Published online Oct 28, 2016. doi: 10.3748/wjg.v22.i40.8991
Peer-review started: June 11, 2016
First decision: July 29, 2016
Revised: August 29, 2016
Accepted: September 28, 2016
Article in press: September 28, 2016
Published online: October 28, 2016
Processing time: 141 Days and 17.8 Hours
To investigate the relationship between pathological oropharyngeal (OP) acid exposure and esophageal motility in patients with extra-esophageal syndromes.
In this prospective study we enrolled consecutive outpatients with extra-esophageal symptoms suspected to be related to gastroesophageal reflux disease (GERD). We enrolled only patients with a reflux symptom index (RSI) score-higher than 13 and with previous lung, allergy and ear, nose and throat evaluations excluding other specific diagnoses. All patients underwent 24-h OP pH-metry with the Dx probe and esophageal high-resolution manometry (HRM). Patients were divided into two groups on the basis of a normal or pathological pH-metric finding (Ryan Score) and all manometric characteristics of the two groups were compared.
We examined 135 patients with chronic extra-esophageal syndromes. Fifty-one were considered eligible for the study. Of these, 42 decided to participate in the protocol. Patients were divided into two groups on the basis of normal or pathological OP acid exposure. All the HRM parameters were compared for the two groups. Significant differences were found in the median upper esophageal sphincter resting pressure (median 71 mmHg vs 126 mmHg, P = 0.004) and the median proximal contractile integral (median 215.5 cm•mmHg•s vs 313.5 cm•mmHg•s, P = 0.039), both being lower in the group with pathological OP acid exposure, and the number of contractions with small or large breaks, which were more frequent in the same group. This group also had a larger number of peristaltic contractions with breaks in the 20 mmHg isobaric contour (38.7% vs 15.38%, P < 0.0001).
In patients with suspected GERD-related extra-esophageal syndromes pathological OP acid exposure was associated with weaker proximal esophageal motility.
Core tip: A new oropharyngeal (OP) pH probe now available is more sensitive than traditional pH sensors for faithfully monitoring the pH of OP reflux, and the latest high-resolution esophageal manometry offers a major advance in defining esophageal motility abnormalities compared to conventional manometry. This study compares these two techniques, for the first time, and indicates that in patients with extra-esophageal syndromes pathological OP acid exposure is associated with weaker proximal esophageal motility.
- Citation: Passaretti S, Mazzoleni G, Vailati C, Testoni PA. Oropharyngeal acid reflux and motility abnormalities of the proximal esophagus. World J Gastroenterol 2016; 22(40): 8991-8998
- URL: https://www.wjgnet.com/1007-9327/full/v22/i40/8991.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i40.8991
Gastro-esophageal reflux disease (GERD) is very common in Western countries, with a prevalence of typical manifestations of 10%-20%[1]. In the last few years, ear, nose and throat (ENT) specialists have increasingly attributed a range of atypical manifestations to GERD. In 2006 an international Consensus Group developed a global classification of GERD manifestations, grouping them as either esophageal or extra-esophageal syndromes[1] .
It is hard to calculate the prevalence of extra-esophageal syndromes because of their multifactorial etiology and the difficulty of establishing a clear cause-effect relationship between reflux and symptoms[2]. The “gold standard” for determining a pathological gastro-esophageal reflux (GER), 24-h esophageal pH-impedance, is not totally reliable for the diagnosis of laryngopharyngeal reflux (LPR) because the standard impedance probes do not have channels reaching the upper esophageal sphincter (UES) and pharynx and traditional pH sensors are poorly reliable when positioned in the hypopharynx[3].
A new oropharyngeal (OP) pH probe, the Dx probe, is now available, and is more sensitive than traditional pH sensors for faithfully monitoring the pH in the oropharynx[4-6]. It is still not clear why in some patients the GER is limited to the distal esophagus while in others it extends to the proximal esophagus and above the UES, where it can cause extra-esophageal manifestations by a direct mechanism[7]. In order to assess whether esophageal motility plays a role in the proximal extension of reflux, various studies have examined patients with extra-esophageal symptoms using 24-h esophageal pH-metry (some also with a proximal pH-probe) and conventional esophageal manometry, but results have been discordant[8-11]. Esophageal high-resolution manometry (HRM) records esophageal motility more reliably than conventional manometry[12].
The aim of this study in patients with extra-esophageal syndromes was to assess, for the first time, the relationship between pathological OP acid exposure, examined with the Dx probe, and esophageal motor characteristics, assessed by HRM.
From October 2011 to March 2013 we prospectively enrolled consecutive patients referred to the gastroenterology outpatient unit of our tertiary center for chronic (> 6 mo) suspected GERD-related extra-esophageal syndromes[1]. In order to increase the probability of identifying a subgroup of patients in this population with pathological OP acid exposure, we enrolled only patients with a reflux symptom index (RSI) score higher than 13[13,14] and with previous lung, allergy and ENT evaluations excluding other specific diagnoses. Other exclusion criteria were: history of thoracic or gastric surgery, dysphagia and known esophageal motility disorders. All the patients underwent 24-h OP pH-monitoring with the Dx probe and esophageal HRM. We compared all the esophageal motility parameters with the OP pH-metry profile.
The protocol was approved by the hospital’s medical ethics committee. Informed consent was obtained for procedures and for data management for scientific purposes.
The RSI is a self-administered nine-item questionnaire for symptoms assessment in patients with suspected LPR. The score for each item ranges from 0 (no problem) to 5 (severe problem), up to a maximum of 45[13]. Schindler et al[14] developed the validated Italian form of the RSI, which is easily administered, highly reproducible, and ensures excellent clinical validity.
For OP pH-monitoring we used the Dx-pH measurement system (Restech - Respiratory Technology Corporation, San Diego, CA, United States). The Dx sensor was calibrated in pH 7 and 4 buffer solutions before use. The probe was inserted transnasally and positioned so that the flashing light-emitting diode at its tip was 5-10 mm below the uvula[5]. Patients were asked to keep a diary during the recording period, indicating the times they spent sleeping or orthostatic and the times when they ate or drank and brushed their teeth; these periods were excluded from the analysis. After the 24-h recording the data were downloaded to a dedicated software program (DataView Lite V3, Respiratory Technology Corporation) and pH tracings were all assessed by a single operator (SP) (GM), who was blinded to the manometric results. Ryan Scores were calculated for the supine and upright positions; these composite scores use 5.0 and 5.5 pH thresholds respectively and combine three parameters: (1) the number of reflux episodes; (2) the duration of the longest episode; and (3) the percentage of time below the defined threshold. Scores higher than 9.41 in the upright position and/or higher than 6.81 in the supine position denoted pathological OP acid exposure[6].
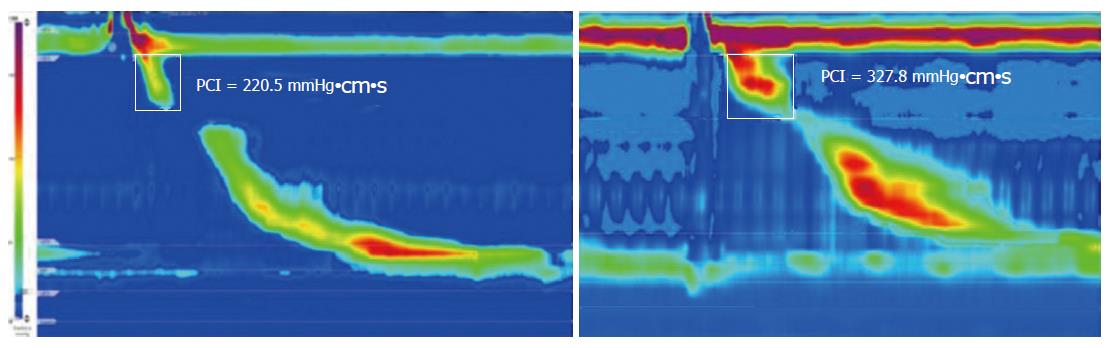
Manometric studies were done using a system (Solar GI HRM, Medical Measurement System, The Netherlands) with a catheter with 36 circumferential solid-state pressure sensors spaced at 1-cm intervals (UniTip High Resolution Kateter 12F, Unisensor, Attikon, Switzerland). Patients fasted overnight then the catheter was placed transnasally, positioned to record from the hypopharynx to the stomach. The manometric protocol was done with patients supine and consisted of a 5-min period to assess basal sphincter pressures, and ten 5-mL water swallows[15-17]. Data were analyzed by a SP, who was blinded to the results of pH tracings, using a dedicated software program (Medical Measurement System Database Software, V8.23a, The Netherlands). All manometric parameters were calculated for each swallow and the contraction patterns were classified according to the Chicago Classification v3.0[16]. The metrics analyzed included: sphincters lengths and resting pressures, lower esophageal sphincter (LES) integrated relaxation pressure (IRP-4s) and distal contractile integral (DCI), as previously defined[15-17]. We also calculated the proximal contractile integral (PCI, Figure 1), applying the same algorithm as for the DCI, to quantify contractile pressure exceeding 20 mmHg for the region spanning from the lower border of the UES to the transition zone (TZ)[18-20]. The individual swallow patterns were classified as peristaltic, premature (distal latency-DL < 4.5 s), hypercontractile (DCI > 8000 mmHg•s•cm), failed (DCI < 100 mmHg•s•cm), weak (DCI < 450 mmHg•s•cm), and fragmented contraction (defect in the 20-mmHg isobaric contour of the peristaltic contraction > 5 cm)[16]. We also evaluated, according to Chicago Classification v2.0, contractions with small defects (between 2 and 5 cm long)[15].
For demographic and clinical characteristics we used a parametric analysis with Student’s t (Table 1) or Fisher’s exact test (Figure 2) to test the significance of differences. For metrics regarding esophageal sphincters and the strength of esophageal contraction (Table 2) first we tested the data distribution with the Kolmogorov-Smirnov test. As the data were not normally distributed we used the median, 95% confidence interval and Mann-Whitney U test for independent samples. For the contraction patterns (Table 3) we used the chi-square test to analyze the differences between the two groups, considering all the subtypes of pattern. As this test gave a significant result (chi-square 26.8, P = 0.0001) we were authorized to make multiple comparisons between each subtype of contraction using Fisher’s exact test. Probability < 5% was considered significant.
| OP pH- | OP pH+ | |
| No. of patients | 18 | 24 |
| Male/female (% of male) | 8/10 (44%) | 6/18 (25%) |
| Mean age (years ± SD) | 52.52 ± 11.71 | 50.51 ± 14.73 |
| Mean BMI (± SD) | 24.50 ± 3.71 | 24.32 ± 3.61 |
| Mean RSI score | 18.00 | 17.80 |
| No. of patients with typical esophageal symptoms | 8/18 (44.44%) | 9/24 (37.50%) |
| OP pH- | OP pH+ | P value | |
| UES length (cm) | 4.2 (4.0-4.7) | 4.3 (4.1-4.8) | |
| UES resting pressure (mmHg) | 126.0 (96.3-59.7) | 71.0 (60.8-110.6) | < 0.05 |
| PCI (cm•mmHg•s) | 313.5 (243-489) | 215.5 (103-290) | < 0.05 |
| DCI (cm•mmHg•s) | 2612 (1121-3195) | 1540 (951-2921) | |
| LES length (cm) | 4.65 (3.8-5.1) | 5.15 (4.1-5.5) | |
| LES resting pressure (mmHg) | 28.0 (19.8-34.1) | 26.0 (20.8-30.8) | |
| LES 4s-IRP (mmHg) | 12.6 (8.0-17.4) | 14.2 (11.4-19.1) |
| OP pH- | OP pH+ | P value | |
| No. of correct swallows | 184 | 240 | |
| No. of peristaltic contractions (without breaks) | 154/182 (84.61%) | 144/235 (61.27%) | < 0.01 |
| No. of failed contractions | 2/184 (1.08%) | 5/240 (2.08%) | |
| No. of peristaltic contractions with small breaks | 21/182 (11.95%) | 68/235 (28.93%) | < 0.01 |
| No. of peristaltic contractions with large breaks | 7/182 (3.84%) | 23/235 (9.78%) | < 0.01 |
| No. of premature contractions | 0 | 0 | |
| No. of rapid contractions | 0 | 0 |
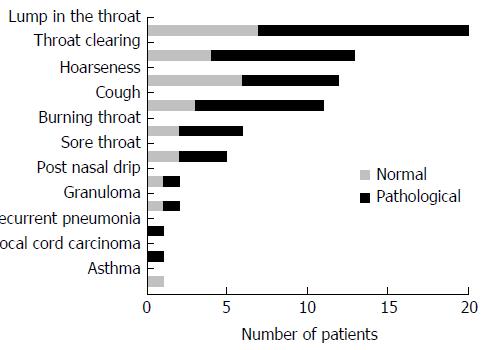
We evaluated 135 patients with chronic extra-esophageal syndromes. Fifty-one were considered eligible. Of these, 42 decided to participate in the protocol; Figure 2 summarizes their main clinical manifestations.
Patients were divided into two groups on the basis of a normal (OP pH-) or pathological (OP pH+) OP acid exposure. The clinical characteristics of the two groups did not significantly differ (Table 1 and Figure 2). All the HRM parameters for the two groups are compared in Tables 2 and 3. Significant differences were found between the two groups in the median UES resting pressure and the median PCI, both lower in patients with pathological OP acid exposure, and the number of contractions with small and large breaks, which were more frequent in the same group.
LPR has been diagnosed increasingly frequently in recent years, but often only on the basis of aspecific laryngoscopic findings, common in asymptomatic people too[21,22]. This over-diagnosis poses an important economic burden for the assessment and treatment of these patients, which often unsatisfactory[23]. Ex adiuvantibus therapy, with double- dose proton pump inhibitors for long periods (3-6 mo), often achieves a partial response due to the placebo effect or to the multifactorial etiology of these symptoms[7,24]. Regrettably, 24-h pH-impedence is not reliable for the diagnosis of LPR because the standard impedance probes do not have channels reaching the UES and pharynx and traditional pH sensors are poorly reliable when positioned in the hypopharynx. In particular, traditional pH sensors, when positioned in the hypopharynx, are prone to drying out and may cause pseudo-reflux due to artifacts[3].
Recently, two new devices that overcome these limitations have been introduced for the detection of LPR: OP pH-metry (Respiratory Technology Corp.)[4-6] and hypopharyngeal multichannel intraluminal impedence (Sandhill Scientific Inc.)[25,26]. We used the OP Dx probe to detect acid reflux in the oropharynx of patients with clinically suspected LPR. This sensor measures the pH of both liquid and aerosolized droplets in the posterior oropharynx, avoids drying, does not require contact with fluid or tissue for electrical continuity and has a teardrop shape with the sensor oriented downward to avoid becoming covered with food or mucus[5]. The Dx probe is more sensitive than traditional pH monitoring for detecting LPR[4]. It can not distinguish healthy volunteers from subjects with laryngeal and reflux symptoms[27], but it can identify patients who respond to medical or surgical treatment of GERD[28,29].
We considered OP acid exposure as normal or pathological according to Ryan scores. These composite scores were calculated by Ayazi et al[6] using the pH thresholds that are best for defining abnormal OP pH.
In this study we included patients with clinically suspected LPR, i.e. with extra-esophageal symptoms and a RSI score higher than 13 and with previous lung, allergy and ENT evaluations excluding other causes of symptoms. In this population we could identify a subgroup in which a pathological LPR was objectively established by 24-h OP pH-monitoring. As far as we know, it is still not clear why some patients have GER limited to the distal esophagus while in others it extends to the proximal esophagus and above the UES. Seeking an answer to this question, different studies have used conventional manometry to assess esophageal motor function in GERD patients. In patients with typical syndromes esophago-gastric junction (EGJ) impairment and ineffective esophageal motility (IEM) were strongly implicated in the development of GERD[30] and the prevalence of these abnormalities rose with the severity of the reflux disease[31]. In contrast, there are few and discordant data about motility abnormalities in patients with extra-esophageal syndromes[8-10,32]. Fouad et al[8] found IEM significantly more often in patients with GERD and chronic cough (41%) or asthma (53%) and numerically more often in patients with GERD and laryngitis (31%) than in patients with heartburn (19%). DiBaise et al[9] reported no significant difference in motility parameters between GERD patients with typical symptoms and those with extra-esophageal symptoms alone. Patti et al[10] reported that in patients who had pH < 4 in the proximal esophagus for more than 3% of the time, the LES was weaker and shorter and UES pressures and peristalsis amplitude lower.
HRM offers a major advance in defining esophageal motility abnormalities[12]. It employs numerous closely spaced pressure sensors, which overcomes the problem of movement-related artifacts for esophageal sphincters and can reveal the segmental character of esophageal peristalsis and the anatomy of the EGJ. Daum et al[33] showed that the frequency of esophageal dysmotility in GERD patients was higher using HRM than conventional manometry.
There are only few HRM studies so far in patients with extra-esophageal syndromes[34,35] and most have been done on patients who had an indirect diagnosis of LPR, based on clinical manifestations, positive response to antisecretory therapy or pathological esophageal pH (more frequently) or pH-impedance monitoring, which is not altogether reliable for detecting LPR. As we discussed above, in most cases the motility has not really been assessed in a population with established LPR. We studied patients with extra-esophageal syndromes using OP pH-monitoring and HRM in order to find out whether there was a motility pattern characteristic of patients with established pathological LPR. Objective identification of LPR is essential to define a population of true patients in which the LPR is proven, not just assumed. We compared all the motility parameters that can be obtained with HRM for patients with pathological OP acid exposure and those with a normal result. The aim of this study was to correlate HRM and OP pH-metry and this is the first comparison of the two techniques. The study did not aim to assess the correlation between manometric features and extra-esophageal syndromes.
The parameters that differed significantly in the two groups were the median UES resting pressure, the median PCI and the number of contractions with small or large breaks. All these parameters are hard to assess with conventional manometry[12,33]. The median UES resting pressure was significantly lower in patients with pathological OP acid exposure. UES incompetence is necessary for LPR[36,37]. A recent study showed that LPR (video-endoscopically documented) could be induced by slow esophageal liquid infusion in patients with a clinical diagnosis of GERD-related extra-esophageal syndromes but not in healthy controls, and that the application of 20-30 mmHg cricoid pressure significantly raised UES intraluminal pressure and prevented the LPR[38]. A new, individually fitted UES assist device (Reza-Band®) to be worn at night has been recently marketed and seems to prevent LPR.
Even though the PCI metric is not included in the Chicago Classification, it has been evaluated in a few published studies, including patients with extra-esophageal symptoms[18-20]. We found it was significantly lower in the group with pathological OP acid exposure. Possibly, therefore, lower proximal esophageal contractile function may lead to less reflux clearance, which would allow the reflux to extend proximally. Clearly, however, it is also possible that a reflux with proximal extension may lead to impairment of upper esophageal motility.
Finally, patients with pathological OP acid exposure had significantly more contractions with small or large breaks in the 20 mmHg isobaric contour between UES and EGJ. The Chicago Classification 2012 distinguishes small (2-5 cm long) and large (> 5 cm) breaks as subtypes of weak peristalsis[15], while the HRM Working Group for Chicago Classification v3.0 proposes considering small breaks as normal and only large breaks as fragmented contractions[16]. However, in our series both types of break were significantly more frequent in the group with pathological OP acid exposure. The significantly larger number of contractions with breaks in these patients might conceivably result in ineffective reflux clearance[39]. The low- pressure segments anatomically correspond to the TZ from striated to smooth esophageal muscle, where the muscle types are imbricated[40]; an area of extreme hypotensive peristalsis correlates with incomplete bolus transit[41]. HRM combined with multichannel impedance may help clarify when the manometric characteristics in patients with pathological OP acid exposure are really associated with delayed reflux clearance. A recent study using this technique in patients with typical GERD reported that those with a pathological number of large breaks had significantly slower reflux clearance (BCT) in the supine position and longer acid exposure time[42].
The motility features of the distal esophagus (DCI, LES resting pressure and 4s-IRP) did not significantly differ in the two groups in this study but this is not really surprising; even in patients with normal distal reflux, the lack of effective proximal reflux clearance might allow a small amount of reflux to flow up from the distal esophagus to the larynx and pharynx, where even a single episode of LPR is considered pathological[25,43].
In conclusion, this study compared, for the first time, the results of OP pH monitoring and esophageal HRM. We found a significant correlation between patho-physiological features, particularly pathological OP acid exposure and esophageal motility. Further studies are now needed to establish whether the motility characteristics we found in patients with pathological OP acid exposure are the cause or consequence of pathological acid reflux.
In the last few years many extra-esophageal manifestations have been increasingly attributed to gastro-esophageal reflux (GER) and laryngopharyngeal reflux (LPR). It is not clear why in some patients GER is limited to the distal esophagus while in others it extends above the upper esophageal sphincter (UES).
A current hotspot is to define the cause-effect relationship between LPR and extra-esophageal syndromes since their diagnosis and treatment pose an important economic burden.
A new oropharyngeal (OP) pH probe is now available which is more sensitive than traditional pH sensors for faithfully monitoring the pH of OP reflux, and high-resolution esophageal manometry offers a major advance in defining esophageal motility abnormalities compared to conventional manometry. This is the first study comparing the two techniques.
The evidence of a correlation between pathological OP acid exposure and weak (altered) esophageal motility could change future therapeutic strategies.
According to the Montreal classification of gastro-esophageal reflux disease (GERD), the manifestations of the disease are divided into esophageal and extra-esophageal syndromes. Extra-esophageal syndromes are further divided into syndromes with an established association with GERD (cough, laryngitis, asthma, dental erosion) and syndromes with a proposed association with GERD (pharyngitis, sinusitis, idiopathic pulmonary fibrosis, recurrent otitis media). “LPR” is the term used to define the reflux of gastric content through the esophagus, reaching the upper UES and pharynx.
This study is deemed worthwhile since the authors investigated the relationship between pathological acid exposure and esophageal motility in patients with extra-esophageal syndromes suspected to be related to GERD. The authors also suggest that pathological OP acid exposure is associated with weaker proximal esophageal motility in patients with suspected GERD-related extra-esophageal syndromes. Although the results and discussions will satiate the readers’ interest, I have some comments mentioned below.
| 1. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920; quiz 1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2368] [Cited by in RCA: 2519] [Article Influence: 126.0] [Reference Citation Analysis (2)] |
| 2. | Vaezi MF, Hicks DM, Abelson TI, Richter JE. Laryngeal signs and symptoms and gastroesophageal reflux disease (GERD): a critical assessment of cause and effect association. Clin Gastroenterol Hepatol. 2003;1:333-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 206] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Wiener GJ, Koufman JA, Wu WC, Cooper JB, Richter JE, Castell DO. Chronic hoarseness secondary to gastroesophageal reflux disease: documentation with 24-h ambulatory pH monitoring. Am J Gastroenterol. 1989;84:1503-1508. [PubMed] |
| 4. | Yuksel ES, Slaughter JC, Mukhtar N, Ochieng M, Sun G, Goutte M, Muddana S, Gaelyn Garrett C, Vaezi MF. An oropharyngeal pH monitoring device to evaluate patients with chronic laryngitis. Neurogastroenterol Motil. 2013;25:e315-e323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Wiener GJ, Tsukashima R, Kelly C, Wolf E, Schmeltzer M, Bankert C, Fisk L, Vaezi M. Oropharyngeal pH monitoring for the detection of liquid and aerosolized supraesophageal gastric reflux. J Voice. 2009;23:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Ayazi S, Lipham JC, Hagen JA, Tang AL, Zehetner J, Leers JM, Oezcelik A, Abate E, Banki F, DeMeester SR. A new technique for measurement of pharyngeal pH: normal values and discriminating pH threshold. J Gastrointest Surg. 2009;13:1422-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Ates F, Vaezi MF. Approach to the patient with presumed extraoesophageal GERD. Best Pract Res Clin Gastroenterol. 2013;27:415-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Fouad YM, Katz PO, Hatlebakk JG, Castell DO. Ineffective esophageal motility: the most common motility abnormality in patients with GERD-associated respiratory symptoms. Am J Gastroenterol. 1999;94:1464-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 129] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | DiBaise JK, Lof J, Quigley EM. Can symptoms predict esophageal motor function or acid exposure in gastroesophageal reflux disease? A comparison of esophageal manometric and twenty-four-hour pH parameters in typical and extraesophageal gastroesophageal reflux disease. J Clin Gastroenterol. 2001;32:128-132. [PubMed] |
| 10. | Patti MG, Debas HT, Pellegrini CA. Clinical and functional characterization of high gastroesophageal reflux. Am J Surg. 1993;165:163-166; discussion 166-168. [PubMed] |
| 11. | Patcharatrakul T, Gonlachanvit S. Gastroesophageal reflux symptoms in typical and atypical GERD: roles of gastroesophageal acid refluxes and esophageal motility. J Gastroenterol Hepatol. 2014;29:284-290. [PubMed] |
| 12. | Pandolfino JE, Roman S. High-resolution manometry: an atlas of esophageal motility disorders and findings of GERD using esophageal pressure topography. Thorac Surg Clin. 2011;21:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice. 2002;16:274-277. [PubMed] |
| 14. | Schindler A, Mozzanica F, Ginocchio D, Peri A, Bottero A, Ottaviani F. Reliability and clinical validity of the Italian Reflux Symptom Index. J Voice. 2010;24:354-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJ; International High Resolution Manometry Working Group. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil. 2012;24 Suppl 1:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 614] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 16. | Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJ, Pandolfino JE; International High Resolution Manometry Working Group. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1373] [Cited by in RCA: 1487] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 17. | Bogte A, Bredenoord AJ, Oors J, Siersema PD, Smout AJ. Normal values for esophageal high-resolution manometry. Neurogastroenterol Motil. 2013;25:762-e579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 18. | Peng L, Patel A, Kushnir V, Gyawali CP. Assessment of upper esophageal sphincter function on high-resolution manometry: identification of predictors of globus symptoms. J Clin Gastroenterol. 2015;49:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Choi WS, Kim TW, Kim JH, Lee SH, Hur WJ, Choe YG, Lee SH, Park JH, Sohn CI. High-resolution Manometry and Globus: Comparison of Globus, Gastroesophageal Reflux Disease and Normal Controls Using High-resolution Manometry. J Neurogastroenterol Motil. 2013;19:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Ren Y, Tang X, Chen F, Deng Z, Wu J, Nei S, Jiang B, Gong W. Myotomy of Distal Esophagus Influences Proximal Esophageal Contraction and Upper Esophageal Sphincter Relaxation in Patients with Achalasia After Peroral Endoscopic Myotomy. J Neurogastroenterol Motil. 2016;22:78-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | de Bortoli N, Nacci A, Savarino E, Martinucci I, Bellini M, Fattori B, Ceccarelli L, Costa F, Mumolo MG, Ricchiuti A. How many cases of laryngopharyngeal reflux suspected by laryngoscopy are gastroesophageal reflux disease-related? World J Gastroenterol. 2012;18:4363-4370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 102] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Milstein CF, Charbel S, Hicks DM, Abelson TI, Richter JE, Vaezi MF. Prevalence of laryngeal irritation signs associated with reflux in asymptomatic volunteers: impact of endoscopic technique (rigid vs. flexible laryngoscope). Laryngoscope. 2005;115:2256-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Francis DO, Rymer JA, Slaughter JC, Choksi Y, Jiramongkolchai P, Ogbeide E, Tran C, Goutte M, Garrett CG, Hagaman D. High economic burden of caring for patients with suspected extraesophageal reflux. Am J Gastroenterol. 2013;108:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 24. | Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328; quiz 329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1136] [Cited by in RCA: 1148] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 25. | Hoppo T, Sanz AF, Nason KS, Carroll TL, Rosen C, Normolle DP, Shaheen NJ, Luketich JD, Jobe BA. How much pharyngeal exposure is “normal”? Normative data for laryngopharyngeal reflux events using hypopharyngeal multichannel intraluminal impedance (HMII). J Gastrointest Surg. 2012;16:16-24; discussion 24-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 26. | Hayat JO, Yazaki E, Moore AT, Hicklin L, Dettmar P, Kang JY, Sifrim D. Objective detection of esophagopharyngeal reflux in patients with hoarseness and endoscopic signs of laryngeal inflammation. J Clin Gastroenterol. 2014;48:318-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Yadlapati R, Adkins C, Jaiyeola DM, Lidder AK, Gawron AJ, Tan BK, Shabeeb N, Price CP, Agrawal N, Ellenbogen M. Abilities of Oropharyngeal pH Tests and Salivary Pepsin Analysis to Discriminate Between Asymptomatic Volunteers and Subjects With Symptoms of Laryngeal Irritation. Clin Gastroenterol Hepatol. 2016;14:535-542.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 28. | Vailati C, Mazzoleni G, Bondi S, Bussi M, Testoni PA, Passaretti S. Oropharyngeal pH monitoring for laryngopharyngeal reflux: is it a reliable test before therapy? J Voice. 2013;27:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Worrell SG, DeMeester SR, Greene CL, Oh DS, Hagen JA. Pharyngeal pH monitoring better predicts a successful outcome for extraesophageal reflux symptoms after antireflux surgery. Surg Endosc. 2013;27:4113-4118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Orlando RC. Overview of the mechanisms of gastroesophageal reflux. Am J Med. 2001;111 Suppl 8A:174S-177S. [PubMed] |
| 31. | Savarino E, Gemignani L, Pohl D, Zentilin P, Dulbecco P, Assandri L, Marabotto E, Bonfanti D, Inferrera S, Fazio V. Oesophageal motility and bolus transit abnormalities increase in parallel with the severity of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2011;34:476-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 32. | Knight RE, Wells JR, Parrish RS. Esophageal dysmotility as an important co-factor in extraesophageal manifestations of gastroesophageal reflux. Laryngoscope. 2000;110:1462-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Daum C, Sweis R, Kaufman E, Fuellemann A, Anggiansah A, Fried M, Fox M. Failure to respond to physiologic challenge characterizes esophageal motility in erosive gastro-esophageal reflux disease. Neurogastroenterol Motil. 2011;23:517-e200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Vardar R, Sweis R, Anggiansah A, Wong T, Fox MR. Upper esophageal sphincter and esophageal motility in patients with chronic cough and reflux: assessment by high-resolution manometry. Dis Esophagus. 2013;26:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Almansa C, Smith JA, Morris J, Crowell MD, Valdramidou D, Lee AS, DeVault KR, Houghton LA. Weak peristalsis with large breaks in chronic cough: association with poor esophageal clearance. Neurogastroenterol Motil. 2015;27:431-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Szczesniak MM, Williams RB, Cook IJ. Mechanisms of esophago-pharyngeal acid regurgitation in human subjects. PLoS One. 2011;6:e22630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Szczesniak MM, Williams RB, Brake HM, Maclean JC, Cole IE, Cook IJ. Upregulation of the esophago-UES relaxation response: a possible pathophysiological mechanism in suspected reflux laryngitis. Neurogastroenterol Motil. 2010;22:381-386, e89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Shaker R, Babaei A, Naini SR. Prevention of esophagopharyngeal reflux by augmenting the upper esophageal sphincter pressure barrier. Laryngoscope. 2014;124:2268-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Pohl D, Ribolsi M, Savarino E, Frühauf H, Fried M, Castell DO, Tutuian R. Characteristics of the esophageal low-pressure zone in healthy volunteers and patients with esophageal symptoms: assessment by high-resolution manometry. Am J Gastroenterol. 2008;103:2544-2549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Meyer GW, Austin RM, Brady CE, Castell DO. Muscle anatomy of the human esophagus. J Clin Gastroenterol. 1986;8:131-134. [PubMed] |
| 41. | Fox M, Hebbard G, Janiak P, Brasseur JG, Ghosh S, Thumshirn M, Fried M, Schwizer W. High-resolution manometry predicts the success of oesophageal bolus transport and identifies clinically important abnormalities not detected by conventional manometry. Neurogastroenterol Motil. 2004;16:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 179] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 42. | Ribolsi M, Balestrieri P, Emerenziani S, Guarino MP, Cicala M. Weak peristalsis with large breaks is associated with higher acid exposure and delayed reflux clearance in the supine position in GERD patients. Am J Gastroenterol. 2014;109:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 43. | Zerbib F, Roman S, Bruley Des Varannes S, Gourcerol G, Coffin B, Ropert A, Lepicard P, Mion F; Groupe Français De Neuro-Gastroentérologie. Normal values of pharyngeal and esophageal 24-hour pH impedance in individuals on and off therapy and interobserver reproducibility. Clin Gastroenterol Hepatol. 2013;11:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Goda K, Lakatos PL S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF