Published online Oct 28, 2016. doi: 10.3748/wjg.v22.i40.8918
Peer-review started: June 29, 2016
First decision: August 8, 2016
Revised: August 24, 2016
Accepted: September 14, 2016
Article in press: September 14, 2016
Published online: October 28, 2016
Processing time: 120 Days and 13.9 Hours
To evaluate the effects of melatonin (Mel) on oxidative stress in an experimental model of bile duct ligation (BDL).
Male Wistar rats (n = 32, weight ± 300 g) were allocated across four groups: CO (sham BDL), BDL (BDL surgery), CO + Mel (sham BDL and Mel administration) and BDL + Mel (BDL surgery and Mel administration). Mel was administered intraperitoneally for 2 wk, starting on postoperative day 15, at a dose of 20 mg/kg.
Mel was effective at the different standards, reestablishing normal liver enzyme levels, reducing the hepatosomatic and splenosomatic indices, restoring lipoperoxidation and antioxidant enzyme concentrations, reducing fibrosis and inflammation, and thereby reducing liver tissue injury in the treated animals.
The results of this study suggest a protective effect of Mel when administered to rats with secondary biliary cirrhosis induced by BDL.
Core tip: Secondary biliary cirrhosis is a late complication of prolonged extrahepatic bile duct obstruction that leads to structural and functional changes in the liver. Melatonin, the main product of the pineal gland, provides hepatic protection in the experimental model of bile duct ligation.
- Citation: Colares JR, Schemitt EG, Hartmann RM, Licks F, Soares MDC, Bosco AD, Marroni NP. Antioxidant and anti-inflammatory action of melatonin in an experimental model of secondary biliary cirrhosis induced by bile duct ligation. World J Gastroenterol 2016; 22(40): 8918-8928
- URL: https://www.wjgnet.com/1007-9327/full/v22/i40/8918.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i40.8918
The liver has a complex structure, allowing it to play a key role in operation and maintenance of several vital functions of the organism, including synthesis activity and excretion of substances. In the liver lobes, hepatocytes are arranged in an orderly fashion out from a central vein, forming the sinusoids, from which they are separated by a narrow space (the space of Disse). This space is the site of the hepatic stellate cells (HSCs), which are known to possess contractile and fibrogenic properties, as well as the ability to synthesize extracellular matrix (ECM)[1-3].
Obstruction of the biliary tract is a congestive process that leads to numerous changes, such as ductular proliferation, stellate cell activation, and accumulation of ECM in the space of Disse. Occurrence of these changes may lead to the development of liver fibrosis, which, in turn, can lead to secondary biliary cirrhosis[4]. Cirrhosis of the liver represents the most advanced stage of fibrosis, in which there is evident loss of structure of the hepatic parenchyma. It is directly associated with development of septa and fibrotic nodules, changes in hepatic blood flow, and high risk of liver failure[5].
Studies have shown that HSCs are directly involved in the process of fibrosis formation and that their activation is influenced by products generated from lipid peroxidation (LPO), formation of reactive oxygen species (ROS), and presence of inflammatory mediators such as tumor necrosis factor-alpha (TNF-α), inducible nitric oxide synthase (iNOS), interleukins, and nuclear factor-kappa B[4,6].
As cirrhosis constitutes a major public health problem[7], much research is being conducted to develop and test different substances that could be used in its treatment. The objective of such substances aims to improve quality of life, increase survival, slow disease progression, and, possibly, mitigate the damage caused by formation of ROS and free radicals (FRs)[8,9].
Prolonged obstruction of the bile duct in rats is an experimental model for induction of secondary biliary cirrhosis[10]. In this model, the characteristic features of the disease are established at approximately 28 d[10]. Studies have demonstrated that the changes occurring in cirrhosis in human patients are similar to those found in experimental models, including jaundice, hepatomegaly, splenomegaly, abnormal gas exchange, and oxidative damage[11-15].
Melatonin (Mel; N-acetyl-5-methoxytryptamine) is the main product synthesized by the pineal gland, which produces Mel in a rhythmic manner, with production inhibited by light, so that its peak production occurs during the dark phase[16,17]. Several effects have been attributed to Mel, including antioxidant capacity, as well as anti-inflammatory and immunomodulatory properties[18-21].
There is an existing important link between cirrhosis, inflammation and oxidative stress; in this sense, treatments are required to protect the liver against these types of damage. Therefore, this present study investigated whether Mel (an anti-inflammatory agent and antioxidant) would afford hepatic-protection in an experimental model of cirrhosis.
All animal procedures were conducted in accordance with the recommendations of the Health Research Ethics Committee of the Research and Graduate Studies Group at the Hospital de Clínicas de Porto Alegre (HCPA) in Brazil (approval number 14-0474), and as recommended in the Guide for the Care and Use of Laboratory Animals[22,23]. The sample comprised male Wistar rats n = 32, weight ± 300 g) that were allocated across four groups: CO [sham bile duct ligation (BDL)], BDL (BDL surgery), CO + Mel (sham BDL and Mel administration) and BDL + Mel (BDL surgery and Mel administration). Cirrhosis was induced surgically by BDL as described by Kountouras et al[10].
