Published online Nov 28, 2016. doi: 10.3748/wjg.v22.i44.9674
Peer-review started: August 9, 2016
First decision: August 29, 2016
Revised: September 29, 2016
Accepted: October 30, 2016
Article in press: October 31, 2016
Published online: November 28, 2016
Processing time: 109 Days and 13.7 Hours
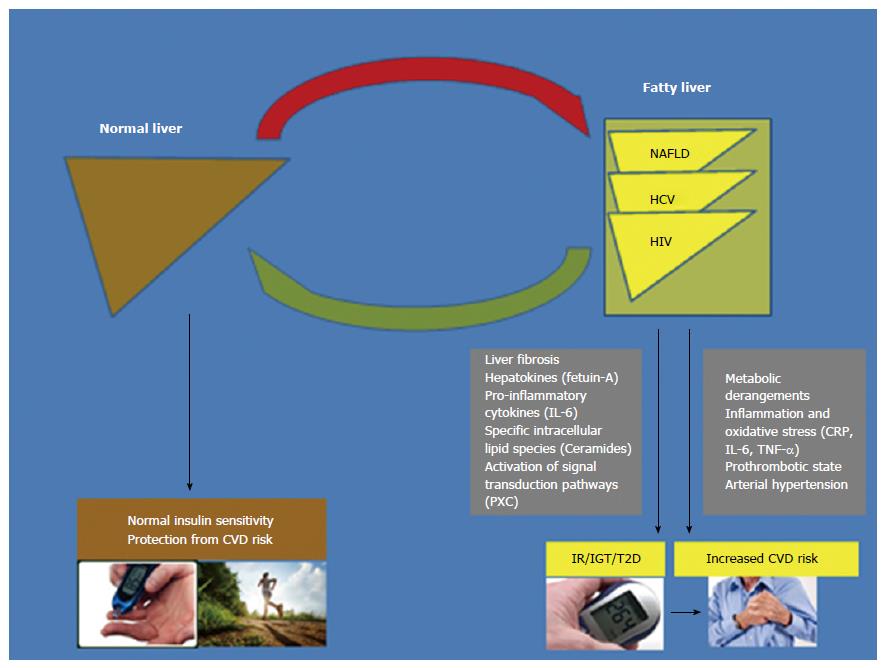
Fatty liver, which frequently coexists with necro-inflammatory and fibrotic changes, may occur in the setting of nonalcoholic fatty liver disease (NAFLD) and chronic infections due to either hepatitis C virus (HCV) or human immunodeficiency virus (HIV). These three pathologic conditions are associated with an increased prevalence and incidence of cardiovascular disease (CVD) and type 2 diabetes (T2D). In this multidisciplinary clinical review, we aim to discuss the ever-expanding wealth of clinical and epidemiological evidence supporting a key role of fatty liver in the development of T2D and CVD in patients with NAFLD and in those with HCV or HIV infections. For each of these three common diseases, the epidemiological features, pathophysiologic mechanisms and clinical implications of the presence of fatty liver in predicting the risk of incident T2D and CVD are examined in depth. Collectively, the data discussed in this updated review, which follows an innovative comparative approach, further reinforce the conclusion that the presence of fatty/inflamed/fibrotic liver might be a shared important determinant for the development of T2D and CVD in patients with NAFLD, HCV or HIV. This review may also open new avenues in the clinical and research arenas and paves the way for the planning of future, well-designed prospective and intervention studies.
Core tip: Normally, the liver is almost devoid of fat and fatty changes often coexist with necro-inflammatory and fibrotic changes in the setting of nonalcoholic fatty liver disease (NAFLD), chronic infection due to hepatitis C virus (HCV) or human immunodeficiency virus (HIV), which have all been associated with an increased prevalence and incidence of cardiovascular disease (CVD) and type 2 diabetes (T2D). On these grounds, in this multidisciplinary clinical review, we discuss the ever-expanding wealth of evidence supporting a key role of fatty liver in the development of T2D and CVD both in patients with NAFLD and in those with HCV or HIV infections.
- Citation: Lonardo A, Ballestri S, Guaraldi G, Nascimbeni F, Romagnoli D, Zona S, Targher G. Fatty liver is associated with an increased risk of diabetes and cardiovascular disease - Evidence from three different disease models: NAFLD, HCV and HIV. World J Gastroenterol 2016; 22(44): 9674-9693
- URL: https://www.wjgnet.com/1007-9327/full/v22/i44/9674.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i44.9674
The storage of excess lipids is not among the physiologic functions of the liver, which normally is almost devoid of fat content[1]. Fatty liver is defined as the presence of triglycerides in more than 5% of the hepatocytes. In many cases, such fatty changes (simple steatosis) may frequently coexist with lipotoxic features, such as necro-inflammation and fibrosis (i.e., steatohepatitis), the extent of which depends on the etiology of such liver changes[2].
Fatty liver may occur in the setting of both metabolic and viral injuries. For example, nonalcoholic fatty liver disease (NAFLD) is defined by fatty changes which occur in individuals without excessive alcohol consumption and who have (or will develop) features of the metabolic syndrome (MetS)[3,4]. Chronic hepatitis C virus (HCV) infection is also commonly associated with fatty liver, which occurs to a variable extent according to the viral genotype and host’s metabolic features[5]. Similarly, human immunodeficiency virus (HIV) infection exhibits fatty liver as a result of multiple viral and host factors, probably including the anti-viral drugs used[6]. Fatty liver associated with these two highly prevalent viral infections has collectively been named “virus-associated fatty liver disease” (VAFLD)[7].
Recent studies have addressed the systemic and cardio-metabolic risks associated with fatty liver due to different etiologies. For example, both NAFLD and VAFLD have been linked with an increased risk of cardiovascular disease (CVD) and type 2 diabetes (T2D). However, in clinical practice, different medical expertise is involved in the diagnosis and management of CVD risk; NAFLD; and HIV mono or co-infection.
In this updated clinical review, we summarize the rapidly expanding body of evidence supporting a contribution of fatty liver per se in the development of CVD and T2D not only in patients with NAFLD but also in those with chronic HCV or HIV infections. For each of these diseases, we extensively discuss the epidemiological burden, pathophysiologic mechanisms and clinical implications of the presence of fatty liver in predicting the risk of developing T2D and CVD. We believe that this review, which follows an innovative multidisciplinary and comparative approach, further reinforces the notion that the fatty/inflamed/fibrotic liver may represent a shared and important determinant for the development of T2D and CVD in patients with any of these three common steatogenic diseases.
The prevalence of NAFLD in the general adult population is approximately 25%-30% in Europe and United States based on imaging studies, i.e., roughly two-fold higher than that identified through serum liver levels; the highest prevalence of disease is observed in Southern American and Middle Eastern countries while the lowest prevalence is found in Africa[8]. An “inverted U” shaped curve describes the prevalence of NAFLD as a function of increasing age, suggesting that younger and older individuals are relatively more spared from NAFLD[9].
The incidence rates of NAFLD have been estimated between 52/1000 person-years in Asia and 28/1000 person-years in Israel, respectively[8]. However, very little is known about the incidence rates of NAFLD in Western countries. A pioneering study conducted in northern Italy found that NAFLD incidence was approximately 2/1000 person-years in a sample of women subjected to hysterectomy[10].
The metabolic predictors of NAFLD are likely to vary based on sex[11]. Compared to women, men are at higher risk of developing NAFLD in most published studies[2,12], and post-menopausal women are no longer spared from NAFLD and its fibrotic evolution, owing to ovarian senescence and estrogen deficiency[13,14].
It is known that abdominal obesity/overweight, T2D and other MetS features are strongly associated with an increased risk of NAFLD. However, the hierarchy of individual MetS features associated with NAFLD risk varies across the different studies. For example, one can report the prevalence of NAFLD in patients with MetS features as opposed to the prevalence of MetS features in patients with NAFLD (Table 1)[8,15]. Of concern, most of the MetS features listed in Table 1 are positively associated with the development and progression of NAFLD[3,16-19].
| From: Younossi et al[8] 2016 | From: Lonardo et al[15] 2015 | ||
| Feature | Strength of associations | Feature | Strength of associations |
| (% Prevalence of a given metabolic condition in those with NAFLD) | (% Prevalence of NAFLD in those with a given metabolic condition) | ||
| Hyperlipidemia | 69% | Obesity | 98% |
| Obesity | 51% | Mixed hyperlipidemia with elevated serum ALT levels | 83% |
| MetS | 42% | T2D | 70% |
| Hypertension | 39% | Mixed hyperlipidemia | 60% |
| T2D | 22% | MetS | 70% |
| Hypertension | 50% | ||
The natural history of NAFLD features both hepatic and extra-hepatic outcomes, which have been discussed in detail elsewhere[20-22]. The extra-hepatic outcomes of NAFLD include T2D/metabolic, CVD and cancer. Simple steatosis, with/without minor degrees of necro-inflammatory changes, is the first step in the histogenesis and natural history of NAFLD. Traditionally, NASH has been postulated to develop in a subset of “bad storers”, leading to the notion that simple steatosis and NASH are two different pathologic conditions with little, if any, mutual interconnections[23,24]. However, recent studies have demonstrated that simple steatosis can progress to NASH and that both conditions progress to advanced fibrosis, though this occurs at a much slower pace for simple steatosis than for NASH[16,25-27]. The severity of hepatic fibrosis, rather than NASH, largely dictates the prognosis of liver-related outcomes in NAFLD[21,28,29].
The often used statement “NAFLD is the hepatic manifestation of the MetS” fails to render the mutual and bidirectional link existing between these two diseases. Notably, such statement also fails to pinpoint that NAFLD is a precursor and almost doubles the risk of developing T2D and MetS over a median follow-up period of 5 years[4,30].
Strong evidence also indicates that NAFLD is associated with an increased risk of subclinical atherosclerosis[31-34]. The entity of this NAFLD-related proatherogenic risk is probably linked to the severity of hepatic fibrosis[35], the degree of fatty liver[36], or both. More importantly, follow-up studies have consistently shown that CVD is the leading cause of mortality in NAFLD patients[37,38]. However, it should also be pointed out that fatal and non-fatal CVD complications are not the only dreadful outcomes in patients with NAFLD. Indeed a variety of other cardiac complications have recently been reported in these patients, including aortic-valve stenosis, cardiac arrhythmias, and increased re-hospitalization rates following discharge for acute heart failure[39-41].
Patients with NAFLD have an increased risk of developing hepatic and extra-hepatic cancers. The risk of hepatocellular carcinoma (HCC) is not only confined to patients with cirrhosis and accordingly, in NAFLD patients, HCC tends to escape those surveillance protocols which are a standard of good clinical practice in individuals with viral chronic liver diseases[42-45]. Further to HCC, increased rates of cholangiocarcinoma have been reported in NAFLD patients[46-48]. Finally, a variety of extra-hepatic cancers have also been reported in patients with NAFLD, including colo-rectal, pancreatic and uterine cancers[49], though the consistency of these associations remains to be definitely proven.
The prevalence of NAFLD is remarkably increased in patients with T2D, ranging from 30% to 75% according to age, ethnicity, the study population and the diagnostic tools used[15].
Several retrospective and prospective studies have shown that NAFLD, as diagnosed either by raised serum liver enzymes or ultrasonography, independently predicts the development of incident T2D and MetS[30,50-52]. Recently, two meta-analyses concluded that NAFLD is associated with a two-fold increased risk of incident T2D and MetS[4,53]. Consistently, an improvement[52,54] or a transient remission of NAFLD significantly decrease risk of new-onset T2D[55].
To date, the only small retrospective cohort study investigating the association between biopsy-proven NAFLD and risk of incident T2D found that most of these NAFLD patients developed T2D over a 13.7-year follow-up, and that T2D risk was almost three-fold higher in patients with NASH than in those with simple steatosis[56].
The presence of T2D also worsens the histological course of NAFLD. Patients with NAFLD and T2D have a high prevalence of NASH and advanced fibrosis[3,15,57-59], and consistently, T2D is a strong predictor of the severity and progression of histologically assessed hepatic fibrosis[25,27,57,60]. Moreover, T2D is also an important predictor of the development of HCC[61,62] and an increased risk of all-cause and liver-related mortality[60,63,64].
Worryingly, T2D patients often exhibit advanced histological disease with normal serum transaminases and may also develop HCC even in the absence of cirrhosis[44,61,65] resulting in restricted therapeutic options and decreased survival[43].
A large number of studies have consistently shown that NAFLD is strongly associated with various markers of subclinical atherosclerosis, independent of traditional CVD risk factors and MetS features[31,34,66]. Epidemiological studies[37,39,67] have also shown that NAFLD is independently associated with a greater severity of coronary stenoses and an increased prevalence of carotid atherosclerotic plaques[66,68,69].
