Published online Nov 14, 2016. doi: 10.3748/wjg.v22.i42.9400
Peer-review started: July 18, 2016
First decision: July 29, 2016
Revised: September 10, 2016
Accepted: October 10, 2016
Article in press: October 10, 2016
Published online: November 14, 2016
Processing time: 118 Days and 19.5 Hours
To identify the frequency, clinicopathological risk factors, and prognostic significance of lymphovascular invasion (LVI) in endoscopically resected small rectal neuroendocrine tumors (NETs).
Between June 2005 and December 2015, 104 cases of endoscopically resected small (≤ 1 cm) rectal NET specimens at Hallym University Sacred Heart Hospital in Korea were retrospectively evaluated. We compared the detected rate of LVI in small rectal NET specimens by two methods: hematoxylin and eosin (H&E) and ancillary immunohistochemical staining (D2-40 and Elastica van Gieson); in addition, LVI detection rate difference between endoscopic procedures were also evaluated. Patient characteristics, prognosis and endoscopic resection results were reviewed by medical charts.
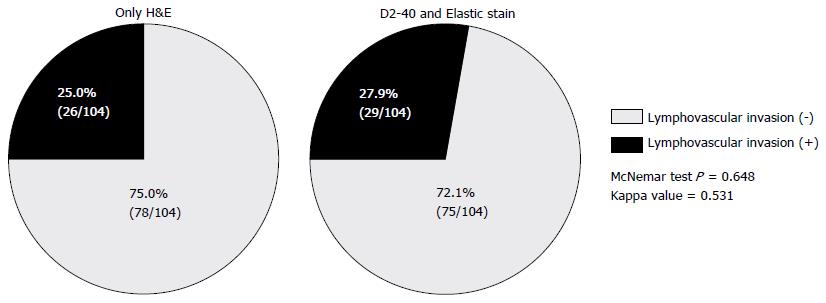
We observed LVI rates of 25.0% and 27.9% through H&E and ancillary immunohistochemical staining. The concordance rate between H&E and ancillary studies was 81.7% for detection of LVI, which showed statistically strong agreement between two methods (κ = 0.531, P < 0.001). Two endoscopic methods were studied, including endoscopic submucosal resection with a ligation device and endoscopic submucosal dissection, and no statistically significant difference in the LVI detection rate was detected between the two (26.3% and 26.8%, P = 0.955). LVI was associated with large tumor size (> 5 mm, P = 0.007), tumor grade 2 (P = 0.006). Among those factors, tumor grade 2 was the only independent predictive factor for the presence of LVI (HR = 4.195, 95%CI: 1.321-12.692, P = 0.015). No recurrence was observed over 28.8 mo regardless of the presence of LVI.
LVI may be present in a high percentage of small rectal NETs, which may not be associated with short-term prognosis.
Core tip: The majority of rectal neuroendocrine tumors (NETs) are small (66%-80% are ≤ 1 cm in diameter) and endoscopic resection techniques have shown successful outcomes. However, lymphovascular invasions, a well-established risk factor for lymph node metastasis, are often found at endoscopically resected specimens and there are no definite guidelines about these cases. Therefore, we investigate the frequency and prognostic significance of lymphovascular invasion (LVI) in small endoscopically resected rectal NETs. We found that LVI may be present in a high percentage of small rectal NETs by two histologic methods; hematoxylin and eosin staining and ancillary immunohistochemical staining (D2-40 and Elastica van Gieson). On the other hands, LVI was not associated with lymph node metastasis or recurrence in small rectal NETs (≤ 1 cm) during a 3 year-follow up period. Although our follow-up period was short, but I'm confident in our studies will be the cornerstone of future researches about significance of LVI in small rectal NETs.
- Citation: Kwon MJ, Kang HS, Soh JS, Lim H, Kim JH, Park CK, Park HR, Nam ES. Lymphovascular invasion in more than one-quarter of small rectal neuroendocrine tumors. World J Gastroenterol 2016; 22(42): 9400-9410
- URL: https://www.wjgnet.com/1007-9327/full/v22/i42/9400.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i42.9400
Rectal neuroendocrine tumors (NETs) arise from enterochromaffin endocrine cells situated within intestinal crypts of Lieberkühn[1], comprising 25% of gastrointestinal NETs with a 5-year overall survival of 88%[2,3]. Despite the comparatively favorable prognosis, rectal NETs are rarely aggressive and distant metastasis is of clinical concern for treating rectal NETs. The clinical or histopathologic indicators for metastasis have been demonstrated including tumor size, muscularis propria invasion, lymphovascular invasion (LVI), mitotic rate, and Ki-67 labeling index in surgically resected specimens[4-11]. Currently, because the majority of rectal NETs are small (66%-80% are ≤ 10 mm in diameter) and found incidentally during screening colonoscopy[12-16], endoscopic resection techniques including endoscopic submucosal resection with ligation (ESMR-L) and endoscopic submucosal dissection (ESD) are applied to treat rectal NETs. ESMR-L and ESD have shown better outcomes in terms of complete resection of rectal NETs when compared with conventional endoscopic mucosal resection[17-21]. However, it is not known which procedure is more feasible for small rectal NETs or for which clinicopathological factors different results will be achieved. Rectal NETs ≤ 10 mm in diameter, confined to the mucosal or submucosal layer, and without LVI can be treated with endoscopic resection[10,18,22-29]. Unexpectedly, lymph node metastasis occurs in 3% of tumors with a diameter of ≤ 10 mm[30]. Given that LVI, as shown by the presence of tumor cells in blood vessels and/or lymphatic channels, is a high risk factor for distant or nodal metastasis and is a poor prognostic factor, LVI should be histologically assessed in specimens obtained by endoscopic resection.
