Y, He L, Xu H, Zhou X, Yan S, Lao L, Liu B. The efficacy and safety study of electro-acupuncture for severe chronic functional constipation: study protocol for a multicenter, randomized, controlled trial. Trials. 2013;14:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Noguchi E. Acupuncture regulates gut motility and secretion via nerve reflexes. Auton Neurosci. 2010;156:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Noguchi E, Ohsawa H, Tanaka H, Ikeda H, Aikawa Y. Electro-acupuncture stimulation effects on duodenal motility in anesthetized rats. Jpn J Physiol. 2003;53:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Li YQ, Zhu B, Rong PJ, Ben H, Li YH. Neural mechanism of acupuncture-modulated gastric motility. World J Gastroenterol. 2007;13:709-716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (2)] |
| 36. | Ohsawa H, Yamaguchi S, Ishimaru H, Shimura M, Sato Y. Neural mechanism of pupillary dilation elicited by electro-acupuncture stimulation in anesthetized rats. J Auton Nerv Syst. 1997;64:101-106. [PubMed] |
| 37. | Koizumi K, Sato A, Terui N. Role of somatic afferents in autonomic system control of the intestinal motility. Brain Res. 1980;182:85-97. [PubMed] |
| 38. | Korobkin AA, Emanuĭlov AI, Korzina MB, Vasil'eva OA, Porseva VV, Masliukov PM. Age-dependent changes of the autonomic neurons expressing vanilloid TRPV1 receptors. Ross Fiziol Zh Im I M Sechenova. 2011;97:1247-1253. [PubMed] |
| 39. | Xu X, Wang P, Zou X, Li D, Fang L, Gong K, Lin Q. The effects of sympathetic outflow on upregulation of vanilloid receptors TRPV(1) in primary afferent neurons evoked by intradermal capsaicin. Exp Neurol. 2010;222:93-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Hughes PA S- Editor: Yu J L- Editor: Wang TQ E- Editor: Zhang DN