Published online Jan 28, 2016. doi: 10.3748/wjg.v22.i4.1650
Peer-review started: July 31, 2015
First decision: August 31, 2015
Revised: September 20, 2015
Accepted: November 24, 2015
Article in press: November 24, 2015
Published online: January 28, 2016
Processing time: 173 Days and 10.5 Hours
Hepatitis C virus (HCV) infection is estimated to affect 130-150 million people globally which corresponds to 2%-3% of the total world population. It remains the leading indication for liver transplant worldwide and has been demonstrated to negatively impact both patient and graft survival following non-hepatic organ transplantation. In the era of interferon-based therapy, although treatment and cure of HCV prior to non-hepatic transplant improved survival, tolerability and low cure rates substantially limited therapy. Interferon (IFN)-based therapy following non-hepatic solid organ transplant, due to the risk of allograft rejection, is generally contraindicated. Rapid advances in IFN-free therapy with direct acting antivirals (DAAs) in the last few years have completely changed the paradigm of hepatitis C therapy. Compared to IFN-based regimens, DAAs have less frequent and less severe adverse effects, shorter durations of therapy, and higher cure rates that are minimally impacted by historically negative predictors of response such as cirrhosis, ethnicity, and post-transplant state. Recent studies have shown that liver transplant (LT) recipients can be safely and effectively treated with DAA combination therapies; although data are limited, many of the principles of therapy in LT may be extrapolated to non-hepatic solid organ transplant recipients. Here we review the data on DAA combination therapies in transplantation, discuss the advantages and disadvantages of pre- vs post-transplant HCV therapy and future directions.
Core tip: Direct acting antiviral (DAA) therapy has the potential to eliminate hepatitis C virus (HCV) from the population of organ transplant candidates and recipients and thereby the negative impact of HCV on outcomes. Among non-hepatic organ transplant patients, the biggest barriers currently are limited safety and efficacy data in this population, particularly in those with advanced renal disease, and global variability of access and reimbursement for DAAs. Future research is needed to better assess safety, efficacy and impact of DAA therapy in non-hepatic solid organ transplant, as well as to explore the safety of using HCV infected donors, with prophylactic therapy, to expand the donor pool.
- Citation: Belga S, Doucette KE. Hepatitis C in non-hepatic solid organ transplant candidates and recipients: A new horizon. World J Gastroenterol 2016; 22(4): 1650-1663
- URL: https://www.wjgnet.com/1007-9327/full/v22/i4/1650.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i4.1650
Hepatitis C virus (HCV) infection affects 130-160 million people globally which corresponds to about 2%-3% of the total world population[1-3]. After acute infection, which is usually asymptomatic, 55% to 85% of the patients go on to develop chronic hepatitis C with a risk of progression to cirrhosis is 15%-45% over 20 to 30 years[1]. Those who develop HCV-related cirrhosis are then at risk of end-stage liver disease and/or hepatocellular carcinoma (HCC) and hepatitis C remains the leading indication for liver transplantation worldwide.
Among candidates for non-hepatic solid organ transplant (SOT), the prevalence of HCV infection varies by organ group and regional epidemiology. In the hemodialysis population, worldwide estimates of prevalence vary between 5% and 60%[4]. Patients with end-stage renal disease (ESRD) on hemodialysis (HD) are at higher risk compared to patients on peritoneal dialysis[5]. The prevalence in dialysis patients is directly related to the duration of dialysis, the type of dialysis and the total volume of blood and blood products transfused[6,7]. The prevalence of HCV in dialysis patients, in Western countries in particular, has declined over the last two decades largely due to blood product screening implemented in the early 1990’s and adherence to infection control practices[8]. Despite this, the prevalence remains higher than in the general population and in 2002 the seroprevalence of HCV in US hemodialysis units was 7.8%[9], at least 4-fold higher than the background rate[1,9,10].
Substantial variability in HCV prevalence exists by country and amongst different dialysis centers within a single country[9,10]. Available data suggest that the prevalence of HCV in hemodialysis units in Asia remains high, at up to 32%[11], even in the era since 2010.
The prevalence of HCV infection in thoracic organ transplant candidates has been less rigorously assessed, but appears to approximate the background population prevalence[12,13]. Based on older studies, the prevalence of HCV infection in cardiac transplant recipients was 11%-18%[14-16]. As in those with end-stage renal disease, the prevalence of HCV in cardiac transplant candidates has likely declined over time since the implementation of routine screening of blood products.
The impact of HCV on the outcomes of non-hepatic SOT has been studied most extensively in renal transplant recipients. The majority of studies demonstrate that in patients with HCV infection, immunosuppression post-transplant accelerates progression and is associated with earlier onset and higher rates of cirrhosis and its complications[17,18]. Nevertheless, some studies have failed to show a clear correlation with fibrosis progression[19-21]. This may be explained by differences in immunosuppressive regimens used in the populations studied as well as small numbers and relatively short duration of follow up in most of these studies[22].
Although hepatitis C does not appear to impact overall survival in renal transplant recipients in the first 5 years post-transplant. Early post-transplant deaths occur in about 1%-2%, up to 5% in older studies, of recipients as a consequence of rapidly progressive fibrosing cholestatic hepatitis and liver failure[18,23-25]. By 10 years after transplant however, survival is decreased in HCV-infected recipients by approximately 15% compared to HCV-uninfected recipients[17,26-32]. The dominant causes of death are due to liver disease[17,20,30] and sepsis[33]. In addition, there is an increased incidence of new onset diabetes, infectious complications, chronic rejection, and proteinuria and membranoproliferative glomerulonephritis in those with HCV[17,29,34].
When the data are examined more closely, the risk of accelerated progression of fibrosis and development of end-stage liver disease and its complications appear to be limited largely to those with advanced fibrosis or cirrhosis at the time of transplant[17,35,36]. As HCV has also been demonstrated to be an independent risk factor for mortality in hemodialysis patients[37,38], the overall survival in those with moderate (METAVIR stage 2) fibrosis or less, is generally improved with renal transplantation compared to remaining on dialysis[39]. In addition, pre-transplant therapy and cure of HCV prior to transplant has been demonstrated to mitigate the impact on post-transplant morbidity and survival[40].