Histologically, rectal NETs are composed of cells with a mixed growth pattern with trabeculae or acini of uniform cells separated by delicate and vascular stroma, which allows for easy recognition. However, marked tumor retraction from the surrounding fibrotic stroma may incorrectly give the false impression that LVI is present[1]. Although this retraction artifact should be accurately histologically distinguished from true lymphatic or vascular invasion, identification of true LVI is not always straightforward on routine hematoxylin and eosin (H&E)-stained slides. Recently, ancillary immunohistochemical staining [D2-40, CD34, CD31, and Elastica van Gieson (EVG)] in addition to H&E histologic examination has been used to evaluate LVI in rectal NETs[31-33]. Through these methods, the high frequency of LVI has been noted in endoscopically resected small rectal NETs[32,33]. However, whether the increased detection rate between H&E and ancillary studies is statistically significant has not been determined.
In the present study, we used 2 methods, H&E and ancillary immunohistochemical staining (D2-40 and EVG), to compare the detected rate of LVI in 104 endoscopically resected small rectal NET specimens and to determine the clinical impact of LVI. In addition, we evaluated differences in the LVI detection rate between endoscopic procedures and prognosis of small rectal NETs with LVI.
Between June 2005 and December 2015, 138 patients with 139 tumors were diagnosed with rectal NET at Hallym University Sacred Heart Hospital in Anyang, Korea. Endoscopic gross tumor size ≤ 10 mm and absence of lymph node involvement or distant metastasis on the abdominal CT were the indications for endoscopic resection. The study inclusion criteria for small rectal NETs were as follows: (1) a tumor ≤ 10 mm, in diameter histologically; (2) a tumor within 15 cm of the anus; (3) no metastasis to lymph nodes or distal organs detected on abdominal computed tomography; and (4) a tumor resected in our institution for the first time. Therefore, the following cases were excluded from this analysis: 2 patients who underwent radical surgical excision with lymph node dissection owing to large tumor (3 cm and 5 cm), 4 who underwent transanal resection based on the decision of the outpatient clinic surgeon regardless of size, 7 who underwent additional transanal resection after incomplete endoscopic resection at other clinics, 12 who did not undergo additional treatment after diagnosis, 4 who were treated at other clinics, 4 who could not be evaluated for LVI owing to an insufficient specimen, and 2 with endoscopically resected tumors exceeding 1 cm (1.2 cm and 1.7 cm). As a result, 103 patients with 104 rectal NETs were included in this study; the related medical records were reviewed retrospectively. This study was conducted with the approval of the ethics committee of Hallym University Sacred Heart Hospital in Anyang, Korea. The study was carried out in accordance with the recommendations of the Declaration of Helsinki.
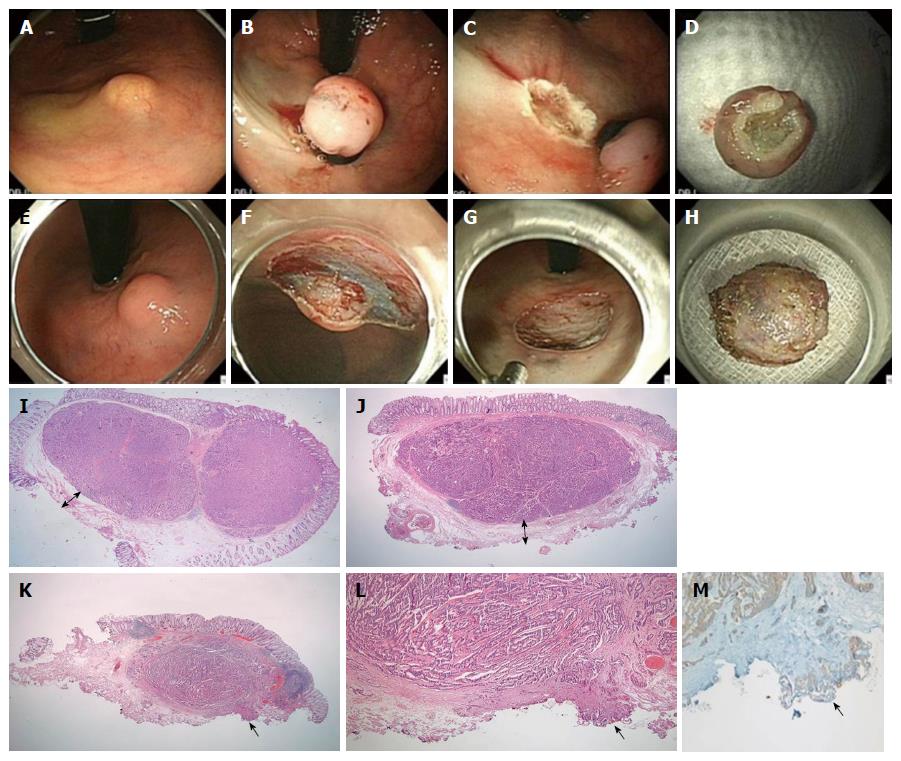
Three techniques were used with a single-channel scope (GIF-H260, Olympus Medical Systems Corp.) and an electrosurgical unit (ERBE VIO 300 D, ERBE Elektromedizin GmbH) after lifting the tumor with a submucosal injection of hypertonic saline solution mixed with a small amount of indigo-carmine and diluted epinephrine (1:10000). These included (1) endoscopic mucosal resection (EMR: conventional snare polypectomy); (2) ESMR-L, Figure 1A-D: Tumor was aspirated into ligator device and followed by deployment of the elastic band; Conventional snare polypectomy done below the band; and (3) endoscopic submucosal resection (ESD; Figure 1E-H): after mucosal incision along outer border of the tumor; submucosal dissection was performed below the tumor with Dual Knife (Electrosurgical Knife ; Olympus).
The 104 endoscopically resected cases were serially sectioned and entirely embedded for histological evaluation. H&E-stained slides from all cases were reviewed by 2 pathologists (MJK and ESN) using a multi-headed microscope. Histological evaluation including tumor size, depth of invasion, lymphatic or vascular invasion, resection margin status, mitotic count, and tumor grade was re-performed using the H&E-stained slides from the time of initial diagnosis. The immunohistochemical (Ki-67, D2-40) and histochemical (EVG) staining and re-evaluation were performed in this study. The pathological grading system of the World Health Organization 2010 criteria for tumors of the digestive system was used for classification of rectal NETs[34]. At least 500 tumor cells were counted to determine the percentage of cells that were positive for Ki-67. Mitotic rates on H&E stain were counted in 50 high power fields (HPFs) (40 × objective, 10 × eyepiece with a field diameter of 0.55 mm and an area of 0.237 mm2; Olympus microscope BX43, Tokyo, Japan), and the mean mitotic count was calculated as the number of mitoses/10 HPFs[35]. The tumors were classified into G1 (a mitotic count of less than 2 per 10 high-power fields and/or < 3% Ki-67) and G2 (a mitotic count of 2-20 per 10 high-power fields and/or 3%-20% Ki-67) according to the WHO 2010 classification and the North American Neuroendocrine Tumor Society guidelines[36]. The resection margin was examined microscopically and its status determined on the basis of the general criteria for cancer involvement in a resection margin (Figure 1I-M). The completeness of resection was classified according to the extension of tumor cells into the resection margin: (1) complete (R0) resection, in which the lateral and vertical resection margins were free of tumor; (2) microscopically incomplete (R1) resection, in which the tumor extended into the lateral or vertical resection margin; and (3) macroscopically incomplete (R2) resection, in which the tumor could not be completely resected according to its endoscopic aspects. The distance between the tumor deepest margin and the endoscopic vertical resection margin was also measured and defined as the “safety resection margin” (Figure 1I and J). The involvement of tumor cells in the resection margin was also confirmed by positive synaptophysin to rule out a squeezing artifact of fibro-connective tissue.
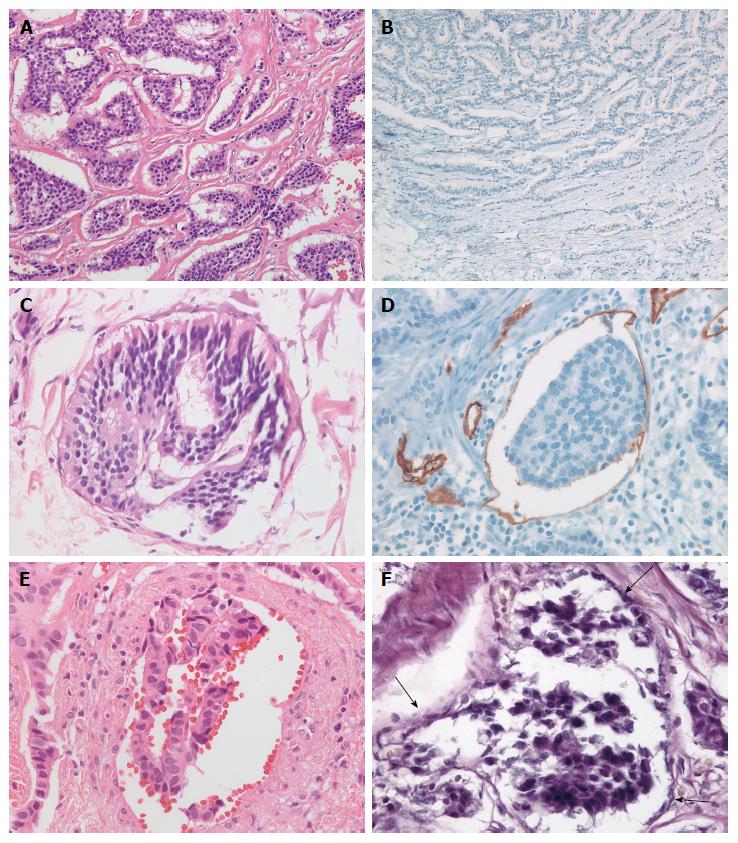
D2-40-stained slides were assessed for lymphatic invasion. A tumor in which a lymphatic vessel showed positive staining of endothelium for D2-40 and surrounded the tumor cells was diagnosed as positive for lymphatic invasion[37]. Venous invasion in H&E sections was defined as a tumor deposit in a space surrounded by a rim of smooth muscle and/or containing red blood corpuscles. Venous invasion in the EVG-stained sections was defined as tumor cells observed in a vein with EVG-stained elastic lamina[38]. Ancillary staining methods are shown in Figure 2.
Immunohistochemical staining was performed on 4-μm-thick formalin-fixed, paraffin-embedded tumor tissue sections using the BenchMark XT automated tissue staining system (Ventana Medical Systems, Inc., Tucson, AZ, United States) according to the manufacturer’s instructions, as described previously. The primary antibodies used were D2-40 (1:100; Dako, Glostrup, Denmark), Ki-67 (1:250, clone MIB-1, Dako), CD31 (1:400, JC/70A, Thermofisher), and synaptophysin (1:2, SP11, Ventana Medical Systems). Each was used in a 40 min incubation at 37 °C; slides were then incubated with a secondary antibody (universal horseradish peroxidase (HRP) Multimer; Ventana Medical System) for 8 min at 37 °C. The tissue sections were then incubated with a chromogendiaminobenzidine (ultraView Universal DAB Kit, Ventana Medical System) and counterstained with hematoxylin.
LVI was assessed using H&E, immunohistochemical, and histochemical stained sections (D2-40 and EVG) individually. The presence of tumor cells within vascular spaces (i.e., lymphatics or small capillaries) surrounding tumors was considered LVI. Furthermore, LVI was divided into lymphatic invasion and vascular invasion depending on the presence or absence of vascular wall smooth muscle on H&E evaluation. The number of the cases positive and negative for lymphatic and vascular invasion, and LVI, was compared among the different staining procedures.
The first follow-up was done 6 mo after endoscopic resection with colonoscopy and abdominal CT. Subsequently, endoscopy and abdominal CT were performed yearly. The follow-up duration was defined as the time from the day of endoscopic resection to the last out-patient visit day.
The Student’s t-test, chi-square test and Fisher’s exact test were used to analyze LVI frequency, clinicopathological factors associated with LVI, and LVI detection rate differences between endoscopic procedures. Multivariate analysis including significant predictors from the univariate analysis was performed by multiple logistic regression analysis and the overall response with a 95%CI was determined. P values < 0.05 were considered significant. Kaplan-Meier analysis was used for evaluation of prognosis. For the kappa value by Kappa statistics, more than 0.5 was considered a strong association between the 2 sets. All statistical analyses were carried out using SPSS (version 18; SPSS Inc., Chicago, Illinois, United States).
A total of 104 cases (66 men and 37 women) with a median age of 47 years (range: 21-80 years) were included in this study. Tumors located in the rectum were an average of 8 cm away from the anal verge. Six (5.8%) patients underwent EMR, 57 (54.8%) underwent ESMR-L, and 41 (39.4%) underwent ESD. The average tumor size was 5.4 ± 2.4 mm (range, 1.2-10 mm), with 62 tumors (59.6%) measuring ≤ 5 mm and 42 tumors (40.4%) measuring 5-10 mm. Three tumors (2.9%) were located at the mucosa and the other 101 (97.1%) at the submucosa. Resection margins were positive in 16 (15.4%) tumors. Procedure-related complications occurred in 1 patient who underwent ESMR-L and experienced perforation of the bowel.
Regular follow-up evaluations were performed on 68 (65.4%) patients. No patient experienced local or distant metastatic tumor recurrence after a mean follow-up of 807 d.
Three patients underwent additional surgery owing to the presence of LVI in our primary histologic reports before this study; among them, 1 patient had lymph node metastasis. This 21-year-old man’s histologic evaluation showed a 5 mm tumor size, a Ki 67 index < 3%, and < 2 mitoses 10 HPFs; however, the vertical margin and lymphatic invasion were positive on the ESD specimen. There were no tumor-related deaths; 2 patients died from other causes. Kaplan-Meier analysis showed that the 5-year overall survival rate was 99%. The patient characteristics are summarized in Table 1.
| Characteristic | n = 104 |
| Gender | |
| Male | 67 |
| Female | 37 |
| Age (yr), median | 47 (range, 21-80) |
| < 60 | 89 |
| ≥ 60 | 15 |
| Distance from anal verge, mean ± SD (cm) | 8.09 ± 3.26 (range, 3-20) |
| Type of endoscopic resection | |
| EMR | 6 |
| ESMR_L | 57 |
| ESD | 41 |
| Tumor size, mean ± SD (mm) | 5.4 ± 2.4 (range, 1.2-10) |
| ≤ 5 | 62 |
| > 5 and ≤ 10 | 42 |
| Tumor depth | |
| Mucosa | 3 |
| Submucosa | 101 |
| Resection margin status | |
| R0 | 88 |
| R1 | 16 |
| Lateral (+) and deep (-) | 1 |
| Lateral (+) and deep (+) | 1 |
| Lateral (-) and deep (+) | 14 |
| Complications | |
| Yes | 1 |
| No | 103 |
| Follow-up | |
| Recurrence | 0 |
| Died | 2 |
Of the 104 specimens examined by H&E, 22 (21.2%) of the tumors were considered to have lymphatic invasion. Conversely, 15 (14.4%) tumors had lymphatic invasion detectable by D2-40. Ancillary staining including D2-40 allowed us to observe 7 lymphatic invasions (8.5%, 7/82) that were not initially detected using H&E. However, the increase of 6.8% compared with H&E was not statistically significant (P = 0.189).
Vascular invasion was detected in 11 (10.6%) of 104 NETs by H&E, whereas it was identified in 16 (15.4%) of 104 tumors by EVG. The detected percentage increased from 10.6% by H&E up to 15.4% by EVG. However, the difference in detection rates was not statistically significant between H&E and EVG (P = 0.227).
As a whole, the presence of LVI was considered positive in 26 (25.0%) of 104 tumors by H&E, and 29 (27.9%) of 104 tumors by ancillary studies. The concordance rate between H&E and ancillary studies was 81.7% for detection of LVI, which showed statistically strong agreement between two methods (κ = 0.531, P < 0.001). D2-40 and EVG staining enhanced LVI detection by 2.9% compared with H&E, however that difference that was not statistically significant (P = 0.648). LVI as assessed by H&E and immunohistochemical or histochemical procedures (D2-40 and EVG) are shown in Table 2 and Figure 3.
| LVI (H&E only) | LVI (D2-40 and EVG) | ||||||
| Total | Present | Absent | P vaule | Present | Absent | P vaule | |
| n = 104 | n = 26 (25.0%) | n = 78 (75.0%) | n = 29 (27.9%) | n = 75 (72.1%) | |||
| D2-40 and EVG | 0.648 | - | |||||
| LVI (+) | 29 (27.9) | 18 (69.2) | 11 (14.1) | - | - | ||
| LVI (-) | 75 (72.1) | 8 (30.8) | 67 (85.9) | - | - | ||
| Age (yr) | 48.20 ± 10.93 | 50.27 ± 11.28 | 47.51 ± 10.80 | 0.282 | 47.86 ± 11.16 | 48.33 ± 10.91 | 0.847 |
| Sex | 1.000 | 0.650 | |||||
| Male | 67 (64.4) | 17 (65.4) | 50 (64.1) | 20 (69.0) | 47 (62.7) | ||
| Female | 37 (35.6) | 9 (34.6) | 28 (35.9) | 9 (31.0) | 28 (37.3) | ||
| AV distance (cm)1 | 8.09 ± 3.25 | 8.35 ± 2.72 | 8.01 ± 3.41 | 0.687 | 7.59 ± 2.87 | 8.25 ± 3.37 | 0.412 |
| Tumor size | 0.038 | 0.007 | |||||
| ≤ 5 mm | 62 (59.6) | 11 (42.3) | 51 (65.4) | 11 (37.9) | 51 (68.0) | ||
| > 5 mm | 42 (40.4) | 15 (57.7) | 27 (34.6) | 18 (62.1) | 24 (32.0) | ||
| Tumor depth | 0.571 | 0.558 | |||||
| Mucosa | 3 (2.9) | 0 (0) | 3 (3.8) | 0 (0) | 3 (4.0) | ||
| Submucosa | 101 (97.1) | 26 (100) | 75 (96.2) | 29 (100) | 72 (96.0) | ||
| Tumor grade | 1.000 | 0.006 | |||||
| Grade 1 | 95 (91.3) | 24 (92.3) | 71 (91.0) | 20 (69.0) | 68 (90.7) | ||
| Grade 2 | 9 (8.7) | 2 (7.7) | 7 (9.0) | 9 (31.0) | 7 (9.3) | ||
| Ki 67% | 1.46 ± 1.01 | 1.08 ± 1.05 | 0.113 | 1.54 ± 1.13 | 1.03 ± 0.97 | 0.023 | |
