Published online Nov 21, 2016. doi: 10.3748/wjg.v22.i43.9586
Peer-review started: July 13, 2016
First decision: August 22, 2016
Revised: September 18, 2016
Accepted: October 10, 2016
Article in press: October 10, 2016
Published online: November 21, 2016
Processing time: 131 Days and 3.1 Hours
To develop a non-invasive model to evaluate significant fibrosis and cirrhosis by investigating the association between serum ceruloplasmin (CP) levels and liver fibrosis in chronic hepatitis B (CHB) patients with normal or minimally raised alanine aminotransferase (ALT).
Serum samples and liver biopsy were obtained from 193 CHB patients with minimally raised or normal ALT who were randomly divided into a training group (n = 97) and a validation group (n = 96). Liver histology was evaluated by the METAVIR scoring system. Receiver operator characteristic curves were applied to the diagnostic value of CP for measuring liver fibrosis in CHB patients. Spearman rank correlation analyzed the relationship between CP and liver fibrosis. A non-invasive model was set up through multivariate logistic regression analysis.
Serum CP levels individualized various fibrosis stages via area under the curve (AUC) values. Multivariate analysis revealed that CP levels were significantly related to liver cirrhosis. Combining CP with serum GGT levels, a CG model was set up to predict significant fibrosis and liver cirrhosis in CHB patients with normal or minimally raised ALT. The AUC, sensitivity, specificity, positive predictive value, and negative predictive value were 0.84, 83.1%, 78.6%, 39.6%, and 96.5% to predict liver cirrhosis, and 0.789, 80.26%, 68.38%, 62.25%, and 84.21% to predict significant fibrosis. This model expressed a higher AUC than FIB-4 (age, ALT, aspartate aminotransferase, platelets) and GP (globulin, platelets) models to predict significant fibrosis (P = 0.019 and 0.022 respectively) and revealed a dramatically greater AUC than FIB-4 (P = 0.033) to predict liver cirrhosis.
The present study showed that CP was independently and negatively associated with liver fibrosis. Furthermore, we developed a novel promising model (CG), based on routine serum markers, for predicting liver fibrosis in CHB patients with normal or minimally raised ALT.
Core tip: To date, few non-invasive approaches have been developed to evaluate liver fibrosis and no studies have proposed measuring ceruloplasmin (CP) levels for predicting liver fibrosis in chronic hepatitis B (CHB) virus patients with normal or minimally raised alanine aminotransferase (ALT). This study showed CP was independently and negatively associated with liver fibrosis. Furthermore, a simple and accurate CG model was developed to predict significant liver fibrosis and cirrhosis in CHB patients with normal or mildly elevated ALT. This model may be a valuable tool to replace liver biopsy in this category of hepatitis B virus-infected patients.
- Citation: Zeng DW, Dong J, Jiang JJ, Zhu YY, Liu YR. Ceruloplasmin, a reliable marker of fibrosis in chronic hepatitis B virus patients with normal or minimally raised alanine aminotransferase. World J Gastroenterol 2016; 22(43): 9586-9594
- URL: https://www.wjgnet.com/1007-9327/full/v22/i43/9586.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i43.9586
Worldwide, an estimated 2 billion people are infected by hepatitis B virus (HBV), with approximately 240 million being chronically infected[1]. The weighted prevalence of hepatitis B surface antigen (HBsAg) in Chinese people aged 1-4 years, 5-14 years, and 15-29 years has been shown to be 0.32%, 0.94%, and 4.38%, respectively[2]. HBV-infected patients can be healthy carriers, or develop chronic hepatitis, which may lead to cirrhosis, end-stage liver failure, or hepatocellular carcinoma (HCC). Anti-HBV therapy reduces the risk of chronic hepatitis B (CHB) developing into cirrhosis or HCC[3].
Based on the current practice guidelines[4-6], a serum alanine aminotransferase (ALT) level greater than or equal to two times the upper normal limit (ULN) - i.e., ALT ≥ 2 × ULN - represents an indication for antiviral treatment in CHB patients. Therefore, patients with ALT < 2 × ULN should be evaluated for hepatic inflammation and fibrosis, and those who show compensated cirrhosis and detectable HBV DNA can be submitted to antiviral treatment, even with normal ALT levels. Therefore, in some conditions, antiviral therapy is based on the stage of liver fibrosis or ALT levels. Indeed, 10%-37% of patients with persistently normal serum ALT levels may progress to advanced fibrosis or cirrhosis[3,7,8]. Therefore, it is essential to evaluate liver fibrosis to make antiviral therapy decisions in CHB patients with ALT < 2 × ULN.
Liver biopsy is still considered the “gold” standard in assessing liver fibrosis. Nevertheless, the biopsy procedure has some limitations such as invasiveness, sampling variability, and cost. Moreover, liver biopsy has been superseded to some extent by the development of imaging techniques such as transient elastography (TE), acoustic radiation force impulse (ARFI), ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI)[9-12]. On the other hand, the high cost of (or poor access to) modern imaging techniques may represent additional limitations in the evaluation of liver fibrosis in developing countries, which can preclude their use.
Serum markers of liver fibrosis and non-invasive predictive models of fibrosis can evaluate fibrosis specifically in HBV patients due to high applicability, inter-laboratory reproducibility, wide availability for repeated assays, and limited cost[13-18]. However, in CHB patients with ALT < 2 × ULN, few non-invasive approaches have been developed to evaluate liver fibrosis[14,19-21].
Ceruloplasmin (CP) is a serum glycoprotein that contains six copper atoms per molecule and is synthesized predominantly in the liver. Serum CP is known as a typical diagnostic biomarker of Wilson’s disease (WD), and is also decreased in marked renal failure or enteric protein loss[22]. In clinical practice, serum CP may be routinely measured by antibody-dependent assays, such as immunoturbidimetric assay or nephelometry, which quantify the whole concentration but do not test the CP activity. In fact, CP activity could be a valuable marker not only in patients with WD but also in hepatitis C virus (HCV)-related cirrhosis patients with hepatic encephalopathy[23]. Interestingly, serum CP has been shown as significantly lower in patients with severe end-stage liver disease compared to patients with other liver diseases, except for WD[24]. However, little is known of its relationship with liver fibrosis. Zeng et al[25] found that serum CP levels were negatively related to hepatic histological stage, and could be used to predict liver fibrosis in CHB-related liver disease. Nobili et al[26] showed that CP was associated with the odds of nonalcoholic steatohepatitis (NASH), inflammation, steatosis, and ballooning but not with fibrosis.
To date, no studies have proposed CP levels as a biological marker to predict fibrosis in CHB patients with ALT < 2 × ULN. Therefore, the purpose of the present study was to analyze the relationship between liver fibrosis and serum CP levels, set up a predictive model based on routine biological parameters to predict fibrosis in HBV-infected patients with minimally raised or steadily normal ALT, and compare its diagnostic value with other non-invasive models such as FIB-4 [aspartate aminotransferase (AST)/ALT/platelets (PLT)/age], APRI (AST/PLT ratio index), PPT [PLT/prothrombin time (PT)/total bile acid (TBA)], GP (globin/PLT), and APPCI [α-fetoprotein (AFP)/PT/PLT/CP] models.
Between January 2009 and January 2016, 193 chronic HBV carriers were systematically enrolled at our hospital (Liver Center, The First Affiliated Hospital of Fujian Medical University). All patients showed positivity for HBsAg for more than 6 mo, with normal or minimally raised ALT and HBV DNA levels of more than 500 IU/mL. ALT was checked at least two times over a 6-mo observation period; our lab reference value was 40 IU/L. Minimally raised ALT levels were considered as ALT levels < 2 × ULN[5]. Patients with the following conditions were excluded from the study: presence of (1) other types of viral hepatitis; (2) HCC; (3) alcoholic liver disease and nonalcoholic fatty liver disease; (4) decompensated cirrhosis; (5) autoimmune hepatitis; (6) concurrent infection with human immunodeficiency virus; (7) hereditary liver diseases; and (8) druginduced liver injury.
None of the patients received antiviral therapy before liver biopsy. All patients were randomly separated into two groups: 97 patients constituted the training group, and 96 were in the validation group. The study protocol was approved by the Institutional Review Board of our hospital.
Using color Doppler ultrasound (ACUSON, Aspen Advanced Ultrasound; Siemens Corporation, Mountain View, CA, United States), liver biopsies were performed in all recruited patients using a 16G Tru-Cut needle (TSK Laboratory, Tochigi, Japan). For most biopsies, liver samples containing more than 11 portal tracts of liver tissue specimens (minimum was 6) with a length of 15 mm to 20 mm were obtained and fixed in 4% neutral formalin before paraffin embedding, and stained with Masson trichrome and hematoxylin-eosin-saffron (HES) stains. The pathologist who reviewed all the biopsy specimens was blinded to the clinical features. Significant fibrosis was defined as fibrosis stage ≥ F2 as defined by the METAVIR scoring system[27].
All serum samples were obtained within 1 wk prior to liver biopsy. Some serum biochemical markers were measured using an automatic biochemistry analyzer, including ALT, AST, albumin, globulins, cholinesterase (CHE), gamma glutamyl transpeptidase (GGT), TBA, total bilirubin (TB), international normalized ratio (INR), PT, PLT, white blood cell count (WBC), and AFP. HBV DNA levels were measured through quantitative PCR assay (PG Company, Shenzhen, China). The test detection range was 500 to 1.0 × 109 IU/mL. HBsAg was quantified via the Architect platform (Abbott Laboratories, Chicago, IL, United States) as per the manufacturer’s instructions, and was calibrated according to the World Health Organization HBsAg standard. Serum CP was tested by the nephelometric immunoassay kit (BN II System; Siemens Healthcare Diagnostics GmbH, Eschborn, Germany) with a lower limit of detection of 200 mg/L.
The whole cohort (193 patients) was randomly divided into a training group and a validation group. Categorical data were expressed as n (%), the abnormal distribution data were presented as median with interquartiles (IQR), and normal distribution data were presented as mean ± standard deviations (SD). Categorical variables were compared by Pearson chi-squared test or Mann-Whitney U test, whereas continuous variates were compared with the two-sample test or Mann-Whitney U test. Correlation analysis was carried out by Spearman’s correlation analysis. Receiver operating characteristic (ROC) curve analysis was applied to assess the optimal diagnostic CP value for significant fibrosis and cirrhosis. Univariate logistic regression analysis was carried out to analyze the important factors of liver fibrosis. Variables were assessed by multivariate logistic regression modeling using the forward selection method. Furthermore, a ROC curve was created, from which area under the ROC curve (AUROC), specificity, sensitivity, negative predictive value (NPV), and positive predictive value (PPV) were measured. The optimal cut-off value was selected based on the maximization of Youden’s index. A two-sided P < 0.05 was considered statistically significant. Statistical analyses were carried out with MedCalc for Windows v9.38 software (MedCalc Software, Mariakerke, Belgium) and SPSS v22.0 statistical software (SPSS Inc, Chicago, IL, United States).
Of the total number of recruited patients (n = 239), 46 were excluded from this study due to previous antivirus therapy, concomitant liver disease, or missing data. Ultimately, 193 patients were included. Among these patients, 138 (71.5%) were male. Demographic, bioclinical features, and liver biopsy data of patients with ALT < 2 × ULN are presented in Table 1. Serum CP levels showed a moderate but significant inverse relationship with liver fibrosis (r = -0.561, P < 0.001). Among all studied variables there were no significant differences between the validation and the training groups (Table 1).
| Variable | All (n = 193) | Training group (n = 97) | Validation group (n = 96) | P value |
| Age, in yr | 39.11 ± 9.31 | 38.70 ± 9.46 | 39.52 ± 9.18 | 0.542 |
| Sex, n (%) | 0.601 | |||
| Male | 138 (71.5) | 71 (73.2) | 67 (69.8) | |
| Female | 55 (28.5) | 26 (26.8) | 29 (30.2) | |
| Total bilirubin, in μmol/L | 12.3 (8.7-18.2) | 11.4 (8.6-16.9) | 12.9 (8.8-18.8) | 0.352 |
| Albumin, in g/L | 42.21 ± 4.16 | 42.46 ± 3.91 | 41.94 ± 4.41 | 0.387 |
| Globulin, in g/L | 27.25 ± 4.36 | 27.54 ± 4.84 | 26.95 ± 3.81 | 0.345 |
| ALT, in IU/L | 37 (27-51) | 35 (26.0-48.5) | 38.5 (28-52) | 0.378 |
| AST, in IU/L | 30 (25.0-37.5) | 30 (26-38) | 29 (25-37) | 0.806 |
| GGT, in IU/L | 27 (18.0-40.5) | 25 (18-38) | 27 (19-43) | 0.577 |
| TBA, in mmol/L | 5.9 (3.85-12.05) | 6.5 (4.10-15.25) | 5.45 (3.73-9.83) | 0.146 |
| ALP, in IU/L | 69 (57.5-82.5) | 69 (58.0-83.5) | 68.5 (57.0-81.8) | 0.990 |
| CHE, in IU/L | 8407.62 ± 2722.19 | 8393.56 ± 2423.51 | 8421.83 ± 3006.82 | 0.943 |
| AFP, in ng/mL | 3.25 (2.19-6.32) | 3.30 (2.30-6.70) | 3.20 (2.03-5.08) | 0.377 |
| PT, in s | 12.4 (11.9-13.1) | 12.4 (11.95-13.15) | 12.3 (11.80-12.97) | 0.061 |
| INR | 1.03 (0.98-1.09) | 1.03 (0.99-1.10) | 1.03 (0.98-1.08) | 0.553 |
| HBsAg levels, in log IU/mL | 3.49 (3.12-3.97) | 3.46 (3.08-3.90) | 3.52 (3.16-4.03) | 0.501 |
| HBV DNA levels, in logIU/mL | 5.27 ± 1.70 | 5.30 ± 1.67 | 5.24 ± 1.74 | 0.791 |
| WBC, in 109/L | 5.63 ± 1.49 | 5.66 ± 1.54 | 5.59 ± 1.44 | 0.723 |
| PLT, in 109/L | 179 (144.5-214.5) | 179 (153.5-214.5) | 177 (128.0-214.8) | 0.208 |
| CP, in mg/L | 195.67 ± 35.62 | 196.86 ± 35.45 | 196.86 ± 35.45 | 0.643 |
| Stage of fibrosis, n (%) | 0.164 | |||
| F1 | 76 (39.38) | 42 (43.30) | 34 (35.42) | |
| F2 | 44 (22.80) | 23 (23.71) | 21 (21.88) | |
| F3 | 42 (21.76) | 18 (18.56) | 24 (25.00) | |
| F4 | 31 (16.06) | 14 (14.43) | 17 (17.70) |
The AUC values, sensitivity, and specificity of serum CP levels for predicting liver fibrosis are presented in Table 2. Serum CP levels produced AUC values of 0.776 and 0.767 for fibrosis stages F2 and F4, respectively. Based on the Youden’s index, the optimal level of serum CP to predict fibrosis was set at 186 mg/L with a specificity of 90.33% and a sensitivity of 54.32%, a NPV of 91.18%, and a PPV of 51.80% for F ≥ 4, while 194 mg/L for F ≥ 2 gave a specificity of 58.98%, sensitivity of 86.84%, NPV of 95.91%, and PPV of 28.83%.
| Fibrosis stages | AUC (95%CI) | Cut-off point | Sensitivity | Specificity | PPV | NPV |
| F ≥ 2 | 0.776 (0.711-0.841) | ≤ 194 | 86.84% | 58.98% | 28.83% | 95.91% |
| F = 4 | 0.767 (0.691-0.843) | ≤ 186 | 54.32% | 90.33% | 51.80% | 91.18% |
Two markers (CP and GGT) were further identified as cirrhosis predictors by multivariate logistic regression analysis. Univariate logistic regression analysis showed a significant relationship between cirrhosis and these serum biomarkers (P < 0.05) in the training group (Table 3). We then constructed a model combining CP and GGT, and its diagnostic value was measured by ROC curve, showing an AUC value of 0.840 in the training group. No significant differences were found between the AUCs of the training and validation groups (Z = 0.646, P = 0.518) (Table 4). When the model was applied to the validation group, the AUC remained high (0.792).
| Variable | ≤F3 | ≥F4 | P value |
| Age, in yr | 38.9 ± 8.9 | 37.6 ± 12.6 | 0.632 |
| Male sex, in % | 70.37% | 77.41% | 0.255 |
| TB, in μmol/L | 11.1 (8.6-17.5) | 13.5 (10.1-15.8) | 0.429 |
| Albumin, in g/L | 42.8 ± 3.7 | 40.5 ± 4.5 | 0.039 |
| Globulin, in g/L | 27.4 ± 5.1 | 28.2 ± 3.2 | 0.590 |
| ALT, in IU/L | 34 (26-46) | 44.5 (24.3-56.8) | 0.281 |
| AST, in IU/L | 29 (24-37) | 32.5 (29.5-44.0) | 0.064 |
| ALP, in IU/L | 69 (58-83) | 70 (61-93) | 0.528 |
| GGT, in IU/L | 24 (17-34) | 55 (33.5-79.3) | < 0.001 |
| TBA, in mmol/L | 6.3 (4.1-14.6) | 9.8 (4.7-20.6) | 0.216 |
| CHE, in IU/L | 8669.1 ± 2421.8 | 6760.2 ± 1737.5 | 0.006 |
| PT, in s | 12.4 (11.9-12.9) | 13.5 (12.4-14.5) | 0.011 |
| INR | 1.03 (0.98-1.08) | 1.12 (1.02-1.20) | 0.019 |
| WBC, in 109/L | 5.69 ± 1.54 | 5.49 ± 1.60 | 0.649 |
| PLT, in 109/L | 183 (163-218) | 155 (131-175) | 0.002 |
| AFP, in ng/mL | 2.9 (2.1-5.0) | 10.75 (5.10-28.45) | < 0.001 |
| HBsAg levels, in logIU/mL | 3.44 (3.05-4.0) | 3.49 (3.39-3.67) | 0.910 |
| HBV DNA levels, in logIU/mL | 5.26 ± 1.69 | 5.57 ± 1.56 | 0.515 |
| CP, in mg/L | 201.9 ± 34.6 | 167.0 ± 24.9 | < 0.001 |
| Variable | Training group (n = 97) | Validation group (n = 96) | ||
| F = 4 | F ≥ 2 | F = 4 | F ≥ 2 | |
| AUC | 0.84 | 0.797 | 0.792 | 0.778 |
| Sensitivity | 83.10% | 83.30% | 74.70% | 79.40% |
| Specificity | 78.60% | 67.30% | 88.20% | 67.70% |
| PPV | 39.60% | 76.94% | 57.70% | 76.29% |
| NPV | 96.50% | 75.48% | 94.20% | 71.51% |
We constructed the following CG model: 3.76-0.034 × CP (mg/L) + 0.013 × GGT (IU/L). The optimal value for predicting cirrhosis from this CG model was -1.38. The values for sensitivity, specificity, PPV, and NPV of the CG model were 83.1%, 78.6%, 39.6%, and 96.5% for the training group, respectively, and 74.7%, 88.2%, 57.7%, and 94.2% for the validation group, respectively. The optimal cut-off value of -2.315 was applied to identify significant fibrosis in the training group, with an AUC of 0.797.
Training and validation groups showed no significant differences in the AUCs (Z = 0.295, P = 0.768). The values for sensitivity, specificity, PPV, and NPV of the CG model were 83.3%, 67.3%, 66.0%, and 84.1% for the training group, respectively, and 79.40%, 67.70%, 76.29%, and 71.51% for the validation group, respectively.
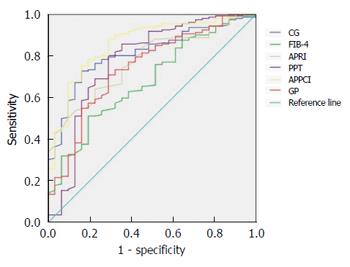
All the models showed very good NPV (> 90%), except FIB-4. CG, PPT, APRI, GP, and APPCI models all had high sensitivity (> 70%). The predictive value of the CG model for cirrhosis (F4) is presented in Table 5. After comparing the AUC of the CG model with that of the other five non-invasive models, we found that the CG model had a significantly greater AUC than FIB-4 (P < 0.05) (Table 5, Figure 1).
| Model | AUC (95%CI) | Youden’s index | Sensitivity | Specificity | PPV | NPV | Z value | P value |
| CG | 0.812 (0.740-0.883) | 0.567 | 72.84% | 83.88% | 46.37% | 94.17% | - | - |
| FIB-4 | 0.679 (0.581-0.777) | 0.318 | 52.23% | 80.65% | 34.06% | 89.82% | 2.138 | 0.0331 |
| APRI | 0.775 (0.698-0.852) | 0.416 | 64.20% | 77.43% | 34.52% | 91.46% | 0.688 | 0.491 |
| PPT | 0.777 (0.671-0.883) | 0.512 | 80.25% | 70.97% | 34.59% | 94.94% | 0.535 | 0.593 |
| APPCI | 0.859 (0.789-0.929) | 0.592 | 75.31% | 83.89% | 47.21% | 94.67% | -0.910 | 0.363 |
| GP | 0.742 (0.647-0.838) | 0.420 | 70.99% | 70.97% | 31.87% | 92.75% | 1.140 | 0.254 |
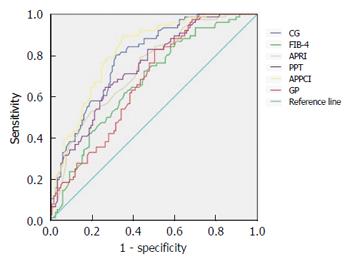
When the CG model was used to identify significant fibrosis in all patients, its predictive value had a higher AUC than the FIB-4 and GP models (P < 0.05), but was not statistically significant from the PPT, APRI, and APPCI models (P > 0.05) (Table 6, Figure 2).
| Model | AUC (95%CI) | Youden’s index | Sensitivity | Specificity | PPV | NPV | Z value | P value |
| CG | 0.789 (0.727-0.852) | 0.486 | 80.26% | 68.38% | 62.25% | 84.21% | - | - |
| FIB-4 | 0.671 (0.595-0.747) | 0.271 | 72.37% | 54.69% | 50.92% | 75.29% | 2.339 | 0.0191 |
| APRI | 0.736 (0.666-0.805) | 0.354 | 78.95% | 56.42% | 54.06% | 80.49% | 1.100 | 0.271 |
| PPT | 0.741 (0.672-0.810) | 0.380 | 64.47% | 73.51% | 61.26% | 76.10% | 1.012 | 0.312 |
| APPCI | 0.818 (0.760-0.877) | 0.544 | 89.47% | 64.96% | 62.39% | 90.47% | -0.661 | 0.509 |
| GP | 0.675 (0.601-0.749) | 0.325 | 82.89% | 49.58% | 51.64% | 81.69% | 2.295 | 0.0221 |
Identifying the degree of liver fibrosis in CHB patients is important for determining antiviral therapeutic options and for monitoring the efficacy of antiviral treatment, especially in patients who have normal or mildly raised ALT[2,5,6].
Liver biopsy remains the “gold” standard in assessing fibrosis, but it has limitations. Non-invasive predictive models of fibrosis are therefore needed. Single markers have been proposed such as platelet count, AST and ALT, gamma-globulins, serum HBsAg levels, CP, red blood cell distribution width, Interleukin-2R, TGF-α, serum Golgi protein 73 (GP73), and miR-122[13,14,16,17,25,28-31]. However, currently, none of these markers are sufficiently liver-specific enough to accurately reflect fibrosis. Thus, serum markers are commonly combined to improve diagnostic sensitivity and specificity.
CP, which is an acute phase protein mostly synthesized in the liver, has been proposed to assess ballooning, steatosis, and inflammation in patients with liver fibrosis that are CHB carriers and afflicted by NASH[25,26]. We previously developed a new non-invasive model of APPCI to predict liver cirrhosis in CHB patients presenting different ALT levels and found that serum CP was a new marker, negatively correlated with liver fibrosis[25]. In the present study, we extended these results to CHB carriers with normal or minimally raised ALT. CP AUCs to predict F ≥ 2 were 0.776 with good sensitivity and NPV%. For predicting F ≥ 4, the AUCs data were 0.767 with good specificity and NPV. The mechanism whereby CP decreases during fibrosis increase could be due to a selective decrease of hepatocyte synthesis, although this group of CHB patients did not exhibit significant signs of global hepatocellular insufficiency. Further studies are needed to elucidate the links between CP and liver fibrosis. From a practical standpoint, CP determination, given its simplicity, may represent a valuable marker of liver fibrosis and may contribute to reducing the need for liver biopsy.
As no single serum parameter can accurately or reliably predict liver fibrosis, combining serum biomarkers has become the preferred approach. Assessing a set of 19 potential biochemical markers of fibrosis, we developed the best logarithmic CG model for predicting liver significant fibrosis and cirrhosis, which consisted of a combination of two common clinical variables (CP and GGT). Our CG model provided AUCs of 0.840 and 0.792 to predict liver cirrhosis in the training and validation groups, respectively. GGT, which is associated with hepatocyte growth factor, has been related to liver fibrosis in CHB patients[32]. GGT could be increased by early cholestasis or an increase of epidermal growth factor, which could explain the relationship between increased GGT and fibrosis severity[33].
Significant differences of serum HBsAg levels have been observed in the process of HBV infection[34-37]. Martinot-Peignoux et al[38] observed no relationship between HBsAg levels and liver fibrosis in treatment-naive CHB patients with hepatitis B e antigen (HBeAg) negativity. Seto et al[39] reported that HBsAg levels can predict liver fibrosis in CHB patients with HBeAg positivity. In our previous study, serum HBsAg levels were found to be significantly related to liver fibrosis during the immune clearance phase.
Depending on the dual standard of both serological and histological profiles, our recent study showed that serum HBsAg level can help identify patients in the immune tolerance phase with potential liver injury, but not in the immune-clearance (IC), low-replicative (LR), and HBeAg-negative hepatitis (ENH) phases[37]. These data can explain the results of our present study in which serum HBsAg level was not an independent predictor of fibrosis, since this retrospective cohort study included both HBeAg(+) and HBeAg(-) CHB patients with various levels of HBV DNA and normal or mildly elevated ALT.
Most non-invasive tests, including FIB-4, European Liver Fibrosis (ELF) score, FibroTest®, HepaScore®, and APRI[40-44], were first developed to assess liver fibrosis in chronic hepatitis C patients. Whether they are also applicable to CHB patients, however, is yet to be demonstrated. Therefore, new non-invasive models were recently developed to detect liver fibrosis in patients with CHB[19,25,45,46]. Compared with FIB-4 and GP, our CG model showed much greater AUC than FIB-4 to the GP model.
To date, the APRI test has been recommended via international guidelines for CHB patient liver biopsy[5,6]. Wang et al[47] were the first to validate the performance of APRI in CHB patients with low serum ALT activity. But they only validated APRI to predict significant fibrosis (≥ F2) with an AUC of 0.77, not to predict liver cirrhosis. In our study, APRI had AUCs of 0.736 and 0.775 for predicting significant fibrosis and cirrhosis, respectively, and APRI could detect CHB-related fibrosis with only moderate sensitivity and specificity.
The current study has several limitations. Firstly, a perfect non-invasive model would require very high sensitivity and specificity, with an AUC of the maximum theoretical value (1.0). In fact, none of the models can achieve both perfect sensitivity and specificity. Indeed, “spectrum bias” due to over-representation of extreme fibrosis stages (F0 and F4) is difficult to avoid. In order to prevent this “spectrum bias”, the adjustment of AUC using the DANA or the Obuchowski methods would be relevant but could not be used in the present study, since we pooled stages F0-F1 given the limited number of F0[45,46]. Secondly, CP levels were tested at different time periods. Thirdly, although an internal validation was carried out from a randomly chosen cohort, a prospective external validation should be performed. Fourthly, in this study, serum CP was tested by nephelometry, which could not distinguish the CP in active or inactive form. In the future, the method of automation of o-dianisidine assay may be preferred to test the CP activity[48].
In conclusion, we established a simple and accurate CG model to predict significant liver fibrosis and cirrhosis in CHB patients with normal or mildly elevated ALT. This model may be a valuable tool for avoiding liver biopsy in this category of HBV-infected patients.
The authors thank Professors Yu-Qing Chen and Li-Hong Chen at Fujian Medical University for their help with the evaluation of liver fibrosis stage.
Chronic hepatitis B (CHB) patients may develop significant fibrosis and even cirrhosis despite normal or mildly elevated serum transaminase levels.
To date, few non-invasive approaches have been developed to evaluate liver fibrosis and no studies have proposed measuring ceruloplasmin (CP) levels for predicting liver fibrosis in CHB patients with normal or minimally raised alanine aminotransferase (ALT).
In the present study, the authors found that CP was independently and negatively associated with liver fibrosis. Furthermore, a simple and accurate CG model was developed to predict significant liver fibrosis and cirrhosis in CHB patients with normal or mildly elevated ALT.
The CG model may be a valuable tool to replace liver biopsy in CHB patients with normal or minimally raised ALT, especially in resource limited settings.
Ceruloplasmin (CP) is a copper-containing glycoprotein synthesized predominantly in the liver, and is a serum ferroxidase that plays an essential role in iron metabolism. It is the major carrier for copper in the blood, accounting for 90% of the circulating copper in normal individuals.
In this manuscript, the authors aim at identify the CP as a non-invasive index to predict liver fibrosis in HBV chronic hepatitis. The topic is interesting, although already investigated in other human models of liver disease. It is globally well-written with a congruous number of patients involved.
| 1. | Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1217] [Cited by in RCA: 1344] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 2. | Hou JL, lai W. The guideline of prevention and treatment for chronic hepatitis B: a 2015 update. Zhonghua Gan Zang Bing Za Zhi. 2015;23:888-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 90] [Reference Citation Analysis (0)] |
| 3. | Yuen MF, Yuan HJ, Wong DK, Yuen JC, Wong WM, Chan AO, Wong BC, Lai KC, Lai CL. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut. 2005;54:1610-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 293] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2411] [Article Influence: 172.2] [Reference Citation Analysis (1)] |
| 5. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 2058] [Article Influence: 205.8] [Reference Citation Analysis (12)] |
| 6. | Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1631] [Article Influence: 163.1] [Reference Citation Analysis (2)] |
| 7. | Martinot-Peignoux M, Boyer N, Colombat M, Akremi R, Pham BN, Ollivier S, Castelnau C, Valla D, Degott C, Marcellin P. Serum hepatitis B virus DNA levels and liver histology in inactive HBsAg carriers. J Hepatol. 2002;36:543-546. [PubMed] |
| 8. | Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, Liaw YF. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 509] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 9. | European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1383] [Article Influence: 125.7] [Reference Citation Analysis (1)] |
| 10. | Park HS, Kim YJ, Yu MH, Choe WH, Jung SI, Jeon HJ. Three-Tesla magnetic resonance elastography for hepatic fibrosis: comparison with diffusion-weighted imaging and gadoxetic acid-enhanced magnetic resonance imaging. World J Gastroenterol. 2014;20:17558-17567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Taniguchi M, Okizaki A, Watanabe K, Imai K, Uchida K, Einama T, Shuke N, Miyokawa N, Furukawa H. Hepatic clearance measured with (99m)Tc-GSA single-photon emission computed tomography to estimate liver fibrosis. World J Gastroenterol. 2014;20:16714-16720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Li SM, Li GX, Fu DM, Wang Y, Dang LQ. Liver fibrosis evaluation by ARFI and APRI in chronic hepatitis C. World J Gastroenterol. 2014;20:9528-9533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Xu Z, Liu L, Pan X, Wei K, Wei M, Liu L, Yang H, Liu Q. Serum Golgi protein 73 (GP73) is a diagnostic and prognostic marker of chronic HBV liver disease. Medicine (Baltimore). 2015;94:e659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (3)] |
| 14. | Deng YQ, Zhao H, Ma AL, Zhou JY, Xie SB, Zhang XQ, Zhang DZ, Xie Q, Zhang G, Shang J. Selected Cytokines Serve as Potential Biomarkers for Predicting Liver Inflammation and Fibrosis in Chronic Hepatitis B Patients With Normal to Mildly Elevated Aminotransferases. Medicine (Baltimore). 2015;94:e2003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Deng H, Qi X, Guo X. Diagnostic Accuracy of APRI, AAR, FIB-4, FI, King, Lok, Forns, and FibroIndex Scores in Predicting the Presence of Esophageal Varices in Liver Cirrhosis: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 2015;94:e1795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Xu WS, Qiu XM, Ou QS, Liu C, Lin JP, Chen HJ, Lin S, Wang WH, Lin SR, Chen J. Red blood cell distribution width levels correlate with liver fibrosis and inflammation: a noninvasive serum marker panel to predict the severity of fibrosis and inflammation in patients with hepatitis B. Medicine (Baltimore). 2015;94:e612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Zeng DW, Liu YR, Dong J, Zhu YY, Li YB, Chen J, Zheng Q, Jiang JJ. Serum HBsAg and HBeAg levels are associated with liver pathological stages in the immune clearance phase of hepatitis B virus chronic infection. Mol Med Rep. 2015;11:3465-3472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Li Q, Song J, Huang Y, Li X, Zhuo Q, Li W, Chen C, Lu C, Qi X, Chen L. The Gamma-Glutamyl-Transpeptidase to Platelet Ratio Does not Show Advantages than APRI and Fib-4 in Diagnosing Significant Fibrosis and Cirrhosis in Patients With Chronic Hepatitis B: A Retrospective Cohort Study in China. Medicine (Baltimore). 2016;95:e3372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Zeng DW, Zhang JM, Liu YR, Dong J, Wu YL, Lin S, Jiang JJ, Zhu YY. A new model for predicting liver cirrhosis in chronic hepatitis B virus carriers with low serum alanine transaminase activity. Clin Res Hepatol Gastroenterol. 2014;38:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Park SH, Kim CH, Kim DJ, Cheong JY, Cho SW, Hwang SG, Lee YJ, Cho M, Yang JM, Kim YB. Development and validation of a model to predict advanced fibrosis in chronic hepatitis B virus-infected patients with high viral load and normal or minimally raised ALT. Dig Dis Sci. 2011;56:1828-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Wang H, Yan R, Zhou Y, Wang MS, Ruo GQ, Cheng MJ. A scoring system for predicting significant fibrosis in chronic hepatitis B patients with normal or mildly elevated alanine aminotransferase levels. J Clin Gastroenterol. 2015;49:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | European Association for Study of Liver. EASL Clinical Practice Guidelines: Wilson’s disease. J Hepatol. 2012;56:671-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 971] [Cited by in RCA: 826] [Article Influence: 59.0] [Reference Citation Analysis (2)] |
| 23. | Marano M, Vespasiani Gentilucci U, Altamura C, Siotto M, Squitti R, Bucossi S, Quintiliani L, Migliore S, Greco F, Scarciolla L. Altered metal metabolism in patients with HCV-related cirrhosis and hepatic encephalopathy. Metab Brain Dis. 2015;30:1445-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Yang X, Tong DJ, Liang J, Zhang YH, Lei JH, He XE, He G. [Ceruloplasmin level of patients with liver disease in China]. Zhonghua Nei Ke Za Zhi. 2005;44:13-15. [PubMed] |
| 25. | Zeng DW, Liu YR, Zhang JM, Zhu YY, Lin S, You J, Li YB, Chen J, Zheng Q, Jiang JJ. Serum ceruloplasmin levels correlate negatively with liver fibrosis in males with chronic hepatitis B: a new noninvasive model for predicting liver fibrosis in HBV-related liver disease. PLoS One. 2013;8:e77942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Nobili V, Siotto M, Bedogni G, Ravà L, Pietrobattista A, Panera N, Alisi A, Squitti R. Levels of serum ceruloplasmin associate with pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2013;56:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3129] [Article Influence: 104.3] [Reference Citation Analysis (1)] |
| 28. | Petta S, Tuttolomondo A, Gagliardo C, Zafonte R, Brancatelli G, Cabibi D, Cammà C, Di Marco V, Galvano L, La Tona G. The Presence of White Matter Lesions Is Associated With the Fibrosis Severity of Nonalcoholic Fatty Liver Disease. Medicine (Baltimore). 2016;95:e3446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Yu SJ, Kim W, Kim D, Yoon JH, Lee K, Kim JH, Cho EJ, Lee JH, Kim HY, Kim YJ. Visceral Obesity Predicts Significant Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Medicine (Baltimore). 2015;94:e2159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 30. | Oztas E, Kuzu UB, Zengin NI, Kalkan IH, Onder FO, Yildiz H, Celik HT, Akdogan M, Kilic MY, Koksal AS. Can Serum ST2 Levels Be Used as a Marker of Fibrosis in Chronic Hepatitis B Infection? Medicine (Baltimore). 2015;94:e1889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Halász T, Horváth G, Pár G, Werling K, Kiss A, Schaff Z, Lendvai G. miR-122 negatively correlates with liver fibrosis as detected by histology and FibroScan. World J Gastroenterol. 2015;21:7814-7823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Myers RP, Tainturier MH, Ratziu V, Piton A, Thibault V, Imbert-Bismut F, Messous D, Charlotte F, Di Martino V, Benhamou Y. Prediction of liver histological lesions with biochemical markers in patients with chronic hepatitis B. J Hepatol. 2003;39:222-230. [PubMed] |
| 33. | Edwards AM, Lucas CM, Baddams HM. Modulation of gamma-glutamyltranspeptidase in normal rat hepatocytes in culture by cell density, epidermal growth factor and agents which alter cell differentiation. Carcinogenesis. 1987;8:1837-1842. [PubMed] |
| 34. | Zeng DW, Zhu YY, Huang Q, Zhang JM, Wu YL, Dong J, Jiang JJ, Liu YR. Hepatitis B surface antigen in late hepatitis B infection. J Med Virol. 2015;87:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Nguyen T, Thompson AJ, Bowden S, Croagh C, Bell S, Desmond PV, Levy M, Locarnini SA. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol. 2010;52:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 282] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 36. | Jaroszewicz J, Calle Serrano B, Wursthorn K, Deterding K, Schlue J, Raupach R, Flisiak R, Bock CT, Manns MP, Wedemeyer H. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol. 2010;52:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 318] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 37. | Zeng DW, Zhang JM, Liu YR, Dong J, Jiang JJ, Zhu YY. A Retrospective Study on the Significance of Liver Biopsy and Hepatitis B Surface Antigen in Chronic Hepatitis B Infection. Medicine (Baltimore). 2016;95:e2503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Martinot-Peignoux M, Carvalho-Filho R, Lapalus M, Netto-Cardoso AC, Lada O, Batrla R, Krause F, Asselah T, Marcellin P. Hepatitis B surface antigen serum level is associated with fibrosis severity in treatment-naïve, e antigen-positive patients. J Hepatol. 2013;58:1089-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 39. | Seto WK, Wong DK, Fung J, Ip PP, Yuen JC, Hung IF, Lai CL, Yuen MF. High hepatitis B surface antigen levels predict insignificant fibrosis in hepatitis B e antigen positive chronic hepatitis B. PLoS One. 2012;7:e43087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 40. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3346] [Article Influence: 145.5] [Reference Citation Analysis (0)] |
| 41. | Vallet-Pichard A, Mallet V, Pol S. FIB-4: a simple, inexpensive and accurate marker of fibrosis in HCV-infected patients. Hepatology. 2006;44:769; author reply 769-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1038] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 43. | Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704-1713. [PubMed] |
| 44. | Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, Kench J, Farrell G, McCaughan GW, Jeffrey GP. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51:1867-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 396] [Article Influence: 18.9] [Reference Citation Analysis (1)] |
| 45. | Zhang YX, Wu WJ, Zhang YZ, Feng YL, Zhou XX, Pan Q. Noninvasive assessment of liver fibrosis with combined serum aminotransferase/platelet ratio index and hyaluronic acid in patients with chronic hepatitis B. World J Gastroenterol. 2008;14:7117-7121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Liu XD, Wu JL, Liang J, Zhang T, Sheng QS. Globulin-platelet model predicts minimal fibrosis and cirrhosis in chronic hepatitis B virus infected patients. World J Gastroenterol. 2012;18:2784-2792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Wang H, Xue L, Yan R, Zhou Y, Wang MS, Cheng MJ, Huang HJ. Comparison of FIB-4 and APRI in Chinese HBV-infected patients with persistently normal ALT and mildly elevated ALT. J Viral Hepat. 2013;20:e3-e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 48. | Siotto M, Pasqualetti P, Marano M, Squitti R. Automation of o-dianisidine assay for ceruloplasmin activity analyses: usefulness of investigation in Wilson’s disease and in hepatic encephalopathy. J Neural Transm (Vienna). 2014;121:1281-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Marano M S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Wang CH