Against this background, a consistent body of evidence also suggests an association between NAFLD and increased incidence of fatal and non-fatal CVD events in NAFLD patients with/without T2D[38,39,67,70]. Indeed, as extensively reviewed elsewhere[34,39], population-based cohort studies, have reported a strong and independent association between NAFLD and increased risk of fatal and non-fatal CVD events independent of multiple cardio-metabolic risk factors[70-81]. Only one of these studies had assessed coronary artery disease (CAD) as a pre-specified outcome; it reported that patients with NAFLD had a higher 10-year risk for CAD as calculated by the Framingham risk score (FRS) than the matched control population, featuring almost the same number of FRS-predicted and actual new CAD events[76]. A recent prospective study, involving 125 patients with ultrasound-diagnosed NAFLD and 250 age- and sex-matched control individuals, followed-up for 10 years, confirmed that the incidence of CVD outcomes was significantly higher in patients with NAFLD[82]. Studies have also highlighted that middle-aged individuals with NAFLD are particularly prone to increased CVD mortality[79,83]. For example, a study enrolling 980 subjects with NAFLD (diagnosed by elevated serum aminotransferase levels) compared to 6594 NAFLD-free individuals, followed up for 8.7 years, reported that the presence of NAFLD was associated with a higher risk of all-cause and CVD mortality, independently of traditional CVD risk factors in the 45-54 age group[83]. A recent Asian cohort study performed on NAFLD patients submitted to coronary angiography paradoxically showed a reduced CVD incidence over the follow-up period; however, the successful coronary re-vascularization procedures and the intensive lipid-lowering therapy might have dramatically improved the prognosis in this specific cohort of patients[84].
Notably, several cohort studies with a reasonably long follow-up that used biopsy-proven NAFLD have clearly shown that this disease is associated with an increased risk of all-cause and cause-specific mortality (mainly CVD, cancer-related and liver-related)[28,29,56,85-87]. Most of these studies showed that the severity of hepatic fibrosis was the main determinant of all-cause and cause-specific mortality, and that CVD was the leading cause of death in patients with NAFLD[28,29,56,85-87]. Despite some studies reporting NASH more closely associated with CVD risk than simple steatosis, a milestone meta-analysis did not find any significant difference in CVD risk among patients with NASH and those with simple steatosis; nevertheless it confirmed an increased CVD risk in patients with NAFLD[53]. Recently, a systematic review and meta-analysis involving 16 observational prospective and retrospective studies with 34043 adult individuals (36.3% with NAFLD) and approximately 2600 CVD outcomes (> 70% CVD deaths) over a median period of 6.9 years found that NAFLD patients had a higher risk of fatal and non-fatal CVD events than NAFLD-free controls[88]. Patients with more “severe” NAFLD were also more likely to develop fatal and non-fatal CVD events[88].
Of relevance, persistent NAFLD on ultrasound also appears to be a risk factor for incident subclinical atherosclerosis, and by treating NAFLD, we may improve vascular health. In fact, a retrospective cohort study of 8020 Japanese adults found that persistent NAFLD was associated with an increased risk of developing subclinical carotid atherosclerosis, and that this association was largely explained by coexisting cardiometabolic risk factors[89]. Consistently, a small randomized clinical trial, by evaluating the effect of a 18-mo treatment with either omega-3 fatty acids or placebo on the carotid intima-media thickness (IMT) progression, found that improvement in two markers of NAFLD severity was independently associated with decreased carotid IMT progression[90].
T2D in NAFLD: Via ectopic fat storage at multiple organ sites, insulin resistance (IR) is a key pathogenic determinant of T2D development in predisposed individuals with NAFLD. In particular, IR results from storage of ectopic fat in the liver and skeletal muscles owing to long-standing excess of energy supply and subsequent infiltration of macrophages into white adipose tissue[91].
Recent research focused on the complex and bidirectional relationship between IR and NAFLD. On the one hand, IR is an established risk factor of NAFLD[3], which occurs owing to unopposed lipogenic pathways being triggered by IR via multiple transcription factors, such as carbohydrate-responsive element-binding protein, liver X receptors, sterol regulatory element-binding protein 1C and upstream stimulatory factors[92]. On the other hand, NAFLD per se is a major determinant of hepatic IR. Evidence for this notion is that in obese T2D patients the presence of NAFLD is associated with more severe atherogenic dyslipidaemia, hyperinsulinaemia and IR in the adipose tissue and the liver compared to NAFLD-free control subjects[93]. A recent study on the molecular effectors of NAFLD-associated IR[94] has shown that fatty liver induce local and systemic chronic inflammation and IR via an altered protein secretory profile, notably including excess fetuin B, and that the prevention of fatty liver is a rational target for reducing the development of impaired glucose disposal in over-nourished individuals. Consistently, strategies aimed at reducing the development of fatty liver via antisense oligonucleotides against β-catenin may protect mice from diet-induced fatty liver and hepatic and peripheral IR[95].
In the setting of obesity or T2DM/pre-diabetes, the presence of NAFLD often develops in concert with homologous fatty changes of the pancreas[96-101]. Nonalcoholic fatty pancreas (NAFP) may be diagnosed by imaging techniques[102], and it is common in the general population[99]. The role of NAFP as a pathogenic mediator for the association between NAFLD and T2D risk has recently been reviewed[103-105].
CVD in NAFLD: In principle, the increased CVD risk seen in patients with NAFLD may result from a shared pathophysiological background, such as systemic IR and MetS. Such a view, however, would conflict with those studies reporting that NAFLD per se, regardless of coexisting MetS features, exposes to excess CVD risk[67]. Consistently, patients with more “severe” NAFLD are exposed to an increased risk of fatal and non-fatal CVD events compared to NAFLD-free controls[88]. In agreement, NASH, rather than simple steatosis, is associated with endothelial damage and over-expression of multiple atherogenic mediators and regulators of blood pressure[106]. Collectively, these data would support that NASH, as opposed to simple steatosis, is associated with a higher CVD risk. However, this conclusion needs to be further confirmed by future larger prospective studies.
Consistent with the notion reported above, the higher CVD risk seen in patients with NAFLD may result from increasing fibrosis stage, steatosis grade or oxidative stress[38]. Two studies indirectly confirmed this contention. The first study found that both all-cause and CVD mortality were higher especially in NAFLD patients with coexisting MetS features; conversely, the risk of mortality of metabolically-normal NAFLD patients was similar to that of the cohort without liver disease[77]. The second study found that the MetS-associated NAFLD was associated with a higher risk of CVD, T2D and increased cardiac mass, whereas NAFLD without MetS [i.e., a condition that was more frequently associated with the I148M variant of the patatin-like phospholipase domain-containing 3 gene (PNPLA3) polymorphism] was not[107].
Evidence for fibrosis stage playing a role in CVD risk derives from a cross-sectional study conducted in 1874 healthy European adolescents belonging to the general population. In that study, NAFLD individuals had more advanced non-invasively assessed liver fibrosis and worse cardiometabolic risk profiles independent of potential confounders[108].
Increasing liver fat content is also associated with worsening atherogenic dyslipidaemia and dysglycaemia; accordingly, it can reasonably be hypothesized that the quantity of fatty liver may dictate the risk of cardiometabolic outcomes in patients with NAFLD[109]. Consistent with this hypothesis, a recent cross-sectional study reported that, independent of NASH, increased liver fat content was associated with increased rates of MetS features in NAFLD patients[110].
Given that free fatty acids may induce systemic/hepatic IR, oxidative stress and increased synthesis of pro-thrombotic markers in cultured human hepatocytes[111], it can also be speculated that increased oxidative stress may be a critical contributor to CVD risk seen in NAFLD patients. Consistently, NASH was associated with increased oxidative stress and subclinical atherosclerosis in a recent study[112].
Finally, it is conceivable that also the chemical nature of hepatic fat content may play a role in the link between NAFLD and CVD risk. A study reported that three chemical compounds (11,12-dihydroxy-eicosatrienoic acid, 13,14-dihydro-15-keto prostaglandin D2 and 20-carboxy arachidonic acid) are more abundant in NASH than in simple steatosis[113]. Moreover, analysis of the human hepatic lipidome showed that similar increases in liver fat content and NASH were associated with a metabolically harmful saturated, ceramide-enriched liver lipidome in “MetS-related NAFLD” but not in “PNPLA3-related NAFLD”, accounting for the finding that the former, rather than the latter, was associated with an increased risk of T2D and CVD[114].
The worldwide prevalence of HCV infection approximates 1.6%, affecting about 115 million individuals, 80 million of whom are deemed to be viremic[115]. Globally, the G1 genotype is the most common and accounts for roughly one in two of all HCV infections in adults, followed by G3, G2, G4, G6, and G5 genotypes[115].
Traditional risk factors for HCV infection are: assumption of intravenous and intranasal illicit drugs; hemodialysis; cancer; paid blood donations; having received blood products prior to 1990; high-risk sexual behavior, tattoos or body piercings, working in health care[116]. Presently, in developed countries, the majority of new HCV infections are observed in individuals who inject drugs and in men who have sex with men[116].
Three to four million people are newly infected annually and approximately 350000 people per year die from HCV-related causes. HIV-HCV co-infected individuals sum up to approximately 2500000 worldwide, half of whom belonging to special populations, such as drug injectors[117].
Given that chronicity is the major complication of acute HCV infection, which can lead to many serious liver-related and extra-hepatic outcomes, HCV to date is a significant global public health burden[118].
Over the last few years, the advent of new direct antiviral agents (DAA) has revolutionized the HCV treatment by increasing sustained viral response (SVR) rates from approximately 50% to > 90%. Given that SVR plays a key role in HCV natural history, a multi-center observational study recently found that the survival rates of HCV-related cirrhotic patients who had attained SVR were essentially similar to those in the general population, justifying DAA treatment being administered as earliest as possible to achieve the maximal benefit in HCV-related, compensated cirrhosis[119].
HCV is a systemic disease featuring a variety of extra-hepatic manifestations, among which T2D plays a leading role[60,120,121]. Consistently, T2D is the second most prevalent extra-hepatic manifestation, affecting up to 15% of HCV-infected patients[122].
Several cross-sectional studies have shown that patients with HCV-related cirrhosis have a higher prevalence of T2D than those with cirrhosis unrelated to HCV infection[123-125]. Similar results have also been reported in the setting of liver transplantation[126-129], and in patients with all stages of chronic HCV, irrespective of the presence of cirrhosis, suggesting that the connection of HCV infection with IR/T2D was independent of the stage of liver disease[130-136]. A cross-sectional study investigating the relationship between HCV and T2D at the population level showed that T2D occurred more often in HCV-infected persons aged more than 40 years[137]. Consistently, other cross-sectional studies, involving T2D patients, showed that these patients had an increased prevalence of HCV infection[138-140].
Stronger evidence for a possible causal link between HCV and T2D came from longitudinal studies and meta-analyses. A United States prospective case-cohort study showed that pre-existing HCV infection increased the risk of new-onset T2D in persons with coexisting T2D risk factors[141]. A subsequent community-based longitudinal study, involving 4958 non-diabetic Taiwanese individuals, confirmed a significant temporal relationship between prior HCV infection and risk of new-onset T2D[142]. A recent meta-analysis of 31 studies (involving a total of 10388 T2D cases among 61843 HCV individuals and 42358 T2D cases among 202130 HCV-free controls) found that the pooled estimates for odds ratios for new-onset T2D among individuals with HCV infection were 1.58 (95%CI: 1.30-1.86)[122]. These findings are strikingly similar to the results of a previous meta-analysis that showed an approximately 1.7-fold excess risk of new-onset T2D in HCV-infected cases as compared to non-infected controls[143].
Interestingly, a recent study has estimated that the direct medical costs associated with HCV-related T2D were 443 million dollars yearly in the United States, making T2D the most expensive extra-hepatic complication of HCV[122]. Moreover, in patients with HCV, the coexistence of T2D is strongly associated with an increased risk of liver fibrosis progression[132,144-148], cirrhosis and HCC development[149-152], poor liver-related outcomes[152], lower response rates to peginteferon/ribavirin treatment[145,153-155], and may also partly account for the increased CVD risk[156,157].
Given the substantial health and economic burden of T2D among patients with HCV, treatment strategies aimed at eradicating HCV should also consider the potential benefits in terms of T2D prevention/resolution. Of note, studies have shown that HCV clearance with interferon-based treatment regimens may improve IR and pancreatic beta cell function[158,159], reduce T2D development[160], and improve renal and CVD outcomes[161,162]. Although new DAAs allow HCV eradication in the vast majority of HCV patients, little is currently known on the effects of DAAs on glucose metabolism[163]. One study showed that SVR obtained with the first-wave protease inhibitor telaprevir was associated with IR improvement[164]. Moreover, preliminary studies showed that DAAs significantly reduced plasma glucose and hemoglobin A1c (HbA1c) levels in both HCV patients with and without T2D[165,166].