| Ki 67 index | 0.627 | 0.213 | |||||
| < 3% | 98 (94.2) | 24 (92.3) | 74 (94.9) | 26 (89.7) | 72 (96.0) | ||
| ≥ 3% | 6 (5.8) | 2 (7.7) | 4 (5.1) | 3 (10.3) | 3 (4.0) | ||
| Mitotic count | 0.65 ± 0.84 | 0.39 ± 0.69 | 0.125 | 0.82 ± 0.88 | 0.32 ± 0.97 | 0.001 | |
| Mitosis/10HPF | 0.357 | 0.005 | |||||
| < 2 | 93 (89.4) | 22 (84.6) | 71 (91.0) | 22 (75.9) | 71 (94.7) | ||
| ≥ 2 | 11 (10.6) | 4 (15.4) | 7 (9.0) | 7 (24.1) | 4 (5.3) | ||
In 19 cases analysis for the presence of LVI using H&E did not match ancillary studies. LVI detected with H&E in 8 cases was not observed in ancillary studies. In addition, 11 cases negative for LVI using H&E were considered positive in ancillary studies. Immunostaining with CD31 and CD34 were performed on the discordant cases. Evaluation with CD31 indicated LVI was absent in the 8 cases detected with H&E, and present in the 11 cases that tested negative with H&E. However, CD34 stained in the delicate fibrovascular connective tissue of all 19 NET cases, of which non-specific staining could not be interpreted as LVI positivity.
Based on the comparative results between H&E and D2-40 and EVG, the results of LVI assessed by D2-40 and EVG showed statistical associations with more numbers of clinicopathological variables of NETs than H&E did. LVI assessed by D2-40 and EVG was significantly associated with tumor size, tumor grade, and mitotic count (P = 0.007, P = 0.006, and P = 0.005, respectively). LVI-positive cases were frequently detected in tumors that were > 5 mm, grade 2, and had a mitotic count ≥ 2. In addition, the mean Ki-67 labeling index and mean mitotic count were higher in tumors with LVI (1.54 ± 1.13 and 0.82 ± 0.88, respectively) than in tumors without LVI (1.03 ± 0.97 and 0.32 ± 0.97, respectively) (P = 0.023 and P = 0.001, respectively). There were no significant differences in LVI between patient’s age (P = 0.847), gender (P = 0.650), tumor distance from anal verge (P = 0.412), or depth of tumor invasion (P = 0.558). The predictive parameters of LVI based on H&E or D2-40 and EVG are shown in Table 2. Unlike D2-40 and EVG, analysis of LVI by H&E was only associated with tumor size (P = 0.038). The results of analysis of LVI using H&E were not related to tumor grade (P = 1.000), Ki-67 labeling index (P = 0.627), and mitotic count/10HPFs (P = 0.357).
Analysis of tumor size and grade for prediction of LVI by multivariate analysis indicated that tumor grade was the only independent predictive factor for LVI in small rectal NET patients treated with endoscopic resection (Table 3). Tumors classified as grade 2 were more likely to have LVI than grade 1 tumors (P = 0.015, hazard ratio = 4.095, 95%CI: 1.321-12.692).
| Lymphovascular invasion | P value | ||
| HR | 95% CI | ||
| Tumor size > 5 mm | 1.694 | 0.639-4.491 | 0.289 |
| Tumor grade Grade 2 | 4.095 | 1.321-12.692 | 0.015 |
We further investigated the possible differences in frequency of detectable LVI, tumor size, resection outcome, and safety margin between ESMR-L and ESD (Table 4). Successful complete resections (R0) by ESMR-L and ESD were achieved in 50 of 57 tumors (success rate, 87.7%) and 34 of 41 tumors (success rate, 82.9%). However, there were no statistically significant differences in the frequency of LVI, tumor size, and resection outcome status between the 2 endoscopic resection methods (P = 0.955, P = 0.192, and P = 0.504, respectively).
| ESMR-L | ESD | P vaule | |
| n = 57 | n = 41 | ||
| LVI | 0.955 | ||
| Absent | 42 (58.3) | 30 (41.7) | |
| Present | 15 (58.2) | 11 (42.3) | |
| Tumor size | 0.192 | ||
| ≤ 5 mm | 38 (66.7) | 22 (53.7) | |
| > 5 mm | 19 (33.3) | 19 (46.3) | |
| Resection outcome | 0.504 | ||
| Complete (R0) | 50 (87.7) | 34 (82.9) | |
| Incomplete (R1) | 7 (12.3) | 7 (17.1) | |
| Safety resection margin (μm) | 725 ± 872 | 322 ± 348 | 0.002 |
Conversely, the vertical safety margin was significantly larger in ESMR-L than ESD (725 ± 872 μm vs 322 ± 348 μm, respectively, P = 0.002). The more successful safety margin was achieved in ESMR-L than in ESD.
The purpose of the present study was to investigate the frequency, risk factors and prognosis of LVI in endoscopically resected small rectal NETs ≤ 1 cm in size, and to compare the therapeutic outcome achieved with ESMR-L and ESD. We have shown that LVI was relatively common in small rectal NETs, with 27.9% exhibiting LVI. Although ancillary studies increased the detection rate of LVI, careful H&E examination was still a reliable method showing high concordance with D2-40 and EVG staining. LVI was associated with large tumor size (> 5 mm), tumor grade 2, and higher mitotic count (≥ 2). Among those factors, tumor grade 2 was the only independent predictive factor for the presence of LVI. No recurrence was observed in patients with small rectal NETs ≤ 1 cm, regardless of the presence of LVI.