There are no long term studies regarding the impact of HCV on outcomes of heart or small bowel or pancreas recipients. Studies in these populations have suggested no difference in patient and graft survival, likely due to short term follow up and/or the relatively small numbers studied[16,41,42]. A retrospective study done between 1987 and 2010 that included 20 patients, showed that hepatitis C did not impact short-term patient and graft survival in cardiac transplant recipients[43]. In the short-term there was also no increased risk of liver failure or accelerated transplant-related coronary artery disease. Another study with 10 patients and mean follow-up of 58 mo, showed similar results[44]. However, there has been some contradictory evidence suggesting that hepatitis C can independently increase the risk of mortality and allograft vasculopathy in cardiac transplant recipients[12,45].
In lung transplant recipients, a recent analysis of the OPTN/UNOS database demonstrated a similar 5 year survival amongst HCV-seropositive and seronegative recipients[46]. The results of this study however are limited by the lack of data on HCV RNA status in the database and the likelihood that the majority of the seropositive recipients were RNA negative based on the authors’ survey of practices amongst US lung transplant centers. Given the data in renal transplant, however there may be an increased risk of HCV-related death beyond 5 years post-transplant in other non-hepatic SOT recipients.
In a single center study of 14 HCV-RNA positive lung transplant recipients, the 5 year survival was similar to HCV negative recipients[47]. Another small retrospective study from Sahi et al[48], also showed no difference in short-term survival between HCV-positive and HCV-negative lung transplant recipients. Despite the limited data on survival of lung transplant recipients with chronic hepatitis C with only small studies and conflicting evidence on outcomes[13,49], hepatitis C remains a relative contraindication to lung transplantation in the update of the International Society for Heart and Lung Transplantation guidelines for the selection of lung transplant candidates, recently published in January of 2015. These suggest that transplant can be considered in patients “without evidence of cirrhosis” and who are on “appropriate therapy” and that such patients be managed in centers with expertise in viral hepatitis. There is no evidence cited to support this recommendation and it gives little guidance to physicians and lung transplant programs as to how best select and manage such patients[50].
The available data on pancreas and pancreas-kidney transplant recipients with hepatitis C is scarce but these patients appear to be at higher risk of graft dysfunction and morbidity[51]. No data exists regarding survival for small bowel transplant recipients with hepatitis C.
Treatment of hepatitis C with interferon based therapy following non-hepatic SOT is generally contraindicated due to a significant risk of rejection, although more recent studies, suggest this risk is only about 5% in renal transplant recipients[52-55]. In life-sustaining (e.g. heart, lung) transplants, however, IFN-based therapy is not recommended and the risks and benefits must be carefully weighed if considering therapy in renal transplant recipients[56,57]. As a result of the risks of therapy post-transplant, focus has largely been on treatment of HCV prior to transplantation. Most of the data on hepatitis C management in non-hepatic organ transplantation is in kidney transplant with very limited evidence in heart and lung transplantation[37].
Table 1 summarizes the results of 3 recently published meta-analyses[58-60] examining the outcomes of HCV therapy in ESRD and predictors of sustained virologic response (SVR). Overall, SVR rates for IFN-based therapy in dialysis patients in these analyses were about 40%. There was no benefit of pegylated over standard interferon in the meta-analyses, although some studies have demonstrated this[61,62]. Although ribavirin is by label contraindicated in those with a creatinine clearance less than 50 mL/min, ribavirin has been used at reduced does in hemodialysis patients. There is little data however on the benefit of adding ribavirin to interferon-based therapy in this setting and it carries an significant risk of severe anemia[4].
| Author | Number of studies included | Patients (n) | Regimen | SVR | Treatment discontinued for adverse effects | Predictors of SVR |
| Alavian et al[60] | 21 | 491 | IFN α 2a or 2b | 39.1% | 29.7% | Age < 40 |
| 12 | 279 | PegIFN α 2a or 2b | 39.3% | 22.6% | ||
| Gordon et al[59] | 20 | 459 | IFN | 41% | 26% | Lower HCV RNA non-cirrhotic elevated ALT genotype 1 |
| 3 | 38 | PegIFN | 37% | 28% | ||
| 2 | 49 | PegIFN/RBV | 43%-97% | |||
| Fabrizi et al[58] | 13 | 539 | IFN | 1OR of no SVR | 1OR dropout | N/A |
| (10 studies) | 0.081 | 0.389 | ||||
| IFN + RBV | (0.029-0.230) | (0.155-0.957) | ||||
| (3 studies) |
The first-generation NS3A protease inhibitors, telaprevir and boceprevir, do not require dose adjustment in renal failure, however, their use in combination with peginterferon and ribavirin is associated with additive side effects, with more anemia and declining renal function of particular concern, and very limited data on safety and efficacy in this population[63,64]. These agents have now been discontinued from most markets worldwide and are listed as contraindicated therapies in international guidelines[65-67]. Although there is no dose adjustment needed for the protease inhibitor simeprevir in advanced renal disease, there are no published data on the efficacy and safety of simeprevir in combination with peginterferon and ribavirin in this population. The combination of sofosbuvir with peginterferon and ribavirin is listed as an interferon containing option for therapy in the 2015 European Association for the Study of the Liver (EASL) guidelines[66], however in those with a creatinine clearance less than 30 mL/min, sofosbuvir is contraindicated due to a lack of safety data.
Dropout rates on interferon-based therapy are high in those with end-stage renal disease, ranging from 19% to 28% in the meta-analyses, and largely due to adverse effects, particularly flu-like symptoms. The positive predictors of response found, were those associated with response in the general population, including low baseline HCV viral load, lesser degrees of hepatic fibrosis, non-1 HCV genotype and age less than 40[59,60]. It has been suggested that specific types of immunosuppressants can have an impact on virological outcomes; however, these data are conflicting and largely examined in the setting of liver transplant[68].
In patients with end-stage heart failure IFN-based therapy, with or without RBV, is contraindicated due to the increased risk of arrhythmias, cardiotoxicity and the risk of RBV-induced anemia can also worsen any underlying cardiac ischemia[41,43,57,69-71]. In lung transplant candidates with end-stage disease, this has been considered by some to be a contraindication to IFN-based therapy due to concerns of intolerability[72]. In carefully selected lung transplant candidates however a small single center study demonstrated the efficacy and safety of IFN and RBV therapy prior to lung transplantation[73].