HCV has been isolated from the myocardium of patients with myocarditis and dilated cardiomyopathy and from carotid atherosclerotic plaques, suggesting a direct role of HCV in cardiac dysfunction and accelerated atherogenesis[167-170]. Moreover, a higher prevalence of traditional CVD risk factors and MetS features, such as IR/T2D and fatty liver, have been reported in patients with HCV compared to uninfected controls[33,60,122,171]. A growing body of evidence also identifies HCV infection as a potential risk factor for subclinical and clinical CVD complications[156,157].
A proof-of-concept study among 217 Japanese HCV-positive patients without overt CAD demonstrated that myocardial perfusion defects, as detected by scintigraphy, are almost universal in HCV patients, are associated with liver disease activity and HCV-RNA levels, and improve following viral eradication[172]. Moreover, functional and morphological myocardial changes (as assessed by cardiac magnetic resonance) are common among HCV patients with end-stage liver disease[173]. A population-based cohort study showed that HCV-infected patients have a higher arterial stiffness than HCV-negative control subjects[174]. In addition, multiple cross-sectional studies, which examined carotid atherosclerosis, provided further strong evidence of a relationship between HCV and subclinical atherosclerosis[156,175-179]. Of note, a recent meta-analysis confirmed an association between HCV infection and carotid atherosclerosis[157]. The severity of hepatic steatosis and fibrosis and HCV viral load are among the strong predictors of carotid atherosclerosis[157,178,179].
Importantly, HCV infection is also associated with increased CVD morbidity and mortality. A large study conducted among US Veterans showed that, despite a more favorable cardio-metabolic risk profile, HCV-infected patients had a 1.25-fold higher risk of CAD than uninfected controls[180]. Similarly, a recent Taiwanese community-based study showed that HCV was significantly associated with ischemic electrocardiogram findings[181], and a United States population study found a significant association between HCV infection and the risk of congestive heart failure[182]. Several case-control studies subsequently confirmed that HCV infection predicted CAD and was independently associated with a greater severity of coronary stenoses[183-186], also among HCV-HIV co-infected patients and dialysis patients[187,188].
Similar findings were reported from studies evaluating the risk of cerebro vascular events among HCV patients[189-191]. For instance, a large Taiwanese population-based cohort study showed that HCV-infected patients had a 1.3-fold higher risk of ischemic stroke than HCV-free individuals[189]. Of note, HCV eradication (with interferon-based therapy) has reduced the long-term risk of ischemic stroke and acute coronary syndrome in HCV patients[161,162,191].
A recent meta-analysis of 8 observational studies confirmed that HCV infection was associated with increased CVD morbidity, and the strength of this association was similar for CVD and cerebro vascular events[157].
Worryingly, some studies also suggested a significant association between HCV and cerebral hemorrhages[192,193], and a recent large Taiwanese cohort study extended the association between HCV and atherosclerosis by showing that HCV-infected patients had an increased risk of peripheral artery disease compared to non-HCV control subjects[194].
Finally, a number of studies reported that HCV infection was independently associated with an increased CVD mortality[157,195-197] also in HIV co-infected patients and in dialysis patients[198,199].
A significant proportion of HCV-related morbidity and mortality may result from the coexistence of T2D and CVD in the development of which HCV may play a pathogenic role; moreover, HCV may also induce fatty liver, which in its turn, further increases CVD risk[200], especially in those with T2D and hypertension[157].
The main reason why HCV is a “successful pathogen” is that it exploits its host’s metabolism to build up viral particles. In so doing, HCV infection has developed a set of metabolic abnormalities collectively referred to as “hepatitis C-associated dysmetabolic syndrome” (HCADS)[171]. HCADS includes fatty liver, hyperuricaemia, hypocholesterolaemia, IR, hypertension and visceral overweight/obesity. Such metabolic disorders may best be interpreted as a “Darwinian strategy”, favoring HCV survival at the expense of the host’s metabolism. The finding of expanded visceral adipose tissue in HCV-infected patients is consistent with the hepatic and extra-hepatic origins of IR discussed above, and prompts further research regarding the potential ability of HCV to infect the adipose tissue[60].
Similar to the pathogenic model of T2D developing in the setting of NAFLD, HCV may exacerbate IR eventually leading to dysfunction/damage of pancreatic beta cells and development of overt T2D in most patients over time[121]. Conversely, HCV eradication may result in IR improvement, although definite evidence for this conclusion is eagerly awaited[201].
HCV antigens, notably including the core protein, play a key role in determining IR by interfering with the AKT signaling pathway, through the action of both pro-inflammatory cytokines [e.g., tumor necrosis factor-alfa and interleukin-6 (IL-6)] and suppressors of cytokine signaling[202]. The site of IR is not only hepatic but also extra-hepatic, mainly in the skeletal muscles, correlates with subcutaneous, rather than visceral adiposity, and is independent of liver fat content[60,171].
The role of specific viral genotypes in the development of IR is also increasingly appreciated. An extensive French study reported that IR was significantly associated with G1 and G4 genotypes, high HCV-RNA viral load and significant hepatic fibrosis, irrespective of fatty liver[134].
T2D and IR are strong predictors of faster progression of liver fibrosis and impaired response rates to HCV treatment[153,203]. Patients with HCV-related cirrhosis and T2D have an increased susceptibility to developing hepatic encephalopathy and HCC; moreover, concurrent T2D accounts for a persistently higher HCC risk even in those patients attaining SVR[204-206]. Theoretically, in patients with chronic HCV infection, a better glycaemic control could improve the prognosis and the response rate to HCV treatment, although direct evidence for this is limited.
Beneficial effects of antiviral treatment on IR and T2D are increasingly identified. By evaluating paired pre-treatment and post-treatment HOMA-estimated IR measurements in 1038 non-diabetic HCV patients, a study found that SVR was associated with a significant IR improvement in HCV patients infected with G1 genotype (but not in those infected with G2/3); IR improvement was independent of body weight, serum transaminase and lipid level changes, suggesting that G1 genotype might directly promote IR, independent of host metabolic factors, and might be improved following HCV eradication[207].
A preliminary clinical trial of 29 patients with T2D who were receiving different interferon-free regimens reported that fasting glucose and HbA1c levels were significantly reduced by the treatment, irrespective of the DAA used, HCV genotype, body weight and HIV status. Consistently, the dosages of hypoglycemic drugs were reduced in about a quarter of these patients[165].
T2D is not the only metabolic disease observed in the setting of HCV infection. Over time, several metabolic features of what is now alluded to as the HCADS have been increasingly identified. The HCV-related fatty liver, which is one of such features, was first identified as a distinct disease entity. Data comparing fatty liver both in the viral setting and NAFLD suggest that IR is a prominent feature specifically associated with HCV infection[7].
All HCV genotypes share multiple “NAFLD-like” steatogenic mechanisms, such as increased availability of lipogenic substrates and de novo lipogenesis; decreased oxidation of fatty substrates and export of fatty substrates. However, G3 genotype induces more prominent derangements in molecular mediators of steatogenesis (e.g., microsomal triglyceride transfer protein; peroxisome proliferator-activated receptor alpha; sterol regulatory element-binding proteins and phosphatase and tensin homologue), thus amplifying those steatogenic mechanisms, which also take place in NAFLD. As a result of this, fatty liver is more frequently associated with G3 genotype and is, therefore, designated as “viral fatty liver” as opposed to fatty liver occurring in HCV non-3 genotypes and particularly G1 genotype, which is more closely associated with host’s features[171,203,208-210].
Among the various features of HCADS, HCV-related fatty liver has a distinct, prominent clinical impact in as much as it accelerates hepatic fibrogenesis; impairs the response rates to interferon-alpha and ribavirin treatments; increases the risk of developing HCC and predisposes to accelerated atherogenesis[33,156,171,179].
Recent UNAIDS estimates indicate than in 2015 there were approximately 2.1 million new cases of HIV infections worldwide, adding up to a total of approximately 37 million people already living with HIV[211]. Against these dramatic features, over the last 15 years, HIV medicine gained impressively positive results in terms of improved life expectancy for HIV-infected adults in Western countries. This finding results from major changes in the natural history of HIV disease and in the efficacy of anti-retroviral therapy (ART)[212,213]. Scale-up of the ART is on a fast-track trajectory that has surpassed expectations. Global coverage of ART reached approximately 46% at the end of 2015 (with the greatest gains in the world’s most affected region, Eastern and Southern Africa)[214]. Several recent studies have suggested that the life expectancy of HIV-infected patients may approach that of the general population, particularly among patients who initiated ART at earlier disease stages[215]. Simultaneously, HIV seroconversion among older age persons is increasingly recognized, partly due to a lower perception of sexual risk in older people[216]. As a result of these changes, what we observe is that HIV-infected persons are more likely to be older and have an increased burden of age-related comorbidities compared to persons of a similar age who have been infected by HIV more recently[217].
While this “graying” of the HIV-infected population is to be welcomed as an indication of improved treatment and survival rates, the increasing number of older HIV-infected patients with increased rates of comorbidities presents new challenges for patient care. Cardio metabolic complications, such as CVD, atherogenic dyslipidemia and glucose intolerance/T2D, are very common in older HIV-infected patients, and justify the onset of multidisciplinary metabolic clinics for the management of patients with chronic HIV-related co-morbidities[218].
Patients with HIV are at higher risk of new-onset T2D compared to the general adult population[219]. The reasons for this increased T2D risk are not entirely understood, but may include the use of some ARTs[220], the presence of HCV co-infection[221], and the HIV-induced systemic chronic inflammation[222,223]. Untreated HIV is associated with higher levels of multiple pro-inflammatory biomarkers, and initiation of ART results in a decline, but does not normalize plasma pro-inflammatory biomarkers[224]. The occurrence of IR, which is causally associated with the development of T2D, was one of the first metabolic disorders associated with HIV disease and treatments[225].
T2D development can be, at least in part, linked to an underlying chronic inflammation. Population-based studies suggested that higher levels of IL-6 and C-reactive protein (CRP) are independently associated with an increased risk of new-onset T2D[226]. Cross-sectional studies also reported a significant and positive association between plasma CRP levels and IR in non-diabetic individuals[227]. Moreover, it has been shown that in healthy volunteers the administration of subcutaneous recombinant human IL-6 induced a dose-dependent increase in fasting glucose levels[228]. A recent retrospective cohort study involving 3695 non-diabetic participants, who took ART and were followed-up for an average of 4.6 years, found that higher circulating CRP and IL-6 levels were significantly associated with an increased risk of new-onset T2D. Other common risk factors for new-onset T2D in this cohort of HIV-infected patients were older age, higher body weight, co-infection with hepatitis B or C, non-smoking status and use of lipid-lowering drugs. All these findings suggest that systemic chronic inflammation may contribute to the development of T2D in patients with HIV[229].
The burden of CVD is increased in HIV-infected patients[230]. The investigators of the Veterans Aging Cohort Study (VACS) recently analyzed the data collected in 82459 Veterans who were followed-up for a mean period of 5.9 years. When these VACS participants were stratified by the presence or absence of traditional CVD risk factors, HIV-infected patients without risk factors had a two-fold greater risk of incident acute myocardial infarction compared to uninfected control individuals and their risk steeply increased with each additional CVD risk factor added[231].
Although CVD has become a major cause of mortality among HIV-infected patients, outnumbering those figures expected based on the high prevalence of CVD risk factors observed in this patient population, controversy still exists regarding the underlying pathogenic mechanisms of accelerated atherosclerosis in HIV.
Such an increased CVD risk may be likely due to both a direct role of HIV and the dysregulated immunological responses caused by chronic HIV infection[231-233]. Tight control of HIV replication and maintenance of a good CD4 count seem to protect from the risk of incident CVD events. However, there is conflicting evidence regarding the association of the CD4 cell count and the HIV-RNA levels with the risk of CVD[234,235]. The trans-activator of transcription (Tat), i.e., a regulatory protein that enhances the efficiency of viral transcription, may represent a pathogenic mechanism by which HIV may directly induce accelerated atherogenesis[236]. Tat induces the expression of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin[237], which are associated with an increased incidence of CVD in healthy individuals[238]. The levels of these circulating endothelial adhesion molecules are markedly increased in HIV-infected patients, and there is a significant relationship of elevated soluble ICAM-1 levels with HIV disease progression and reduced CD4 cell count[239]. Similarly to Gp120, Tat may also decrease endothelium-dependent vasodilation and nitric oxide synthase secretion in porcine coronary arteries[240].