Only a few studies have investigated the frequency, risk factors, and prognostic significance of pathologically proven LVI in rectal NETs ≤ 1 cm in size after endoscopic resection[32,33]. Prevalence of LVI in rectal NETs is 0-20% by H&E examination[17,19,24,25,32,39]. Immunohistochemical analysis is not currently recommended for routine use to identify LVI in NETs. However, for accurate and reliable diagnosis of LVI, we applied additional staining using D2-40 and EVG to confirm the presence of LVI after H&E examination.
Staining of elastic tissue during microscopic assessment has been proposed as being a more sensitive means of revealing venous invasion within the tumor[38]. D2-40 is the best selective immunohistochemical marker for staining lymphatic endothelium[37]. In the present study, LVI was identified in 29 (27.9%) of 104 tumors by D2-40 and EVG, and 26 (25.0%) of 104 tumors by H&E. Although staining with D2-40 and EVG raised the detection rate of LVI by 2.9%, the difference was not statistically significant. Rather, H&E showed a high concordance rate with ancillary studies (81.7%).
There have been only two studies of D2-40 and EVG staining for identification of LVI[32,33]. Those studies also reported a high frequency of LVI (46.7% and 22.4%) in endoscopically resected small rectal NETs[32,33]. Taken together with our study, the frequency of LVI appears to be high in even small NETs. However, in those studies, the detection rate for LVI using H&E staining alone was much lower (1.1% and 10.2%) than the rate detected with D2-40 and EVG staining[32,33]. The wide range of frequencies reported may be due to difficulties evaluating LVI H&E-stained sections due to retraction artifacts in the tumor. We also had 19 results (18.3%) in which the results of H&E and ancillary staining were discordant. The absence of D2-40 and EVG in 8 (42.1%) out of the 19 discordant cases was also confirmed by the absence of CD31 staining, which indicated a retraction artifact from the surrounding fibrotic stroma. Although the immunohistochemical and/or special staining used in our study was not demonstrated as a statistically significant indicator for the identification of LVI, the ancillary studies may be of help to differentiate retraction artifacts. The high concordance rate in our study between H&E and ancillary studies may be because two pathologists carefully re-evaluated H&E-stained slides from all cases and discussed the findings of LVI using a multi-headed microscope. The previous studies did not describe the number of pathologist participating in slide review[32,33].
The metastatic potential and aggressive behavior of a rectal NET are generally proportional to tumor size[30]. A close relationship has been noted between tumor size or LVI and risk of metastasis even in small rectal NETs. LVI-positive tumors have significantly larger tumor size (median 5 mm) than those without LVI (median 4 mm)[32]. The metastasis rate of early stage rectal NETs (10 mm or less in size) was 9.7% (58/595)[2]. Three tumors (25%) out of 12 with lymph node metastasis were less than 10 mm[5]. In our study, LVI was frequently detected in tumor size > 5 mm in univariate analysis. However, the multivariate analysis failed to demonstrate the correlation between tumor size and LVI.
LVI was also associated with tumor grade 2 and increased mitotic count (≥ 2). Tumor grade 2 was the only independent predictive factor for the presence of LVI. The majority of small rectal NETs are NET G1 (97.6%-100%)[8,25,32,33,39,40]. Few studies have demonstrated a correlation between LVI and grade 2 in small rectal NETs, although NETs G1 and G2 exhibit significant differences in patient survival. The present study included 16 NETs classified as G2 (15%). Interestingly, a significant association between grade 2 and LVI was only found in the LVI results assessed by D2-40 and EVG but not by H&E staining. Thus, immunohistochemically confirmed LVI may more precisely reflect on clinicopathological features of such tumors.
Strategies for treating small rectal NETs ≤ 1 cm in size with LVI remain controversial, and clear-cut indications for local resection and additional surgery have not been established. Patients with rectal NETs without metastasis have a good prognosis if they undergo endoscopic resection; the 3-year survival rate is 100%[23]. In the present study, an excellent prognosis was found in the small rectal NETs. There was no recurrence or metastasis in patients with LVI during follow-up periods of 28.8 mo in our study. Similarly, no metastasis or recurrence has been reported in the small rectal NETs with LVI but without additional surgery over the 5 years median follow-up period[32,33]. Furthermore, recurrences have not been observed following the removal of tumors 20 mm in size and positive for LVI, but not in any tumors < 20 mm, even if they were positive for LVI during a 10-year period[39]. In contrast, a delayed localized recurrence has been unexpectedly reported 23 years after endoscopic resection of 4 mm sized rectal G1 NET[41], and the size of a lymph node metastasis has remained unchanged during 7 years of follow-up[42], suggesting that the metastatic lymph node growth rate may be extremely low in some cases. However, there are no definite guidelines for regular follow-up of LVI-positive small rectal NETs[10,27]. While small rectal NETs seem to have a favorable short-term prognosis, the long-term prognosis may be difficult to determine.
Complete resection of rectal NETs is difficult to achieve with conventional endoscopic resection techniques because these tumors often extend into the submucosa. We found that ESMR-L (725 ± 872 μm) showed a larger safety resection margin than ESD (725 ± 872 μm vs 322 ± 348 μm) despite similar rates of complete resection between two methods (ESMR-L 87.7% and ESD 82.9%). It may be that ESMR-L gets more submucosal tissue below NETs because submucosal aspiration is done by negative pressure. The short procedure time of ESMR-L may result in a smaller coagulation effect in the submucosa than ESD[21]. We found that there was no statistical difference in LVI detection rates between two endoscopic methods. The subsequent surgical resection with lymph node dissection for small rectal NETs with LVI after endoscopic resection has no worldwide accepted consensus. This study showed excellent outcomes of endoscopic resection with LVI. After endoscopic resection is completely achieved through ESMR-L and ESD, close follow-up should be pursued in cases with LVI[23].
The approximately 3-year follow-up period may be a limitation of our study. Nevertheless, some significant findings emerged from our results. The present study demonstrated that LVI in small rectal NETs may be high, and that this may not be associated with lymph node metastasis or recurrence in small rectal NETs (≤ 1 cm) during a 3 year-follow up period. Application of ancillary studies may be help differentiate retraction artifacts from true LVI, which may contribute to a close association with clinicopathological characteristics of rectal NETs.