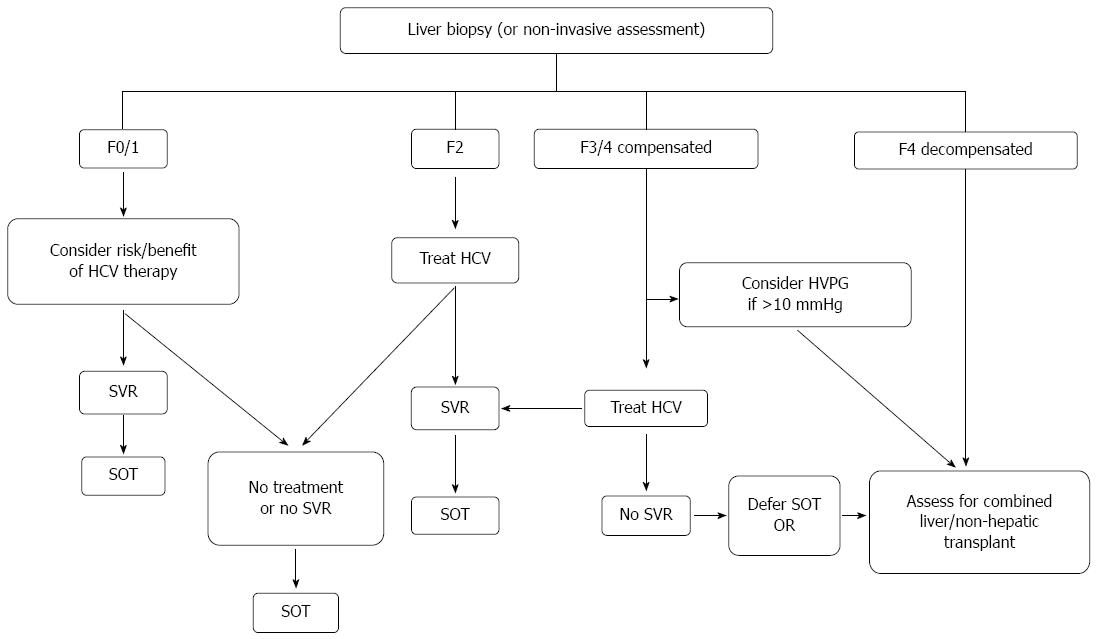
The epidemiologic, natural history and treatment outcomes data led to the development of international guidelines on the management of hepatitis C in renal and non-hepatic transplant candidates and recipients[56,57]. As part of the evaluation for transplantation candidacy, liver biopsy is recommended in current guidelines to confirm or exclude the presence of cirrhosis, unless the latter has already been documented on clinical, radiographic and/or biochemical grounds. All non-hepatic, non-cardiac organ transplant candidates, should be considered potential candidates for therapy of HCV. With interferon-based therapy however, the numerous adverse effects, particularly in those with end-stage organ disease, along with the overall poor responses to therapy requires careful consideration of the risks and benefits in the individual. There is clearly a greater urgency for therapy in those with more advanced fibrosis, but the success and tolerability in such patients in decreased. Figure 1 summarizes the current recommendations for assessment, transplant listing and HCV therapy in non-hepatic SOT.
IFN-free regimens with direct acting antivirals (DAAs) have numerous advantages including, shorter duration of therapy, far fewer adverse effects and higher cure rates when compared to interferon-based therapy. There are now data on DAA regimens for all HCV genotypes with recommendations published in international guidelines[65,66]. Recommendations from EASL and AASLD/IDSA guidelines for treatment of naïve patients with chronic HCV, by genotype, with interferon free regimens are summarized in Table 2.
| SOF + RBV[65,66] | SOF + LDV[65,66] | PTV/r + OMB + DSV[65,66] | PTV/r + OMB[65,66] | SOF + SMV[65,66] | SOF + DCV[66] | |
| (SVR) | (SVR) | (SVR) | (SVR) | (SVR) | (SVR) | |
| Without cirrhosis | ||||||
| Genotype 1a | 8-12 wk | 12 wk with RBV | 12 wk without RBV | 12 wk without RBV | ||
| (93%-99%) | (95%-97%) | (93%-100%) | (95%-100%) | |||
| Genotype 1b | 8-12 wk | 12 wk without RBV | 12 wk without RBV (93%-100%) | 12 wk without RBV | ||
| (93%-99%) | (98%-99%) | (95%-100%) | ||||
| Genotype 2 | 12 wk | |||||
| (97%) | ||||||
| Genotype 3 | 24 wk | 12 wk without RBV | ||||
| (94%) | (89-97%) | |||||
| Genotype 4 | 12 wk | 12 wk with RBV | 12 wk without RBV | 12 wk without RBV | ||
| (95%) | (89%-97%) | |||||
| Genotype 5 or 6 | 12 wk | 12 wk without RBV | ||||
| (89%-97%) | ||||||
| With cirrhosis | ||||||
| Genotype 1a | 12 wk without RBV (95%) | 24 wk with RBV | 12 wk without RBV | 12 wk with or 24 wk without RBV | ||
| (92%) | (93%) | |||||
| Genotype 1b | 12 wk without RBV | 12 wk with RBV | ||||
| (96%) | ||||||
| Genotype 2 | 16-20 wk | 12 wk without RBV | ||||
| (78%-83%) | ||||||
| Genotype 3 | 24 wk | 24 wk with RBV | ||||
| (92%) | ||||||
| Genotype 4 | 12 wk with RBV or 24 wk without RBV | 24 wk with RBV | 12 wk without RBV | 12 wk with RBV or 24 wk without RBV | ||
| Genotype 5 or 6 | 12 wk with RBV or 24 wk without RBV | 12 wk with RBV or 24 wk without RBV | ||||
Current DAAs target three viral non-structural proteins that are vital in viral replication: the NS3/4A protease, the NS5A protein and the NS5B polymerase[74]. Simeprevir (SMV)[75] and paritaprevir (PTV)[76] are NS3/4A protease inhibitors, ledipasvir (LDV)[77], daclatasvir (DCV)[78] and ombitasvir (OMV)[76] are NS5A inhibitors and sofosbuvir (SOF)[77] and dasabuvir (DSV)[76] are nucleoside and non-nucleoside, respectively, NS5B polymerase inhibitors. Ritonavir (RTV), used in combination with PTV acts as a booster via CYP3A inhibition[76]. The most robust data are in genotypes 1, 2 and 3, however there are limited data in genotype 4, 5 and 6 with additional studies ongoing, including with next generation molecules[79].