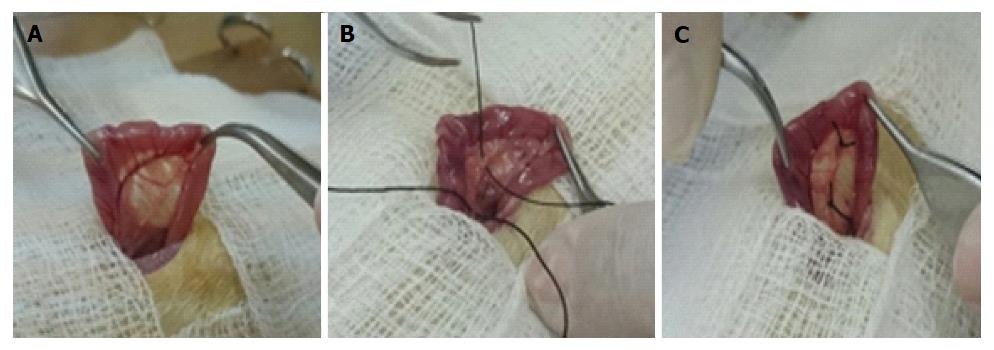
During the experiment, the animals were kept in boxes lined with wood shavings, under a 12-h light/dark cycle and controlled temperature conditions (18-22 °C), with free access to water and chow. As shown in Figure 1A, animals in the CO and CO + Mel groups only underwent localization and manipulation of the bile duct (sham surgery). Figure 1B and C show the procedures performed in the BDL and BDL + Mel groups respectively: after localization of the bile duct, it was isolated and tied off with two knots made with 3-0 silk thread. All animals were euthanized at 29 d after the start of the experiment[24].
Treatment started on day 15 after BDL surgery. Mel was administered at a dose of 20 mg/kg body weight, always at 7:00 p.m., away from light.
After the blood was collected through the retro-orbital plexus and placed in assay tubes with heparin, it was centrifuged at 4000 rpm for 10-min time. The precipitate was displaced and the plasma was removed with pipette (Labsystems 4500, 100-200 μL) for the different analyses of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (AP) via commercial kit Labtest®.
For the preparation of the homogenate we used 9 mL of phosphate buffered solution (1.15% KCl) per gram of tissue (liver) and phenylmethylsulfonyl fluoride at a concentration of 100 mmol/L in isopropanol (10 μL/mL of KCl). The tissue was homogenized in ULTRA-TURRAX for 40 s at 0-2 °C and subsequently centrifuged for 10 min at 3000 rpm in a refrigerated centrifuge. The precipitate was discarded and the supernatant removed and frozen at -80 °C for subsequent biochemical analyses[25].
Activity of the liver enzymes AST and ALT, which are markers of hepatocyte integrity, were measured by the ultraviolet kinetic method. AP was measured by the colorimetric method. All tests were performed in plasma, under routine HCPA laboratory methods, using a Liquiform Labs® test commercial kit.
The liver and spleen were resected and weighed for derivation of the hepatosomatic index (HSI) and splenosomatic index (SSI), which were calculated as the percentage of total organ (liver and spleen) weight divided by the body weight of the animal: HSI = liver weight (g)/rat weight (g) × 100; SSI = spleen weight (g)/rat weight (g) × 100[26].
Liver tissue samples were placed in test tubes containing a mixture of trichloroacetic acid (TCA) 10% and thiobarbituric acid (TBA) 0.67%, heated at 100 °C in a water bath for 15 min, and cooled on ice for approximately 5 min. TBA reacts with LPO products to form a Schiff base, whereas TCA is used to denature proteins present and acidify the reaction. After cooling the samples, 1.5 mL of n-butyl alcohol was added to extract the formed pigment. Samples were stirred for 45 s and centrifuged for 10 min at 3000 rpm. Finally, the stained product present in the top fraction was read in a spectrophotometer at a wavelength of 535 nm. The TBARS concentration obtained was expressed as nmol/mg protein[27].
Superoxide dismutase: The activity of superoxide dismutase (SOD) is defined by its ability to inhibit the reaction of superoxide radicals with adrenaline, and was monitored spectrophotometrically at 560 nm. Results were expressed as USOD/mg protein[28].
Catalase: The activity of Catalase (CAT) was determined by measuring the decrease in absorption in action medium containing 50 mmol/L phosphate buffered saline (pH 7.2) and 0.3 mol/L hydrogen peroxide. The enzyme activity was assayed spectrophotometrically at 240 nm and expressed as pmol/mg protein[29].
Glutathione peroxidase: The activity of the antioxidant enzyme glutathione peroxidase (GPx) was assessed by the NADPH oxidation rate in the presence of reduced glutathione (GSH) and glutathione reductase. Sodium azide was added to inhibit CAT activity. The enzyme activity was measured spectrophotometrically at 340 nm and expressed as nmol/min/mg protein[30].
Glutathione S-transferase: The glutathione S-transferase (GST) activity assay is based on an enzyme reaction which at 30 °C catalyzes the formation of 1 μmol DNP-SG using a GSH concentration of 1 mmol/L and chloro dinitrobenzene (CDNB). The enzyme activity was measured spectrophotometrically at 340 nm and expressed as μmol/min/mg protein[31].