Small rectal NETs have a favorable prognosis and successful outcomes following endoscopic resection. However, a low but real risk of metastasis remains, as in our results, and there are several cases of recurrence during long-term observation. Therefore, careful histologic examination for LVI and prospective studies with long-term follow up are needed to determine the natural course of small, endoscopically resected rectal NETs.
Rectal neuroendocrine tumors (NETs) arise from enterochromaffin endocrine cells and are found incidentally during sigmoidoscopy or colonoscopy. On endoscopy, they typically appear as sessile, subepithelial tumors covered with yellow, discolored epithelium. Rectal NETs ≤ 10 mm in diameter, within the mucosal or submucosal layer, can be treated with endoscopic resection and have a good prognosis. However, lymphovascular invasion (LVI), a well-established risk factor for lymph node metastasis, is often found in endoscopically resected specimens, and there are no definite guidelines about these cases. Therefore, the authors investigated the frequency and prognostic significance of LVI in small, endoscopically resected rectal NETs.
Immunohistochemical analysis is not currently recommended for routine use to identify LVI in NETs. However, for accurate and reliable diagnosis of LVI, the authors undertook additional immunohistochemical staining using D2-40 and Elastica van Gieson staining to confirm the presence of LVI.
The authors observed LVI rates of 25% and 27.9%, higher than previously reported, through hematoxylin and eosin (H&E) and additional immunohistochemical staining. On the other hand, LVI was not associated with lymph node metastasis or recurrence in small rectal NETs (≤ 1 cm) during a 3 year-follow up period.
After endoscopic resection of rectal NETs, even in small tumors (≤ 10 mm), careful histologic examination for LVI is needed. Furthermore, long-term prospective studies are required to determine the natural course of endoscopically resected rectal NETs.
In this article, the authors analyzed the frequency of LVI in endoscopically resected small rectal NETs by precise methods and compared these results with conventional H&E staining. By these methods, they found that the frequency of LVI was higher than the previously reported ratio. Although they could not determine the relationship between LVI and clinical outcome, such as survival and recurrence, this study provides very important insights for future study.
| 1. | Riddell RH, Petras PE, Williams GT, Sobin LH. Tumors of the Intestines. In: Rosai J, editor. Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology; 2003: 279-321. . |
| 2. | Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer. 2005;103:1587-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 222] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1877] [Article Influence: 81.6] [Reference Citation Analysis (1)] |
| 4. | Fahy BN, Tang LH, Klimstra D, Wong WD, Guillem JG, Paty PB, Temple LK, Shia J, Weiser MR. Carcinoid of the rectum risk stratification (CaRRS): a strategy for preoperative outcome assessment. Ann Surg Oncol. 2007;14:396-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Fujimoto Y, Oya M, Kuroyanagi H, Ueno M, Akiyoshi T, Yamaguchi T, Muto T. Lymph-node metastases in rectal carcinoids. Langenbecks Arch Surg. 2010;395:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Shields CJ, Tiret E, Winter DC. Carcinoid tumors of the rectum: a multi-institutional international collaboration. Ann Surg. 2010;252:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Yoon SN, Yu CS, Shin US, Kim CW, Lim SB, Kim JC. Clinicopathological characteristics of rectal carcinoids. Int J Colorectal Dis. 2010;25:1087-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Kasuga A, Chino A, Uragami N, Kishihara T, Igarashi M, Fujita R, Yamamoto N, Ueno M, Oya M, Muto T. Treatment strategy for rectal carcinoids: a clinicopathological analysis of 229 cases at a single cancer institution. J Gastroenterol Hepatol. 2012;27:1801-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Yamagishi D, Matsubara N, Noda M, Yamano T, Tsukamoto K, Kuno T, Hamanaka M, Kobayashi M, Ikeuchi H, Matsuda I. Clinicopathological characteristics of rectal carcinoid patients undergoing surgical resection. Oncol Lett. 2012;4:910-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Caplin M, Sundin A, Nillson O, Baum RP, Klose KJ, Kelestimur F, Plöckinger U, Papotti M, Salazar R, Pascher A. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology. 2012;95:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 197] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 11. | Zhou X, Xie H, Xie L, Li J, Fu W. Factors associated with lymph node metastasis in radically resected rectal carcinoids: a systematic review and meta-analysis. J Gastrointest Surg. 2013;17:1689-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Bernick PE, Klimstra DS, Shia J, Minsky B, Saltz L, Shi W, Thaler H, Guillem J, Paty P, Cohen AM. Neuroendocrine carcinomas of the colon and rectum. Dis Colon Rectum. 2004;47:163-169. [PubMed] |
| 13. | Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1203] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 14. | Scherübl H. Rectal carcinoids are on the rise: early detection by screening endoscopy. Endoscopy. 2009;41:162-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Taghavi S, Jayarajan SN, Powers BD, Davey A, Willis AI. Examining rectal carcinoids in the era of screening colonoscopy: a surveillance, epidemiology, and end results analysis. Dis Colon Rectum. 2013;56:952-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 16. | Kim JY, Kim KS, Kim KJ, Park IJ, Lee JL, Myung SJ, Park Y, Park YS, Yu CS, Kim JC. Non-L-cell immunophenotype and large tumor size in rectal neuroendocrine tumors are associated with aggressive clinical behavior and worse prognosis. Am J Surg Pathol. 2015;39:632-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Lee DS, Jeon SW, Park SY, Jung MK, Cho CM, Tak WY, Kweon YO, Kim SK. The feasibility of endoscopic submucosal dissection for rectal carcinoid tumors: comparison with endoscopic mucosal resection. Endoscopy. 2010;42:647-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Kim HH, Park SJ, Lee SH, Park HU, Song CS, Park MI, Moon W. Efficacy of endoscopic submucosal resection with a ligation device for removing small rectal carcinoid tumor compared with endoscopic mucosal resection: analysis of 100 cases. Dig Endosc. 2012;24:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Kim KM, Eo SJ, Shim SG, Choi JH, Min BH, Lee JH, Chang DK, Kim YH, Rhee PL, Kim JJ. Treatment outcomes according to endoscopic treatment modalities for rectal carcinoid tumors. Clin Res Hepatol Gastroenterol. 2013;37:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Zhou X, Xie H, Xie L, Li J, Cao W, Fu W. Endoscopic resection therapies for rectal neuroendocrine tumors: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Choi CW, Kang DH, Kim HW, Park SB, Jo WS, Song GA, Cho M. Comparison of endoscopic resection therapies for rectal carcinoid tumor: endoscopic submucosal dissection versus endoscopic mucosal resection using band ligation. J Clin Gastroenterol. 2013;47:432-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Konishi T, Watanabe T, Kishimoto J, Kotake K, Muto T, Nagawa H. Prognosis and risk factors of metastasis in colorectal carcinoids: results of a nationwide registry over 15 years. Gut. 2007;56:863-868. [PubMed] |
| 23. | Park CH, Cheon JH, Kim JO, Shin JE, Jang BI, Shin SJ, Jeen YT, Lee SH, Ji JS, Han DS. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy. 2011;43:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Suzuki S, Ishii N, Uemura M, Deshpande GA, Matsuda M, Iizuka Y, Fukuda K, Suzuki K, Fujita Y. Endoscopic submucosal dissection (ESD) for gastrointestinal carcinoid tumors. Surg Endosc. 2012;26:759-763. [PubMed] |
| 25. | Kim GU, Kim KJ, Hong SM, Yu ES, Yang DH, Jung KW, Ye BD, Byeon JS, Myung SJ, Yang SK. Clinical outcomes of rectal neuroendocrine tumors ≤ 10 mm following endoscopic resection. Endoscopy. 2013;45:1018-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Park SB, Kim HW, Kang DH, Choi CW, Kim SJ, Nam HS. Advantage of endoscopic mucosal resection with a cap for rectal neuroendocrine tumors. World J Gastroenterol. 2015;21:9387-9393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Anthony LB, Strosberg JR, Klimstra DS, Maples WJ, O’Dorisio TM, Warner RR, Wiseman GA, Benson AB, Pommier RF. The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (nets): well-differentiated nets of the distal colon and rectum. Pancreas. 2010;39:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 28. | Kulke MH, Shah MH, Benson AB, Bergsland E, Berlin JD, Blaszkowsky LS, Emerson L, Engstrom PF, Fanta P, Giordano T. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw. 2015;13:78-108. [PubMed] |
| 29. | O’Toole D, Kianmanesh R, Caplin M. ENETS 2016 Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Tumors: An Update. Neuroendocrinology. 2016;103:117-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Mani S, Modlin IM, Ballantyne G, Ahlman H, West B. Carcinoids of the rectum. J Am Coll Surg. 1994;179:231-248. [PubMed] |
| 31. | Ikeda K, Kojima M, Saito N, Sakuyama N, Koushi K, Watanabe T, Sugihara K, Akimoto T, Ito M, Ochiai A. Current status of the histopathological assessment, diagnosis, and reporting of colorectal neuroendocrine tumors: A Web survey from the Japanese Society for Cancer of Colon and Rectum. Pathol Int. 2016;66:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Sekiguchi M, Sekine S, Sakamoto T, Otake Y, Nakajima T, Matsuda T, Taniguchi H, Kushima R, Ohe Y, Saito Y. Excellent prognosis following endoscopic resection of patients with rectal neuroendocrine tumors despite the frequent presence of lymphovascular invasion. J Gastroenterol. 2015;50:1184-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 33. | Nakamura K, Osada M, Goto A, Iwasa T, Takahashi S, Takizawa N, Akahoshi K, Ochiai T, Nakamura N, Akiho H. Short- and long-term outcomes of endoscopic resection of rectal neuroendocrine tumours: analyses according to the WHO 2010 classification. Scand J Gastroenterol. 2016;51:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Edge SB, Byrd DR, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE. AJCC Cancer StagingManual, 7th edn. New York: Springer, 2010. . |
| 35. | Voss SM, Riley MP, Lokhandwala PM, Wang M, Yang Z. Mitotic count by phosphohistone H3 immunohistochemical staining predicts survival and improves interobserver reproducibility in well-differentiated neuroendocrine tumors of the pancreas. Am J Surg Pathol. 2015;39:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Kim JY, Hong SM. Recent Updates on Neuroendocrine Tumors From the Gastrointestinal and Pancreatobiliary Tracts. Arch Pathol Lab Med. 2016;140:437-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (2)] |
| 37. | Arnaout-Alkarain A, Kahn HJ, Narod SA, Sun PA, Marks AN. Significance of lymph vessel invasion identified by the endothelial lymphatic marker D2-40 in node negative breast cancer. Mod Pathol. 2007;20:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Vass DG, Ainsworth R, Anderson JH, Murray D, Foulis AK. The value of an elastic tissue stain in detecting venous invasion in colorectal cancer. J Clin Pathol. 2004;57:769-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Shigeta K, Okabayashi K, Hasegawa H, Ishii Y, Ochiai H, Tsuruta M, Mukai M, Kameyama K, Uraoka T, Yahagi N. Long-term outcome of patients with locally resected high- and low-risk rectal carcinoid tumors. J Gastrointest Surg. 2014;18:768-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Holinga J, Khalid A, Fasanella K, Sanders M, Davison J, McGrath K. Metastatic risk of diminutive rectal carcinoid tumors: a need for surveillance rectal ultrasound? Gastrointest Endosc. 2012;75:913-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Judd S, Nangia S, Levi E, Antaki F. Rectal carcinoid tumor: a delayed localized recurrence 23 years after endoscopic resection. Endoscopy. 2014;46 Suppl 1 UCTN:E555-E556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Kim SH, Yang DH, Lee JS, Park S, Lee HS, Lee H, Park SH, Kim KJ, Ye BD, Byeon JS. Natural course of an untreated metastatic perirectal lymph node after the endoscopic resection of a rectal neuroendocrine tumor. Intest Res. 2015;13:175-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Yoshitomi H S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF