Published online Oct 28, 2016. doi: 10.3748/wjg.v22.i40.8949
Peer-review started: June 28, 2016
First decision: August 22, 2016
Revised: September 2, 2016
Accepted: September 28, 2016
Article in press: September 28, 2016
Published online: October 28, 2016
Processing time: 124 Days and 2.7 Hours
To evaluate the diagnostic performance of computed tomography (CT) volumetry for discriminating the fibrosis stage in patients with nonalcoholic fatty liver disease (NAFLD).
A total of 38 NAFLD patients were enrolled. On the basis of CT imaging, the volumes of total, left lateral segment (LLS), left medial segment, caudate lobe, and right lobe (RL) of the liver were calculated with a dedicated liver application. The relationship between the volume percentage of each area and fibrosis stage was analyzed using Spearman’s rank correlation coefficient. A receiver operating characteristic (ROC) curve analysis was performed to determine the accuracy of CT volumetry for discriminating fibrosis stage.
The volume percentages of the caudate lobe and the LLS significantly increased with the fibrosis stage (r = 0.815, P < 0.001; and r = 0.465, P = 0.003, respectively). Contrarily, the volume percentage of the RL significantly decreased with fibrosis stage (r = -0.563, P < 0.001). The volume percentage of the caudate lobe had the best diagnostic accuracy for staging fibrosis, and the area under the ROC curve values for discriminating fibrosis stage were as follows: ≥ F1, 0.896; ≥ F2, 0.929; ≥ F3, 0.955; and ≥ F4, 0.923. The best cut-off for advanced fibrosis (F3-F4) was 4.789%, 85.7% sensitivity and 94.1% specificity.
The volume percentage of the caudate lobe calculated by CT volumetry is a useful diagnostic parameter for staging fibrosis in NAFLD patients.
Core tip: This is a retrospective study to elucidate the morphological change in nonalcoholic fatty liver disease (NAFLD) using computed tomography (CT) volumetry and to evaluate the diagnostic performance of CT volumetry for discriminating the fibrosis stage in NAFLD. The volume percentages of the caudate lobe calculated by CT volumetry were significantly increased with the increase in fibrosis stage in NAFLD. The volume percentage of the caudate lobe is a useful diagnostic parameter for staging fibrosis in patients with NAFLD. The evaluation of liver volume using CT volumetry is useful for predicting the fibrosis stage in NAFLD.
- Citation: Fujita N, Nishie A, Asayama Y, Ishigami K, Ushijima Y, Takayama Y, Okamoto D, Shirabe K, Yoshizumi T, Kotoh K, Furusyo N, Hida T, Oda Y, Fujioka T, Honda H. Fibrosis in nonalcoholic fatty liver disease: Noninvasive assessment using computed tomography volumetry. World J Gastroenterol 2016; 22(40): 8949-8955
- URL: https://www.wjgnet.com/1007-9327/full/v22/i40/8949.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i40.8949
Nonalcoholic fatty liver disease (NAFLD) is currently the most prevalent liver disease worldwide with a prevalence of 20%-30%[1,2]. The spectrum of NAFLD ranges from simple steatosis to nonalcoholic steatohepatitis (NASH), which can progress to end-stage cirrhosis[1,3]. It has been reported that advanced fibrosis or cirrhosis of NAFLD represents a clear worsening of prognosis[4]. Monitoring the fibrosis stage is therefore important in the treatment of NAFLD.
A liver biopsy is considered the reference standard for the diagnosis of NAFLD, but repeating a liver biopsy is not desirable because of the risk of complications and the costs[5]. A noninvasive tool for the diagnosis of NAFLD would be clinically useful, and the utility of magnetic resonance (MR) imaging for detecting the fibrosis stage in NAFLD has been reported[6-8]. To the best of our knowledge, there have been no studies describing the diagnostic feasibility of computed tomography (CT) imaging for assessment of the fibrosis stage of NAFLD.
Generally, the morphology of the liver changes as the fibrosis stage advances, and its pattern depends on the primary disease. The morphological change of the liver as the fibrosis stage advances in other liver diseases such as chronic viral hepatitis, alcoholic hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis and autoimmune hepatitis has been well documented[9], but the morphologic change of the liver caused by NAFLD is still unclear. Multidetector CT (MDCT) has been demonstrated to measure liver volume correctly, and CT volumetry has been used widely- especially as a method for preoperatively assessing the volume of the liver[10]. It would be clinically useful to be able to use CT volumetry to elucidate this morphological change and to predict the fibrosis stage in NAFLD noninvasively.
Our purposes in the present study were thus to elucidate the morphological change in NAFLD using CT volumetry and to evaluate the diagnostic performance of CT volumetry for discriminating the fibrosis stage in NAFLD.
Our institutional review board approved this study, and the requirements for informed consent were waived due to the retrospective design.
We enrolled 38 patients who underwent contrast-enhanced CT and were diagnosed as having NAFLD based on histological findings from March 2004 to August 2013 at our institution. All patients had no alcohol consumption habit and no evidence of a specific cause for liver disease such as viral hepatitis B or C, hemochromatosis, or autoimmune or cholestatic liver disease. Of the 38 patients, 20 were men and 18 were women. The mean age was 50.7 years, ranging from 20 to 82 years. The mean body mass index was 26.6 kg/m2, ranging from 20.3 to 36.8 kg/m2. The prevalence of diabetes mellitus was 36.8% (14 patients); that of dyslipidemia was 18.4% (7 patients), and that of hypertension was 31.6% (12 patients). Twenty-eight patients were diagnosed as having NAFLD based on liver biopsy, and the diagnoses of the other 10 patients were based on a surgically resected specimen. Of the 10 patients who underwent surgery, liver resection was performed in three patients for hepatocellular carcinoma (HCC), and liver transplantation was performed in seven patients for decompensated cirrhosis.
Because we used a retrospective design, various types of CT scanners and different CT protocols had been used. Dynamic CT studies were performed with a 4-slice (Aquilion: Toshiba Medical Systems, Tokyo: n = 9; or Somatom Plus 4 Volume Zoom: Siemens-Asahi Medical Technologies, Tokyo: n = 2), 64-slice (Aquilion: Toshiba Medical Systems: n = 16; or Brilliance 64: Philips, Cleveland OH, United States: n = 5), 256-slice (Brilliance iCT: Philips: n = 4) or 320-slice (Aquilion One: Toshiba Medical Systems: n = 2) MDCT scanner. Each patient received intravenous nonionic contrast material containing 300 mgI/mL or 370 mgI/mL iopamidol (Iopamiron; Bayer, Osaka, Japan) by an automated power injector, and the portal phase was acquired. The details of the CT protocols are shown in Table 1. The interval between the preoperative MDCT study and the biopsy or surgery ranged from 1 to 244 d (mean ± SD, 44.0 ± 56.7 d).
| CT scanner | 4-slice MDCT | 64- or 256- or 320- slice MDCT | ||||||
| No. of patients | 4 | 2 | 5 | 5 | 2 | 10 | 4 | 6 |
| Contrast material (mgI/mL) | 370 | 300 | 370 | 370 | 370 | 370 | 300 | 370 |
| Contrast material dose | 100 mL | 100 mL | 600 mgI/kg | 100 mL | 100 mL | 600 mgI/kg | 100 mL | 600 mgI/kg |
| Injection rate | 2.5 mL/s | 2.5 mL/s | 20 s1 | 3 mL/s | 2.5 mL/s | 30 s1 | 2.5 mL/s | 20 s1 |
| Portal phase delay (s) | 70 | 70 | 55 | 70 | 70 | 60 | 60 | 70 |
| Tube voltage (kVp) | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 |
| Tube current (mAs) | 300 | 300 | 300 | Auto | Auto | Auto | Auto | Auto |
| Reconstruction thickenss (mm) | 5 | 3 | 3 | 5 | 3 | 1 | 5 | 2 |
Two radiologists (Nishie A and Fujita N, with 21 and 12 years of experience in abdominal imaging, respectively) who were blinded to the clinical and pathologic results measured the liver segment volumes on portal phase images in a consensus fashion. The portal phase data were transferred to a workstation (Intellispace Portal 6.0, Philips) and analyzed with a dedicated liver application (Liver Analysis: Philips).
First, the total liver and vessels (hepatic and portal venous trees) were segmented automatically. If liver lesions such as a hepatic cyst, cavernous hemangioma or HCC were present, the lesions were segmented semi-automatically and subtracted from the liver volume. In this study, we subtracted the volume of live lesions with a diameter < 5 cm.
Second, we calculated the volumes of the total, the left lateral segment (LLS), the left medial segment (LMS), the caudate lobe, and the right lobe (RL) of the liver semi-automatically using the Philips “Liver Analysis” application by the position of 10 anatomical landmarks: inferior vena cava, right portal bifurcation, right hepatic vein, middle hepatic vein, umbilical fissure, left portal bifurcation, tip left liver, superficial ligamentum venosum, deep ligamentum venosum, and superior deep ligamentum venosum. If corrections were necessary, we corrected the data manually on the application to achieve a precise final result. We then determined the volume percentage of the LLS (LLS volume/total volume), LMS (LMS volume/total volume), caudate lobe (caudate lobe volume/total volume) and RL (RL volume/total volume).
All of the liver specimens were reviewed by one pathologist (Hida T) who was blinded to the patients’ information. The criteria for the fibrosis severity of NAFLD was based on the classification by Brunt et al[11], and liver fibrosis was staged as follows: stage 0 (F0), no fibrosis; stage 1 (F1), zone 3 perisinusoidal/pericellular fibrosis; stage 2 (F2), zone 3 perisinusoidal/pericellular fibrosis with focal or extensive periportal fibrosis; stage 3 (F3), zone 3 perisinusoidal/pericellular fibrosis and portal fibrosis with focal or extensive bridging fibrosis; stage 4 (F4), liver cirrhosis[11].
We analyzed the correlation between the volume percentage of each region and the fibrosis stage by Spearman’s rank correlation test. A correlation was considered strong if the absolute value of the correlation coefficient (r) was > 0.7, moderate if the r was 0.4-0.7, weak if the r was 0.2-0.4, and absent if the r was < 0.2. The diagnostic accuracy of volume percentage was assessed by a receiver operating characteristic curve analysis. We calculated the area under the curve (Az value) and the optimal cutoff value for differentiating ≥ F1 from F0, ≥ F2 from ≤ F1, ≥ F3 from ≤ F2, and F4 from ≤ F3. Standard definitions were used for the calculation of the sensitivity, specificity, accuracy, positive predictive value and negative predictive value. JMP 11.0.0 software (SAS Institute, Cary, NC, United States) was used for the analyses. P values < 0.05 were considered significant.
Of the 38 patients with histological data, the distribution of fibrosis stage was as follows: F0 in 28.9% (11/38), F1 in 13.2% (5/38), F2 in 2.6% (1/38), F3 in 23.7% (9/38), and F4 in 31.6% (12/38).
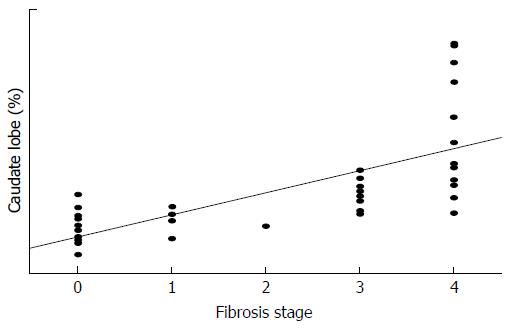
Table 2 provides the correlations between liver volumes and fibrosis stage. With the increase in the liver fibrosis stage, the volume percentages of the LLS and the caudate lobe increased significantly (P = 0.003, r = 0.465 and P < 0.001, r = 0.815, respectively) and that of the RL decreased significantly (P < 0.001, r = -0.563). A strong correlation was observed between the liver fibrosis stage and the volume percentage of the caudate lobe (Figure 1). There was no correlation between fibrosis stage and the total volume or the volume percentage of the LMS.
| Parameter | F0 (n = 11) | F1 (n = 5) | F2 (n = 1) | F3 (n = 9) | F4 (n = 12) | r | P value |
| Total volume (cm3) | 1252.3 ± 155.1 | 1280.4 ± 240.0 | 1235.8 | 1364.5 ± 320.0 | 1030.2 ± 366.1 | -0.193 | NS |
| LLS (%) | 18.3 ± 1.9 | 22.1 ± 3.9 | 21.5 | 25.3 ± 8.8 | 26.5 ± 8.8 | 0.465 | 0.003 |
| LMS (%) | 12.6 ± 1.7 | 12.0 ± 3.7 | 16.2 | 11.3 ± 2.8 | 11.1 ± 3.5 | -0.248 | NS |
| Caudate lobe (%) | 3.0 ± 1.2 | 3.7 ± 0.8 | 3.2 | 5.3 ± 1.0 | 9.3 ± 4.1 | 0.815 | < 0.001 |
| RL (%) | 66.1 ± 2.2 | 62.1 ± 5.6 | 59.1 | 58.0 ± 10.4 | 52.7 ± 10.5 | -0.563 | < 0.001 |
Table 3 summarizes the diagnostic performance of the volume percentage of the caudate lobe for predicting the fibrosis stage. The volume percentage of the caudate lobe was the best to discriminate ≥ F3 from ≤ F2, with an Az value of 0.955.
| F0 vs ≥ F1 | ≤ F1 vs ≥ F2 | ≤ F2 vs ≥ F3 | ≤ F3 vs F4 | |
| Az value | 0.896 | 0.929 | 0.955 | 0.923 |
| Cutoff value | 3.957 | 4.789 | 4.789 | 5.834 |
| Sensitivity (%) | 88.9 | 81.8 | 85.7 | 83.3 |
| Specificity (%) | 90.9 | 93.8 | 94.1 | 88.4 |
| Accuracy (%) | 89.4 | 86.8 | 89.4 | 86.8 |
| PPV (%) | 96.0 | 94.7 | 94.7 | 76.9 |
| NPV (%) | 76.9 | 78.9 | 84.2 | 92.0 |
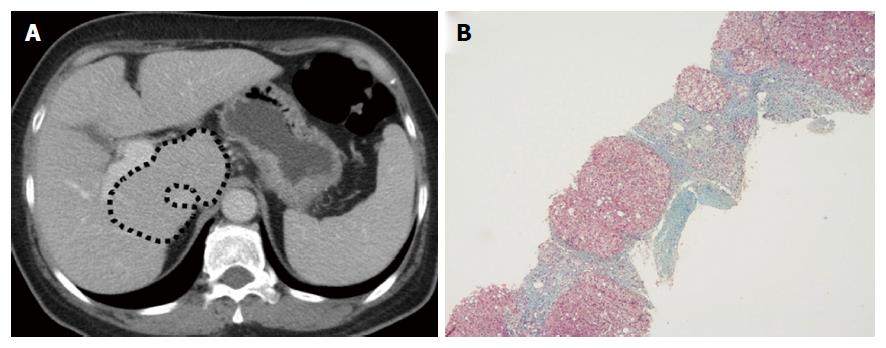
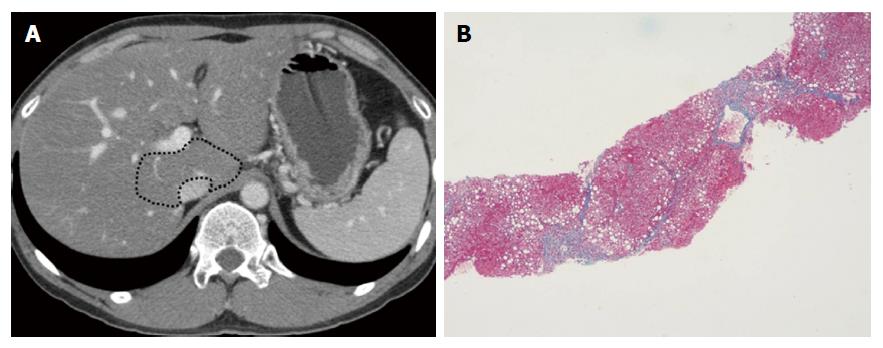
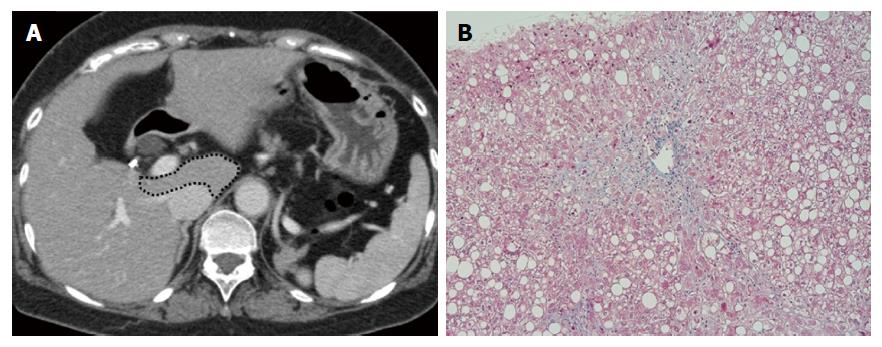
Figures 2-4 present representative patient images.
The major causes of liver fibrosis include hepatitis B and C, alcohol abuse, primary sclerosing cholangitis, primary biliary cirrhosis and autoimmune hepatitis. In such diseases, the morphologic change of the liver with the advance in fibrosis stage has been well reported[9]. However, to the best of our knowledge, the morphologic change of the liver with the advanced stage of NAFLD is still unclear. In the present study, as the fibrosis stage advanced, the volume percentage of the LLS and the caudate lobe increased significantly, and that of the RL decreased significantly in the NAFLD patients.
Focal hypertrophy of the caudate lobe or LLS and atrophy of the RL are common findings in liver cirrhosis[9]. However, a strong correlation was observed between fibrosis stage and the volume percentage of the caudate lobe in our study. Ozaki et al[12] recently reported that hypertrophy of the caudate lobe progressed more in alcoholism and NASH patients than in virus-related etiologies in patients with liver cirrhosis, Child-Pugh Class A. It was also reported that enlargement of the caudate lobe was a more frequent finding in alcoholic cirrhosis than virus-induced cirrhosis[13]. Considering our present findings and the above reports, we suggest that hypertrophy of the caudate lobe is a characteristic change of NAFLD as well as alcoholism, despite their distinctly different clinical histories.
It has been reported that fibrosis in NASH worsened in 30%-40% and 5%-25% of NASH cases that advanced to liver cirrhosis over a period of 5-10 years[14,15]. Advanced fibrosis (≥ F3) or cirrhosis (F4) of NASH represents a clear worsening of prognosis[4]. Liver biopsy has been considered the reference standard in the assessment of liver fibrosis in NALFD, but it is invasive and cannot be repeated frequently. Several noninvasive imaging methods have thus been developed to estimate liver fibrosis in NAFLD. Kim et al[6] reported that MR elastography was a useful diagnostic tool for detecting advanced fibrosis in NAFLD (≥ F3, Az value = 0.954). Ding et al[7] reported the usefulness of T1 mapping on Gd-EOB-DTPA-enhanced MR imaging. They asserted that both the T1 relaxation times of the liver parenchyma and the decreased rate were useful to diagnose advanced fibrosis in NAFLD (≥ F3, Az value = 0.95 and Az value = 0.95, respectively).
However, these imaging techniques are not widely spread and may not be suitable for routine imaging examinations. The results of our present study indicate that the volume percentage of the caudate lobe has a high diagnostic performance for the fibrous staging of NAFLD (Az value = 0.955). The Az value that we obtained in this study is equivalent to that of previous studies using MR imaging[6,7]. This suggests that routine CT imaging using a combination of CT volumetry would be an effective and noninvasive way to diagnose hepatic fibrosis in NAFLD.
CT volumetry is now widely used for the preoperative volumetric assessment of the liver[10]. Traditionally, CT volumetry is performed by manually tracing the liver and by the summation of the liver volume in axial sections. However, such manual methods are operator-dependent and require a significant amount of time and attention. Automated and semi-automated versions of CT volumetry have been proposed, but automatic CT volumetry may tend to fail for CT images that are low-contrast and have missing edges due to similar intensities of adjacent organs. In the present study, we used a semi-automated CT volumetry method that provides more flexibility than an automated method. Indeed, Gotra et al[16] reported that a semi-automated method substantially shortened the interaction time while preserving high repeatability and agreement with manual volumetry.
Our study has some limitations. First, our population of 38 patients was small, and the number of cases with each degree of fibrosis was not uniform. Second, we diagnosed the fibrosis stage of NALFD based on a liver biopsy in 28 of the 38 patients. Assessments of liver biopsy results can have high inter- and intraobserver variability[17], and the reproducibility of the histological fibrosis staging could not be examined in this study. Third, we used a retrospective design, and various types of CT scanners and different CT protocols were used.
In conclusion, the volume percentages of the LLS and the caudate lobe calculated by CT volumetry were significantly increased and that of the RL was significantly decreased with the increase in fibrosis stage in NAFLD. The volume percentage of the caudate lobe is a useful diagnostic parameter for staging fibrosis in patients with NAFLD. The evaluation of liver volume using CT volumetry is useful for predicting the fibrosis stage in NAFLD.
We thank Dr. Yoshihiko Maehara, Department of Surgery and Science, Kyushu University, for providing the clinical information for this manuscript.
Nonalcoholic fatty liver disease (NAFLD) is currently the most prevalent liver disease worldwide. Advanced fibrosis or cirrhosis of NAFLD represents a clear worsening of prognosis. Monitoring the fibrosis stage is important in the treatment of NAFLD. The morphological change of the liver as the fibrosis stage advances in NAFLD is still unclear. In this study, we elucidated the morphological change in NAFLD using computed tomography (CT) volumetry and evaluated the diagnostic performance of CT volumetry for discriminating the fibrosis stage in NAFLD.
CT volumetry has been used as a method for assessing the volume of the liver. The results of this study contribute to clarifying the diagnostic potential of CT volumetry for the fibrosis stage in NAFLD.
These results indicate that the volume percentage of the caudate lobe has a high diagnostic performance for the fibrous staging of NAFLD (≥ F3 from ≤ F2, Az value = 0.955).
This study suggests that the evaluation of liver volume using CT volumetry is useful for predicting the fibrosis stage in NAFLD.
CT volumetry: A method that enables assessment the volume of the liver.
Recommend of the manuscript is to be accepted, despite several limitations of the study that you already mentioned on the DISCUSSION section.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Souftas VD S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
| 1. | Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann Epidemiol. 2007;17:863-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 2718] [Article Influence: 123.5] [Reference Citation Analysis (3)] |
| 3. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3739] [Article Influence: 155.8] [Reference Citation Analysis (4)] |
| 4. | Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Heuman D, Coterrell A, Fisher RA. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 366] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 5. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1759] [Article Influence: 70.4] [Reference Citation Analysis (1)] |
| 6. | Kim D, Kim WR, Talwalkar JA, Kim HJ, Ehman RL. Advanced fibrosis in nonalcoholic fatty liver disease: noninvasive assessment with MR elastography. Radiology. 2013;268:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 7. | Ding Y, Rao SX, Meng T, Chen C, Li R, Zeng MS. Usefulness of T1 mapping on Gd-EOB-DTPA-enhanced MR imaging in assessment of non-alcoholic fatty liver disease. Eur Radiol. 2014;24:959-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Wu Z, Matsui O, Kitao A, Kozaka K, Koda W, Kobayashi S, Ryu Y, Minami T, Sanada J, Gabata T. Usefulness of Gd-EOB-DTPA-enhanced MR imaging in the evaluation of simple steatosis and nonalcoholic steatohepatitis. J Magn Reson Imaging. 2013;37:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Dodd GD, Baron RL, Oliver JH, Federle MP. Spectrum of imaging findings of the liver in end-stage cirrhosis: part I, gross morphology and diffuse abnormalities. AJR Am J Roentgenol. 1999;173:1031-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Lim MC, Tan CH, Cai J, Zheng J, Kow AW. CT volumetry of the liver: where does it stand in clinical practice? Clin Radiol. 2014;69:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2702] [Cited by in RCA: 2918] [Article Influence: 108.1] [Reference Citation Analysis (2)] |
| 12. | Ozaki K, Matsui O, Kobayashi S, Minami T, Kitao A, Gabata T. Morphometric changes in liver cirrhosis: aetiological differences correlated with progression. Br J Radiol. 2016;89:20150896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Okazaki H, Ito K, Fujita T, Koike S, Takano K, Matsunaga N. Discrimination of alcoholic from virus-induced cirrhosis on MR imaging. AJR Am J Roentgenol. 2000;175:1677-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1647] [Cited by in RCA: 1731] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 15. | Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 663] [Article Influence: 31.6] [Reference Citation Analysis (1)] |
| 16. | Gotra A, Chartrand G, Massicotte-Tisluck K, Morin-Roy F, Vandenbroucke-Menu F, de Guise JA, Tang A. Validation of a semiautomated liver segmentation method using CT for accurate volumetry. Acad Radiol. 2015;22:1088-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1580] [Article Influence: 65.8] [Reference Citation Analysis (0)] |