GSH reduced: To prepare the homogenate for measuring levels of GSH reduced, for every 1 g of tissue, 20 mL of perchloric acid (2 mmol/L) + EDTA (4 mmol/L) was diluted in 1 mL H2O. The levels GSH were evaluated spectrophotometrically at 412 nm by quantifying intracellular levels of GSH from modification of 2-nitrobenzoic acid and expressed as μmol/mg protein[32].
After anatomical dissection of the liver of each animal, approximately 2 cm were removed for histological evaluation. The tissues were isolated and immersed in 10% buffered formalin for 24 h for fixation, followed by histological processing (dehydration in a graded alcohol series of six concentrations, clearing in xylol at two concentrations, and embedding in paraffin at 64 °C). The resulting paraffin blocks were attached to a microtome (Leitz® 1512) and slices of 3 μm thickness were obtained. These specimens were placed in a histological bath at 50 °C. For the staining step, the slides were immersed in vats containing hematoxylin-eosin (HE) and Picrosirius red (5 min in each stain). After the hydration stage, the sample was covered with a coverslip and fixed with Canada Balsam or the blade, finalizing the preparation process. The slides were examined by a pathologist who was blinded to group allocation and were photographed under a NIKON LABOPHOT binocular microscope at 200 × magnification.
For immunohistochemistry, liver tissue samples were fixed in 10% formalin and placed in a histological tissue processor (ANCAP), through a graded ethanol series and two vats of xylene, for dehydration. Specimens were then embedded and blocks were cooled, modeled, and attached to a microtome (Leitz® 1512) to obtain slices 4 μm thick. The resulting slides were incubated with mouse anti-iNOS (SC-7271; Santa Cruz Biotechnology, Santa Cruz, CA, United States) and TNF-α polyclonal antibodies (SC-52746; Santa Cruz Biotechnology) at a dilution of 1:200 overnight at 4°C, followed by incubation with the secondary antibody (SC-2005; Santa Cruz Biotechnology) at 1:300 for 30 min at room temperature. The slides were analyzed by a pathologist who was blinded to group allocation and were photographed under a NIKON LABOPHOT binocular microscope at 200 × magnification. Digital images were analyzed in Image-Pro Plus version 4.5 (Media Cybernetics, Rockville, MD, United States). The expression level was determined by multiplying the average density of the image by the percent area positively stained by the antibodies [brown colored areas obtained by the peroxidase + diaminobenzidine reaction].
The present study was accomplished in the HCPA with the approval of the project (No. 14-0474).
All experimental design, collections of biological samples and analyses carried out were in accordance with ethical principles of the Committee Ethics on Animal Use (CEUA-HCPA).
Quantitative data are presented as mean ± SD error. The comparison between groups was performed by one-way analysis of variance followed by the Student-Newman-Keuls procedure. P < 0.05 was considered as statistically significant.
Evaluation of liver enzyme activity performed in plasma showed a significant increase in all enzymes in the BDL group compared with the control groups, as well as a significant reduction of these values in the BDL + Mel group compared to the BDL group. AST levels increased 379% in the BDL group compared to the CO group, and were 72% reduced in the BDL + Mel group compared to the BDL group. ALT, a specific marker of liver damage, was 186% increased in the BDL group in relation to the CO group and 60% lower in the BDL + Mel group compared to the BDL group. AP levels were 211% higher in the BDL group compared to the CO group and 72% lower in the BDL + Mel group compared to the BDL group (P < 0.001) (Table 1).
| Group | AST (U/L) | ALT (U/L) | AP (U/L) |
| CO | 88.8 ± 0.07 | 37.0 ± 1.9 | 122.4 ± 13.5 |
| CO + Mel | 90.4 ± 8.4 | 38.8 ± 3.2 | 111.6 ± 8.1 |
| BDL | 425.8 ± 46.6e | 105.8 ± 13.5e | 381.2 ± 35.5e |
| BDL + Mel | 117.5 ± 18.8f | 42.0 ± 3.4f | 104.3 ± 11.03f |
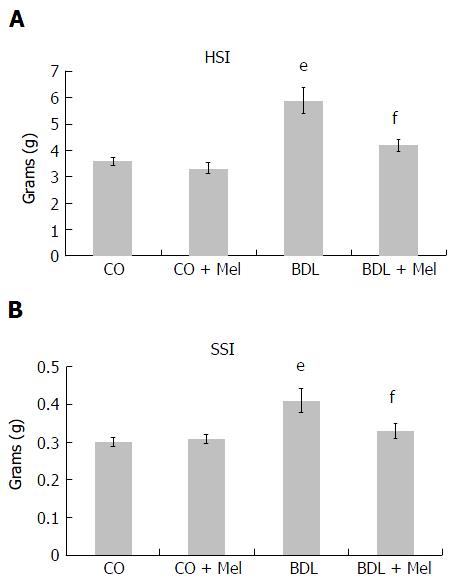
Analysis of HSI and SSI showed significant increases in the BDL group compared to control animals (CO and CO + Mel), as well as a significant decrease in the BDL + Mel group compared to the cirrhotic group (BDL) (Figure 2A and B).
The evaluation of LPO and GSH levels was performed on homogenized liver.
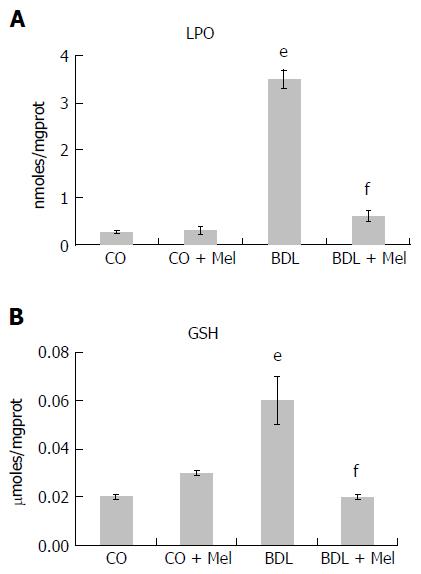
The LPO analysis revealed a significant increase in LPO markers in the BDL group compared to the CO and CO + Mel groups, and administration of Mel to BDL + Mel animals was associated with a significant decrease in damage in this group. GSH levels were increased in the BDL group compared to the control groups (CO and CO + Mel), and reduced in BDL + Mel compared to BDL (Figure 3A and B).
Evaluation of SOD, CAT, GPx and GST activity revealed reductions in SOD and CAT in BDL animals compared to controls (CO and CO + Mel), as well as functional recovery of these enzymes in the BDL + Mel group compared to the BDL group. Activity of GPx and GST were increased in the BDL group compared to both control groups (CO and CO + Mel), and decreased in the BDL + Mel group compared with BDL (Table 2).
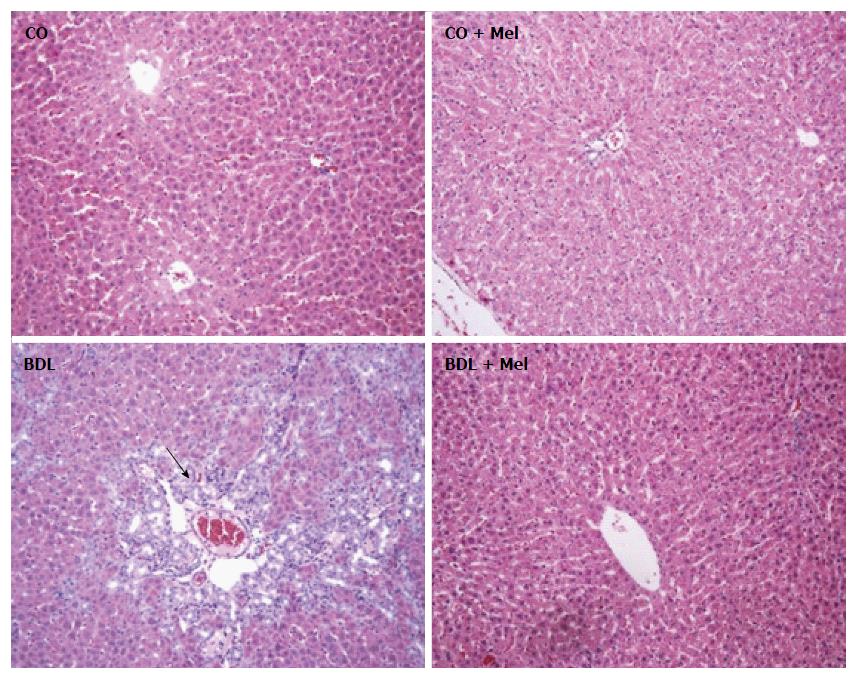
HE staining: In the control groups (CO and CO + Mel), histological analysis by HE staining revealed normal liver parenchyma with clearly defined hepatocyte cords. In the BDL group, there was tissue disorganization with loss of hepatocyte cords and inflammatory infiltration. In the cirrhotic group treated with Mel (BDL + Mel), restructuring of these patterns was observed, with formation of hepatocyte cords arising from a centrilobular vein (Figure 4).
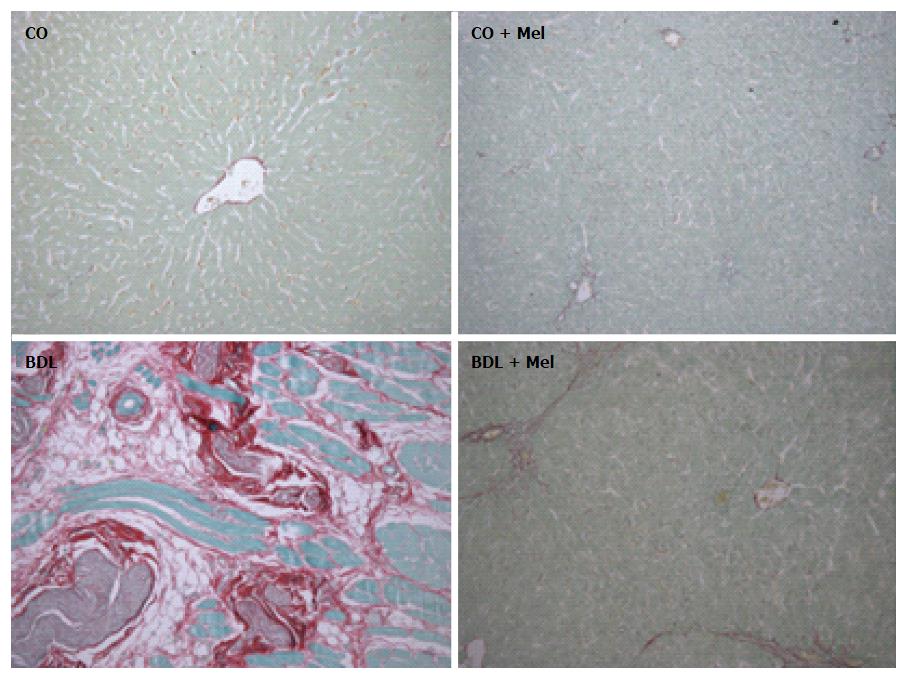
Picrosirius staining: Assessment of liver fibrosis in Picrosirius-stained sections revealed absence of fibrotic septa in the control groups (CO and CO + Mel). In animals subjected to BDL, there was positive labeling consistent with presence of fibrotic septa. However, in the BDL + Mel group, fibrosis was minimal (Figure 5).
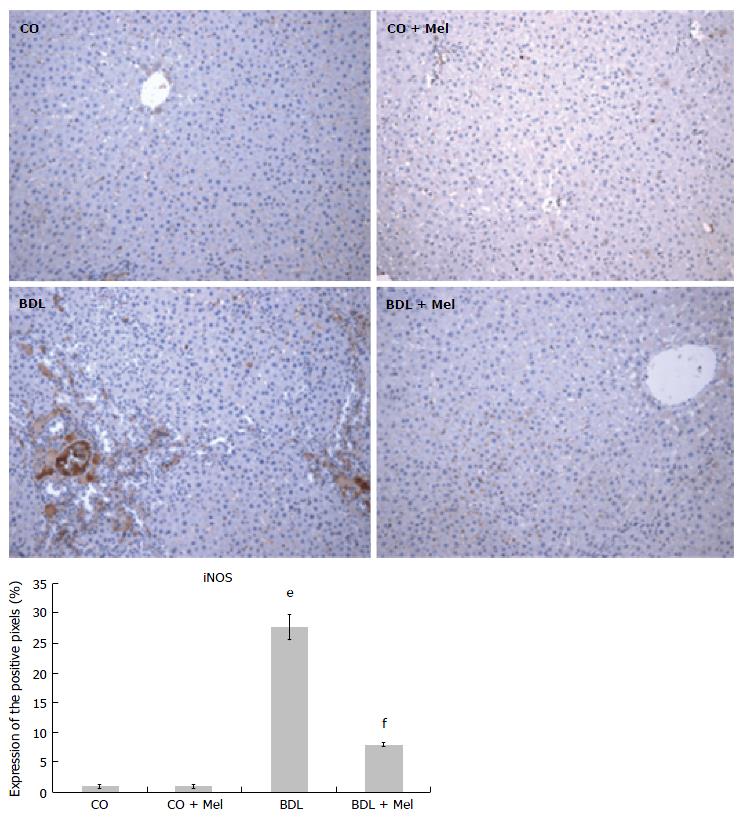
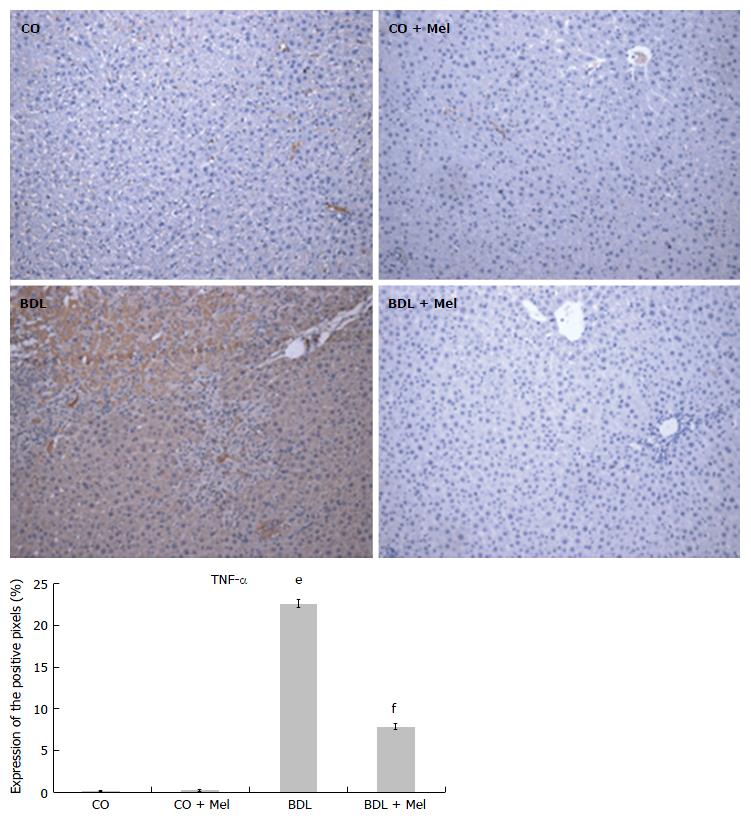
Liver specimens from the BDL group exhibited strong positive staining for iNOS (Figure 6) and TNF-α (Figure 7), whereas specimens from the CO and CO + Mel groups did not stain. Treatment with Mel reduced iNOS and TNF-α positivity. Likewise, iNOS and TNF-α expression was significantly reduced in BDL + Mel compared to the BDL group (P < 0.001; Figures 6 and 7).
The BDL model is widely used to reproduce secondary biliary cirrhosis in animals, as it induces changes that closely resemble those seen in cirrhosis in humans and in experimental cirrhosis induced by carbon tetrachloride (CCl4)[4,10,12].
Liver integrity can be evaluated by measuring levels of the enzymes AST, ALT, and AP. Increases in these markers suggest liver dysfunction[32]. In the present study, animals subjected to BDL exhibited higher levels of AST, ALT, and AP than animals in all other groups. Our findings also demonstrated that administration of Mel to animals with cirrhosis induced by BDL reduced the liver damage caused by duct ligation. These results corroborate the findings of a previous study conducted by Bona et al[4], using the CCl4 model of cirrhosis, in which animals exhibited a significant increase in AST, ALT and AP levels and equally significant reductions of these markers after treatment with the antioxidant quercetin. Shu et al[9] demonstrated that administration of tanshinone IIA, the active ingredient of Salvia miltiorrhiza, reduced ALT and AST levels in an experimental model of cirrhosis in rats.
The terms hepatomegaly and splenomegaly refer, respectively, to enlargement of the liver and spleen. Hepatomegaly is often associated with hepatobiliary diseases. Splenomegaly, in turn, is associated with numerous chronic diseases of the liver[33,34,35]. In our study, both the HSI and SSI were significantly increased in the BDL group compared with both control groups, and both indices decreased to near control levels when administered Mel in the BDL + Mel group.
The splenomegaly observed in the BDL model is due to portal hypertension as a result of enlargement of the splenic veins. Hepatomegaly, in turn, is secondary to biliary retention and subsequent obstruction of biliary drainage, which ultimately leads to liver fibrosis[13,14,36]. Using a model of liver damage induced by administration of polychlorinated biphenyls, Oliveira et al[33] found that splenomegaly was minimal in exposed animals given the antioxidant quercetin. LPO causes disorganization of cell membranes, resulting in an increase in membrane permeability and consequent extravasation of enzymes, leading to cell death[37]. Studies have demonstrated that MDA levels may be associated with increased LPO[5].
Studies report that, in the pathophysiology of biliary cirrhosis, liver damage is maximized by the action of FRs[12]. This phenomenon was also observed in the present study by measuring LPO, which was significantly higher in the cirrhotic group (BDL) when compared to the other groups and, accordingly, may have been associated with a process of cell membrane damage. Furthermore, the BDL + Mel group exhibited a significant decrease in LPO as compared with the BDL group, which suggests a protective role of Mel against LPO induced by BDL. These data corroborate a previous study by Bona et al[4] (2012), in which LPO was found to be increased in a model of CCl4-induced cirrhosis, and quercetin treatment appeared to decrease LPO significantly.
CAT catalyzes the breakdown of H2O2 into water and O2. SOD is regarded as the first line of defense against ROS formation, and decreases in its activity could be related to increased LPO and heightened consumption of the enzyme in an attempt to decrease oxidative damage from ROS dismutation and H2O2 formation[38].
In the present study, activity of the antioxidant enzymes SOD and CAT was significantly decreased in the BDL group compared to all others, and Mel administration was able to restore activity of these enzymes to near-control levels. These data suggest that treatment with Mel attenuated FR formation secondary to liver damage resulting from BDL-induced cirrhosis. These data corroborate the findings of Bona et al[4] (2012), a study in which rats with CCl4-induced cirrhosis exhibited an increase in antioxidant enzymes after treatment with quercetin.
Levels of the other enzymes evaluated (GPx and GST), as well as of GSH, were increased in the BDL group compared to the other groups, and decreased significantly to near-control values in the BDL + Mel group. The increases observed in cirrhotic animals may be associated with enzyme activation in an attempt to clear FRs and minimize oxidative damage from the disease, while the reduction in these levels in the group administered Mel suggests decreased FR formation[39]. These data corroborate the findings of Amália et al[38], who observed that, in a model of CCl4-induced cirrhosis, GPx, GST and GSH levels were increased, and treatment with quercetin appeared to decrease these values.
Changes in the hepatic parenchyma, as well as formation of fibrotic septa and necrosis, are often associated with the cirrhotic process[4]. In our study, we observed loss of tissue organization in the BDL group when assessed by HE staining, demonstrating cellular disorganization with loss of hepatocyte cords and presence of inflammatory infiltrate. In the BDL + Mel group, a restructuring effect was observed, with tissue organization resembling that seen in the CO and CO + Mel groups.
Ferrari et al[40] demonstrated that rats with cirrhosis, whether induced by BDL or by CCl4, exhibit necrosis, fibrotic nodules, inflammatory infiltrate and cellular changes. Tieppo et al[14] also observed that rats subjected to BDL exhibit hepatic changes with ductular proliferation and fibrosis, findings that improved in cirrhotic rats treated with quercetin.
Fibrosis is the end result of long-term liver injury. Evaluation of fibrotic area in Picrosirius-stained slides revealed increased collagen deposition in the BDL group, in contrast to the BDL + Mel animals, in which collagen deposition was minimal. These data corroborate various studies which observed increased collagen deposition in the liver of rats with cirrhosis induced by CCl4 and BDL[4,14]. Saleh et al[41] administered the natural marine compound Sepia officinalis, known for its major antioxidant, antibacterial and antitumor effects, and observed a reduction in collagen deposition in animals subjected to bile duct ligation.
Increased production of TNF-α and iNOS is related to acute and chronic inflammatory processes. In the present study, we found higher TNF-α and iNOS expression in animals subjected to BDL, as well as decreased expression of these parameters in animals administered Mel. These findings corroborate those of Gonçalves Schemitt et al[42], who observed lower expression of TNF-α and iNOS in animals treated with glutamine in an experimental model of fulminant hepatic failure.
In view of the evidence presented herein, we suggest that the antioxidant and anti-inflammatory effects of Mel acted to restore serum levels of liver enzymes and the HSI and SSI, decrease LPO, restore antioxidant enzymes, and attenuate collagen deposition, inflammation and tissue damage in the livers of animals subjected to BDL. However, other pathways of Mel action should be studied to elucidate the protective mechanisms involved in this experimental model.
The authors would like to thank the Coordination for the Higher-Level Personnel (CAPES), the Research Support Foundation of Rio Grande do Sul (FAPERGS), the National Council of Research Development (CNPq), the Federal University of Rio Grande do Sul (UFRGS) and the Lutheran University of Brazil (ULBRA) for their support.
Liver cirrhosis is characterized by the appearance of septa and fibrotic nodules. Bile duct ligation (BDL) in rats is an effective experimental model of secondary biliary cirrhosis induction. Melatonin (Mel) has proven to be a potent antioxidant in different experimental models.
Experiments previous studies have proved that Mel presents itself as a potent antioxidant in different experimental models.
This is the first study evaluating the antioxidant capacity of Mel in a surgical model of secondary biliary cirrhosis, in order to evaluate its possible therapeutic efficacy.
Despite the secondary biliary cirrhosis affect, a significant number of patients, still do not have an effective treatment. These data indicate that Mel administration may be a target for further study and suggest its applicability for patients in order to better support the life of the same.
N-acetyl-5-methoxytryptamine, a physiologic hormone synthesized in a rhythmic manner by the pineal gland and with production inhibited by light. Exogenous administration has been related to its antioxidant capacity, and anti-inflammatory and immunomodulatory properties.
This manuscript is a good research article. The study is interesting and appropriate because it provides novel information about the beneficial effects of Mel on a model of cirrhosis. The authors studied the antioxidant and anti-inflammatory effects of a treatment with the indoleamine and evaluated the possible reversion of the structural changes induced in the liver by BDL.
| 1. | Zimmerman HJ. Hepatotoxicity: The adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott Williams and Wilkins, 1999: 428-433. . |
| 2. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1607] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 3. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2191] [Article Influence: 121.7] [Reference Citation Analysis (1)] |
| 4. | Bona S, Filippin LI, Di Naso FC, de David C, Valiatti B, Isoppo Schaun M, Xavier RM, Marroni NP. Effect of antioxidant treatment on fibrogenesis in rats with carbon tetrachloride-induced cirrhosis. ISRN Gastroenterol. 2012;2012:762920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1313] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 6. | Guimarães EL, Franceschi MF, Grivicich I, Dal-Pizzol F, Moreira JC, Guaragna RM, Borojevic R, Margis R, Guma FC. Relationship between oxidative stress levels and activation state on a hepatic stellate cell line. Liver Int. 2006;26:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Sherlock S. Chronic hepatitis and cirrhosis. Hepatology. 1984;4:25S-28S. [PubMed] |
| 8. | Zhang Y, He Y, Yu H, Ma F, Wu J, Zhang X. Liquiritigenin Protects Rats from Carbon Tetrachloride Induced Hepatic Injury through PGC-1α Pathway. Evid Based Complement Alternat Med. 2015;2015:649568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Shu M, Hu XR, Hung ZA, Huang DD, Zhang S. Effects of tanshinone IIA on fibrosis in a rat model of cirrhosis through heme oxygenase-1, inflammation, oxidative stress and apoptosis. Mol Med Rep. 2016;13:3036-3042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Kountouras J, Billing BH, Scheuer PJ. Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Br J Exp Pathol. 1984;65:305-311. [PubMed] |
| 11. | Chang SW, Ohara N. Increased pulmonary vascular permeability in rats with biliary cirrhosis: role of thromboxane A2. Am J Physiol. 1993;264:L245-L252. [PubMed] |
| 12. | Tieppo J, Vercelino R, Dias AS, Marroni CA, Marroni N. [Common bile duct ligation as a model of hepatopulmonary syndrome and oxidative stress]. Arq Gastroenterol. 2005;42:244-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Vercelino R, Tieppo J, Dias AS, Marroni CA, Garcia E, Meurer L, Picada JN, Marroni NP. N-acetylcysteine effects on genotoxic and oxidative stress parameters in cirrhotic rats with hepatopulmonary syndrome. Basic Clin Pharmacol Toxicol. 2008;102:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Tieppo J, Cuevas MJ, Vercelino R, Tuñón MJ, Marroni NP, González-Gallego J. Quercetin administration ameliorates pulmonary complications of cirrhosis in rats. J Nutr. 2009;139:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Silveira KC, Viau CM, Colares JR, Saffi J, Marroni NP, Porawski M. Cirrhosis induces apoptosis in renal tissue through intracellular oxidative stress. Arq Gastroenterol. 2015;52:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Espino J, Pariente JA, Rodríguez AB. Role of melatonin on diabetes-related metabolic disorders. World J Diabetes. 2011;2:82-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, Reiter RJ. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71:2997-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 789] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 18. | Reiter RJ, Calvo JR, Karbownik M, Qi W, Tan DX. Melatonin and its relation to the immune system and inflammation. Ann N Y Acad Sci. 2000;917:376-386. [PubMed] |
| 19. | Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27:189-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 456] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 20. | Neto JAS, de Castro BF. Melatonina, ritmos biológicos e sono-uma revisão da literatura. Rev Bras Neurol. 2008;44:5-11. |
| 21. | Rosa DP, Bona S, Simonetto D, Zettler C, Marroni CA, Marroni NP. Melatonin protects the liver and erythrocytes against oxidative stress in cirrhotic rats. Arq Gastroenterol. 2010;47:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Goldim JR, Raymundo MM. Pesquisa em saúde e direitos dos animais. 2 ed. Porto Alegre: HCPA; 1997. . |
| 23. | Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. 8th ed. Washington, D.C.: The national academies press; 2011. . |
| 24. | Grigorov I, Bogojević D, Jovanović S, Petrović A, Ivanović-Matić S, Zolotarevski L, Poznanović G, Martinović V. Hepatoprotective effects of melatonin against pronecrotic cellular events in streptozotocin-induced diabetic rats. J Physiol Biochem. 2014;70:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Llesuy SF, Milei J, Molina H, Boveris A, Milei S. Comparison of lipid peroxidation and myocardial damage induced by adriamycin and 4’-epiadriamycin in mice. Tumori. 1985;71:241-249. [PubMed] |
| 26. | Lizama MAP, Takemoto RM, Ranzani-Paiva MJT, Ranzani-Paiva MJT, da Silva Ayroza LM, Pavanelli GC. Relação parasito-hospedeiro em peixes de pisciculturas da região de Assis, Estado de São Paulo, Brasil. 1. Oreochromis niloticus (Linnaeus, 1757). Acta Sci Biol Sci. 2007;29:223-231. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-310. [PubMed] |
| 28. | Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170-3175. [PubMed] |
| 29. | Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707-716. [PubMed] |
| 30. | Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114-121. [PubMed] |
| 31. | Mannervik B, Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231-235. [PubMed] |
| 32. | Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882-888. [PubMed] |
| 33. | Oliveira CR, Ceolin J, de Oliveira RR, Schemitt EG, Colares JR, Bauermann LF, Costabeber IH, Morgan-Martins MI, Mauriz JL, da Silva J. Efecto de La quercetina sobre La lesión hepática inducida por bifenilos policlorados en ratas. Nutr Hosp. 2014;29:1141-1148. |
| 34. | Vera-Méndez FJ, Trujillo-Santos AJ, Cano-Sánchez A, Delgado-Romero B. [Toxicity and causes of change of antiretroviral regimen among immigrant patients with HIV infection]. Rev Clin Esp. 2011;211:66-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Vargas VP, Hurtado MR, Villalobos AJA. Esplenomegalia. Rev Fac Med. 2013;56:37-45. |
| 36. | Yaari A, Sikuler E, Keynan A, Ben-Zvi Z. Bromosulfophthalein disposition in chronically bile duct obstructed rats. J Hepatol. 1992;15:67-72. [PubMed] |
| 37. | Mason RP, Walter MF, Mason PE. Effect of oxidative stress on membrane structure: small-angle X-ray diffraction analysis. Free Radic Biol Med. 1997;23:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Amália PM, Possa MN, Augusto MC, Francisca LS. Quercetin prevents oxidative stress in cirrhotic rats. Dig Dis Sci. 2007;52:2616-2621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Ferreira ICFR, Abreu RMV. Stress oxidativo, Antioxidantes e Fitoquímicos. Bioanálise. 2007;2:32-39. |
| 40. | Ferrari RS, Tieppo M, Rosa DP, Forgiarini Jr LA, Dias AS, Marroni NP. Lung and liver changes due to the induction of cirrhosis in two experimental models. Arq Gastroenterol. 2013;50:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Saleh H, Soliman AM, Mohamed AS, Marie MA. Antioxidant Effect of Sepia Ink Extract on Extrahepatic Cholestasis Induced by Bile Duct Ligation in Rats. Biomed Environ Sci. 2015;28:582-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 42. | Gonçalves Schemitt E, Raskopf Colares J, Minuzzo Hartmann R, Morgan-Martins MI, Marroni CA, Tuñón MJ, Possa Marroni N. Efecto de la glutamina en el estrés oxidativo y la inflamación en un modelo de rata con insuficiencia hepática fulminante. Nutr Hosp. 2016;33:92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Chen WX, Maarman G, Vega-Naredo I S- Editor: Qi Y L- Editor: Filipodia E- Editor: Zhang FF