Published online Nov 21, 2016. doi: 10.3748/wjg.v22.i43.9515
Peer-review started: June 24, 2016
First decision: August 22, 2016
Revised: September 4, 2016
Accepted: September 28, 2016
Article in press: September 28, 2016
Published online: November 21, 2016
Processing time: 148 Days and 16.6 Hours
To investigate the anti-inflammatory effect and the possible mechanisms of an agonist of cannabinoid (CB) receptors, WIN55-212-2 (WIN55), in mice with experimental colitis, so as to supply experimental evidence for its clinical use in future.
We established the colitis model in C57BL/6 mice by replacing the animals’ water supply with 4% dextran sulfate sodium (DSS) for 7 consecutive days. A colitis scoring system was used to evaluate the severity of colon local lesion. The plasma levels of proinflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), and the myeloperoxidase (MPO) activity in colon tissue were measured. The expressions of cannabinoid receptors, claudin-1 protein, p38 mitogen-activated protein kinase (p38MAPK) and its phosphorylated form (p-p38) in colon tissue were determined by immunohistochemistry and Western blot. In addition, the effect of SB203580 (SB), an inhibitor of p38, was investigated in parallel experiments, and the data were compared with those from intervention groups of WIN55 and SB alone or used together.
The results demonstrated that WIN55 or SB treatment alone or together improved the pathological changes in mice with DSS colitis, decreased the plasma levels of TNF-α, and IL-6, and MPO activity in colon. The enhanced expression of claudin-1 and the inhibited expression of p-p38 in colon tissues were found in the WIN55-treated group. Besides, the expression of CB1 and CB2 receptors was enhanced in the colon after the induction of DSS colitis, but reduced when p38MAPK was inhibited.
These results confirmed the anti-inflammatory effect and protective role of WIN55 on the mice with experimental colitis, and revealed that this agent exercises its action at least partially by inhibiting p38MAPK. Furthermore, the results showed that SB203580, affected the expression of CB1 and CB2 receptors in the mouse colon, suggesting a close linkage and cross-talk between the p38MAPK signaling pathway and the endogenous CB system.
Core tip: Inflammatory bowel disease demands more effective therapies, and cannabinoids are beneficial in alleviating inflammation in some diseases. In this study, we reported the anti-inflammatory effect and protective role of WIN55-212-2, an agonist of cannabinoid receptors, on the mice with dextran sulfate sodium-induced experimental colitis, and revealed that this agent exerts its action at least partially by inhibiting p38 mitogen-activated protein kinase.
- Citation: Feng YJ, Li YY, Lin XH, Li K, Cao MH. Anti-inflammatory effect of cannabinoid agonist WIN55, 212 on mouse experimental colitis is related to inhibition of p38MAPK. World J Gastroenterol 2016; 22(43): 9515-9524
- URL: https://www.wjgnet.com/1007-9327/full/v22/i43/9515.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i43.9515
Inflammatory bowel disease (IBD), including ulcerative colitis and Crohn’s disease, is a group of chronic disorders characterized by recurrent intestinal inflammation and overactive immune responses[1,2]. Generally, for lifelong IBD in patients, disease flare-up periods alternate with disease remission periods principally represented by different degrees of intestinal inflammation. Since p38 mitogen-activated protein kinases (p38MAPKs) signaling pathways participate in the regulation of recurrent inflammatory progression[3], the activation of p38MAPKs can greatly promote the process of inflammation by up-regulating certain proinflammatory genes’ expression, such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1, IL-6, etc[3,4]. Furthermore, excessive activation of p38MAPKs can cause recurrent attack of intestinal inflammation and facilitate forming of vicious cycles[5]. In order to circumvent the vicious circle caused by p38MAPKs, some p38 inhibitors have been applied in preclinical and clinical studies. However, results of these studies show that inhibition of p38MAPKs by p38 inhibitors is unacceptable in clinical practice due to obvious side effects. These side effects include infections, headache, toxicity to the central nervous system, gastrointestinal tract symptoms, hepatotoxicity, cardiotoxicity, etc[6]. More effective therapeutic approaches and agents need to be explored and developed.
The endogenous cannabinoid system (ECS) was discovered in the late 1990s. Since then, it has been brought into focus due to its huge potential in regulating gastrointestinal inflammation, abdominal pain, diarrhea, etc[7]. WIN55-212-2(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinyl-methyl)pyrrolo(1,2,3-de)-1,4-benzoxazin-6-yl]-1-napthalenylmethanone (WIN55) is a typical cannabinoid receptor agonist and can activate ECS via acting on the Gi/o coupled membrane receptors: cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2), the two main typical cannabinoid receptors[8,9]. WIN55 has been reported as beneficial in treating gastrointestinal inflammatory disorders; albeit, its pharmacological mechanism has not been demonstrated clearly[10,11]. In this report, we designed experiments to explore the effect of WIN55 on the C57BL/6 mice with dextran sulfate sodium (DSS)-induced colitis, examined the changes of p38 activity during the treatment of WIN55 and SB203580, (4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine, SB), an inhibitor of p38, and investigated the interplay between ECS and p38MAPK.
C57BL/6 mice (half males and half females, 6-8 wk old, 18-24 g) were purchased from the Experimental Animal Center of Second Medical University, Shanghai, China, and housed for 2 wk prior to experiments under standard conditions (temperature 24 ± 1 °C; humidity 55%; 12:12 h light-dark cycle) with free access to laboratory food and tap water. All experimental procedures complied with international guidelines for the care and use of laboratory animals and approved by the Animal Ethics Committee of Tongji University, Shanghai, China.
Colitis was induced in the C57BL/6 mice by replacing tap water with the solution of 4% (wt/vol) DSS (reagent grade: 36-50000 Da; MP Biomedicals, Illkirch, France) from day 1 to day 7, according to the literature[12-14]. SB and WIN55 were obtained from Tocris Bioscience (Ellisville, MO, United States) and dissolved in a vehicle containing 2% dimethyl sulfoxide and sterile saline. The mice were assigned to 6 groups with 8 mice in each group, and they were given different treatments: (1) mice drinking DSS water and receiving vehicle intraperitoneally (i.p.) once daily for 7 d (DSS + Veh group); (2) mice drinking DSS water and receiving WIN55 (5 mg/kg) i.p. daily for 7 d starting from DSS treatment through the end of experiment (DSS + WIN group)[15]; (3) mice drinking DSS water and receiving SB (5 μmol/kg) i.p. beginning from 60 h after the DSS treatment and continuing until the last day (DSS+SB group)[16]; (4) mice drinking DSS water and receiving both WIN55 and SB in the same dose and same manner as above (DSS + WIN + SB group); (5) mice drinking normal water and receiving vehicle i.p. for 7 d (Control group); and (6) mice drinking normal water and receiving WIN55 i.p. for 7 d (WIN55 group). During the 7-d period, the body weight, stool and general conditions of the mice were observed daily and the consumption of DSS-containing liquid was monitored every day to ensure the proper intake of DSS by mice.
All of the C57BL/6 mice were anesthetized with isoflurane and sacrificed by decapitation on day 7. Soon after the execution, blood samples were collected via the carotid aorta into heparinized Eppendorf tubes. Colon specimens were carefully dissected and removed from the sacrificed mice. Plasma samples were obtained by centrifugation of the blood for 10 min at 12000 ×g, 4 °C, and stored at -80 °C until examination. Colon samples were weighted and measured for length, then opened longitudinally and cleaned by clearing stool and rinsing with normal saline. After macroscopic inspection, colon samples were divided into several portions; one portion was put into 10% neutral buffered formalin immediately for histopathological examination, and the other portions were placed in liquid nitrogen for shock-freezing followed by storage at -80 °C until next examination.
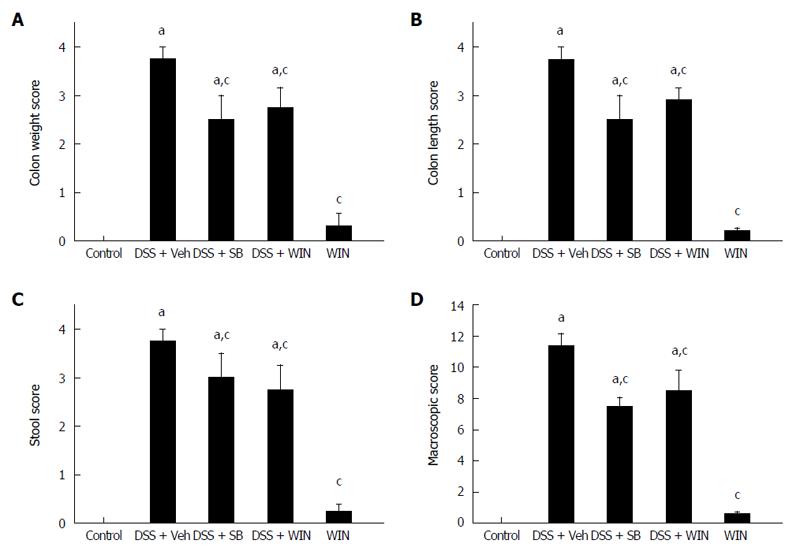
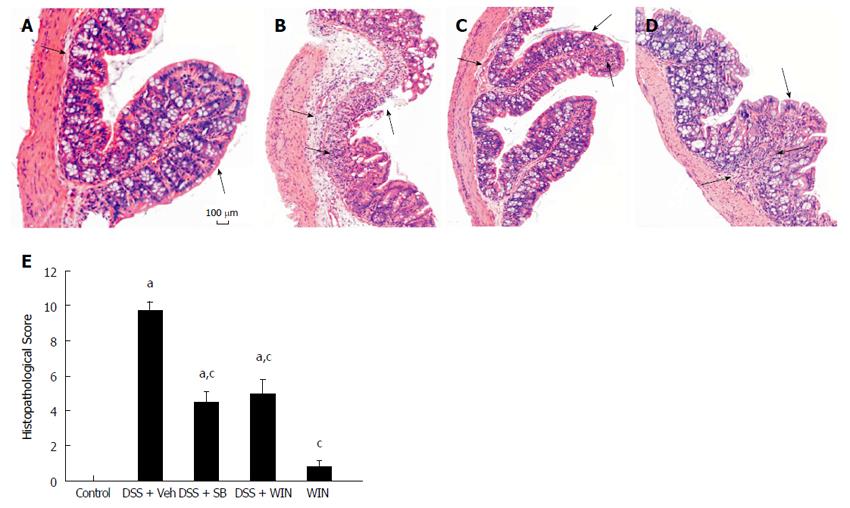
Different colitis score systems, including macroscopical score (MS) and histopathological score (HS), were used to evaluate pathological changes of the experimental colitis[17,18]. MS (with a total maximum score of 12) was evaluated by indications of stool, colon weight and colon length. Scores on stool varied from 0 (normal) to 3 (diarrhea) according to stool properties (shape, moisture, viscosity). When blood was discovered in stool, 1 point was added. Scores on both colon weight and length were determined by calculating the weight increase and length shortening in comparison to controls, as described[17]. HS (with a maximum score of 11) was evaluated by inflammation extent (0-2 score), damage in crypt architecture (0-2 score), hyperemia/edema (0-3 score), as well as the infiltration of inflammatory cells in the colon tissues (0-3 score). Presence of ulceration and/or crypt abscess, respectively, led to 1 point being added[18]. All the histological evaluations were performed and scored by an experienced researcher blinded to the experimental plans.
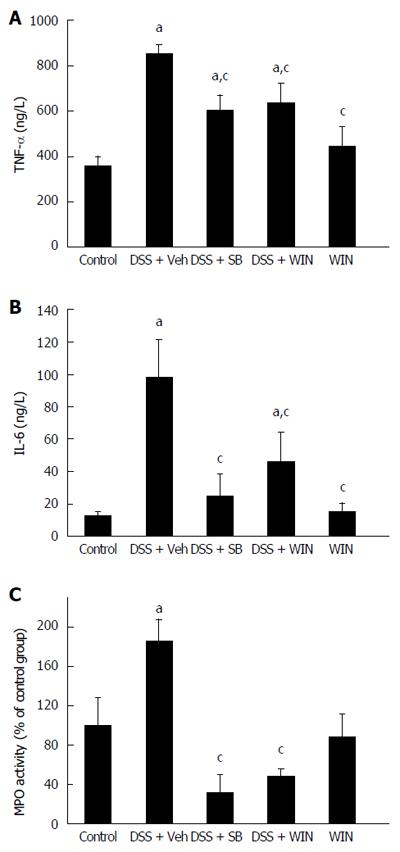
The plasma levels of proinflammatory cytokines, such as TNF-α and IL-6, were determined by using mouse-specific enzyme-linked immunosorbent assay (ELISA) kits (Cat No. MTA00 and M6000B; R&D Systems, Minneapolis, MN, United States). The ELISA examination procedures were followed by the recommended manufacturer protocols, and all the samples of blood supernatant were measured in duplicate. Results were presented in the form of ng/L or in percentile of the control group.
myeloperoxidase (MPO) activity in colon tissues, as an indicator of neutrophil infiltration, was measured as described[19]. Briefly, 150-200 mg of colon tissues was homogenized with a homogenizer and separated on ice to obtain protein components. Some volume of homogenate was removed for protein determination by the BCA™ protein assay, and the other part was manipulated using the MPO assay procedure. The absorbance was measured at 540 nm on a microplate reader, and MPO activity expressed as milliunits per milligram of wet weight of colon tissue and calculated as percentage of the control.
The expression of CB1 and CB2 receptors in colon tissues were detected by immunohistochemistry as described[20]. Gut specimens were obtained from proximal colon tissues approximately 3-4 cm to the anus. The manipulation was conducted according to the manufacturer’s instructions. The first antibody was rabbit anti-CB1 receptor or rabbit anti-CB2 receptor polyclonal antibody (1:20 dilution for both; Enzo, Plymouth Meeting, PA, United States). The streptavidin-peroxidase goat anti-rabbit antibody (Zymed Labs, San Francisco, CA, United States) was used as secondary antibody, and a streptavidin-biotin complex immunohistochemical staining kit (Boster, Wuhan, Hubei, China) was used for visualization. The pictures were then documented digitally on the Leica DM2500 microscope and saved as results.
Colon tissues from the different experimental groups were homogenized and lysed using the lysate buffer containing aprotinin (2 μg/mL) and phenylmethanesulfonyl fluoride (1 mol/L). The lysate was analyzed by western blotting according to the method described[21]. The protein detection procedure adopted the enhanced chemiluminescence method (Cat No. SC-2048; Santa Cruz Biotechnology, Dallas, TX, United States). Specific polyclonal antibodies were used to detect the related protein expression: rabbit anti-p38 (1:350 dilution), rabbit anti-p-p38 (1:400 dilution) (Santa Cruz Biotechnology), rabbit anti- claudin-1 (1:250 dilution) (Invitrogen, Camarillo, CA, United States), rabbit anti-CB1 receptor (1:250 dilution) and rabbit anti-CB2 receptor (1:250 dilution) (Enzo). Rabbit anti-β-actin (1:2000 dilution) polyclonal antibody (Abmart, Shanghai, China) acted as loading control in the western blotting analyses, and goat anti-rabbit IgG-HRP (1:5000 dilution; Abmart) was used as secondary antibody. Semi-quantitative statistical analysis of the western blot strips was conducted with the software ImageJ 1.44p.
Data of each experimental group are presented as mean ± SEM. Statistical analysis was performed by one-way analysis of variance (ANOVA) and post-hoc testing for multiple comparisons by using SPSS 13.0 software (SPSS Co. Ltd., Shanghai, China). Values of P < 0.05 were considered to be significant.
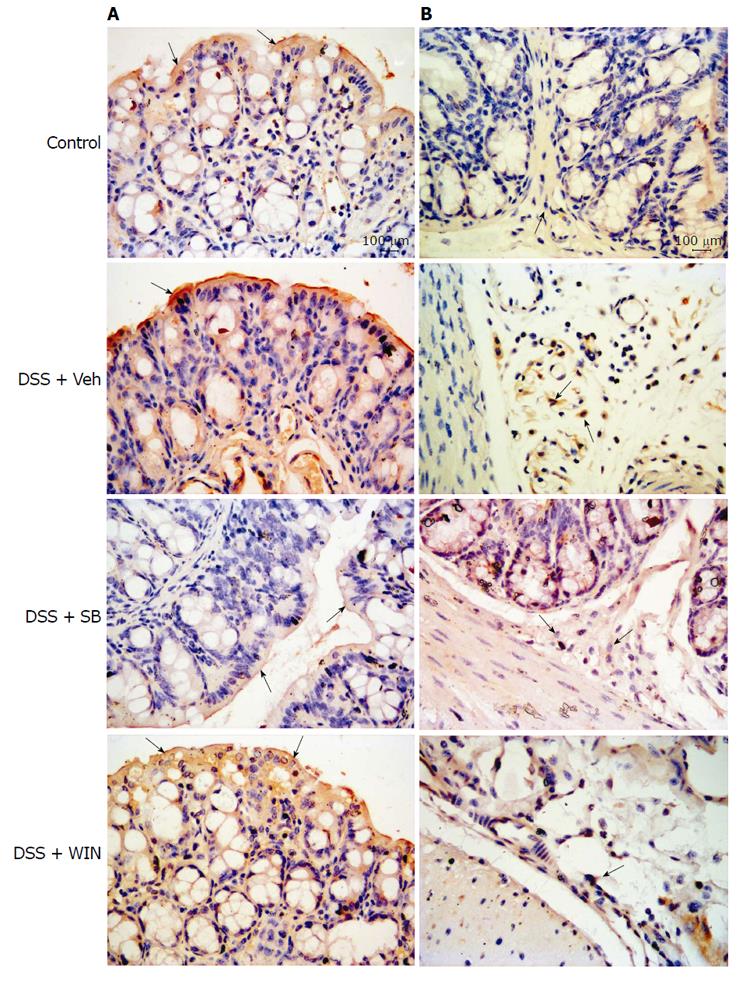
As shown in Figure 1, after drinking 4% DSS solution for 7 d, C57BL/6 mice showed signs of severe colitis with obviously increased colon weight, shortened colon length and stool changes, which were evaluated by using the colitis scoring systems. The histological damages in epithelium and edema, as well as infiltration of inflammatory cells in the sub-mucosa tissues were indicated by arrows in Figure 2 in animals of different groups. These pathological changes, both MS and HS, were significantly inhibited by SB or WIN55 treatment alone or both. There were no apparent differences among animals in the SB and WIN55 treatment groups. Furthermore, no raised anti-inflammatory effect was observed with the use of SB plus WIN55 (DSS + WIN + SB) (data not shown).
TNF-α and IL-6 are inflammatory indicators reflecting the severity of experimental colitis. Examination showed that the plasma levels of TNF-α and IL-6 were elevated significantly after the induction of colitis (P < 0.05). Compared to the colitis group, mice receiving SB or WIN55 treatment had a reduced TNF-α and IL-6 level in plasma (P < 0.05). No evident difference was observed between the SB group and the WIN55 group (Figure 3A and B). By detecting and analyzing MPO activity of colon tissues, the results revealed that MPO activity in colon tissues were elevated in the mice of the colitis group. Along with the improvement of local inflammation, mice in the SB or WIN55 treatment groups showed lower levels of MPO activity with statistical significance at P < 0.05, respectively (Figure 3C).
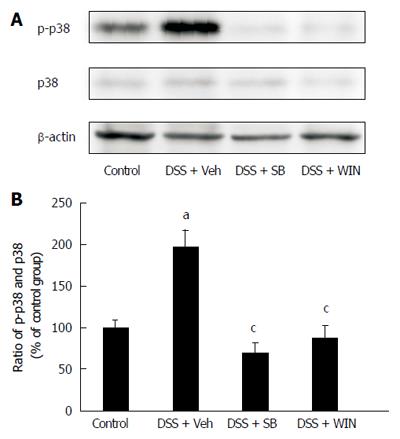
In order to evaluate the activation of p38MAPK, p38 as well as p-p38 were investigated by western blotting. Results showed p-p38 expression was significantly boosted in the colon tissues of DSS treatment mice (P < 0.05), and this expression enhancement was inhibited by using SB or WIN55 (P < 0.05), respectively (Figure 4).
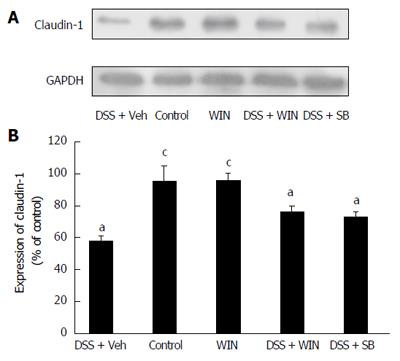
The protein of claudin-1 in colon tissues was investigated by western blotting. Results showed expression of claudin-1 decreased in the colon tissues of DSS-treated mice (P < 0.05). The decreased expression of claudin-1 was reversed both by the treatment of WIN55 and SB (P < 0.05) (Figure 5).
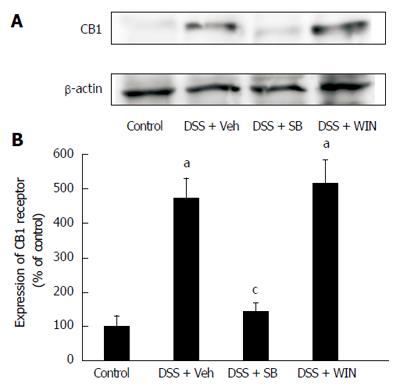
By immunohistochemistry examination, expression of CB1 and CB2 receptors in colon tissues was found, as shown in Figure 6 by marked brown-yellow staining and indicated by arrows. The semi-quantitative analysis data which represented the average optical density (AOD) of the positive staining are shown in Table 1. By comparing the AOD in different groups, we found that the expression of CB1 and CB2 receptors was significantly enhanced in the DSS + Veh group when compared to that in the control group (P < 0.05). SB, but not WIN55, showed an inhibiting effect on the expression of CB receptors.
By western blotting analysis, the increased expression of CB1 receptor in colon tissues was also found in the DSS + Veh group (P < 0.05), and the enhancement was reversed by use of SB (P < 0.05), but not by WIN55 (Figure 7). The expression of CB2 receptor was too trivial to be detected by western blotting assay.
In the pathophysiological process of colitis, a variety of signaling pathways are involved. Among these signaling pathways, p38MAPK is recognized as an extremely important pathway related to inflammatory response. Activation of p38MAPK can greatly promote the recruitment, activation and deployment of immune cells and aggravate inflammation by up-regulating the expression of some pro-inflammatory cytokines, such as TNF-α, IL-1, IL-6, IL-8, etc[3]. The fact that levels of TNF-α and IL-6 in plasma, as well as MPO activity in colon tissue, increase in animals with experimental colitis indicates that colitis is likely a systemic disease with manifestations in multiple organs. In this study, p38MAPK activation has been confirmed after induction of the experimental colitis which was accompanied by increased expression of p-p38, the phosphorylated form of p38. It is known that p38MAPK can be activated by various factors, especially the cytokines, such as IL-1 and TNF-α[3], and conversely, the activation of p38MAPK further promotes the production of the pro-inflammatory cytokines, resulting in a cytokine activation cascade and development of inflammatory response[22]. Inhibiting p38MAPK by its inhibitor SB can effectively decrease the expression of p-p38 and alleviate the intestinal inflammation[16], which was confirmed by our present study. It is interesting to discover in this study that WIN55 shows an evident anti-inflammatory effect and inhibiting role in p38MAPK activation. A decreased expression of p-p38 in DSS-induced colitis was observed after administering WIN55 in animals. At the same time, the proinflammatory cytokines, such as TNF-α and IL-6, shown in this study, were reduced by the treatment of WIN55. It is well known that SB exerts its anti-inflammatory effect by inhibiting p38 activation directly; however, WIN55 exerts its roles mainly by activating the ECS. Activation of ECS by WIN55 improves the pathological changes in colon tissues significantly, which we have evaluated adequately using the pathological scoring systems. Furthermore, WIN55-mediated reduction of the expression of p-p38 in the DSS-induced colitis suggests that the anti-inflammatory effect of ECS is related with the inhibition of p38MAPK, which might be one of the mechanisms underlying its anti-inflammatory roles.
In order to further explore the role of WIN55 in improving the gut pathological changes in DSS-induced colitis, we tested the expression of claudin-1 in colon tissues. As the most important components of the gut tight junctions, the claudin proteins participate in establishing the paracellular barrier and controlling intestinal permeability[23]. In this experiment, the destroyed epithelial barrier and a decreased expression of claudin-1 were observed in the colitis model, and these changes were greatly alleviated by treatment of WIN55 as well as of SB.
Although p38 inhibitors show obvious anti-inflammatory effect in colitis, they are not acceptable in clinical treatment due to their severe side effects, such as infection, hepatotoxicity, cardiotoxicity, headache, gastrointestinal tract symptoms, etc[6]. Inhibiting p38MAPK by ECS might be a good way to avoid such side effects. In addition, the newly developed specific agonists of CBs show diminished psychoactive side effects, such as drug addiction, euphoria, depression, alteration of sensory perception, etc when compared with the traditional CBs[10], which promotes the possibility of them using in clinic.
Another interesting finding in this study is that the induction of DSS colitis boosts the expression of CB1 and CB2 receptors in the mouse colon, and p38MAPK inhibition attenuates this effect. WIN55 exerts no effect on expression of CB1 and CB2 receptors, and the results from western blotting are in accord with those from immunohistochemistry on the expression of CB receptors. For the first time the changes of expression of CB receptors have been evaluated in the DSS-induced mouse colitis, with or without the regulation of p38MAPK signaling pathway. We speculate that p38MAPK and ECS are closely related. However, the detailed mechanism of the interplay between p38MAPK and ECS is still unclear which will be further investigated in depth in our future study.
In summary, by using a DSS-induced mouse colitis model, we confirmed the anti-inflammatory effect of the cannabinoid receptor agonist WIN55, and revealed that this agent exercises its action by inhibiting p38MAPK. Furthermore, we found that SB203580, a p38MAPK inhibitor, affected the expression of CB1 and CB2 receptors in the colon tissues of mice with colitis. The results provide fresh evidence suggesting a close linkage and cross-talk between the p38MAPK signaling pathway and the ECS, and shed light on the action mechanism of WIN55. However, further investigation is justified until new, effective therapeutic agents are developed.
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease, is a group of chronic disorders characterized by recurrent intestinal inflammation and overactive immune responses. Generally, for lifelong IBD in patients, disease flare-up periods alternate with disease remission periods principally represented by different degrees of intestinal inflammation. It places a heavy burden on the patients and society, and more effective therapies for the disease are needed. Cannabinoids (CBs) are reported to be beneficial in alleviating inflammation in some diseases. It is worthy to evaluate the anti-inflammatory efficacy and related mechanism of action of potential CB candidates in UC, which may also offer hints for new directions in UC therapy. In this study, the authors investigated the anti-inflammatory effect and the possible mechanism of an agonist of cannabinoid receptors, WIN55-212-2 (WIN55), in mice with dextran sulfate sodium (DSS)-induced experimental colitis, in order to supply experimental evidence for its possible use in clinic.
Excessive activation of p38 mitogen-activated protein kinases (p38MAPKs) can cause recurrent attack of intestinal inflammation and facilitate forming of vicious cycles. Inhibition of p38MAPKs by p38 inhibitors is unacceptable in clinical practice due to obvious side effects. WIN55 has been reported as beneficial for treating some gastrointestinal inflammatory disorders, but the effective mechanisms are far from being elucidated. This study explored whether the anti-inflammatory effect of WIN55 is related with p38MAPKs inhibition.
This study provides experimental evidence that WIN55 or SB203580 (SB), an inhibitor of p38, treatment alone or together improved the pathological changes in mice with DSS-induced colitis, decreased the myeloperoxidase (MPO) activity in colon and the levels of TNF-α and IL-6 in plasma. The enhanced expression of claudin-1 and the inhibited expression of p38 phosphorylated form (p-p38) in colon tissues were found in the WIN55-treated group. In addition, the expression of CB1 and CB2 receptors was enhanced in the colon after the induction of DSS colitis but reduced when p38MAPK was inhibited. These results confirmed the anti-inflammatory effect and protective role of WIN55 in mice with experimental colitis, and revealed that this agent exercises its action at least partially by inhibiting p38MAPK. Furthermore, the results showed that SB affected the expression of CB1 and CB2 receptors in the mouse colon, suggesting a close linkage and cross-talk between the p38MAPK signaling pathway and the endogenous cannabinoid system (ECS).
The cannabinoid agonist WIN55 showed greater anti-inflammatory and organ protective efficacy for experimental colitis, which offers hints for new directions in UC therapy. And, the experimental results suggest that the p38MAPK signaling pathway may be a possible therapeutic target in UC.
WIN55, as a typical cannabinoid receptor agonist, can activate the ECS via acting on the Gi/o coupled membrane receptors: CB1 and CB2, the two main typical cannabinoid receptors.
The authors studied an animal model of DSS-induced colitis and the anti-inflammatory effects of WIN55-212-2 on inflammation. The manuscript is well written and the experiments thoughtfully designed. The results reveal the anti-inflammatory effect and protective role of WIN55 on mice with experimental colitis, and that this agent exerts its action at least partially by inhibiting p38MAPK.
| 1. | Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 751] [Cited by in RCA: 1135] [Article Influence: 94.6] [Reference Citation Analysis (32)] |
| 2. | Gómez-Gómez GJ, Masedo Á, Yela C, Martínez-Montiel Mdel P, Casís B. Current stage in inflammatory bowel disease: What is next? World J Gastroenterol. 2015;21:11282-11303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Feng YJ, Li YY. The role of p38 mitogen-activated protein kinase in the pathogenesis of inflammatory bowel disease. J Dig Dis. 2011;12:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1545] [Cited by in RCA: 2086] [Article Influence: 173.8] [Reference Citation Analysis (1)] |
| 5. | Docena G, Rovedatti L, Kruidenier L, Fanning A, Leakey NA, Knowles CH, Lee K, Shanahan F, Nally K, McLean PG. Down-regulation of p38 mitogen-activated protein kinase activation and proinflammatory cytokine production by mitogen-activated protein kinase inhibitors in inflammatory bowel disease. Clin Exp Immunol. 2010;162:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Dambach DM. Potential adverse effects associated with inhibition of p38alpha/beta MAP kinases. Curr Top Med Chem. 2005;5:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | De Petrocellis L, Di Marzo V. An introduction to the endocannabinoid system: from the early to the latest concepts. Best Pract Res Clin Endocrinol Metab. 2009;23:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | González C, Herradón E, Abalo R, Vera G, Pérez-Nievas BG, Leza JC, Martín MI, López-Miranda V. Cannabinoid/agonist WIN 55,212-2 reduces cardiac ischaemia-reperfusion injury in Zucker diabetic fatty rats: role of CB2 receptors and iNOS/eNOS. Diabetes Metab Res Rev. 2011;27:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Li YY, Li YN, Ni JB, Chen CJ, Lv S, Chai SY, Wu RH, Yüce B, Storr M. Involvement of cannabinoid-1 and cannabinoid-2 receptors in septic ileus. Neurogastroenterol Motil. 2010;22:350-e88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Izzo AA, Sharkey KA. Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Ther. 2010;126:21-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 326] [Article Influence: 20.4] [Reference Citation Analysis (1)] |
| 11. | Di Sabatino A, Battista N, Biancheri P, Rapino C, Rovedatti L, Astarita G, Vanoli A, Dainese E, Guerci M, Piomelli D. The endogenous cannabinoid system in the gut of patients with inflammatory bowel disease. Mucosal Immunol. 2011;4:574-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Larsson MH, Rapp L, Lindström E. Effect of DSS-induced colitis on visceral sensitivity to colorectal distension in mice. Neurogastroenterol Motil. 2006;18:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Klompus M, Ho W, Sharkey KA, McKay DM. Antisecretory effects of neuropeptide Y in the mouse colon are region-specific and are lost in DSS-induced colitis. Regul Pept. 2010;165:138-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Jurjus AR, Khoury NN, Reimund JM. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods. 2004;50:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 203] [Article Influence: 9.2] [Reference Citation Analysis (2)] |
| 15. | Petrella C, Agostini S, Alema’ GS, Casolini P, Carpino F, Giuli C, Improta G, Linari G, Petrozza V, Broccardo M. Cannabinoid agonist WIN55,212 in vitro inhibits interleukin-6 (IL-6) and monocyte chemo-attraactant protein-1 (MCP-1) release by rat pancreatic acini and in vivo induces dual effects on the course of acute pancreatitis. Neurogastroenterol Motil. 2010;22:1248-1256, e323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Hollenbach E, Neumann M, Vieth M, Roessner A, Malfertheiner P, Naumann M. Inhibition of p38 MAP kinase- and RICK/NF-kappaB-signaling suppresses inflammatory bowel disease. FASEB J. 2004;18:1550-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 190] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Kimball ES, Wallace NH, Schneider CR, D’Andrea MR, Hornby PJ. Vanilloid receptor 1 antagonists attenuate disease severity in dextran sulphate sodium-induced colitis in mice. Neurogastroenterol Motil. 2004;16:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | D’Argenio G, Valenti M, Scaglione G, Cosenza V, Sorrentini I, Di Marzo V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006;20:568-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Li YY, Ochs S, Gao ZR, Malo A, Chen CJ, Lv S, Gallmeier E, Göke B, Schäfer C. Regulation of HSP60 and the role of MK2 in a new model of severe experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G981-G989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Kimball ES, Wallace NH, Schneider CR, D’Andrea MR, Hornby PJ. Small intestinal cannabinoid receptor changes following a single colonic insult with oil of mustard in mice. Front Pharmacol. 2010;1:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Li K, Feng JY, Li YY, Yuece B, Lin XH, Yu LY, Li YN, Feng YJ, Storr M. Anti-inflammatory role of cannabidiol and O-1602 in cerulein-induced acute pancreatitis in mice. Pancreas. 2013;42:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Broom OJ, Widjaya B, Troelsen J, Olsen J, Nielsen OH. Mitogen activated protein kinases: a role in inflammatory bowel disease? Clin Exp Immunol. 2009;158:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Iraha A, Chinen H, Hokama A, Yonashiro T, Kinjo T, Kishimoto K, Nakamoto M, Hirata T, Kinjo N, Higa F. Fucoidan enhances intestinal barrier function by upregulating the expression of claudin-1. World J Gastroenterol. 2013;19:5500-5507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Chatterjee N S- Editor: Yu J L- Editor: Filipodia E- Editor: Wang CH