Published online Nov 28, 2016. doi: 10.3748/wjg.v22.i44.9822
Peer-review started: September 10, 2016
First decision: September 28, 2016
Revised: October 6, 2016
Accepted: October 30, 2016
Article in press: October 31, 2016
Published online: November 28, 2016
Processing time: 78 Days and 18.2 Hours
To evaluate portal vein (PV) stenosis and stent patency after hepatobiliary and pancreatic surgery, using abdominal computed tomography (CT).
Percutaneous portal venous stenting was attempted in 22 patients with significant PV stenosis (> 50%) - after hepatobiliary or pancreatic surgery - diagnosed by abdominal CT. Stents were placed in various stenotic lesions after percutaneous transhepatic portography. Pressure gradient across the stenotic segment was measured in 14 patients. Stents were placed when the pressure gradient across the stenotic segment was > 5 mmHg or PV stenosis was > 50%, as observed on transhepatic portography. Patients underwent follow-up abdominal CT and technical and clinical success, complications, and stent patency were evaluated.
Stent placement was successful in 21 patients (technical success rate: 95.5%). Stents were positioned through the main PV and superior mesenteric vein (n = 13), main PV (n = 2), right and main PV (n = 1), left and main PV (n = 4), or main PV and splenic vein (n = 1). Patients showed no complications after stent placement. The time between procedure and final follow-up CT was 41-761 d (mean: 374.5 d). Twenty stents remained patent during the entire follow-up. Stent obstruction - caused by invasion of the PV stent by a recurrent tumor - was observed in 1 patient in a follow-up CT performed after 155 d after the procedure. The cumulative stent patency rate was 95.7%. Small in-stent low-density areas were found in 11 (55%) patients; however, during successive follow-up CT, the extent of these areas had decreased.
Percutaneous transhepatic stent placement can be safe and effective in cases of PV stenosis after hepatobiliary and pancreatic surgery. Stents show excellent patency in follow-up abdominal CT, despite development of small in-stent low-density areas.
Core tip: Portal vein (PV) stenosis can occur after hepatobiliary and pancreatic surgery. Portal hypertension due to PV stenosis induces clinical manifestations in many organs, including intractable ascites, esophageal and gastrointestinal bleeding, and liver dysfunction. Percutaneous transhepatic stent placement can be safe and effective in cases of PV stenosis since stents show excellent patency on follow-up abdominal computed tomography despite the presence of small in-stent low-density areas.
- Citation: Jeon UB, Kim CW, Kim TU, Choo KS, Jang JY, Nam KJ, Chu CW, Ryu JH. Therapeutic efficacy and stent patency of transhepatic portal vein stenting after surgery. World J Gastroenterol 2016; 22(44): 9822-9828
- URL: https://www.wjgnet.com/1007-9327/full/v22/i44/9822.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i44.9822
Acquired portal vein (PV) stenosis is most commonly observed after liver transplantation (LT)[1-3]. In the non-transplant population, PV stenosis can present with pancreatitis[4] or tumor encasement[5], or as a postsurgical complication[6]. Portal hypertension from PV stenosis induces clinical manifestations in many organs, including intractable ascites, esophageal and gastrointestinal bleeding, and liver dysfunction[7].
Treatment options for patients with PV stenosis or occlusions are limited. In the past, PV complications were managed surgically, such as by thrombectomy or surgical revision. However, surgical management of these complications has been limited by technical difficulties due to the development of postsurgical fibrosis and the length of the involved venous structures[8]. Percutaneous interventional procedures have gained worldwide acceptance for alleviating the symptoms of portal hypertension, due to their minimal invasiveness and high success rates with low number of complications[9]. Abdominal computed tomography (CT) and Doppler ultrasound tests have been the main modalities of patient follow-up after this procedure; the reported stent patency rate has been relatively good, ranging between 60 and 100% during the first year[5,7,10,11]. Abdominal CT scans allow the visualization not only of the status of the original lesion, but also of changes in the PV stent. This study aimed to evaluate the therapeutic efficacy and stent patency of PV stenting after hepatobiliary and pancreatic surgery, using abdominal CT.
From January 2011 to December 2012 at a single institution, percutaneous transhepatic stent placement was attempted in 22 patients [11 women, 11 men; mean age: 65.6 years (range: 26-78 years)] with significant PV stenosis (> 50% of the main PV diameter) diagnosed using abdominal CT after hepatobiliary or pancreatic surgery [pylorus-preserving pancreatoduodenectomy (n = 7), Whipple procedure (n = 6), distal pancreatectomy (n = 2), hepatectomy or hepatic segmentectomy (n = 6), and deceased donor liver transplant (n = 1)] (Table 1).
| patient No. | Age/sex | Surgery | Locations |
| 1 | 65/F | DDLT | Main PV |
| 2 | 74/F | Whipple’s op | PV-SMV |
| 3 | 58/M | Distal pancreatectomy + Spl-SMV bypass | PV-SMV |
| 4 | 73/M | Whipple’s op | PV-SMV |
| 5 | 61/F | Whipple’s op | PV-SMV |
| 6 | 63/F | PPPD | PV-SMV |
| 7 | 75/F | PPPD | PV-SMV |
| 8 | 68/F | PPPD | PV-SMV |
| 9 | 65/F | PPPD | PV-SMV |
| 10 | 70/M | PPPD | PV-SMV |
| 11 | 55/M | PPPD | PV-SMV |
| 12 | 70/F | PPPD | Main PV |
| 13 | 69/F | Whipple’s op | PV-SMV |
| 14 | 54/M | Right anterior segmentectomy | Right PV |
| 15 | 76/M | Right hemicolectomy + PPPD | PV-SpV |
| 16 | 26/M | Right lobectomy | Left PV |
| 17 | 55/F | Right + caudate lobectomy | Left PV |
| 18 | 66/M | Right + caudate lobectomy | Left PV |
| 19 | 74/M | Subtotal pancreatectomy, Splenectomy, partial nephrectomy | PV-SMV |
| 20 | 79/F | Trisegmentectomy + caudate lobectomy | Left PV |
| 21 | 68/M | Whipple’s op | PV-SMV |
| 22 | 78/M | Central segmentectomy | Right PV |
The reasons for surgery included pancreatic cancer (n = 7), common bile duct cancer (n = 3), Klatskin tumor (n = 2), colon cancer with duodenal invasion (n = 1), colon cancer with liver metastasis (n = 1), hepatocellular carcinoma (n = 1), gall bladder cancer (n = 1), gastrointestinal stromal tumor (n = 1), ampulla of Vater cancer (n = 1), chronic pancreatitis (n = 1), intrahepatic bile duct stone (n = 1), and hepatic failure after toxic hepatitis (n = 1). Clinical manifestations in patients with PV stenosis included intestinal angina-like abdominal pain (disabling mid-epigastric or central abdominal pain within 10-15 min after eating) and anorexia refractory to medical treatment, ascites, increased Jackson-Pratt (JP) drain output, and abnormal liver function test (LFT). The interval between surgery and stent placement was 5-274 d (mean: 34 d) (Table 2).
| Clinical manifestations | No. of patients |
| Intestinal angina-like abdominal pain refractory to medical treatment | |
| Only pain | 2 |
| Worsening of PVS during the follow-up (> 2 wk) | 4 |
| Worsening of PVS during the follow-up (> 2 wk) + abnormal LFT | 1 |
| PVT after PCD | 1 |
| Abnormal LFT | 1 |
| Fail to PV anastomosis during the operation | 1 |
| Anorexia refractory to medical treatment (with increased JPDO) | 1 |
| Ascites | 4 |
| Increased JPDO | 6 |
| Abnormal LFT | 1 |
One patient was diagnosed with asymptomatic left PV stenosis after right hepatectomy, when the pressure gradient on transhepatic portography was 5 mmHg. She had undergone balloon angioplasty only and was excluded from this study.
Informed consent was obtained from each patient. This retrospective study was approved by our institutional review board. All patients received local anesthesia consisting on subcutaneous injection of lidocaine and an intravenous injection of pentanyl (pentanyl citrate, Myung Moon Pharmaceutical; Seoul, Korea). Percutaneous transhepatic puncture of the intrahepatic PV was performed with a 21-gauge needle (PTC US needle, A&A M.D.; Seongnam, South Korea) under sonographic and fluoroscopic guidance. The needle was exchanged for a 5-French coaxial dilator, and an 8-French sheath was placed over a 0.035-inch guidewire. A 0.035-inch angled hydrophilic guidewire (Radifocus, Terumo, Tokyo, Japan) and a 5-French cobra catheter were used to traverse the PV stenosis. Direct main portography was performed with 12 mL of contrast medium injected into the patient’s splenic or superior mesenteric vein (SMV) and the pressure gradient across the stenosis was measured. The criteria for PV stenting were as follows: stenosis > 50% of the main PV diameter revealed by transhepatic portography, or a pressure gradient across the stenosis > 5 mmHg (Table 3).
| Criteria | No. of patients |
| PVS > 50% with measuring of PrG | |
| PrG > 5 mmHg | 11 |
| PrG = 5 mmHg | |
| Contrast stagnation with collaterals formations | 2 |
| Kinking of PV (stenosis and acute angulation) | 1 |
| PVS > 50% without measuring of PrG | |
| Contrast stagnation with collaterals formations | 3 |
| Kinking of PV | 3 |
| Stenosis and partial PVT | 1 |
Stent placement was performed using stents from the following vendors: Smart (Cordis; Miami Lakes, FL, United States), Zilver (Cook; Bloomington, IN, United States), Protégé (Covidien; St. Paul, MN, United States), Hercules (S&G Biotech Inc.; Seongnam, South Korea), Complete SE (Medtronic Inc.; Minneapolis, MN, United States), and Express LD (Boston Scientific; Natick, MA, United States). Stents of the same diameter or with a diameter 1-2 mm larger than that of the non-stenotic extrahepatic PV were used.
Balloon angioplasty following stent placement was not routinely performed; however, balloon angioplasty was performed in cases in which the deployed stent showed an hourglass deformity of < 50% its normal diameter or the pressure gradient across the stenosis was > 5 mmHg. Angioplasty was carefully performed using a balloon catheter of the same (or smaller than the deployed stent to prevent PV rupture.
After transhepatic portography and stent placement, the PV pressure gradient was measured to evaluate whether the stenosis or occlusion was resolved. The transhepatic parenchymal track was embolized with several 0.035-inch stainless steel coils (Cook; Bloomington, IN, United States), gelfoam slurry (Cutanplast, Mascia Brunelli; Milano, Italy), or n-butyl cyanoacrylate (NBCA; Histoacryl, B. Braun; Melsungen, Germany) to prevent bleeding. No routine anticoagulation therapy was administered after the procedure.
The following parameters were retrospectively documented: technical success, clinical success, development of complications, PV flow after stent placement, and pressure gradient across the area before and after stent placement. Technical success was defined as successful stent placement in the intended location in the PV with subsequent improvement in portal venous flow and < 30% residual stenosis. Clinical success was defined as improvement on patient clinical manifestations, such as nausea, abdominal pain, abnormal liver function, and ascites. Major complications included those requiring increased level of care or additional surgical or interventional manipulation and those resulting in adverse sequelae or death. Minor complications defined self-limiting events.
Patency of the PV stents was evaluated with follow-up abdominal CT 3-6 mo after the procedure and, after that, annually. According to clinician discretion, however, the follow-up schedule could be altered. Axial CT image of portal venous phase (5 mm-thick slice) was routinely used for evaluation. In cases in which the luminal changes caused by the stents were not fully visualized with axial images, some of the axial images were transferred to a workstation in which a PC-based three-dimensional reconstruction software (Aquarius iNtuition 4.4, TeraRecon; San Mateo, CA, United States) was installed. Three-dimensional image reconstruction was performed, which included volume-rendering and multiplanar or curved planar reformation along or across the venous segments of interest. Small in-stent low-density areas, such as the ones observed after carotid stenting[12], were observed between the stent wall and a contrast-filled PV on follow-up CT scans. Extent, location, and relative time of discovery were recorded.
Portal venous pressure gradient was analyzed before and after stent placement using the Wilcoxon rank test; cumulative stent patency rates were calculated using the Kaplan-Meier estimation. Statistical analysis was conducted using the SPSS software (version 18.0); a P < 0.05 indicates a statistically significant difference.
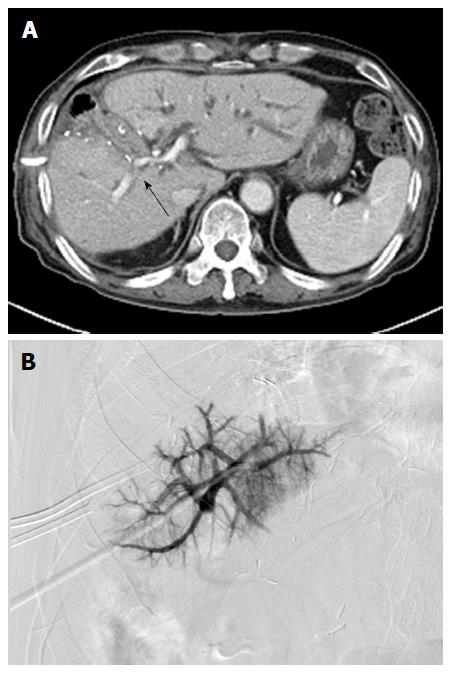
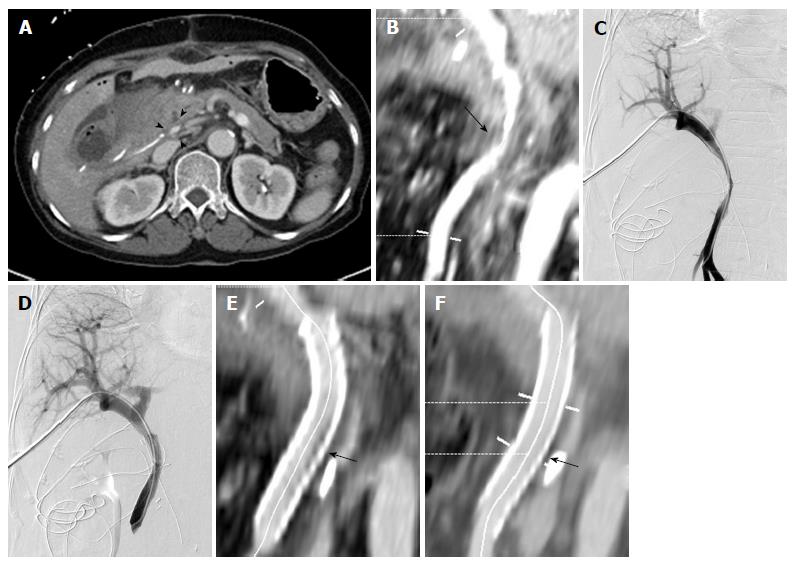
Stenting was successful in 21 out of 22 patients (technical success rate: 95.5%). Guidewire selection failed in 1 patient due to severe PV stenosis (Figure 1). All patients in whom PV stenting was successful (n = 21) showed improvement of clinical manifestations (e.g., disappearance of intestinal angina, ascites, and abnormal LFT, and decreased JP drain output). Remarkably, 6 patients showed increased JP drain output during the early follow-up periods after surgery, with JP drain output decreasing rapidly within 1 wk after stenting. Therefore, the clinical success rate was of 100%. The used stents were 37-80 mm long and 8-14 mm in diameter. In 1 patient, 2 stents were used due to stent misplacement. Twenty-one self-expandable and 1 balloon-expandable stents were used, including the Smart (Cordis, Cordis, Inc.; Miami Lakes, FL, United States; n = 8), Zilver (Cook; Bloomington, IN, United States; n = 7), Protégé (Covidien; St. Paul, MN, United States; n = 3), Hercules (S&G Biotech Inc.; Seongnam, South Korea, n = 2), Complete SE (Medtronic Inc.; Minneapolis, MN, United States, n = 1), and Express LD (Boston Scientific; Natick, MA, United States; n = 1).
Stents were positioned as follows: through main PV and SMV (n = 13), through main PV (n = 2), through right PV and main PV (n = 1), through left PV and main PV (n = 4), and through main PV and splenic vein (n = 1).
The transhepatic parenchymal track was embolized with coils (n = 15), gelfoam slurry (n = 6) or n-butyl cyanoacrylate (n = 1) to prevent bleeding. There were no procedure-related complications, such as perihepatic hematoma, hemobilia, hemoperitoneum, or pneumoperitoneum.
Pre- and post-stenting pressure gradients across the stenosis (measured in 14 patients) were 8.7 ± 3.4 mmHg (range: 5-16 mmHg) and 2.1 ± 1.1 mmHg (range: 1-5 mmHg), respectively. The decrease in pressure gradient after the procedure was statistically significantly (Wilcoxon rank test; P < 0.05).
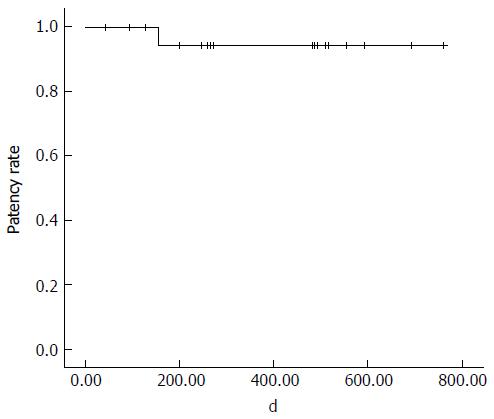
The first follow-up CT examination was performed 1-162 d (mean: 40.1 d) after the procedure; sequential follow-up CT were performed in all patients. The interval between procedure and final follow-up CT was 41-761 d (mean: 374.5 d). Twenty stents remained patent during the entire follow-up period. One stent obstruction was identified on a follow-up CT scan 155 d after the procedure; this obstruction was caused by invasion of the PV stent by a recurrent tumor. In a study population, 8/17 patients with malignant tumors showed local recurrence of tumors, but 7 stents that were not directly invaded by the tumor remained patent during the entire follow-up period. The cumulative stent patency rate was 95.7% (Kaplan-Meier estimation; Figure 2). Follow-up CT performed 83-409 d (mean: 180.6 ± 99.5 d) after the procedure revealed small in-stent low-density areas in 11 patients (55%). In all cases, these areas were visualized in patients in whom the stents were placed through PV and SMV, within 25% of the luminal diameter. During consecutive follow-up CT, the extent of these areas slightly decreased (Figure 3). These low-density areas were located in the proximal and distal edge (n = 9) or mid portion (n = 2) of stents.
Postoperative PV stenosis is a surgical complication usually observed after concurrent resection and anastomosis of the PV after hepatobiliary and pancreatic surgery[7,13], and LT[1,9]. Percutaneous intervention through transhepatic approach was firstly introduced in LT patients by Olcott et al[14]. This technique has gained worldwide acceptance for the treatment of PV stenosis after LT due to their minimal invasiveness, low number of complications, and high success rate[15,16]. Nonetheless, the reported stenosis recurrence rate following balloon angioplasty alone has been relatively high (28.6%-36.8%). Stents have usually been used to treat recurrent and elastic portal venous stenoses following balloon angioplasty, as this procedure presents several potential complications, such as PV rupture or recoil[15,17,18]. Therefore, Ko et al[11] suggested performing primary stent placement rather than balloon angioplasty in the early post-transplantation period (< 1 mo) to minimize the potential need of repeat surgery and the risk of anastomotic rupture during balloon angioplasty. In our study, primary stenting was performed in all patients in the same way as done in the study by Ko et al[11], given that the interval between operation and stenting was relatively short. PV stent placement success in non-transplant populations was similar to that in the LT population[7,19,20].
In our study, all patients showed minor symptoms and signs of portal hypertension, such as nausea, intestinal angina-like pain, abnormal LFT, ascites, and increased JP drain output (no variceal bleeding was recorded). PV stenosis symptoms were identified after relatively short postoperative follow-up periods (within 2 mo, with the exception of 2 patients). Conservative management and follow-up was performed in patients presenting with intestinal angina only (n = 5, Table 1); however, PV stenosis in these cases was not improved during the follow-up period. Six patients presenting JP drains showed serosanguineous drainage output increase; these findings were different manifestations of ascites. Considering these observations, patients with significant PV stenosis presenting only minor symptoms, such as intestinal angina, ascites, or increased JP drain output, were recommended to be treated early. Woodrum at al[20] reported on a patient that presented with intestinal angina symptoms, with a mesenteric venogram showing near occlusion of the splenic vein and SMV. After PV stent placement, the patient’s symptoms were completely relieved, which is in accordance with the results of our study.
The long-term patency of PV stents has been previously investigated. Reported stent patency was of 60%-100% during 1-2 years follow-up periods[5,7,10,11]. Kim et al[7] performed a stent patency comparison between groups of patients presenting benign and malignant stenosis and observed that the mean patency period in the benign stenosis group was higher than that of the tumor recurrence group. The shorter patency period in the tumor recurrence group seemed to be caused by a shorter survival period rather than by tumor ingrowth into the stent due to tumor recurrence. In our study, even in those cases in which local tumor recurrence was observed, the stent remained patent unless direct tumor invasion to the stent occurred; however, further follow-up was needed for these patients.
A pressure gradient > 5 mmHg across a stenosis has been considered “significant” in some reports[3,18,21]. However, no standard definition regarding a significant pressure gradient currently exists. In our study, the pressure gradient in 3 patients was < 6 mmHg. We observed aggravation of stenosis in one of these patients, kinking of PV in another, and diffuse stenosis (> 50%) in the third one. Several researchers have also reported clinically successful cases of stent placement following balloon angioplasty for the treatment of PV stenosis, using a pressure gradient < 6 mmHg[2,15,22]. Therefore, we consider that treatment is beneficial in patients who have symptoms related to PV inflow abnormalities or portal hypertension, even when the change in pressure gradient is not significant.
In some cases, small in-stent low-density areas were identified on follow-up CT scans, such as after carotid stenting[12]. The size of these areas decreased during consecutive follow-up CT. The exact pathology of these lesions was not fully understood but did not alter the patency of the stents. Additional studies are needed to clarify these observations.
The main limitations of this study are its retrospective nature and the small number of patients. Another limiting factor is that all procedures were usually performed based on clinical manifestations observed on CT and PV pressure gradient, not on clinical manifestations, such as intestinal angina and ascites; however, risk reduction of PV hypertension should also be considered in cases in which early intervention was recommended.
In conclusion, percutaneous transhepatic stent placement can be safe and effective in the treatment of PV stenosis developed after hepatobiliary and pancreatic surgery. Importantly, stents show excellent patency in follow-up abdominal CT scans, despite the development of small in-stent low-density areas.
Acquired portal vein (PV) stenosis is sometimes observed after hepatobiliary and pancreatic surgery, but treatment options for patients with PV stenosis or occlusions are limited. Surgical management of these complications has been limited by technical difficulties due to the development of postsurgical fibrosis and the lengths of the involved venous structures.
Percutaneous interventional procedures have gained worldwide acceptance for their ability to alleviate the symptoms of portal hypertension because of their minimal invasiveness and high success rates, with low number of complications.
This study aimed to evaluate the therapeutic efficacy and patency of PV stenting after hepatobiliary and pancreatic surgery, using abdominal CT.
Percutaneous transhepatic stent placement can be safe and effective for the treatment of PV stenosis after hepatobiliary and pancreatic surgery.
Pressure gradient: pressure changes in two different vessels (e.g., the PV and superior mesenteric vein). This can be checked between anatomical stenoses.
The authors report their experience with PV/SMV stenting in patients who had previous HPB surgical resections. The study is well written and interesting as there is very little in the medical literature on this topic.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fusai G, Garcia-Olmo D, Tarazov PG S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Funaki B, Rosenblum JD, Leef JA, Hackworth CA, Szymski GX, Alonso EM, Piper JB, Whitington PF. Portal vein stenosis in children with segmental liver transplants: treatment with percutaneous transhepatic venoplasty. AJR Am J Roentgenol. 1995;165:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Funaki B, Rosenblum JD, Leef JA, Hackworth CA, Szymski GX, Alonso EM. Angioplasty treatment of portal vein stenosis in children with segmental liver transplants: mid-term results. AJR Am J Roentgenol. 1997;169:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Raby N, Karani J, Thomas S, O’Grady J, Williams R. Stenoses of vascular anastomoses after hepatic transplantation: treatment with balloon angioplasty. AJR Am J Roentgenol. 1991;157:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 132] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Mathias K, Bolder U, Löhlein D, Jäger H. Percutaneous transhepatic angioplasty and stent implantation for prehepatic portal vein obstruction. Cardiovasc Intervent Radiol. 1993;16:313-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Yamakado K, Nakatsuka A, Tanaka N, Fujii A, Terada N, Takeda K. Malignant portal venous obstructions treated by stent placement: significant factors affecting patency. J Vasc Interv Radiol. 2001;12:1407-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Vogelzang RL, Reddy SG, Braun MA, Nemcek AA. Extrahepatic portal venous stenosis: treatment with percutaneous transhepatic stent placement. J Vasc Interv Radiol. 1996;7:269-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Kim KR, Ko GY, Sung KB, Yoon HK. Percutaneous transhepatic stent placement in the management of portal venous stenosis after curative surgery for pancreatic and biliary neoplasms. AJR Am J Roentgenol. 2011;196:W446-W450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Woo DH, Laberge JM, Gordon RL, Wilson MW, Kerlan RK. Management of portal venous complications after liver transplantation. Tech Vasc Interv Radiol. 2007;10:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Vignali C, Cioni R, Petruzzi P, Cicorelli A, Bargellini I, Perri M, Urbani L, Filipponi F, Bartolozzi C. Role of interventional radiology in the management of vascular complications after liver transplantation. Transplant Proc. 2004;36:552-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Tsukamoto T, Hirohashi K, Kubo S, Tanaka H, Hamba H, Shuto T, Higaki I, Takemura S, Kinoshita H. Percutaneous transhepatic metallic stent placement for malignant portal vein stenosis. Hepatogastroenterology. 2003;50:453-455. [PubMed] |
| 11. | Ko GY, Sung KB, Yoon HK, Lee S. Early posttransplantation portal vein stenosis following living donor liver transplantation: percutaneous transhepatic primary stent placement. Liver Transpl. 2007;13:530-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Okahara M, Kiyosue H, Kashiwagi J, Ueda S, Hori Y, Mori H. Small in-stent Low Density on CT Angiography after Carotid Artery Stenting. Interv Neuroradiol. 2008;14 Suppl 2:41-46. [PubMed] |
| 13. | Hwang S, Sung KB, Park YH, Jung DH, Lee SG. Portal vein stenting for portal hypertension caused by local recurrence after pancreatoduodenectomy for periampullary cancer. J Gastrointest Surg. 2007;11:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Olcott EW, Ring EJ, Roberts JP, Ascher NL, Lake JR, Gordon RL. Percutaneous transhepatic portal vein angioplasty and stent placement after liver transplantation: early experience. J Vasc Interv Radiol. 1990;1:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Park KB, Choo SW, Do YS, Shin SW, Cho SG, Choo IW. Percutaneous angioplasty of portal vein stenosis that complicates liver transplantation: the mid-term therapeutic results. Korean J Radiol. 2005;6:161-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Wang JF, Zhai RY, Wei BJ, Li JJ, Jin WH, Dai DK, Yu P. Percutaneous intravascular stents for treatment of portal venous stenosis after liver transplantation: midterm results. Transplant Proc. 2006;38:1461-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Shibata T, Itoh K, Kubo T, Maetani Y, Shibata T, Togashi K, Tanaka K. Percutaneous transhepatic balloon dilation of portal venous stenosis in patients with living donor liver transplantation. Radiology. 2005;235:1078-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Funaki B, Rosenblum JD, Leef JA, Zaleski GX, Farrell T, Lorenz J, Brady L. Percutaneous treatment of portal venous stenosis in children and adolescents with segmental hepatic transplants: long-term results. Radiology. 2000;215:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 19. | Novellas S, Denys A, Bize P, Brunner P, Motamedi JP, Gugenheim J, Caroli FX, Chevallier P. Palliative portal vein stent placement in malignant and symptomatic extrinsic portal vein stenosis or occlusion. Cardiovasc Intervent Radiol. 2009;32:462-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Woodrum DA, Bjarnason H, Andrews JC. Portal vein venoplasty and stent placement in the nontransplant population. J Vasc Interv Radiol. 2009;20:593-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Buell JF, Funaki B, Cronin DC, Yoshida A, Perlman MK, Lorenz J, Kelly S, Brady L, Leef JA, Millis JM. Long-term venous complications after full-size and segmental pediatric liver transplantation. Ann Surg. 2002;236:658-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 200] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Zajko AB, Sheng R, Bron K, Reyes J, Nour B, Tzakis A. Percutaneous transluminal angioplasty of venous anastomotic stenoses complicating liver transplantation: intermediate-term results. J Vasc Interv Radiol. 1994;5:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 81] [Article Influence: 2.5] [Reference Citation Analysis (0)] |