In addition to the above-mentioned humoral effects, cellular immune activation may also play a role in CVD development among HIV-infected patients[241]. Monocytes are readily infected by HIV and may adhere to the endothelial surface and, eventually, penetrate in the sub-endothelial space and intima[242]. Furthermore, monocytes expressing CD14+ and CD16+ are also prone to a greater pro-inflammatory activity once infected by HIV[243].
Uncertainty still remains as to the extent to which traditional CVD risk factors, immune risk factors, and non-traditional behavioral risk factors contribute to CVD development in HIV-infected individuals. Importantly, the circulating monocyte activation marker sCD163 is associated with high-risk morphology coronary atherosclerotic plaques and arterial inflammation[244,245]. Finally, in specific populations of HIV patients, non-traditional behavioral risk factors, such as cocaine use, may also contribute to accelerated atherogenesis via multiple mechanisms[246].
In HIV-infected patients the etiology of CVD and T2D reflects the complex and intertwined interactions of multiple factors present in this particularly high-risk patient population, such as the higher prevalence of traditional and non-traditional CVD risk factors, the effects of HIV infection, and the effects of ARTs. In this context, it is reasonable to assume that fatty liver itself might also play a pathogenic role in the development of CVD in patients with HIV.
The adverse effect of the HIV infection on the re-distribution of adipose tissue is known as “lipo-hypertrophy”, which mainly consists of the expansion of ectopic fat depots such as abdomen, liver, dorso-cervical region (sometimes with buffalo hump), trunk and heart, which may all contribute to the development of T2D and other MetS features[247].
In patients in whom fatty liver occurs in the context of HIV and/or HCV (i.e., VAFLD), the development of fatty liver may result from a complex interplay between host and viral factors, such as sex, lifestyle habits, IR, MetS, HCV genotype, viral load and ART use[210,248]. In HIV-infected patients, the potential steatogenic effect of ARTs remains controversial[249,250]. Although VAFLD is highly prevalent in HCV/HIV-coinfected patients[251], the natural history of fatty liver observed in these patients remains incompletely understood. A recent study reported that fatty liver was a precursor lesion to hepatic fibrosis[252], suggesting that the effective management of fatty liver might also result in the reduction of hepatic fibrosis progression in patients with HCV/HIV co-infection[253]. Moreover, IR is a predictor of reduced SVR in both HCV-monoinfected and HCV/HIV-coinfected patients[155]. All these findings further underline the importance of improving our understanding of the independent contribution of fatty liver to the pathophysiology of CVD and T2D in this patient population.
To date, there are very limited data regarding the clinical, biochemical and liver histological features of HIV-related VAFLD; moreover, it remains uncertain whether VAFLD differs in terms of clinical presentation and histological severity from primary NAFLD. A small cross-sectional study showed that, compared to age- and sex-matched patients with primary NAFLD, those with HIV-related VAFLD not only had a more severe metabolic profile but also more severe forms of liver disease[254].
Presently, large prospective studies on VAFLD in HIV are lacking. A very small series of 10 HIV mono-infected VAFLD patients, who were treated either with maraviroc (MRV) plus atazanavir (n = 7) or with vitamin E (n = 3), found that at baseline the histologic degree of hepatic steatosis was 20% and decreased to approximately 10% after treatment. The extent of steatosis improved in all patients, except for two patients who gained body weight. A reduction in hepatic fat content assessed histologically and with magnetic resonance at 48 wk was found both in the atazanavir/MRV and, to a lesser extent, in the Vitamin E treatment groups. The results of this preliminary study suggest a possible role of MRV in the treatment of fatty liver in HIV-infected patients (Guaraldi G et al, unpublished data). However, future large randomized clinical trials are needed to confirm these findings.
As lipodystrophy may predispose HIV-infected patients to develop T2D and other MetS features, new prevention and management strategies should be evaluated. For example, avoidance of some ART regimens, containing stavudine and zidovudine, might help in preventing the development of lipoatrophy, but no strategies have proven effective against lipohypertrophy[255]. For patients with established lipoatrophy, switching from ART regimens, containing either stavudine or zidovudine, to an alternative ART regimen could be an empirical choice. Tesamorelin, a synthetic growth-hormone-releasing analogue, has been approved for the treatment of lipohypertrophy. This drug may reduce both abdominal visceral adiposity[256] and liver fat content[257], but its long-term effects on the risk of T2D and CVD remain uncertain.
In this narrative review, we have tested the hypothesis that fatty liver represents a shared pathogenic mechanism for the development of CVD and T2D not only in patients with NAFLD but also in those with chronic HCV or HIV infections. To do so, we have followed an innovative multidisciplinary comparative approach, by extending the widely accepted comparison of NAFLD with chronic HCV infection[60,258] to HIV infection.
What we have found is that, the published data are biologically consistent in identifying NAFLD as a major player in the development of T2D and CVD (as schematically shown in Figure 1). Indeed, the clinical burden of NAFLD is not only restricted to liver-related morbidity or mortality, but there is now growing evidence that NAFLD is a multisystem disease, affecting multiple extra-hepatic organs and regulatory, inflammatory and metabolic pathways[259]. Strong evidence indicates that CVD complications frequently dictate the clinical outcomes of NAFLD patients and, therefore, screening for CVD is mandatory in all patients with NAFLD, at least by utilizing a detailed risk factor assessment[15,259-261]. Furthermore, convincing evidence also supports a link between NAFLD and the risk of new-onset T2D[60,259]. Collectively, accumulating evidence suggests that NAFLD is not a simple marker of CVD and T2D, but also plays a part in the pathophysiology of these cardiometabolic complications[38,60,259,261]. NAFLD, especially in its necro-inflammatory variant (NASH), exacerbates systemic/hepatic IR, predisposes to atherogenic dyslipidaemia, and releases several pro-inflammatory, pro-coagulant, pro-oxidant and pro-fibrogenic mediators that play important roles in the development of CVD and T2D[38,60,259,261].
Likewise, HCV and HIV are two globally prevalent pathogens and leading causes of mortality and morbidity worldwide. Patients with chronic HCV infection or HIV infection are at higher risk of developing T2D and CVD[60,157,262,263]. Interestingly, fatty liver is often observed in patients with chronic HCV or HIV infections[60,264] and a wide array of similarities with NAFLD further supports a link of HCV or HIV infections with the risk of T2D and CVD.
On the grounds of data examined here, it is therefore reasonable to assume that fatty liver might be a shared pathological condition which plays a key role in the development of T2D and CVD not only in patients with NAFLD but also in those with chronic HCV or HIV infections. However, further research is required to further corroborate the prognostic role of fatty liver per se in the development of T2D and CVD in patients with chronic HCV or HIV infections, and to better elucidate the differences and similarities between NAFLD and VAFLD. Moreover, it remains uncertain whether specific algorithms for CVD/T2D risk assessment[37] should be implemented and validated both in patients with NAFLD and in those with VAFLD.
In the meantime, the findings of this review may disclose new avenues in the clinical and research arenas. Our findings also pave the way for planning future prospective and intervention studies of clinical relevance because, for example, with the success of ARTs, HIV-related co-morbidities, like T2D and CVD, are of increasing concern[263].
| 1. | Petäjä EM, Yki-Järvinen H. Definitions of Normal Liver Fat and the Association of Insulin Sensitivity with Acquired and Genetic NAFLD-A Systematic Review. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Lonardo A, Lombardini S, Scaglioni F, Carulli L, Ricchi M, Ganazzi D, Adinolfi LE, Ruggiero G, Carulli N, Loria P. Hepatic steatosis and insulin resistance: does etiology make a difference? J Hepatol. 2006;44:190-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Ballestri S, Nascimbeni F, Romagnoli D, Lonardo A. The independent predictors of non-alcoholic steatohepatitis and its individual histological features.: Insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol Res. 2016;46:1074-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 4. | Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, Roverato A, Guaraldi G, Lonardo A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:936-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 562] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 5. | Adinolfi LE, Rinaldi L, Guerrera B, Restivo L, Marrone A, Giordano M, Zampino R. NAFLD and NASH in HCV Infection: Prevalence and Significance in Hepatic and Extrahepatic Manifestations. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Guaraldi G, Stentarelli C, Orlando G, Zona S, Carli F, Ballestri S, Lonardo A, Squillace N, Loria P. Nonalcoholic fatty liver disease in HIV-infected persons: epidemiology and the role of nucleoside reverse transcriptase inhibitors. J Acquir Immune Defic Syndr. 2010;53:278; author reply 278-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Guaraldi G, Lonardo A, Ballestri S, Zona S, Stentarelli C, Orlando G, Carli F, Carulli L, Roverato A, Loria P. Human immunodeficiency virus is the major determinant of steatosis and hepatitis C virus of insulin resistance in virus-associated fatty liver disease. Arch Med Res. 2011;42:690-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7912] [Article Influence: 791.2] [Reference Citation Analysis (5)] |
| 9. | Bertolotti M, Lonardo A, Mussi C, Baldelli E, Pellegrini E, Ballestri S, Romagnoli D, Loria P. Nonalcoholic fatty liver disease and aging: epidemiology to management. World J Gastroenterol. 2014;20:14185-14204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 232] [Cited by in RCA: 236] [Article Influence: 19.7] [Reference Citation Analysis (1)] |
| 10. | Bruno S, Maisonneuve P, Castellana P, Rotmensz N, Rossi S, Maggioni M, Persico M, Colombo A, Monasterolo F, Casadei-Giunchi D. Incidence and risk factors for non-alcoholic steatohepatitis: prospective study of 5408 women enrolled in Italian tamoxifen chemoprevention trial. BMJ. 2005;330:932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 179] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Lonardo A, Trande P. Are there any sex differences in fatty liver? A study of glucose metabolism and body fat distribution. J Gastroenterol Hepatol. 2000;15:775-782. [PubMed] |
| 12. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2325] [Article Influence: 155.0] [Reference Citation Analysis (1)] |
| 13. | Turola E, Petta S, Vanni E, Milosa F, Valenti L, Critelli R, Miele L, Maccio L, Calvaruso V, Fracanzani AL. Ovarian senescence increases liver fibrosis in humans and zebrafish with steatosis. Dis Model Mech. 2015;8:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Klair JS, Yang JD, Abdelmalek MF, Guy CD, Gill RM, Yates K, Unalp-Arida A, Lavine JE, Clark JM, Diehl AM. A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology. 2016;64:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 160] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 15. | Lonardo A, Bellentani S, Argo CK, Ballestri S, Byrne CD, Caldwell SH, Cortez-Pinto H, Grieco A, Machado MV, Miele L. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig Liver Dis. 2015;47:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 352] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 16. | Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643-654.e1-9; quiz e39-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 1300] [Article Influence: 118.2] [Reference Citation Analysis (1)] |
| 17. | Dixon JB, Bhathal PS, O‘Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91-100. [PubMed] |
| 18. | Wei JL, Leung JC, Loong TC, Wong GL, Yeung DK, Chan RS, Chan HL, Chim AM, Woo J, Chu WC. Prevalence and Severity of Nonalcoholic Fatty Liver Disease in Non-Obese Patients: A Population Study Using Proton-Magnetic Resonance Spectroscopy. Am J Gastroenterol. 2015;110:1306-1314; quiz 1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 245] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 19. | Glass LM, Dickson RC, Anderson JC, Suriawinata AA, Putra J, Berk BS, Toor A. Total body weight loss of ≥ 10 % is associated with improved hepatic fibrosis in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2015;60:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 20. | Lonardo A, Sookoian S, Chonchol M, Loria P, Targher G. Cardiovascular and systemic risk in nonalcoholic fatty liver disease - atherosclerosis as a major player in the natural course of NAFLD. Curr Pharm Des. 2013;19:5177-5192. [PubMed] |
| 21. | Ballestri S, Nascimbeni F, Romagnoli D, Baldelli E, Lonardo A. The Role of Nuclear Receptors in the Pathophysiology, Natural Course, and Drug Treatment of NAFLD in Humans. Adv Ther. 2016;33:291-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism. 2016;65:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 325] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 23. | Lonardo A, Bellentani S, Ratziu V, Loria P. Insulin resistance in nonalcoholic steatohepatitis: necessary but not sufficient - death of a dogma from analysis of therapeutic studies? Expert Rev Gastroenterol Hepatol. 2011;5:279-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Yilmaz Y. Review article: is non-alcoholic fatty liver disease a spectrum, or are steatosis and non-alcoholic steatohepatitis distinct conditions? Aliment Pharmacol Ther. 2012;36:815-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (2)] |
| 25. | Pais R, Charlotte F, Fedchuk L, Bedossa P, Lebray P, Poynard T, Ratziu V. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59:550-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 396] [Article Influence: 30.5] [Reference Citation Analysis (1)] |
| 26. | Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, Chim AM, Yu J, Sung JJ, Chan HL. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 498] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 27. | McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 817] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 28. | Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389-397.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2304] [Cited by in RCA: 2326] [Article Influence: 211.5] [Reference Citation Analysis (2)] |
| 29. | Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1775] [Article Influence: 161.4] [Reference Citation Analysis (2)] |
| 30. | Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. 2015;47:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 521] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 31. | Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, Erbel R, Blankstein R, Feldman T, Al-Mallah MH. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230:258-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 32. | Fargion S, Porzio M, Fracanzani AL. Nonalcoholic fatty liver disease and vascular disease: state-of-the-art. World J Gastroenterol. 2014;20:13306-13324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 144] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (1)] |
| 33. | Loria P, Marchesini G, Nascimbeni F, Ballestri S, Maurantonio M, Carubbi F, Ratziu V, Lonardo A. Cardiovascular risk, lipidemic phenotype and steatosis. A comparative analysis of cirrhotic and non-cirrhotic liver disease due to varying etiology. Atherosclerosis. 2014;232:99-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 34. | Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1521] [Article Influence: 95.1] [Reference Citation Analysis (9)] |
| 35. | Chen Y, Xu M, Wang T, Sun J, Sun W, Xu B, Huang X, Xu Y, Lu J, Li X. Advanced fibrosis associates with atherosclerosis in subjects with nonalcoholic fatty liver disease. Atherosclerosis. 2015;241:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Sapmaz F, Uzman M, Basyigit S, Ozkan S, Yavuz B, Yeniova A, Kefeli A, Asilturk Z, Nazligül Y. Steatosis Grade is the Most Important Risk Factor for Development of Endothelial Dysfunction in NAFLD. Medicine (Baltimore). 2016;95:e3280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Lonardo A, Ballestri S, Targher G, Loria P. Diagnosis and management of cardiovascular risk in nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2015;9:629-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Lonardo A, Sookoian S, Pirola CJ, Targher G. Non-alcoholic fatty liver disease and risk of cardiovascular disease. Metabolism. 2016;65:1136-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 39. | Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:1724-1745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 204] [Cited by in RCA: 205] [Article Influence: 17.1] [Reference Citation Analysis (1)] |
| 40. | Valbusa F, Bonapace S, Grillo C, Scala L, Chiampan A, Rossi A, Zoppini G, Lonardo A, Arcaro G, Byrne CD. Nonalcoholic Fatty Liver Disease Is Associated With Higher 1-year All-Cause Rehospitalization Rates in Patients Admitted for Acute Heart Failure. Medicine (Baltimore). 2016;95:e2760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Mantovani A, Rigamonti A, Bonapace S, Bolzan B, Pernigo M, Morani G, Franceschini L, Bergamini C, Bertolini L, Valbusa F. Nonalcoholic Fatty Liver Disease Is Associated With Ventricular Arrhythmias in Patients With Type 2 Diabetes Referred for Clinically Indicated 24-Hour Holter Monitoring. Diabetes Care. 2016;39:1416-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 42. | Giannini EG, Marabotto E, Savarino V, Trevisani F, di Nolfo MA, Del Poggio P, Benvegnù L, Farinati F, Zoli M, Borzio F. Hepatocellular carcinoma in patients with cryptogenic cirrhosis. Clin Gastroenterol Hepatol. 2009;7:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 628] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 44. | Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, Bellentani S. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 479] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 45. | Sangiovanni A, Colombo M. Surveillance for hepatocellular carcinoma: a standard of care, not a clinical option. Hepatology. 2011;54:1898-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Michelini E, Lonardo A, Ballestri S, Costantini M, Caporali C, Bonati ME, Bertolotti M, Iori R, Loria P. Is cholangiocarcinoma another complication of insulin resistance: a report of three cases. Metab Syndr Relat Disord. 2007;5:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 427] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 48. | Reddy SK, Hyder O, Marsh JW, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Aldrighetti L. Prevalence of nonalcoholic steatohepatitis among patients with resectable intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2013;17:748-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Sanna C, Rosso C, Marietti M, Bugianesi E. Non-Alcoholic Fatty Liver Disease and Extra-Hepatic Cancers. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 50. | Fukuda T, Hamaguchi M, Kojima T, Hashimoto Y, Ohbora A, Kato T, Nakamura N, Fukui M. The impact of non-alcoholic fatty liver disease on incident type 2 diabetes mellitus in non-overweight individuals. Liver Int. 2016;36:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 51. | Shah RV, Allison MA, Lima JA, Bluemke DA, Abbasi SA, Ouyang P, Jerosch-Herold M, Ding J, Budoff MJ, Murthy VL. Liver fat, statin use, and incident diabetes: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2015;242:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Sung KC, Wild SH, Byrne CD. Resolution of fatty liver and risk of incident diabetes. J Clin Endocrinol Metab. 2013;98:3637-3643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 53. | Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 934] [Article Influence: 62.3] [Reference Citation Analysis (1)] |
| 54. | Yamazaki H, Tsuboya T, Tsuji K, Dohke M, Maguchi H. Independent Association Between Improvement of Nonalcoholic Fatty Liver Disease and Reduced Incidence of Type 2 Diabetes. Diabetes Care. 2015;38:1673-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 55. | Hashimoto Y, Hamaguchi M, Fukuda T, Nakamura N, Ohbora A, Kojima T, Fukui M. BMI history and risk of incident fatty liver: a population-based large-scale cohort study. Eur J Gastroenterol Hepatol. 2016;28:1188-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1647] [Cited by in RCA: 1734] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 57. | Leite NC, Villela-Nogueira CA, Cardoso CR, Salles GF. Non-alcoholic fatty liver disease and diabetes: from physiopathological interplay to diagnosis and treatment. World J Gastroenterol. 2014;20:8377-8392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 74] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (3)] |
| 58. | Goh GB, Pagadala MR, Dasarathy J, Unalp-Arida A, Sargent R, Hawkins C, Sourianarayanane A, Khiyami A, Yerian L, Pai RK. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin. 2015;3:141-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 59. | Nascimbeni F, Aron-Wisnewsky J, Pais R, Tordjman J, Poitou C, Charlotte F, Bedossa P, Poynard T, Clément K, Ratziu V. Statins, antidiabetic medications and liver histology in patients with diabetes with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 60. | Ballestri S, Nascimbeni F, Romagnoli D, Baldelli E, Targher G, Lonardo A. Type 2 Diabetes in Non-Alcoholic Fatty Liver Disease and Hepatitis C Virus Infection--Liver: The “Musketeer” in the Spotlight. Int J Mol Sci. 2016;17:355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Oda K, Uto H, Mawatari S, Ido A. Clinical features of hepatocellular carcinoma associated with nonalcoholic fatty liver disease: a review of human studies. Clin J Gastroenterol. 2015;8:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 62. | Reeves HL, Zaki MY, Day CP. Hepatocellular Carcinoma in Obesity, Type 2 Diabetes, and NAFLD. Dig Dis Sci. 2016;61:1234-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 63. | Zoppini G, Fedeli U, Gennaro N, Saugo M, Targher G, Bonora E. Mortality from chronic liver diseases in diabetes. Am J Gastroenterol. 2014;109:1020-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 64. | Wild SH, Morling JR, McAllister DA, Kerssens J, Fischbacher C, Parkes J, Roderick PJ, Sattar N, Byrne CD. Type 2 diabetes and risk of hospital admission or death for chronic liver diseases. J Hepatol. 2016;64:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 65. | Ertle J, Dechêne A, Sowa JP, Penndorf V, Herzer K, Kaiser G, Schlaak JF, Gerken G, Syn WK, Canbay A. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128:2436-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 388] [Article Influence: 25.9] [Reference Citation Analysis (1)] |
| 66. | Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol. 2008;49:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 313] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 67. | Mantovani A, Ballestri S, Lonardo A, Targher G. Cardiovascular Disease and Myocardial Abnormalities in Nonalcoholic Fatty Liver Disease. Dig Dis Sci. 2016;61:1246-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 68. | Pais R, Giral P, Khan JF, Rosenbaum D, Housset C, Poynard T, Ratziu V. Fatty liver is an independent predictor of early carotid atherosclerosis. J Hepatol. 2016;65:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 69. | Ampuero J, Gallego-Durán R, Romero-Gómez M. Association of NAFLD with subclinical atherosclerosis and coronary-artery disease: meta-analysis. Rev Esp Enferm Dig. 2015;107:10-16. [PubMed] |
| 70. | Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, Arcaro G. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 415] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 71. | Jepsen P, Vilstrup H, Mellemkjaer L, Thulstrup AM, Olsen JH, Baron JA, Sørensen HT. Prognosis of patients with a diagnosis of fatty liver--a registry-based cohort study. Hepatogastroenterology. 2003;50:2101-2104. [PubMed] |
| 72. | Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 708] [Article Influence: 37.3] [Reference Citation Analysis (1)] |
| 73. | Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579-1584. [PubMed] |
| 74. | Haring R, Wallaschofski H, Nauck M, Dörr M, Baumeister SE, Völzke H. Ultrasonographic hepatic steatosis increases prediction of mortality risk from elevated serum gamma-glutamyl transpeptidase levels. Hepatology. 2009;50:1403-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 75. | Zhou YJ, Li YY, Nie YQ, Huang CM, Cao CY. Natural course of nonalcoholic fatty liver disease in southern China: a prospective cohort study. J Dig Dis. 2012;13:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 76. | Treeprasertsuk S, Leverage S, Adams LA, Lindor KD, St Sauver J, Angulo P. The Framingham risk score and heart disease in nonalcoholic fatty liver disease. Liver Int. 2012;32:945-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 77. | Younossi ZM, Otgonsuren M, Venkatesan C, Mishra A. In patients with non-alcoholic fatty liver disease, metabolically abnormal individuals are at a higher risk for mortality while metabolically normal individuals are not. Metabolism. 2013;62:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 78. | Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 639] [Article Influence: 49.2] [Reference Citation Analysis (1)] |
| 79. | Pisto P, Santaniemi M, Bloigu R, Ukkola O, Kesäniemi YA. Fatty liver predicts the risk for cardiovascular events in middle-aged population: a population-based cohort study. BMJ Open. 2014;4:e004973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 80. | Pickhardt PJ, Hahn L, Muñoz del Rio A, Park SH, Reeder SB, Said A. Natural history of hepatic steatosis: observed outcomes for subsequent liver and cardiovascular complications. AJR Am J Roentgenol. 2014;202:752-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 81. | Moon SH, Hong SP, Cho YS, Noh TS, Choi JY, Kim BT, Lee KH. Hepatic FDG uptake is associated with future cardiovascular events in asymptomatic individuals with non-alcoholic fatty liver disease. J Nucl Cardiol. 2015; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 82. | Fracanzani AL, Tiraboschi S, Pisano G, Consonni D, Baragetti A, Bertelli C, Norata D, Valenti L, Grigore L, Porzio M. Progression of carotid vascular damage and cardiovascular events in non-alcoholic fatty liver disease patients compared to the general population during 10 years of follow-up. Atherosclerosis. 2016;246:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 83. | Dunn W, Xu R, Wingard DL, Rogers C, Angulo P, Younossi ZM, Schwimmer JB. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol. 2008;103:2263-2271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 250] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 84. | Wong VW, Wong GL, Yeung JC, Fung CY, Chan JK, Chang ZH, Kwan CT, Lam HW, Limquiaco J, Chim AM. Long-term clinical outcomes after fatty liver screening in patients undergoing coronary angiogram: A prospective cohort study. Hepatology. 2016;63:754-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 85. | Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121. [PubMed] |
| 86. | Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 570] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 87. | Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 566] [Article Influence: 35.4] [Reference Citation Analysis (1)] |
| 88. | Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1051] [Article Influence: 105.1] [Reference Citation Analysis (0)] |
| 89. | Sinn DH, Cho SJ, Gu S, Seong D, Kang D, Kim H, Yi BK, Paik SW, Guallar E, Cho J. Persistent Nonalcoholic Fatty Liver Disease Increases Risk for Carotid Atherosclerosis. Gastroenterology. 2016;151:481-488.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 90. | Bhatia L, Scorletti E, Curzen N, Clough GF, Calder PC, Byrne CD. Improvement in non-alcoholic fatty liver disease severity is associated with a reduction in carotid intima-media thickness progression. Atherosclerosis. 2016;246:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 91. | Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016;126:12-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 997] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 92. | Wang Y, Viscarra J, Kim SJ, Sul HS. Transcriptional regulation of hepatic lipogenesis. Nat Rev Mol Cell Biol. 2015;16:678-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 522] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 93. | Lomonaco R, Bril F, Portillo-Sanchez P, Ortiz-Lopez C, Orsak B, Biernacki D, Lo M, Suman A, Weber MH, Cusi K. Metabolic Impact of Nonalcoholic Steatohepatitis in Obese Patients With Type 2 Diabetes. Diabetes Care. 2016;39:632-638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 94. | Meex RC, Hoy AJ, Morris A, Brown RD, Lo JC, Burke M, Goode RJ, Kingwell BA, Kraakman MJ, Febbraio MA. Fetuin B Is a Secreted Hepatocyte Factor Linking Steatosis to Impaired Glucose Metabolism. Cell Metab. 2015;22:1078-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 190] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 95. | Popov VB, Jornayvaz FR, Akgul EO, Kanda S, Jurczak MJ, Zhang D, Abudukadier A, Majumdar SK, Guigni B, Petersen KF. Second-generation antisense oligonucleotides against β-catenin protect mice against diet-induced hepatic steatosis and hepatic and peripheral insulin resistance. FASEB J. 2016;30:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 96. | Sepe PS, Ohri A, Sanaka S, Berzin TM, Sekhon S, Bennett G, Mehta G, Chuttani R, Kane R, Pleskow D. A prospective evaluation of fatty pancreas by using EUS. Gastrointest Endosc. 2011;73:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 97. | Ou HY, Wang CY, Yang YC, Chen MF, Chang CJ. The association between nonalcoholic fatty pancreas disease and diabetes. PLoS One. 2013;8:e62561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 98. | Wu WC, Wang CY. Association between non-alcoholic fatty pancreatic disease (NAFPD) and the metabolic syndrome: case-control retrospective study. Cardiovasc Diabetol. 2013;12:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 99. | Wang CY, Ou HY, Chen MF, Chang TC, Chang CJ. Enigmatic ectopic fat: prevalence of nonalcoholic fatty pancreas disease and its associated factors in a Chinese population. J Am Heart Assoc. 2014;3:e000297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 100. | Uygun A, Kadayifci A, Demirci H, Saglam M, Sakin YS, Ozturk K, Polat Z, Karslioglu Y, Bolu E. The effect of fatty pancreas on serum glucose parameters in patients with nonalcoholic steatohepatitis. Eur J Intern Med. 2015;26:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 101. | Yamazaki H, Tsuboya T, Katanuma A, Kodama Y, Tauchi S, Dohke M, Maguchi H. Lack of Independent Association Between Fatty Pancreas and Incidence of Type 2 Diabetes: 5-Year Japanese Cohort Study. Diabetes Care. 2016;39:1677-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 102. | Pezzilli R, Calculli L. Pancreatic steatosis: Is it related to either obesity or diabetes mellitus? World J Diabetes. 2014;5:415-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 103. | Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem. 2012;81:767-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 474] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 104. | Loria P, Lonardo A, Anania F. Liver and diabetes. A vicious circle. Hepatol Res. 2013;43:51-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 105. | Sharma RB, Alonso LC. Lipotoxicity in the pancreatic beta cell: not just survival and function, but proliferation as well? Curr Diab Rep. 2014;14:492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 106. | Sookoian S, Gianotti TF, Rosselli MS, Burgueño AL, Castaño GO, Pirola CJ. Liver transcriptional profile of atherosclerosis-related genes in human nonalcoholic fatty liver disease. Atherosclerosis. 2011;218:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 107. | Käräjämäki AJ, Bloigu R, Kauma H, Kesäniemi YA, Koivurova OP, Perkiömäki J, Huikuri H, Ukkola O. Non-alcoholic fatty liver disease with and without metabolic syndrome: Different long-term outcomes. Metabolism. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 108. | Lawlor DA, Callaway M, Macdonald-Wallis C, Anderson E, Fraser A, Howe LD, Day C, Sattar N. Nonalcoholic fatty liver disease, liver fibrosis, and cardiometabolic risk factors in adolescence: a cross-sectional study of 1874 general population adolescents. J Clin Endocrinol Metab. 2014;99:E410-E417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 109. | Adams LA. NAFLD. Accurate quantification of hepatic fat--is it important? Nat Rev Gastroenterol Hepatol. 2015;12:126-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 110. | Arulanandan A, Ang B, Bettencourt R, Hooker J, Behling C, Lin GY, Valasek MA, Ix JH, Schnabl B, Sirlin CB. Association Between Quantity of Liver Fat and Cardiovascular Risk in Patients With Nonalcoholic Fatty Liver Disease Independent of Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2015;13:1513-1520.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 111. | Soardo G, Donnini D, Domenis L, Catena C, De Silvestri D, Cappello D, Dibenedetto A, Carnelutti A, Bonasia V, Pagano C. Oxidative stress is activated by free fatty acids in cultured human hepatocytes. Metab Syndr Relat Disord. 2011;9:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 112. | Leach NV, Dronca E, Vesa SC, Sampelean DP, Craciun EC, Lupsor M, Crisan D, Tarau R, Rusu R, Para I. Serum homocysteine levels, oxidative stress and cardiovascular risk in non-alcoholic steatohepatitis. Eur J Intern Med. 2014;25:762-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 113. | Loomba R, Quehenberger O, Armando A, Dennis EA. Polyunsaturated fatty acid metabolites as novel lipidomic biomarkers for noninvasive diagnosis of nonalcoholic steatohepatitis. J Lipid Res. 2015;56:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 114. | Luukkonen PK, Zhou Y, Sädevirta S, Leivonen M, Arola J, Orešič M, Hyötyläinen T, Yki-Järvinen H. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J Hepatol. 2016;64:1167-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 356] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 115. | Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1381] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 116. | Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58-S68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 630] [Article Influence: 52.5] [Reference Citation Analysis (1)] |
| 117. | Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, Yanny I, Razavi H, Vickerman P. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16:797-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 539] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 118. | Aghemo A, Dore GJ, Hatzakis A, Wedemeyer H, Razavi H. Estimating HCV disease burden - volume 3 (editorial). J Viral Hepat. 2015;22 Suppl 4:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 119. | Bruno S, Di Marco V, Iavarone M, Roffi L, Crosignani A, Calvaruso V, Aghemo A, Cabibbo G, Viganò M, Boccaccio V. Survival of patients with HCV cirrhosis and sustained virologic response is similar to the general population. J Hepatol. 2016;64:1217-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 120. | Negro F, Forton D, Craxì A, Sulkowski MS, Feld JJ, Manns MP. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology. 2015;149:1345-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 282] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 121. | Lonardo A, Adinolfi LE, Petta S, Craxì A, Loria P. Hepatitis C and diabetes: the inevitable coincidence? Expert Rev Anti Infect Ther. 2009;7:293-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 122. | Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic Manifestations of Hepatitis C: A Meta-analysis of Prevalence, Quality of Life, and Economic Burden. Gastroenterology. 2016;150:1599-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 307] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 123. | Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135-1139. [PubMed] |
| 124. | Caronia S, Taylor K, Pagliaro L, Carr C, Palazzo U, Petrik J, O’Rahilly S, Shore S, Tom BD, Alexander GJ. Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30:1059-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 263] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 125. | Zein NN, Abdulkarim AS, Wiesner RH, Egan KS, Persing DH. Prevalence of diabetes mellitus in patients with end-stage liver cirrhosis due to hepatitis C, alcohol, or cholestatic disease. J Hepatol. 2000;32:209-217. [PubMed] |
| 126. | Knobler H, Stagnaro-Green A, Wallenstein S, Schwartz M, Roman SH. Higher incidence of diabetes in liver transplant recipients with hepatitis C. J Clin Gastroenterol. 1998;26:30-33. [PubMed] |
| 127. | Bigam DL, Pennington JJ, Carpentier A, Wanless IR, Hemming AW, Croxford R, Greig PD, Lilly LB, Heathcote JE, Levy GA. Hepatitis C-related cirrhosis: a predictor of diabetes after liver transplantation. Hepatology. 2000;32:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 102] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 128. | Khalili M, Lim JW, Bass N, Ascher NL, Roberts JP, Terrault NA. New onset diabetes mellitus after liver transplantation: the critical role of hepatitis C infection. Liver Transpl. 2004;10:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 129. | Saliba F, Lakehal M, Pageaux GP, Roche B, Vanlemmens C, Duvoux C, Dumortier J, Salamé E, Calmus Y, Maugendre D. Risk factors for new-onset diabetes mellitus following liver transplantation and impact of hepatitis C infection : an observational multicenter study. Liver Transpl. 2007;13:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 130. | Knobler H, Schihmanter R, Zifroni A, Fenakel G, Schattner A. Increased risk of type 2 diabetes in noncirrhotic patients with chronic hepatitis C virus infection. Mayo Clin Proc. 2000;75:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 189] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 131. | Petit JM, Bour JB, Galland-Jos C, Minello A, Verges B, Guiguet M, Brun JM, Hillon P. Risk factors for diabetes mellitus and early insulin resistance in chronic hepatitis C. J Hepatol. 2001;35:279-283. [PubMed] |
| 132. | Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, McCaughan GW, George J. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected]. Gastroenterology. 2003;125:1695-1704. [PubMed] |
| 133. | Lecube A, Hernández C, Genescà J, Esteban JI, Jardí R, Simó R. High prevalence of glucose abnormalities in patients with hepatitis C virus infection: a multivariate analysis considering the liver injury. Diabetes Care. 2004;27:1171-1175. [PubMed] |
| 134. | Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault MP, Sobesky R, Martinot-Peignoux M, Maylin S, Nicolas-Chanoine MH. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 378] [Article Influence: 21.0] [Reference Citation Analysis (2)] |
| 135. | Harrison SA. Correlation between insulin resistance and hepatitis C viral load. Hepatology. 2006;43:1168; author reply 1168-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 136. | Hsu CS, Liu CJ, Liu CH, Wang CC, Chen CL, Lai MY, Chen PJ, Kao JH, Chen DS. High hepatitis C viral load is associated with insulin resistance in patients with chronic hepatitis C. Liver Int. 2008;28:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 137. | Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592-599. [PubMed] |
| 138. | Gray H, Wreghitt T, Stratton IM, Alexander GJ, Turner RC, O’Rahilly S. High prevalence of hepatitis C infection in Afro-Caribbean patients with type 2 diabetes and abnormal liver function tests. Diabet Med. 1995;12:244-249. [PubMed] |
| 139. | Simó R, Hernández C, Genescà J, Jardí R, Mesa J. High prevalence of hepatitis C virus infection in diabetic patients. Diabetes Care. 1996;19:998-1000. [PubMed] |
| 140. | Mason AL, Lau JY, Hoang N, Qian K, Alexander GJ, Xu L, Guo L, Jacob S, Regenstein FG, Zimmerman R. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 449] [Article Influence: 16.6] [Reference Citation Analysis (3)] |
| 141. | Mehta SH, Brancati FL, Strathdee SA, Pankow JS, Netski D, Coresh J, Szklo M, Thomas DL. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 290] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 142. | Wang CS, Wang ST, Yao WJ, Chang TT, Chou P. Hepatitis C virus infection and the development of type 2 diabetes in a community-based longitudinal study. Am J Epidemiol. 2007;166:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 143. | White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49:831-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 306] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 144. | Fartoux L, Poujol-Robert A, Guéchot J, Wendum D, Poupon R, Serfaty L. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut. 2005;54:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 278] [Article Influence: 13.2] [Reference Citation Analysis (3)] |
| 145. | D’Souza R, Sabin CA, Foster GR. Insulin resistance plays a significant role in liver fibrosis in chronic hepatitis C and in the response to antiviral therapy. Am J Gastroenterol. 2005;100:1509-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 167] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 146. | Muzzi A, Leandro G, Rubbia-Brandt L, James R, Keiser O, Malinverni R, Dufour JF, Helbling B, Hadengue A, Gonvers JJ. Insulin resistance is associated with liver fibrosis in non-diabetic chronic hepatitis C patients. J Hepatol. 2005;42:41-46. [PubMed] |
| 147. | Petta S, Cammà C, Di Marco V, Alessi N, Cabibi D, Caldarella R, Licata A, Massenti F, Tarantino G, Marchesini G. Insulin resistance and diabetes increase fibrosis in the liver of patients with genotype 1 HCV infection. Am J Gastroenterol. 2008;103:1136-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 148. | Veldt BJ, Poterucha JJ, Watt KD, Wiesner RH, Hay JE, Rosen CB, Heimbach JK, Janssen HL, Charlton MR. Insulin resistance, serum adipokines and risk of fibrosis progression in patients transplanted for hepatitis C. Am J Transplant. 2009;9:1406-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 149. | Veldt BJ, Chen W, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, de Knegt RJ, Zeuzem S, Manns MP, Hansen BE. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47:1856-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 219] [Article Influence: 12.2] [Reference Citation Analysis (4)] |
| 150. | Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 426] [Article Influence: 23.7] [Reference Citation Analysis (2)] |
| 151. | Hung CH, Wang JH, Hu TH, Chen CH, Chang KC, Yen YH, Kuo YH, Tsai MC, Lu SN, Lee CM. Insulin resistance is associated with hepatocellular carcinoma in chronic hepatitis C infection. World J Gastroenterol. 2010;16:2265-2271. [PubMed] |
| 152. | Nkontchou G, Bastard JP, Ziol M, Aout M, Cosson E, Ganne-Carrie N, Grando-Lemaire V, Roulot D, Capeau J, Trinchet JC. Insulin resistance, serum leptin, and adiponectin levels and outcomes of viral hepatitis C cirrhosis. J Hepatol. 2010;53:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 153. | Romero-Gómez M, Del Mar Viloria M, Andrade RJ, Salmerón J, Diago M, Fernández-Rodríguez CM, Corpas R, Cruz M, Grande L, Vázquez L. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636-641. [PubMed] |
| 154. | Poustchi H, Negro F, Hui J, Cua IH, Brandt LR, Kench JG, George J. Insulin resistance and response to therapy in patients infected with chronic hepatitis C virus genotypes 2 and 3. J Hepatol. 2008;48:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 155. | Dai CY, Huang JF, Hsieh MY, Hou NJ, Lin ZY, Chen SC, Hsieh MY, Wang LY, Chang WY, Chuang WL. Insulin resistance predicts response to peginterferon-alpha/ribavirin combination therapy in chronic hepatitis C patients. J Hepatol. 2009;50:712-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 156. | Adinolfi LE, Zampino R, Restivo L, Lonardo A, Guerrera B, Marrone A, Nascimbeni F, Florio A, Loria P. Chronic hepatitis C virus infection and atherosclerosis: clinical impact and mechanisms. World J Gastroenterol. 2014;20:3410-3417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 157. | Petta S, Maida M, Macaluso FS, Barbara M, Licata A, Craxì A, Cammà C. Hepatitis C Virus Infection Is Associated With Increased Cardiovascular Mortality: A Meta-Analysis of Observational Studies. Gastroenterology. 2016;150:145-155.e4; quiz e15-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 197] [Article Influence: 19.7] [Reference Citation Analysis (1)] |
| 158. | Kawaguchi T, Ide T, Taniguchi E, Hirano E, Itou M, Sumie S, Nagao Y, Yanagimoto C, Hanada S, Koga H. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 190] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 159. | Conjeevaram HS, Wahed AS, Afdhal N, Howell CD, Everhart JE, Hoofnagle JH. Changes in insulin sensitivity and body weight during and after peginterferon and ribavirin therapy for hepatitis C. Gastroenterology. 2011;140:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 160. | Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Kawamura Y, Yatsuji H, Sezaki H, Hosaka T, Hirakawa M. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49:739-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 210] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 161. | Hsu YC, Lin JT, Ho HJ, Kao YH, Huang YT, Hsiao NW, Wu MS, Liu YY, Wu CY. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59:1293-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 162. | Hsu YC, Ho HJ, Huang YT, Wang HH, Wu MS, Lin JT, Wu CY. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut. 2015;64:495-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 163. | Grasso A, Malfatti F, Testa R. Are metabolic factors still important in the era of direct antiviral agents in patients with chronic hepatitis C? World J Gastroenterol. 2013;19:6947-6956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 164. | Serfaty L, Forns X, Goeser T, Ferenci P, Nevens F, Carosi G, Drenth JP, Lonjon-Domanec I, DeMasi R, Picchio G. Insulin resistance and response to telaprevir plus peginterferon α and ribavirin in treatment-naive patients infected with HCV genotype 1. Gut. 2012;61:1473-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 165. | Pavone P, Tieghi T, d’Ettorre G, Lichtner M, Marocco R, Mezzaroma I, Passavanti G, Vittozzi P, Mastroianni CM, Vullo V. Rapid decline of fasting glucose in HCV diabetic patients treated with direct-acting antiviral agents. Clin Microbiol Infect. 2016;22:462.e1-462.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 166. | Meissner EG, Lee YJ, Osinusi A, Sims Z, Qin J, Sturdevant D, McHutchison J, Subramanian M, Sampson M, Naggie S. Effect of sofosbuvir and ribavirin treatment on peripheral and hepatic lipid metabolism in chronic hepatitis C virus, genotype 1-infected patients. Hepatology. 2015;61:790-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 167. | Matsumori A, Yutani C, Ikeda Y, Kawai S, Sasayama S. Hepatitis C virus from the hearts of patients with myocarditis and cardiomyopathy. Lab Invest. 2000;80:1137-1142. [PubMed] |
| 168. | Boddi M, Abbate R, Chellini B, Giusti B, Solazzo V, Soft F, Pratesi G, Pratesi C, Gensini G, Zignego AL. HCV infection facilitates asymptomatic carotid atherosclerosis: preliminary report of HCV RNA localization in human carotid plaques. Dig Liver Dis. 2007;39 Suppl 1:S55-S60. [PubMed] |
| 169. | Sanchez MJ, Bergasa NV. Hepatitis C associated cardiomyopathy: potential pathogenic mechanisms and clinical implications. Med Sci Monit. 2008;14:RA55-RA63. [PubMed] |
| 170. | Boddi M, Abbate R, Chellini B, Giusti B, Giannini C, Pratesi G, Rossi L, Pratesi C, Gensini GF, Paperetti L. Hepatitis C virus RNA localization in human carotid plaques. J Clin Virol. 2010;47:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 171. | Lonardo A, Adinolfi LE, Restivo L, Ballestri S, Romagnoli D, Baldelli E, Nascimbeni F, Loria P. Pathogenesis and significance of hepatitis C virus steatosis: an update on survival strategy of a successful pathogen. World J Gastroenterol. 2014;20:7089-7103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 75] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 172. | Maruyama S, Koda M, Oyake N, Sato H, Fujii Y, Horie Y, Murawaki Y. Myocardial injury in patients with chronic hepatitis C infection. J Hepatol. 2013;58:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 173. | Omran DA, Behairy NH, Zakaria KS, Nabil MM, Said K. Functional and morphological myocardial changes in hepatitis C virus patients with end-stage liver disease. Scand J Gastroenterol. 2015;50:1135-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 174. | Tomiyama H, Arai T, Hirose K, Hori S, Yamamoto Y, Yamashina A. Hepatitis C virus seropositivity, but not hepatitis B virus carrier or seropositivity, associated with increased pulse wave velocity. Atherosclerosis. 2003;166:401-403. [PubMed] |
| 175. | Ishizaka N, Ishizaka Y, Takahashi E, Tooda Ei, Hashimoto H, Nagai R, Yamakado M. Association between hepatitis C virus seropositivity, carotid-artery plaque, and intima-media thickening. Lancet. 2002;359:133-135. [PubMed] |
| 176. | Targher G, Bertolini L, Padovani R, Rodella S, Arcaro G, Day C. Differences and similarities in early atherosclerosis between patients with non-alcoholic steatohepatitis and chronic hepatitis B and C. J Hepatol. 2007;46:1126-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 177. | Mostafa A, Mohamed MK, Saeed M, Hasan A, Fontanet A, Godsland I, Coady E, Esmat G, El-Hoseiny M, Abdul-Hamid M. Hepatitis C infection and clearance: impact on atherosclerosis and cardiometabolic risk factors. Gut. 2010;59:1135-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 178. | Petta S, Torres D, Fazio G, Cammà C, Cabibi D, Di Marco V, Licata A, Marchesini G, Mazzola A, Parrinello G. Carotid atherosclerosis and chronic hepatitis C: a prospective study of risk associations. Hepatology. 2012;55:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 179. | Adinolfi LE, Restivo L, Zampino R, Guerrera B, Lonardo A, Ruggiero L, Riello F, Loria P, Florio A. Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis. 2012;221:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 180. | Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49:225-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 181. | Lin MS, Guo SE, Chen MY, Huang TJ, Huang JC, Hu JH, Lin YS. The impact of hepatitis C infection on ischemic heart disease via ischemic electrocardiogram. Am J Med Sci. 2014;347:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 182. | Younossi ZM, Stepanova M, Nader F, Younossi Z, Elsheikh E. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 183. | Vassalle C, Masini S, Bianchi F, Zucchelli GC. Evidence for association between hepatitis C virus seropositivity and coronary artery disease. Heart. 2004;90:565-566. [PubMed] |
| 184. | Alyan O, Kacmaz F, Ozdemir O, Deveci B, Astan R, Celebi AS, Ilkay E. Hepatitis C infection is associated with increased coronary artery atherosclerosis defined by modified Reardon severity score system. Circ J. 2008;72:1960-1965. [PubMed] |
| 185. | Satapathy SK, Kim YJ, Kataria A, Shifteh A, Bhansali R, Cerulli MA, Bernstein D. Higher Prevalence and More Severe Coronary Artery Disease in Hepatitis C Virus-infected Patients: A Case Control Study. J Clin Exp Hepatol. 2013;3:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 186. | Pothineni NV, Delongchamp R, Vallurupalli S, Ding Z, Dai Y, Hagedorn CH, Mehta JL. Impact of hepatitis C seropositivity on the risk of coronary heart disease events. Am J Cardiol. 2014;114:1841-1845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 187. | McKibben RA, Haberlen SA, Post WS, Brown TT, Budoff M, Witt MD, Kingsley LA, Palella FJ, Thio CL, Seaberg EC. A Cross-sectional Study of the Association Between Chronic Hepatitis C Virus Infection and Subclinical Coronary Atherosclerosis Among Participants in the Multicenter AIDS Cohort Study. J Infect Dis. 2016;213:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 188. | Yelken B, Gorgulu N, Caliskan Y, Elitok A, Cimen AO, Yazici H, Oflaz H, Turkmen A, Sever MS. Association between chronic hepatitis C infection and coronary flow reserve in dialysis patients with failed renal allografts. Transplant Proc. 2009;41:1519-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 189. | Liao CC, Su TC, Sung FC, Chou WH, Chen TL. Does hepatitis C virus infection increase risk for stroke? A population-based cohort study. PLoS One. 2012;7:e31527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 190. | Adinolfi LE, Restivo L, Guerrera B, Sellitto A, Ciervo A, Iuliano N, Rinaldi L, Santoro A, Li Vigni G, Marrone A. Chronic HCV infection is a risk factor of ischemic stroke. Atherosclerosis. 2013;231:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 191. | Hsu CS, Kao JH, Chao YC, Lin HH, Fan YC, Huang CJ, Tsai PS. Interferon-based therapy reduces risk of stroke in chronic hepatitis C patients: a population-based cohort study in Taiwan. Aliment Pharmacol Ther. 2013;38:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 192. | Karibe H, Niizuma H, Ohyama H, Shirane R, Yoshimoto T. Hepatitis C virus (HCV) infection as a risk factor for spontaneous intracerebral hemorrhage: hospital based case-control study. J Clin Neurosci. 2001;8:423-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 193. | Tseng CH, Muo CH, Hsu CY, Kao CH. Increased Risk of Intracerebral Hemorrhage Among Patients With Hepatitis C Virus Infection. Medicine (Baltimore). 2015;94:e2132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 194. | Hsu YH, Muo CH, Liu CY, Tsai WC, Hsu CC, Sung FC, Kao CH. Hepatitis C virus infection increases the risk of developing peripheral arterial disease: a 9-year population-based cohort study. J Hepatol. 2015;62:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 195. | Guiltinan AM, Kaidarova Z, Custer B, Orland J, Strollo A, Cyrus S, Busch MP, Murphy EL. Increased all-cause, liver, and cardiac mortality among hepatitis C virus-seropositive blood donors. Am J Epidemiol. 2008;167:743-750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 196. | Lee MH, Yang HI, Wang CH, Jen CL, Yeh SH, Liu CJ, You SL, Chen WJ, Chen CJ. Hepatitis C virus infection and increased risk of cerebrovascular disease. Stroke. 2010;41:2894-2900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 197. | Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, Wang CH, Chen WJ, Chen CJ. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 430] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 198. | Fernández-Montero JV, Barreiro P, de Mendoza C, Labarga P, Soriano V. Hepatitis C virus coinfection independently increases the risk of cardiovascular disease in HIV-positive patients. J Viral Hepat. 2016;23:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 199. | Fabrizi F, Dixit V, Messa P. Impact of hepatitis C on survival in dialysis patients: a link with cardiovascular mortality? J Viral Hepat. 2012;19:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 200. | Negro F. Facts and fictions of HCV and comorbidities: steatosis, diabetes mellitus, and cardiovascular diseases. J Hepatol. 2014;61:S69-S78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 201. | Vanni E, Bugianesi E, Saracco G. Treatment of type 2 diabetes mellitus by viral eradication in chronic hepatitis C: Myth or reality? Dig Liver Dis. 2016;48:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 202. | Banerjee S, Saito K, Ait-Goughoulte M, Meyer K, Ray RB, Ray R. Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate-1 and impairs the downstream akt/protein kinase B signaling pathway for insulin resistance. J Virol. 2008;82:2606-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 203. | Adinolfi LE, Restivo L, Zampino R, Lonardo A, Loria P. Metabolic alterations and chronic hepatitis C: treatment strategies. Expert Opin Pharmacother. 2011;12:2215-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 204. | Jepsen P, Watson H, Andersen PK, Vilstrup H. Diabetes as a risk factor for hepatic encephalopathy in cirrhosis patients. J Hepatol. 2015;63:1133-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 205. | El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology. 2016;64:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 323] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 206. | Singal AG, El-Serag HB. Hepatocellular Carcinoma From Epidemiology to Prevention: Translating Knowledge into Practice. Clin Gastroenterol Hepatol. 2015;13:2140-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 413] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 207. | Thompson AJ, Patel K, Chuang WL, Lawitz EJ, Rodriguez-Torres M, Rustgi VK, Flisiak R, Pianko S, Diago M, Arora S. Viral clearance is associated with improved insulin resistance in genotype 1 chronic hepatitis C but not genotype 2/3. Gut. 2012;61:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 208. | Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 774] [Article Influence: 31.0] [Reference Citation Analysis (3)] |
| 209. | Poynard T, Ratziu V, McHutchison J, Manns M, Goodman Z, Zeuzem S, Younossi Z, Albrecht J. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 433] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 210. | Lonardo A, Loria P, Adinolfi LE, Carulli N, Ruggiero G. Hepatitis C and steatosis: a reappraisal. J Viral Hepat. 2006;13:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 211. | Global-AIDS-update-2016_en.pdf [Internet]. unaids.org [cited 2016-06-30]. Available from: http://wwwunaidsorg/sites/default/files/media_asset/global-AIDS-update-2016_enpdf. |
| 212. | Sabin CA. Do people with HIV infection have a normal life expectancy in the era of combination antiretroviral therapy? BMC Med. 2013;11:251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 213. | Greene M, Justice AC, Lampiris HW, Valcour V. Management of human immunodeficiency virus infection in advanced age. JAMA. 2013;309:1397-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 214. | De Cock KM, El-Sadr WM, Ghebreyesus TA. Game changers: why did the scale-up of HIV treatment work despite weak health systems? J Acquir Immune Defic Syndr. 2011;57 Suppl 2:S61-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 215. | Rodger AJ, Lodwick R, Schechter M, Deeks S, Amin J, Gilson R, Paredes R, Bakowska E, Engsig FN, Phillips A. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS. 2013;27:973-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 293] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 216. | Cooperman NA, Arnsten JH, Klein RS. Current sexual activity and risky sexual behavior in older men with or at risk for HIV infection. AIDS Educ Prev. 2007;19:321-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 217. | Guaraldi G, Zona S, Brothers TD, Carli F, Stentarelli C, Dolci G, Santoro A, Beghetto B, Menozzi M, Mussini C. Aging with HIV vs. HIV seroconversion at older age: a diverse population with distinct comorbidity profiles. PLoS One. 2015;10:e0118531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 218. | Guaraldi G, Orlando G, Squillace N, De Santis G, Pedone A, Spaggiari A, De Fazio D, Vandelli M, De Paola M, Bertucelli C. Multidisciplinary approach to the treatment of metabolic and morphologic alterations of HIV-related lipodystrophy. HIV Clin Trials. 2006;7:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 219. | Samaras K. The burden of diabetes and hyperlipidemia in treated HIV infection and approaches for cardiometabolic care. Curr HIV/AIDS Rep. 2012;9:206-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 220. | Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, Visscher BR, Margolick JB, Dobs AS. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 627] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 221. | Reingold J, Wanke C, Kotler D, Lewis C, Tracy R, Heymsfield S, Tien P, Bacchetti P, Scherzer R, Grunfeld C. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr. 2008;48:142-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 222. | Stankov MV, Behrens GM. Contribution of inflammation to fat redistribution and metabolic disturbances in HIV-1 infected patients. Curr Pharm Des. 2010;16:3361-3371. [PubMed] |
| 223. | Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2178] [Cited by in RCA: 2723] [Article Influence: 181.5] [Reference Citation Analysis (0)] |
| 224. | Baker JV, Neuhaus J, Duprez D, Kuller LH, Tracy R, Belloso WH, De Wit S, Drummond F, Lane HC, Ledergerber B. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr. 2011;56:36-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 225. | Feeney ER, Mallon PW. Insulin resistance in treated HIV infection. Best Pract Res Clin Endocrinol Metab. 2011;25:443-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 226. | Herder C, Brunner EJ, Rathmann W, Strassburger K, Tabák AG, Schloot NC, Witte DR. Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes: the Whitehall II study. Diabetes Care. 2009;32:421-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 227. | Festa A, D’Agostino R, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000;102:42-47. [PubMed] |
| 228. | Tsigos C, Papanicolaou DA, Kyrou I, Defensor R, Mitsiadis CS, Chrousos GP. Dose-dependent effects of recombinant human interleukin-6 on glucose regulation. J Clin Endocrinol Metab. 1997;82:4167-4170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 170] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 229. | Béténé A Dooko C, De Wit S, Neuhaus J, Palfreeman A, Pepe R, Pankow JS, Neaton JD. Interleukin-6, high sensitivity C-reactive protein, and the development of type 2 diabetes among HIV-positive patients taking antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;67:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 230. | Zanni MV, Schouten J, Grinspoon SK, Reiss P. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol. 2014;11:728-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 231. | Paisible AL, Chang CC, So-Armah KA, Butt AA, Leaf DA, Budoff M, Rimland D, Bedimo R, Goetz MB, Rodriguez-Barradas MC. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr. 2015;68:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 232. | Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 1076] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 233. | Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506-2512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1257] [Cited by in RCA: 1279] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 234. | Marin B, Thiébaut R, Bucher HC, Rondeau V, Costagliola D, Dorrucci M, Hamouda O, Prins M, Walker S, Porter K. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009;23:1743-1753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 235. | Baker JV, Peng G, Rapkin J, Abrams DI, Silverberg MJ, MacArthur RD, Cavert WP, Henry WK, Neaton JD. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22:841-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 298] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 236. | Debaisieux S, Rayne F, Yezid H, Beaumelle B. The ins and outs of HIV-1 Tat. Traffic. 2012;13:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 237. | Dhawan S, Puri RK, Kumar A, Duplan H, Masson JM, Aggarwal BB. Human immunodeficiency virus-1-tat protein induces the cell surface expression of endothelial leukocyte adhesion molecule-1, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 in human endothelial cells. Blood. 1997;90:1535-1544. [PubMed] |
| 238. | Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Boerwinkle E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96:4219-4225. [PubMed] |
| 239. | Puppo F, Brenci S, Scudeletti M, Lanza L, Bosco O, Indiveri F. Elevated serum levels of circulating intercellular adhesion molecule-1 in HIV infection. AIDS. 1993;7:593-594. [PubMed] |
| 240. | Paladugu R, Fu W, Conklin BS, Lin PH, Lumsden AB, Yao Q, Chen C. Hiv Tat protein causes endothelial dysfunction in porcine coronary arteries. J Vasc Surg. 2003;38:549-555; discussion 555-556. [PubMed] |
| 241. | Shikuma CM, Barbour JD, Ndhlovu LC, Keating SM, Norris PJ, Budoff M, Parikh N, Seto T, Gangcuangco LM, Ogata-Arakaki D. Plasma monocyte chemoattractant protein-1 and tumor necrosis factor-α levels predict the presence of coronary artery calcium in HIV-infected individuals independent of traditional cardiovascular risk factors. AIDS Res Hum Retroviruses. 2014;30:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 242. | Kedzierska K, Crowe SM. The role of monocytes and macrophages in the pathogenesis of HIV-1 infection. Curr Med Chem. 2002;9:1893-1903. [PubMed] |
| 243. | Palmer CS, Anzinger JJ, Zhou J, Gouillou M, Landay A, Jaworowski A, McCune JM, Crowe SM. Glucose transporter 1-expressing proinflammatory monocytes are elevated in combination antiretroviral therapy-treated and untreated HIV+ subjects. J Immunol. 2014;193:5595-5603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 244. | Zanni MV, Abbara S, Lo J, Wai B, Hark D, Marmarelis E, Grinspoon SK. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS. 2013;27:1263-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 245. | Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, Corsini E, Abdelbaky A, Zanni MV, Hoffmann U. Arterial inflammation in patients with HIV. JAMA. 2012;308:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 393] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 246. | Chakko S, Myerburg RJ. Cardiac complications of cocaine abuse. Clin Cardiol. 1995;18:67-72. [PubMed] |
| 247. | Carr A. HIV protease inhibitor-related lipodystrophy syndrome. Clin Infect Dis. 2000;30 Suppl 2:S135-S142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 141] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 248. | Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in patients coinfected with human immunodeficiency virus/hepatitis C virus: a meta-analysis of the risk factors. Hepatology. 2010;52:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 249. | Guaraldi G, Squillace N, Stentarelli C, Orlando G, D’Amico R, Ligabue G, Fiocchi F, Zona S, Loria P, Esposito R. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 183] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 250. | Crum-Cianflone N, Dilay A, Collins G, Asher D, Campin R, Medina S, Goodman Z, Parker R, Lifson A, Capozza T. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr. 2009;50:464-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 251. | Castéra L, Loko MA, Le Bail B, Coffie P, De Ledinghen V, Trimoulet P, Winnock M, Dabis F, Neau D. Hepatic steatosis in HIV-HCV coinfected patients in France: comparison with HCV monoinfected patients matched for body mass index and HCV genotype. Aliment Pharmacol Ther. 2007;26:1489-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 252. | Woreta TA, Sutcliffe CG, Mehta SH, Brown TT, Higgins Y, Thomas DL, Torbenson MS, Moore RD, Sulkowski MS. Incidence and risk factors for steatosis progression in adults coinfected with HIV and hepatitis C virus. Gastroenterology. 2011;140:809-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 253. | McGovern BH. Hepatic steatosis in HIV/HCV-coinfected patients: time to reevaluate! Gastroenterology. 2011;140:772-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 254. | Vodkin I, Valasek MA, Bettencourt R, Cachay E, Loomba R. Clinical, biochemical and histological differences between HIV-associated NAFLD and primary NAFLD: a case-control study. Aliment Pharmacol Ther. 2015;41:368-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 255. | Brown TT, Glesby MJ. Management of the metabolic effects of HIV and HIV drugs. Nat Rev Endocrinol. 2011;8:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 256. | Falutz J, Allas S, Blot K, Potvin D, Kotler D, Somero M, Berger D, Brown S, Richmond G, Fessel J. Metabolic effects of a growth hormone-releasing factor in patients with HIV. N Engl J Med. 2007;357:2359-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 257. | Stanley TL, Feldpausch MN, Oh J, Branch KL, Lee H, Torriani M, Grinspoon SK. Effect of tesamorelin on visceral fat and liver fat in HIV-infected patients with abdominal fat accumulation: a randomized clinical trial. JAMA. 2014;312:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 258. | Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126:586-597. [PubMed] |
| 259. | Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1516] [Cited by in RCA: 2257] [Article Influence: 205.2] [Reference Citation Analysis (1)] |
| 260. | Nascimbeni F, Pais R, Bellentani S, Day CP, Ratziu V, Loria P, Lonardo A. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013;59:859-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 260] [Article Influence: 20.0] [Reference Citation Analysis (4)] |
| 261. | European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3279] [Article Influence: 327.9] [Reference Citation Analysis (6)] |
| 262. | Nix LM, Tien PC. Metabolic syndrome, diabetes, and cardiovascular risk in HIV. Curr HIV/AIDS Rep. 2014;11:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 263. | Hemkens LG, Bucher HC. HIV infection and cardiovascular disease. Eur Heart J. 2014;35:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 264. | Vallet-Pichard A, Mallet V, Pol S. Nonalcoholic fatty liver disease and HIV infection. Semin Liver Dis. 2012;32:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Dajani A, Hamaguchi M, Wan YL S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF