Published online Nov 14, 2016. doi: 10.3748/wjg.v22.i42.9411
Peer-review started: June 16, 2016
First decision: July 12, 2016
Revised: July 18, 2016
Accepted: August 19, 2016
Article in press: August 19, 2016
Published online: November 14, 2016
Processing time: 149 Days and 17.8 Hours
To evaluate the usefulness of different parameters to differentiate Crohn’s disease (CD) from primary intestinal lymphoma (PIL).
The medical records of 85 patients with CD and 56 patients with PIL were reviewed retrospectively. Demographic, clinical, laboratory, endoscopic, and computed tomographic enterography (CTE) parameters were collected. The univariate value of each parameter was analyzed. A differentiation model was established by pooling all the valuable parameters. Diagnostic efficacy was analyzed, and a receiver operating characteristic (ROC) curve was plotted.
The demographic and clinical parameters that showed significant values for differentiating CD from PIL included age of onset, symptom duration, presence of diarrhea, abdominal mass, and perianal lesions (P < 0.05). Elevated lactate dehydrogenase and serum β2-microglobulin levels suggested a PIL diagnosis (P < 0.05). The endoscopic parameters that showed significant values for differentiating CD from PIL included multiple-site lesions, longitudinal ulcer, irregular ulcer, and intraluminal proliferative mass (P < 0.05). The CTE parameters that were useful in the identification of the two conditions included involvement of ≤ 3 segments, circular thickening of the bowel wall, wall thickness > 8 mm, aneurysmal dilation, stricture with proximal dilation, “comb sign”, mass showing the “sandwich sign”, and intussusceptions (P < 0.05). The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of the differentiation model were 91.8%, 96.4%, 93.6%, 97.5%, and 88.5%, respectively. The cutoff value was 0.5. The area under the ROC curve was 0.989.
The differentiation model that integrated the various parameters together may yield a high diagnostic efficacy in the differential diagnosis between CD and PIL.
Core tip: Crohn’s disease and primary intestinal lymphoma (PIL) have overlapping clinical manifestations. Misdiagnosis of PIL would lead to disastrous outcomes in patients. Consequently, the differential diagnosis between these two conditions has perplexed clinical practitioners for decades. In this article, we evaluated the usefulness of different parameters, including clinical manifestations, laboratory tests, endoscopic features, and computed tomographic enterographic characteristics for differentiating these two conditions and established an objective differentiation model that would yield a high diagnostic efficacy in order to avoid misdiagnosis of PIL. This is a first study which focuses on the differential diagnosis of these two diseases.
- Citation: Zhang TY, Lin Y, Fan R, Hu SR, Cheng MM, Zhang MC, Hong LW, Zhou XL, Wang ZT, Zhong J. Potential model for differential diagnosis between Crohn's disease and primary intestinal lymphoma. World J Gastroenterol 2016; 22(42): 9411-9418
- URL: https://www.wjgnet.com/1007-9327/full/v22/i42/9411.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i42.9411
Crohn’s disease (CD) is a chronic inflammatory granulomatous disorder that can affect any segment of the gastrointestinal (GI) tract, especially the terminal ileum and ileocecal region. The incidence of CD in China is increasing in recent years[1]. However, the incidence of noncaseating granuloma as a gold standard criterion for the diagnosis of CD is quite low in terms of pathological findings, especially those from biopsy[2]. Moreover, the clinical manifestations of CD and other GI diseases in terms of symptoms, and endoscopic and radiological findings overlap, making the diagnosis of CD an even more complicated issue[3].
The estimated incidence of extranodal lymphomas in the GI tract is approximately 5%-20%[4]. On the other hand, primary GI lymphomas only account for 1%-4% of all GI cancers[5]. In the past decade, the incidence of primary GI lymphomas has been increasing worldwide[6]. The so-called primary intestinal lymphoma (PIL) includes about 30% of primary GI lymphomas that occur in the small intestine, ileocecal region, and colorectum. Derived from submucosal lymphoid tissue, PILs were mostly classified as a subtype of non-Hodgkin’s lymphoma. The clinical features of PIL lack specificity, and definitive diagnosis often relies on histological confirmation. However, in clinical practice, qualified specimens are sometimes difficult to acquire through endoscopy due to the small size and superficial nature of PILs. Furthermore, for some lesions confined to the deep small intestine, accurate localization and diagnosis without surgery are challenging[7]. Consequently, the preoperative diagnostic rate of PIL is low (30.5% as estimated)[8].
Therefore, it is important to establish a correct differential diagnosis between CD and PIL. One concern is that PIL has a malignant potential to some extent, and a delayed diagnosis may lead to lethal outcomes. However, these two conditions have overlapping characteristics in terms of symptoms, and laboratory, endoscopic, and radiological findings. An inconvenient truth is that the risk of misdiagnosis could not be neglected in some intricate cases[9,10].
In the past decade, the advent of new technology such as double-balloon enteroscopy (DBE) and CTE provided an unprecedented opportunity for us to have better knowledge and visualization of small bowel lesions, and the bowel wall and extralumen. DBE and CTE, when properly performed and accurately interpreted, are helpful in the diagnosis and management of small bowel disease and act as a good complement to other examinations[11-13]. Undoubtedly, they would play an important role in the preoperative differential diagnosis between CD and PIL.
In recent years, we have done much work in the differential diagnosis between CD and PIL. In this study, we retrospectively enrolled patients with CD and PIL treated in our hospital and evaluated the diagnostic values of clinical, laboratory, endoscopic, and CTE parameters.
A retrospective study of a single center was designed. The medical records of inpatients with CD and PIL treated from August 1, 2005, to March 31, 2016 in Ruijin Hospital, Shanghai, China were reviewed. None of the cases was complicated with both CD and intestinal lymphoma or intestinal lymphoma in preexisting CD. Patients with CD were included in our final cohort if they met the following criteria: (1) newly diagnosed with CD with lower GI tract involvement; (2) aged >18 years; (3) with a definite diagnosis for at least 1 year; (4) had a Montreal classification of L1, L2, or L3 (with an involvement of the ileum, colon, or both)[14]; (5) underwent endoscopy and CTE at least once during hospitalization; and (6) had an exclusion of other concomitant GI diseases. Diagnosis of PIL was based on Dawson standards as follows[15]: (1) no enlargement of the peripheral or mediastinal lymph nodes; (2) normal white blood cell count; (3) predominance of alimentary tract lesions with only regional lymph node involvement; and (4) no involvement of the liver and spleen. Patients with PIL were included in our final cohort if they met the following criteria: (1) newly diagnosed as having PIL; (2) aged >18 years; (3) had a definitive histological diagnosis through endoscopy or surgery; (4) underwent endoscopy and CTE for at least once during hospitalization; and (5) had an exclusion of other concomitant GI diseases.
All the data of the enrolled patients with CD and PIL were reviewed. Parameters regarding demographic information, clinical manifestations, laboratory data, endoscopic feature, and CTE characteristics were collected prior to treatment.
The demographic parameters included sex, age of onset, height, and weight. Clinical manifestations were symptom duration, abdominal pain, diarrhea, abdominal distension, nausea, hematochezia, fever, weight loss, abdominal mass, perianal lesions, history of GI surgery, perforation, and extraintestinal manifestations. The laboratory data documented were as follows: hemoglobin (Hb) level, hematocrit (Hct) level, platelet (Plt) count, albumin (Alb) level, elevated erythrocyte sedimentation rate (ESR), elevated C-reactive protein (CRP) level, elevated lactate dehydrogenase (LDH) level, and elevated serum β2-microglobulin (β2-MG) level.
DBE was performed if the lesion was confined to the small intestine, while colonoscopy was performed for patients with only colonic involvement. Endoscopic features included multiple-site lesions, pseudo-polyp formation, aphthoid ulcer, longitudinal ulcer, irregular ulcer, intraluminal proliferative mass, bowel stricture, and anorectal involvement.
All of our enrolled patients had undergone CTE at least once and was evaluated by an experienced radiologist independently. CTE characteristics mainly included involvement of ≤ 3 segments, circular thickness of the bowel wall, wall thickness of > 8 mm, target sign, enhancement after a contrast-enhanced scan, aneurysmal dilation, stricture with proximal dilation, abscess, phlegmon, ascites, “comb sign”, enlargement of the abdominal lymph nodes, enhanced density of the peri-intestinal fat, mass showing the “sandwich sign”, and intussusceptions.
SPSS 19.0 was used for the data analyses and screening for potential valuable parameters for differential diagnosis between CD and PIL. Continuous variables were expressed as mean ± SD, and a comparison was performed by using the Student t test if the data had a normal distribution. Median values (upper and lower quartiles) were calculated, and the Wilcoxon rank sum test was used to analyze the data that did not have a normal distribution. Binary categorical variables were expressed as frequency and percentage values, while comparisons were made using the Chi-square or Fisher’s exact test. A probability (P) value of < 0.05 was considered to be statistically significant. Then, continuous variables were converted to binary categorical variables based on the Youden index. All of the parameters with significant differences in differentiating diagnosis were graded, with CD1 and PIL-1. A differentiation model was created by adding all of the scores of the valuable parameters. The total score was calculated, and a receiver operating characteristic (ROC) curve was plotted. The cutoff value was obtained from the Youden index. Sensitivity, specificity, accuracy, PPV, and NPV were calculated to evaluate the diagnostic efficacy of the model.
The demographic, clinical, and laboratory features of CD and PIL are listed in Table 1. No significant difference was found with respect to the patients’ sex, height, and weight. The CD patients were significantly younger than the PIL patients in terms of age of onset (32.0 ± 9.9 years vs 52.8 ± 16.3 years, P < 0.05). The CD patients had a longer symptom duration before definite diagnosis than the PIL patients [median time, 16 (11-19) mo vs 2 (1-6) mo, P < 0.05]. Based on the Youden index, age of onset of < 40 years was graded 1, while symptom duration of < 12.5 mo was graded 1. For clinical manifestations, the incidence of diarrhea and perianal lesions in CD was significantly higher than that in PIL (P < 0.05). In contrast, the incidence of an abdominal mass in PIL was significantly higher than that in CD (P < 0.05). For other clinical parameters, including abdominal pain, abdominal distension, nausea, hematochezia, fever, weight loss, history of GI surgery, perforation, and extraintestinal manifestations, no significant difference was found between these two conditions. As shown by the laboratory data, the mean levels of hemoglobin, hematocrit, platelet, and albumin had no significant differences between CD and PIL. Furthermore, the levels of serum inflammatory markers such as ESR and CRP tended to elevate in more CD patients than PIL patients, but the differences were not significant. LDH level was found to be elevated in the PIL patients (8/56), much higher than that in the CD patients (2/85; P < 0.05). Moreover, we found that 21.4% (12/56) of the patients with PIL had an elevated β2-MG level, while none of the CD patients had elevated serum β2-MG levels.
| Parameters | CD | PIL | P value | Score |
| n = 85 | n = 56 | |||
| Gender (male/female) | 48/37 | 32/24 | 0.937 | N/A |
| Age of onset | 32.0 ± 9.9 | 52.8 ± 16.3 | < 0.001 | 1 |
| Height (cm) | 166.1 ± 6.6 | 164.6 ± 7.3 | 0.189 | N/A |
| Weight (kg) | 59.3 ± 9.4 | 57.7 ± 10.2 | 0.346 | N/A |
| Symptom duration (mo) | 16 (11-19) | 2 (1-6) | < 0.001 | -1 |
| Abdominal pain | 64 (75.3) | 39 (69.6) | 0.459 | N/A |
| Diarrhea | 60 (70.6) | 15 (26.8) | < 0.001 | 1 |
| Abdominal distension | 29 (34.1) | 21 (37.5) | 0.681 | N/A |
| Nausea | 12 (14.1) | 13 (23.2) | 0.166 | N/A |
| Hematochezia | 22 (25.9) | 11 (19.6) | 0.392 | N/A |
| Fever | 13 (15.3) | 15 (26.8) | 0.094 | N/A |
| Weight loss | 42 (49.4) | 32 (57.1) | 0.368 | N/A |
| Abdominal mass | 5 (5.9) | 9 (16.1) | 0.048 | -1 |
| Perianal lesions | 37 (43.5) | 2 (3.6) | < 0.001 | 1 |
| History of GI surgery | 12 (14.1) | 4 (7.1) | 0.189 | N/A |
| Perforation | 4 (4.7) | 5 (8.9) | 0.515 | N/A |
| Extraintestinal manifestation | 6 (7.1) | 2 (3.6) | 0.614 | N/A |
| Hemoglobin(g/L) | 106.9 ± 17.8 | 109.7 ± 22.4 | 0.408 | N/A |
| Hematocrit | 35.1 ± 3.9 | 33.9 ± 5.7 | 0.127 | N/A |
| Platelet (1 × 109/L) | 263.7 ± 96.2 | 261.2 ± 116.5 | 0.890 | N/A |
| Albumin (g/L) | 29.7 ± 6.1 | 31.3 ± 7.0 | 0.151 | N/A |
| Elevated ESR | 53 (62.4) | 28 (50.0) | 0.147 | N/A |
| Elevated CRP | 59 (69.4) | 31 (55.4) | 0.089 | N/A |
| Elevated lactate dehydrogenase | 2 (2.4) | 8 (14.3) | 0.018 | -1 |
| Elevated serum β2-microglobulin | 0 (0.0) | 12 (21.4) | < 0.001 | -1 |
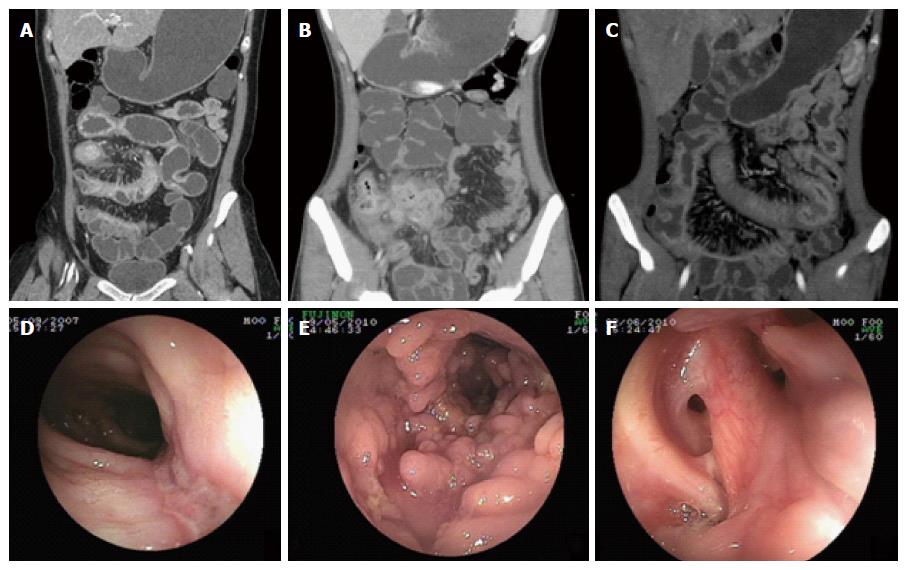
The endoscopic parameters of CD and PIL are summarized in Table 2. Lesions of CD tended to involve multiple sites compared with those of PIL (P < 0.05). The morphology of ulcers under endoscopy differed between CD and PIL patients. Longitudinal ulcers (Figure 1D) were more apparent in the CD patients (P < 0.05), whereas irregular ulcers (Figure 2F) were more common in the PIL patients (P < 0.05). We also found that intraluminal proliferative mass (Figure 2E) was more frequent in the PIL patients than in the CD patients (P < 0.05). Other parameters, including pseudo-polyp formation (Figure 1E), aphthoid ulcer, bowel stricture, and anorectal involvement (Figure 1F), showed no significant differences between the two diseases.
| Parameters | CD | PIL | P value | Score |
| n = 85 | n = 56 | |||
| Multiple-site lesions | 73 (85.9) | 19 (33.9) | < 0.001 | 1 |
| Pseudo-polyp formation | 26 (30.6) | 10 (17.9) | 0.090 | N/A |
| Aphthoid ulcer | 37 (43.5) | 21 (37.5) | 0.477 | N/A |
| Longitudinal ulcer | 69 (81.2) | 5 (8.9) | < 0.001 | 1 |
| Irregular ulcer | 31 (36.5) | 32 (57.1) | 0.016 | -1 |
| Intraluminal proliferative mass | 11 (12.9) | 31 (55.4) | < 0.001 | -1 |
| Bowel stricture | 27 (31.8) | 15 (26.8) | 0.527 | N/A |
| Anorectal involvement | 13 (15.3) | 4 (7.1) | 0.146 | N/A |
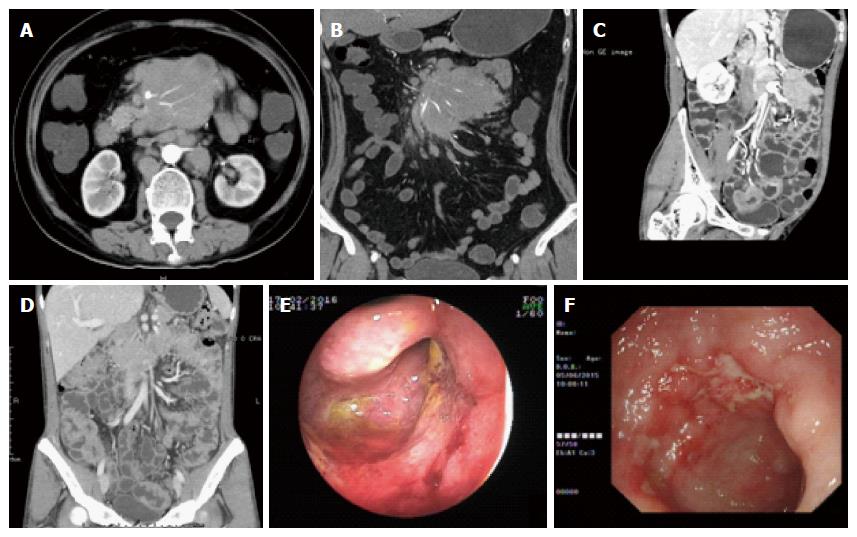
For the CTE parameters listed in Table 3, we found that involvement of ≤ 3 segments, circular thickening of the bowel wall (Figure 2D), wall thickness > 8 mm, and aneurysmal dilation (Figure 2C) were significantly more prevalent in the PIL patients than in the CD patients (P < 0.05). Stricture with proximal dilation (Figure 1A) in CD was more common compared with that in PIL. In terms of parenteral manifestations, the “comb sign” (stretching and densifying of the distal mesenteric artery; Figure 1C) indicated a diagnosis of CD (P < 0.05). On the other hand, mass showing the “sandwich sign” (enhanced vessel and nonenhanced fat inside the mesenteric mass; Figure 2A and B) and intussusceptions were more frequently observed in PIL than in CD (P < 0.05), suggesting that these parameters were indicative of a PIL diagnosis. No significant difference was found between these two groups in terms of target sign, abscess enhancement after a contrast-enhanced scan, phlegmon (Figure 1B), ascites, enlargement of abdominal lymph nodes, and enhanced density of peri-intestinal fat.
| Parameters | CD | PIL | P value | Score |
| n = 85 | n = 56 | |||
| Involvement of ≤ 3 segments | 31 (36.5) | 45 (80.4) | < 0.001 | -1 |
| Circular thickening of bowel wall | 33 (38.8) | 35 (62.5) | 0.006 | -1 |
| Wall thickness of > 8 mm | 21 (24.7) | 45 (80.4) | < 0.001 | -1 |
| Aneurysmal dilation | 5 (5.9) | 27 (48.2) | < 0.001 | -1 |
| Target sign | 25 (29.4) | 12 (21.4) | 0.292 | N/A |
| Enhancement after a contrast-enhanced scan | 67 (78.8) | 37 (66.1) | 0.092 | N/A |
| Stricture with proximal dilation | 19 (22.4) | 4 (7.1) | 0.017 | 1 |
| Abscess | 7 (8.2) | 2 (3.6) | 0.449 | N/A |
| Phlegmon | 8 (9.4) | 2 (3.6) | 0.324 | N/A |
| Ascites | 5 (5.9) | 7 (12.5) | 0.174 | N/A |
| “Comb sign” | 61 (71.8) | 18 (32.1) | < 0.001 | 1 |
| Enlargement of the abdominal lymph nodes | 42 (49.4) | 36 (64.3) | 0.082 | N/A |
| Enhanced density of the peri-intestinal fat | 30 (35.3) | 26 (46.4) | 0.186 | N/A |
| Mass showing the “sandwich sign” | 2 (2.4) | 9 (16.1) | < 0.001 | -1 |
| Intussusceptions | 0 (0.0) | 3 (5.4) | 0.031 | -1 |
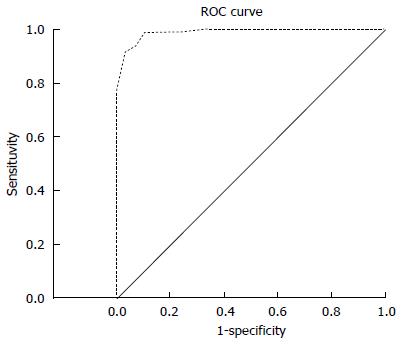
The total score was calculated by pooling all of the valuable differential parameters together. A differentiating diagnostic model was established, with high sensitivity (91.8%), specificity (96.4%), accuracy (93.6%), PPV (97.5%), and NPV (88.5%). A ROC curve was plotted (Figure 3). Based on the Youden index, a diagnostic point of 0.5 was obtained (P > 0.5, predictable diagnosis of CD; P < 0.5, diagnosis of PIL) and the area under the ROC curve was 0.989.
The incidence of CD, which was formerly considered as a Western disease, was estimated to have increased by threefold during the past two decades in China, making it a major health concern in the nation[16,17]. Differential diagnosis between CD and PIL is challenging because of the overlapping manifestations of the two conditions[18]. A correct and timely differential diagnosis is of great importance for the contradictory medications and sometimes lethal prognosis of PIL[19]. In addition, correct differential diagnosis through routine clinical examinations without surgery should be a goal worth pursuing by clinicians. To the best of our knowledge, no diagnostic algorithm has been established between CD and PIL that collated numerous parameters. In the present study, we analyzed the differential diagnostic values of demographic, clinical, endoscopic, and CTE data of CD and PIL patients. Then, using valuable parameters, we investigated a differentiation model for a more objective and easier facilitation of differential diagnosis between CD and PIL.
From among various demographic and clinical factors, in this study we found that five factors, including age of onset, symptom duration, diarrhea, abdominal mass, and perianal lesions, were most helpful in the differential diagnosis of CD and PIL. Among these factors, age of onset of < 40 years, diarrhea, and perianal lesions favored a diagnosis of CD, whereas symptom duration of < 12.5 mo and abdominal mass favored a diagnosis of PIL. For the other demographic and clinical parameters, no significant differences were found between the two groups, which further confirm that CD and PIL exhibit overlapping manifestations and symptoms. The results of our study were similar to those of Zou et al[20] and Wang et al[21].
Our study showed that CD and PIL patients displayed no significant difference in routine laboratory test results, including hemoglobin level, hematocrit level, platelet count, albumin level, elevated ESR, and elevated CRP level. On the contrary, elevated LDH and serum β2-MG levels exhibited significant differences between CD and PIL. These may be attributed to the hematologic malignant nature of PIL. The two above-mentioned laboratory parameters only require a blood sample from the patient, thus highlighting their convenience, replicability, minimal invasiveness, and high specificity in the diagnosis of PIL. Thus, in the process of differentiating CD from PIL, measurement of serum LDH and β2-MG levels should be a routine laboratory test.
Endoscopy is the first choice of clinical practitioners for detecting bowel lesions and evaluating therapeutic response. In this study, we found that CD tended to have multiple-site involvement compared with PIL. Moreover, the morphology of bowel ulcers differs between CD and PIL patients. The ulcers of CD patients were longitudinal and stretched across several intestinal folds. In contrast, the morphology of PIL ulcers was characterized by irregular ulcers. In addition, we found that intraluminal proliferative mass was more common in PIL than in CD, probably because of its malignant nature.
CTE is an emerging noninvasive technology for the diagnosis and evaluation of small bowel lesions. It offers an unparalleled tool to detect bowel wall lesions and extra-enteric complications, which is a necessary complement to endoscopy. Our study illustrated that inside the lumen, the presence of involvement of ≤ 3 segments, circular thickening of the bowel wall, wall thickness of > 8 mm, and aneurysmal dilation indicated a probable diagnosis of PIL. These are the characteristic imaging findings of PIL, which may be attributed to the proliferation of lymphoma cells without damage of normal intestinal wall cells. As a result, in spite of diffuse thickening of the bowel wall, the incidences of stricture and obstruction are relatively low. On the other hand, proliferation of lymphoma cells brings invasion to the nervous plexus of the bowel wall, leading to a decreased muscular tension of the bowel wall and aneurysmal dilation[22]. Compared with that in PIL, the chronic intestinal inflammation in CD would end up with fibrosis. Consequently, stricture with proximal dilation would occur. Regarding extra-enteric manifestations, our study showed that the “comb sign” was more likely found in the CD patients, while the mass showing the sandwich sign and intussusceptions was more likely found in the PIL patients. Our findings further proved that PIL had characteristic features on CTE which provides a new prospective that extraluminal manifestations should not be neglected in differentiating CD from PIL.
Although a series of differentiating parameters had been proposed, none of them had high sensitivity and specificity at the same time. For this reason, differentiating between these two conditions through a single parameter is difficult. Thus, we established a diagnostic model that combines all of the valuable parameters. Through later analysis, we proved that the sensitivity, specificity, accuracy, PPV, and NPV of our model were 91.8%, 96.4%, 93.6%, 97.5%, and 88.5%, respectively. Based on the Youden index, the cutoff value was 0.5 and the area under the curve was 0.989. These prove that our differentiation model produced a high diagnostic efficacy. In addition, it is easy for clinicians to apply in clinical practice. The differentiation model could help avoid a misdiagnosis between CD and PIL.
Positive positron emission tomography/computed tomography (PET/CT) plays an important role in the diagnosis and therapeutic evaluation of lymphomas[23]. However, we did not take PET/CT parameters into consideration in our differentiation model. Our concern was that the CD patients were already at an increased risk of malignancies[24]. Besides, PET/CT is too expensive. Thus, it is not suitable for all CD patients to undergo PET/CT examination. We recommend that only intricate cases with a grade of nearly 0.5 in our differentiation model should proceed to PET/CT examination. If a more accurate diagnosis can not be made, surgery should be earnestly considered.
This study has some limitations. First, this study is retrospective; thus, diseases other than CD and PIL were not included in the analysis. Consequently, clinicians could use our model to avoid misdiagnosing PIL as CD when they had excluded other diseases, which may hamper its application. In the future, a prospective study should be conducted to discriminate bowel ulcers. Second, the number of patients enrolled was limited, and our differentiation model should be further proved. We are looking forward to conducting a multicenter collaboration to enlarge the sample size and modify or further prove our differentiation model.
In conclusion, CD and PIL have overlapping features, which continuously perplex clinicians. However, some valuable parameters can be used to differentiate these two conditions, including clinical manifestations, laboratory test results, endoscopic features, and CTE characteristics. Our differential model integrated various parameters to yield a high diagnostic efficacy, which should be further proved.
Crohn’s disease (CD) and primary intestinal lymphoma (PIL) have overlapping clinical manifestations which sometimes make differential diagnosis difficult. Misdiagnosis of PIL could cause disastrous outcomes in patients. For lesions in the deep small intestine, qualified tissues for accurate diagnosis is difficult to acquire without surgery.
Some studies focused on the differential diagnosis of small bowel ulcers. However, currently, none of these studies focused on the differential diagnosis between CD and PIL without histology. Some valuable parameters have been proposed, but no scoring system has been established for differential diagnosis.
In this study, the authors evaluated the usefulness of various parameters, including clinical manifestations, laboratory test results, endoscopic features, and computed tomographic enterographic characteristics, for differentiating CD from PIL. A scoring system was established. These will help clinical practitioners to avoid misdiagnosis of these two conditions.
This scoring system would provide a more objective and convenient tool for the differential diagnosis between CD and PIL.
It is an interesting retrospective study on the clinical, laboratory, endoscopic, and CTE features of CD and PIL. The authors defined some important parameters that are important in the differential diagnosis of these two entities.
| 1. | Ooi CJ, Makharia GK, Hilmi I, Gibson PR, Fock KM, Ahuja V, Ling KL, Lim WC, Thia KT, Wei SC. Asia Pacific Consensus Statements on Crohn’s disease. Part 1: Definition, diagnosis, and epidemiology: (Asia Pacific Crohn’s Disease Consensus--Part 1). J Gastroenterol Hepatol. 2016;31:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | Mazor Y, Karban A, Nesher S, Weiss B, Leshinsky-Silver E, Levine A, Eliakim R. Granulomas in Crohn’s disease: are newly discovered genetic variants involved? J Crohns Colitis. 2010;4:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Gecse KB, Bemelman W, Kamm MA, Stoker J, Khanna R, Ng SC, Panés J, van Assche G, Liu Z, Hart A. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut. 2014;63:1381-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 296] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 4. | Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Gurney KA, Cartwright RA. Increasing incidence and descriptive epidemiology of extranodal non-Hodgkin lymphoma in parts of England and Wales. Hematol J. 2002;3:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Müller AM, Ihorst G, Mertelsmann R, Engelhardt M. Epidemiology of non-Hodgkin’s lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol. 2005;84:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 354] [Article Influence: 16.1] [Reference Citation Analysis (7)] |
| 7. | Arora N, Manipadam MT, Pulimood A, Ramakrishna BS, Chacko A, Kurian SS, Nair S. Gastrointestinal lymphomas: pattern of distribution and histological subtypes: 10 years experience in a tertiary centre in South India. Indian J Pathol Microbiol. 2011;54:712-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 8. | Chen JH, Ho CL, Chen YC, Chao TY, Kao WY. Clinicopathological analysis and prognostic factors of 11 patients with primary non-Hodgkin lymphoma of the small intestine in a single institute. Oncol Lett. 2014;8:876-880. [PubMed] |
| 9. | McCullough JE, Kim CH, Banks PM. Mantle zone lymphoma of the colon simulating diffuse inflammatory bowel disease. Role of immunohistochemistry in establishing the diagnosis. Dig Dis Sci. 1992;37:934-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Camera L, Della Noce M, Cirillo LC. [Primary ileo-cecal lymphoma mimicking Crohn’s disease. Report of a case]. Radiol Med. 1997;94:122-124. [PubMed] |
| 11. | Sun B, Rajan E, Cheng S, Shen R, Zhang C, Zhang S, Wu Y, Zhong J. Diagnostic yield and therapeutic impact of double-balloon enteroscopy in a large cohort of patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2006;101:2011-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 12. | Barlow JM, Goss BC, Hansel SL, Kolbe AB, Rackham JL, Bruining DH, Fletcher JG. CT enterography: technical and interpretive pitfalls. Abdom Imaging. 2015;40:1081-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Baker ME, Hara AK, Platt JF, Maglinte DD, Fletcher JG. CT enterography for Crohn’s disease: optimal technique and imaging issues. Abdom Imaging. 2015;40:938-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Spekhorst LM, Visschedijk MC, Alberts R, Festen EA, van der Wouden EJ, Dijkstra G, Weersma RK. Performance of the Montreal classification for inflammatory bowel diseases. World J Gastroenterol. 2014;20:15374-15381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 15. | Dawson IM, Cornes JS, Morson BC. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg. 1961;49:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 474] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 16. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3599] [Article Influence: 257.1] [Reference Citation Analysis (6)] |
| 17. | Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145:158-165.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 615] [Article Influence: 47.3] [Reference Citation Analysis (3)] |
| 18. | Liu YY, Chen MK, Cao Z, Liu SZ, Ding BJ. Differential diagnosis of intestinal tuberculosis from Crohn’s disease and primary intestinal lymphoma in China. Saudi J Gastroenterol. 2014;20:241-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Peng JC, Zhong L, Ran ZH. Primary lymphomas in the gastrointestinal tract. J Dig Dis. 2015;16:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Zou N, Lv H, Qian JM. The clinical differences and similarities between Crohn’s disease and primary intestinal lymphoma. Chin J Dig. 2006;26:364-367. [DOI] [Full Text] |
| 21. | Wang CD, Zheng MY. Analysis of Clinical features between Crohn’s disease and primary intestinal lymphoma. Chin J Dig. 2011;31:406-408. [DOI] [Full Text] |
| 22. | Ghai S, Pattison J, Ghai S, O’Malley ME, Khalili K, Stephens M. Primary gastrointestinal lymphoma: spectrum of imaging findings with pathologic correlation. Radiographics. 2007;27:1371-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Tari A, Asaoku H, Kunihiro M, Tanaka S, Yoshino T. Usefulness of positron emission tomography in primary intestinal follicular lymphoma. World J Gastroenterol. 2013;19:1992-1996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Benjelloun el B, Abkari M, Ousadden A, Ait Taleb K. Squamous cell carcinoma associated anal fistulas in Crohn’s disease unique case report with literature review. J Crohns Colitis. 2013;7:e232-e235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Konturek PC, Meshikhes AN S- Editor: Qi Y L- Editor: Ma JY E- Editor: Wang CH