The majority of the data on HCV therapy in transplantation are in liver transplant candidates and recipients. The safety and tolerability in addition to high efficacy have been demonstrated for several interferon-free regimens in decompensated cirrhotics awaiting liver transplant and are currently recommended in international guidelines[65,66]. These include LDV/SOF with RBV for 12 wk or LDV/SOF for 24 wk in genotype 1, 4, 5 or 6[80,81] and SOF/RBV for up to 48 wk in genotypes 2 and 3[82].
In those with recurrent HCV following liver transplant, and genotype 1, 4, 5 or 6, LDV/SOF with RBV for 12 wk is recommended[65,66]. In the SOLAR 1 and 2 studies, the SVR12 in genotype 1 was 96%-98% in those with F0-3 or Child Pugh A cirrhosis and 88%-89% in those with Child Pugh B or C cirrhosis[83,84]. Adverse effects, largely attributable to RBV, are seen with this regimen, so the alternative of LDV/SOF for 24 wk can be considered, particularly in those with decompensated liver disease. Although data are limited, on the combination of SOF and DCV post-transplant, overall SVR has been more than 90%, including in those with fibrosing cholestatic HCV[85-87] and this combination can be considered in all genotypes[66]. All of these combinations can be used post-transplant without concerns regarding drug interactions and the need to adjust immunosuppressant doses (Table 3).
| SOF | SMV | LDV | PTV/r + OMB + DSV | DCV | |
| Cyclosporine | * | *** | ** | ** | * |
| Tacrolimus | * | ** | ** | ** | * |
| Sirolimus | * | ** | ** | ** | * |
| MMF | * | * | * | ** | * |
| Azathioprine | * | * | * | * | * |
| Prednisone | * | ** | * | ** | * |
| Fluconazole | * | *** | * | * | * |
| Voriconazole | * | *** | * | *** | ** |
| Posaconazole | * | *** | * | *** | ** |
| PPI | * | * | ** | ** | * |
Based on the real world data from observational studies[88,89], the combination of SOF/SMV can be considered, however, there is a significant interaction with cyclosporine, which must not be used in combination, and the relatively poor performance of this regimen in the phase 3 trials in non-transplant patients[90,91], raises concerns that this combination may not be as potent as others. The combination of OMV/PTV/RTV with DSV and ribavirin has been studied in 34 post-liver transplant patients with genotype 1 HCV recurrence and mild to moderate fibrosis (Metavir F0-2)[92]. Dose adjustments were needed for cyclosporine and tacrolimus due to the drug-drug interactions between ritonavir. There are several other drug interactions with ritonavir and other medications commonly taken by liver transplant recipients (Table 3) and these must be carefully considered if this regimen is used for treatment in this population. Although the efficacy and tolerability of this regimen in the study was excellent, with an SVR achieved in 33 of 34 and only 1 discontinuation for adverse effects, the safety and efficacy in patients with more advanced HCV infection post-liver transplant are unknown.
Of the interferon free DAA combinations studied to date in the post liver transplant population, all have an excellent safety and tolerability profile and hence a significantly lower discontinuation rate when compared to IFN-based regimens[93]. SMV may cause rash, pruritus, gastrointestinal upset and muscle pain, but these rarely lead to discontinuation[75]. Photosensitivity can occur, but usually when SMV is combined with IFN and RBV[75]. Fatigue and headache are the most common side-effects of LDV/SOF, but are generally mild and occur in at most 10%-20%[77]. OMV/PTV/RTV + DSV as well as DCV can lead to fatigue, gastrointestinal symptoms, pruritus, skin reactions and insomnia, but again, discontinuations for adverse effects are uncommon[76,78]. When RBV is, or must be, used as part of an interferon-free regimen, significant anemia (hemoglobin < 100 g/L)[94] is more common and quality of life decreased compared to the RBV-free regimens[95,96]. This is an important consideration, particularly in those with end-stage organ disease.
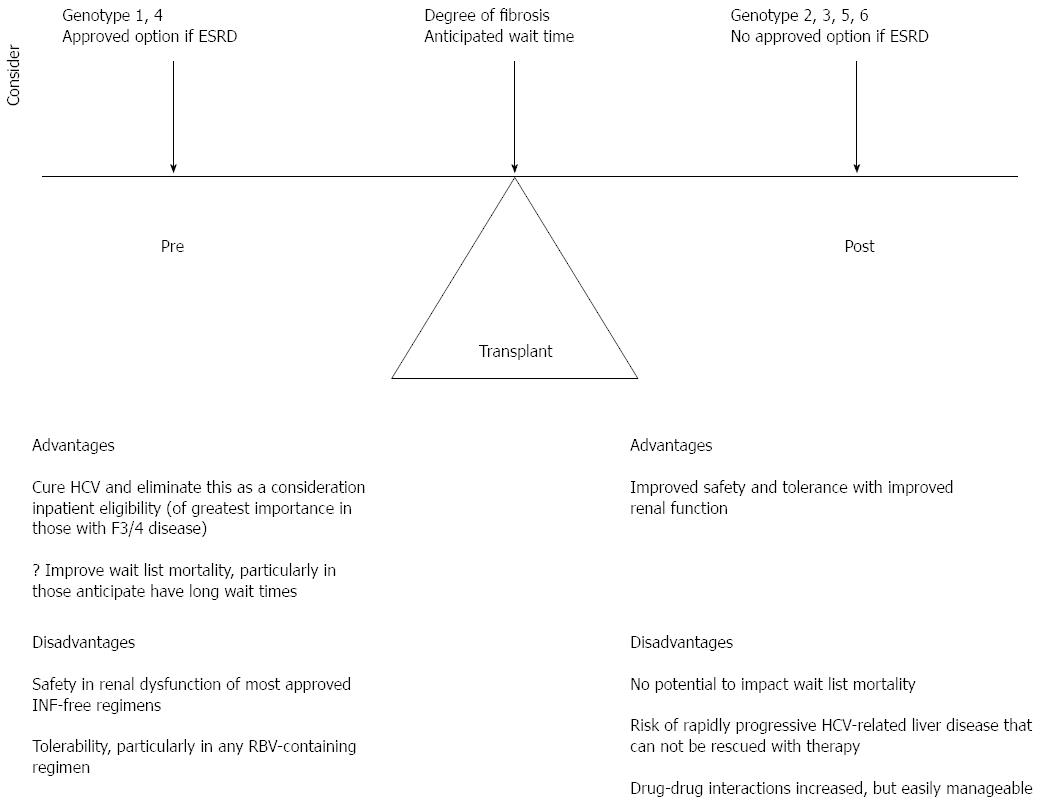
There are very limited data on the treatment of HCV following non-hepatic transplant, with to our knowledge only 1 published case report using SMV/SOF and a case series of 17 patients treated with SOF and RBV[97,98]. However, these 2 reports and all of the data in historically “difficult-to-cure” populations in the interferon era, such as cirrhotics, HIV co-infected, prior null responders, and post-liver transplant patients, demonstrate cure rates comparable to uncomplicated HCV treatment naive patients when interferon free DAA therapy is used. This supports the extrapolation of data in liver transplantation to the treatment of hepatitis C in non-hepatic solid organ transplant recipients. In liver transplant, there remains controversy as to the optimal timing of HCV therapy, pre or post-transplant in order to maximize survival benefit and quality of life. The timing of therapy is also a major consideration in non-hepatic SOT. Considerations include the degree of hepatic fibrosis, the HCV genotype, treatment history and access to interferon free therapy, the presence or absence of renal dysfunction; for those pre-transplant the degree of sensitization and anticipated wait time as well as the immunosuppressive plan. Figure 2 summarizes these considerations in the non-hepatic SOT candidate or recipient with HCV and the advantages and disadvantages of HCV therapy prior to or after transplant.
Renal dysfunction is the primary barrier to therapy prior to renal transplant. Most of the currently recommended interferon free regimens are sofosbuvir-based and the safety of sofosbuvir in those with a creatinine clearance under 30 mL/min is under investigation[99], but remains uncertain at this time. Table 4 summarizes the dosing considerations for DAA therapy in those with renal dysfunction. OMV/PTV/RTV with DSV, and with or without RBV was recently shown to be effective in patients with HCV genotype 1 and stage 5 chronic kidney disease (creatinine clearance less than 15 mL/min)[100]. Data on the investigational combination of grazoprevir, an NS3 protease inhibitor and elbasvir, an NS5A inhibitor, was recently presented from the phase III trial examining the efficacy and safety of for 12 wk in patients with stage 4 or 5 chronic kidney disease, most of whom were on hemodialysis[101]. The SVR12 was 94% overall and adverse effects in the treatment group were similar to placebo. These data are extremely encouraging in this historically difficult to cure population who experienced numerous adverse effects of therapy. As a result of these results, the combination was granted expedited breakthrough designation for this indication from the United States Food and Drug Administration.
| DAA agent or combination | Issues in renal dysfunction |
| Sofosbuvir | Contraindication in GFR < 30 mL/min due to insufficient safety data[127] |
| Simeprevir | No dose adjustment[75] |
| Ledipasvir | Limited data; no theoretical concerns[127] |
| PTV/r + OMB + DSV | Safe and effective and no dose adjustment in ESRD[100] |
| Daclatasvir | No dose adjustment[78] |
| Elbasvir/Grazoprevir | Safe and effective and no dose adjustment in ESRD[101] |
In an ideal world, pretransplant therapy is preferable in order to document cure of HCV and improve patient eligibility for non-hepatic SOT. For most thoracic organ transplant candidates, this should be the goal. In renal transplant candidates, and any other non-hepatic SOT candidate with advanced renal dysfunction, until the licensing of therapies such as grazoprevir/elbasvir, with proven safety and efficacy in end stage renal disease, and/or additional data on the use of sofosbuvir in this population, in general therapy should be deferred until post-transplant. In those with genotype 1 or 4 and access to OMV/PTV/RTV, with or without DSV and RBV, as applicable to the HCV genotype, therapy can be considered prior to transplant, with cautious use of ribavirin and considerations of the drug interactions. Pre transplant therapy is of higher priority in those with advanced (F3 or Child Pugh A cirrhosis) fibrosis. In this group, deferral to post renal transplant therapy may carry the risk of rapidly progressive fibrosis, including fibrosing cholestatic hepatitis C, and decompensation. Although cure rates with current DAA therapy are high, the risk of failure remains highest in those with decompensated liver disease and there is concern that should this occur, patients may not be rescued with HCV therapy.
For those with genotype 2, 3, 5 and 6, as all currently available IFN-free therapies are SOF-based, until additional safety data are available, therapy should be deferred in those with creatinine clearance < 30 mL/min until after SOT. In the setting of advanced (F3 or Child Pugh A cirrhosis) fibrosis, the risks and benefits of deferral of therapy and transplantation must be carefully assessed. Non-hepatic SOT in this setting should be done in consultation with a liver transplant program and patients should be assessed for liver transplant candidacy as a back-up should they develop rapidly progressive HCV-related liver disease post non-hepatic SOT that cannot be rescued with therapy.
In post-transplant populations, the other key consideration influencing the choice of therapy is the potential for drug interactions; although renal dysfunction may also be a concern in this group. Most novel DAAs are metabolized via cytochrome P 450 3A (CYP3A) and therefore drug interactions, particularly with calcineurin inhibitors, remain a concern[102].
SMV should not be co-administered with cyclosporine as previous studies have shown a significant increase in SMV levels due to inhibition of organic anion transporting polypeptide 1B1 (OATP1B1), P-glycoprotein and CYP3A by cyclosporine[75]. However, SMV can be used concomitantly with tacrolimus, despite up to 2-fold increase in SMV concentrations with the latter, requiring close drug monitoring[102]. SMV can increase or decrease sirolimus levels; therefore, close monitoring of sirolimus levels is also recommended[75,102]. SMV can be safely used with mycophenolate mofetil (MMF) and azathioprine[102].
SOF can be safely co-administered with all immunosuppressants used in the transplant setting, including tacrolimus, cyclosporine, sirolimus, MMF and azathioprine[77,102]. When SOF is used in combination with LDV, monitoring is required for co-administration with tacrolimus, sirolimus and cyclosporine as there is theoretical possibility of an interaction, and data are insufficient[102]. There have been no clinically relevant drug interactions seen in studies to date, however in those treated with advanced liver disease, calcineurin inhibitor levels have been noted to drop and require increased dosing, an effect thought to be related to improved liver function and metabolism. No dose adjustment is necessary for when DCV is co-administered with cyclosporine, tacrolimus, sirolimus and MMF[78].
OMV/PTV/RTV + DSV requires dose adjustment with sirolimus, MMF, tacrolimus and cyclosporine[76,102,103]. The cyclosporine dose needs to be decreased to one fifth the dose used prior to HCV therapy and tacrolimus decreased to 0.5 mg weekly or 0.2 mg every 3 d with close monitoring of levels. No dose adjustment is necessary when used concomitantly with azathioprine[102].
When protease inhibitor containing regimens (PTV/r or SMV) are used concomitantly with prednisone, a substrate of CYP3A4, prednisone exposure may increase due to CYP3A4 inhibition[102]. No dose adjustments are recommended, but patients should be monitored clinically.
Importantly, a careful review of all patient medications and potential interactions should take place before starting any DAA regimen and special caution should be taken with certain drugs, particularly antibiotics, antifungals, antiretrovirals, anticonvulsants, antipsychotics, sedatives, oral contraceptives, antiarrhythmics and antihypertensives[75-78,102].
Use of anti-infectives is particularly common following SOT. Most commonly used antibiotics can be administered safely with new DAA regimens with the exception of the macrolide clarithromycin which cannot be co-administered with OMV/PTV/RTV + DSV, and rifampin which is contraindicated with any all the current DAA regimens due to significant interactions[75-78,102]. Antifungals such as fluconazole, posaconazole and voriconazole cannot be co-administered with SMV. OMV/PTV/RTV + DSV is contraindicated to be used with posaconazole or voriconazole and DCV must be used with caution in combination with these antifungal agents[78,102]. Of note, proton-pump inhibitors, such as omeprazole and pantoprazole, by increasing the gastric pH, can lead to decreased absorption of LDV[77].
In patients with end-stage organ failure, donor organ shortage is a growing concern and there is increasing use of increased infectious risk, as well as otherwise medically marginal, donors. Transplantation of HCV-positive donor organs into HCV-negative recipients will almost invariably leads to chronic hepatitis C in the immunosuppressed host[104-106] and historically this has been associated with an aggressive course with a high risk of death from infectious complications and fibrosing cholestatic HCV[107,108]. As such, guidelines currently recommend against transplanting an HCV positive organ into an HCV negative recipient[56,57].
In those already infected with HCV, some groups have found no difference in patient and graft survival when using HCV positive kidneys into HCV positive recipients[109,110], while several recent large studies have demonstrated a significant increased risk of death in HCV-positive recipients receiving an HCV-positive kidney or heart transplant[12,109,111,112]. Despite the decrement in survival compared to receiving an HCV negative graft, there remains an overall survival advantage to receiving an HCV-positive kidney transplant over remaining on dialysis[113]. The waiting time on the renal transplant list is also reduced significantly in the United States, by approximately 1 year. In spite of the overall benefit, HCV-positive kidneys continue to be underutilized in the United States[114].
With highly effective interferon-free DAA therapy for HCV, it is easy to imagine an era on the horizon where HCV in donors will be approached in a manner similar to a donor with positive blood cultures; there may be a slightly increased risk of donor derived transmission of infection, but with safe effective therapy, this risk could be effectively mitigated and the donor pool further expanded. This approach however will require further data before such recommendations can be made.
Although historical data demonstrate that hepatitis C has a negative long-term impact in both patient and graft survival in non-hepatic solid organ transplant recipients, we are on the precipice of a new reality. Previous IFN-based regimens limited HCV treatment to pre-transplantation, and resulted in only a minority eligible for therapy, poor tolerability and resulted in only a small proportion of patients being able to complete treatment and achieve SVR.
The era of DAAs has been a major advance in hepatitis C management and prognosis, particularly in patients who were historically “difficult to cure”. Recent studies show that liver transplant recipients can be safely and effectively treated with the new DAA regimens and although data are limited, there is every reason to believe these data can be extrapolated to non-hepatic solid organ transplantation recipients. This is expected to essentially eliminate the risk of hepatic and non-hepatic post-transplantation complications of hepatitis C. In patients with moderate to severe renal impairment, there are some emerging data on safety and efficacy of DAAs and further data are eagerly awaited. Until there are new licensed therapies, or additional data on current IFN-free therapies, those with ESRD should in general have HCV therapy deferred until post-transplant when renal function improves. This is a complete reversal of the historical paradigm of therapy restricted essentially to the pre-transplant period in non-hepatic SOT.
Despite some potential for drug-drug interactions remaining a concern, these are generally predictable and easily managed in a group of patients in whom management of drug interactions is a routine consideration.
In future directions, although dedicated prospective studies on IFN-free therapies are unlikely to be undertaken in non-hepatic SOT, these patients will undoubtedly be treated based on the existing data. It will be important to see results of observational studies published in order to confirm the efficacy and safety in this population. The potential for utilization of HCV - positive organ donors, even into HCV - negative recipients is an intriguing possibility that will require future study. Within this defined population of non-hepatic SOT, it should be our goal, and easily achievable, to eradicate HCV.
| 1. | World Health Organization. Hepatitis C: Fact sheet NO164. 2015. Available from: http://www.who.int/mediacentre/factsheets/fs164/en/. |
| 2. | Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 945] [Article Influence: 63.0] [Reference Citation Analysis (3)] |
| 3. | Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1854] [Article Influence: 142.6] [Reference Citation Analysis (3)] |
| 4. | Ozer Etik D, Ocal S, Boyacioglu AS. Hepatitis C infection in hemodialysis patients: A review. World J Hepatol. 2015;7:885-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Chan SE, Schwartz JM, Rosen HR. Treatment of hepatitis C in solid organ transplantation. Drugs. 2004;64:489-498. [PubMed] |
| 6. | Chan TM, Lok AS, Cheng IK, Chan RT. Prevalence of hepatitis C virus infection in hemodialysis patients: a longitudinal study comparing the results of RNA and antibody assays. Hepatology. 1993;17:5-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 110] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Genescà J, Vila J, Córdoba J, Sauleda S, Quer J, Esteban JI, Esteban R, Piera L, Guardia J. Hepatitis C virus infection in renal transplant recipients: epidemiology, clinical impact, serological confirmation and viral replication. J Hepatol. 1995;22:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Espinosa M, Martn-Malo A, Ojeda R, Santamara R, Soriano S, Aguera M, Aljama P. Marked reduction in the prevalence of hepatitis C virus infection in hemodialysis patients: causes and consequences. Am J Kidney Dis. 2004;43:685-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial. 2005;18:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 286] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 10. | Fissell RB, Bragg-Gresham JL, Woods JD, Jadoul M, Gillespie B, Hedderwick SA, Rayner HC, Greenwood RN, Akiba T, Young EW. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2004;65:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 310] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 11. | Liu YB, Xie JZ, Zhong CJ, Liu K. Hepatitis C virus infection among hemodialysis patients in Asia: a meta-analysis. Eur Rev Med Pharmacol Sci. 2014;18:3174-3182. [PubMed] |
| 12. | Gasink LB, Blumberg EA, Localio AR, Desai SS, Israni AK, Lautenbach E. Hepatitis C virus seropositivity in organ donors and survival in heart transplant recipients. JAMA. 2006;296:1843-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (7)] |
| 13. | Cotler SJ, Jensen DM, Kesten S. Hepatitis C virus infection and lung transplantation: a survey of practices. J Heart Lung Transplant. 1999;18:456-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Cadranel JF, Di Martino V, Dorent R, Bernard B, Hoang C, Myara A, Pauwels A, Ghoussoub JJ, Perrin M, Grippon P. Effects of ursodeoxycholic acid (ursodiol) treatment on chronic viral hepatitis in heart transplant patients: results of a prospective, double-blind, placebo-randomized study. Transplantation. 2003;75:977-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Cadranel JF, Grippon P, Lunel F, Desruennes M, Leger P, Azar N, Moussalli J, Pauwels A, Cabrol A, Salmon P. Chronic liver dysfunction in heart transplant recipients, with special reference to viral B, C, and non-A, non-B, non-C hepatitis. A retrospective study in 80 patients with follow-up of 60 months. Transplantation. 1991;52:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Lunel F, Cadranel JF, Rosenheim M, Dorent R, Di-Martino V, Payan C, Fretz C, Ghoussoub JJ, Bernard B, Dumont B. Hepatitis virus infections in heart transplant recipients: epidemiology, natural history, characteristics, and impact on survival. Gastroenterology. 2000;119:1064-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Mathurin P, Mouquet C, Poynard T, Sylla C, Benalia H, Fretz C, Thibault V, Cadranel JF, Bernard B, Opolon P. Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology. 1999;29:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 406] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 18. | Zylberberg H, Carnot F, Mamzer MF, Blancho G, Legendre C, Pol S. Hepatitis C virus-related fibrosing cholestatic hepatitis after renal transplantation. Transplantation. 1997;63:158-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 66] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Alric L, Di-Martino V, Selves J, Cacoub P, Charlotte F, Reynaud D, Piette JC, Péron JM, Vinel JP, Durand D. Long-term impact of renal transplantation on liver fibrosis during hepatitis C virus infection. Gastroenterology. 2002;123:1494-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Legendre C, Garrigue V, Le Bihan C, Mamzer-Bruneel MF, Chaix ML, Landais P, Kreis H, Pol S. Harmful long-term impact of hepatitis C virus infection in kidney transplant recipients. Transplantation. 1998;65:667-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 182] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Kamar N, Rostaing L, Selves J, Sandres-Saune K, Alric L, Durand D, Izopet J. Natural history of hepatitis C virus-related liver fibrosis after renal transplantation. Am J Transplant. 2005;5:1704-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Vallet-Pichard A, Fontaine H, Mallet V, Pol S. Viral hepatitis in solid organ transplantation other than liver. J Hepatol. 2011;55:474-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Delladetsima JK, Boletis JN, Makris F, Psichogiou M, Kostakis A, Hatzakis A. Fibrosing cholestatic hepatitis in renal transplant recipients with hepatitis C virus infection. Liver Transpl Surg. 1999;5:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Lam PW, Wachs ME, Somberg KA, Vincenti F, Lake JR, Ferrell LD. Fibrosing cholestatic hepatitis in renal transplant recipients. Transplantation. 1996;61:378-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Toth CM, Pascual M, Chung RT, Graeme-Cook F, Dienstag JL, Bhan AK, Cosimi AB. Hepatitis C virus-associated fibrosing cholestatic hepatitis after renal transplantation: response to interferon-alpha therapy. Transplantation. 1998;66:1254-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Kokado Y, Takahara S, Ichimaru N, Toki K, Wang JD, Permpongkosol S, Sagawa S, Ichikawa Y, Akiyama T, Yoshimura N. Clinical outcome of HCV infection after renal transplantation. Transplant Proc. 2000;32:1940-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Scott DR, Wong JK, Spicer TS, Dent H, Mensah FK, McDonald S, Levy MT. Adverse impact of hepatitis C virus infection on renal replacement therapy and renal transplant patients in Australia and New Zealand. Transplantation. 2010;90:1165-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 28. | Morales JM, Fabrizi F. Hepatitis C and its impact on renal transplantation. Nat Rev Nephrol. 2015;11:172-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 29. | Domínguez-Gil B, Morales JM. Transplantation in the patient with hepatitis C. Transpl Int. 2009;22:1117-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Hanafusa T, Ichikawa Y, Kishikawa H, Kyo M, Fukunishi T, Kokado Y, Okuyama A, Shinji Y, Nagano S. Retrospective study on the impact of hepatitis C virus infection on kidney transplant patients over 20 years. Transplantation. 1998;66:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 144] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Periera BJ, Wright TL, Schmid CH, Levey AS. The impact of pretransplantation hepatitis C infection on the outcome of renal transplantation. Transplantation. 1995;60:799-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 170] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Sezer S, Ozdemir FN, Akcay A, Arat Z, Boyacioglu S, Haberal M. Renal transplantation offers a better survival in HCV-infected ESRD patients. Clin Transplant. 2004;18:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Gane E, Pilmore H. Management of chronic viral hepatitis before and after renal transplantation. Transplantation. 2002;74:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Carbone M, Cockwell P, Neuberger J. Hepatitis C and kidney transplantation. Int J Nephrol. 2011;2011:593291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Roth D, Gaynor JJ, Reddy KR, Ciancio G, Sageshima J, Kupin W, Guerra G, Chen L, Burke GW. Effect of kidney transplantation on outcomes among patients with hepatitis C. J Am Soc Nephrol. 2011;22:1152-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 36. | Izopet J, Rostaing L, Sandres K, Cisterne JM, Pasquier C, Rumeau JL, Duffaut M, Durand D, Puel J. Longitudinal analysis of hepatitis C virus replication and liver fibrosis progression in renal transplant recipients. J Infect Dis. 2000;181:852-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Fabrizi F, Bunnapradist S, Martin P. Treatment of hepatitis C in potential kidney and heart transplant patients. Clin Liver Dis. 2005;9:487-503, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Goodkin DA, Bragg-Gresham JL, Koenig KG, Wolfe RA, Akiba T, Andreucci VE, Saito A, Rayner HC, Kurokawa K, Port FK. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol. 2003;14:3270-3277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 581] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 39. | Bloom RD, Sayer G, Fa K, Constantinescu S, Abt P, Reddy KR. Outcome of hepatitis C virus-infected kidney transplant candidates who remain on the waiting list. Am J Transplant. 2005;5:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | González-Roncero F, Gentil MA, Valdivia MA, Algarra G, Pereira P, Toro J, Sayago M, Mateos J. Outcome of kidney transplant in chronic hepatitis C virus patients: effect of pretransplantation interferon-alpha2b monotherapy. Transplant Proc. 2003;35:1745-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Wells JT, Lucey MR, Said A. Hepatitis C in transplant recipients of solid organs, other than liver. Clin Liver Dis. 2006;10:901-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Miguel M, Sampaio MS, Kuo HT, Poommipanit N, Martin P, Bunnapradist S. Influence of preexisting hepatitis C virus antibody positivity in simultaneous pancreas-kidney transplant recipients. Transplantation. 2010;90:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Lin MH, Chou NK, Chi NH, Chen YS, Yu HY, Huang SC, Ko WJ, Chou HW, Wang SS. The outcome of heart transplantation in hepatitis C-positive recipients. Transplant Proc. 2012;44:890-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Shafii AE, Su JW, Smedira NG, Navia JL, Taylor DO, Starling RC, Gonzalez-Stawinski G. The effect of recipient hepatitis C virus infection on outcomes following heart transplantation. Transplant Proc. 2010;42:1784-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Haji SA, Starling RC, Avery RK, Mawhorter S, Tuzcu EM, Schoenhagen P, Cook DJ, Ratliff NB, McCarthy PM, Young JB. Donor hepatitis-C seropositivity is an independent risk factor for the development of accelerated coronary vasculopathy and predicts outcome after cardiac transplantation. J Heart Lung Transplant. 2004;23:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 46. | Fong TL, Cho YW, Hou L, Hutchinson IV, Barbers RG, Herrington CS. Outcomes after lung transplantation and practices of lung transplant programs in the United States regarding hepatitis C seropositive recipients. Transplantation. 2011;91:1293-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Doucette K, Weinkauf J, Jackson K, Lien D. Survival following lung transplantation is not impacted by hepatitis c infection. Am J Transplant. 2012;12:27-542. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Sahi H, Zein NN, Mehta AC, Blazey HC, Meyer KH, Budev M. Outcomes after lung transplantation in patients with chronic hepatitis C virus infection. J Heart Lung Transplant. 2007;26:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Carreno MC, Piedad UG, Maite L, Andrés V, Francisca P, Cesar B, Alvarez MJ. Hepatitis C virus infection after lung transplantation: dim prognosis. J Heart Lung Transplant. 2001;20:224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, Lederer DJ, Mulligan MJ, Patterson GA, Singer LG. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1122] [Cited by in RCA: 953] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 51. | Honaker MR, Stratta RJ, Lo A, Egidi MF, Shokouh-Amiri MH, Grewal HP, Alloway RR, Gaber LW, Hardinger KL, Gaber AO. Impact of hepatitis C virus status in pancreas transplantation: a case controlled study. Clin Transplant. 2002;16:243-251. [PubMed] |
| 52. | Sharma RK, Bansal SB, Gupta A, Gulati S, Kumar A, Prasad N. Chronic hepatitis C virus infection in renal transplant: treatment and outcome. Clin Transplant. 2006;20:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Pageaux GP, Hilleret MN, Garrigues V, Bismuth M, Audin-Mamlouk H, Zarski JP, Mourad G. Pegylated interferon-alpha-based treatment for chronic hepatitis C in renal transplant recipients: an open pilot study. Transpl Int. 2009;22:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Aljumah AA, Saeed MA, Al Flaiw AI, Al Traif IH, Al Alwan AM, Al Qurashi SH, Al Ghamdi GA, Al Hejaili FF, Al Balwi MA, Al Sayyari AA. Efficacy and safety of treatment of hepatitis C virus infection in renal transplant recipients. World J Gastroenterol. 2012;18:55-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | Sanai FM, Mousa D, Al-Mdani A, Al-Shoail G, Al-Ashgar H, Al Meshari K, Al-Qahtani A, Saadeh M, Bzeizi KI, Aleid H. Safety and efficacy of peginterferon-α2a plus ribavirin treatment in renal transplant recipients with chronic hepatitis C. J Hepatol. 2013;58:1096-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008;S1-S99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 57. | Levitsky J, Doucette K. Viral hepatitis in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:147-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 58. | Fabrizi F, Ganeshan SV, Lunghi G, Messa P, Martin P. Antiviral therapy of hepatitis C in chronic kidney diseases: meta-analysis of controlled clinical trials. J Viral Hepat. 2008;15:600-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Gordon CE, Uhlig K, Lau J, Schmid CH, Levey AS, Wong JB. Interferon treatment in hemodialysis patients with chronic hepatitis C virus infection: a systematic review of the literature and meta-analysis of treatment efficacy and harms. Am J Kidney Dis. 2008;51:263-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Alavian SM, Tabatabaei SV. Meta-analysis of factors associated with sustained viral response in patients on hemodialysis treated with standard or pegylated interferon for hepatitis C infection. Iran J Kidney Dis. 2010;4:181-194. [PubMed] |
| 61. | Okoh EJ, Bucci JR, Simon JF, Harrison SA. HCV in patients with end-stage renal disease. Am J Gastroenterol. 2008;103:2123-2134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 62. | Liu CH, Kao JH. Treatment of hepatitis C virus infection in patients with end-stage renal disease. J Gastroenterol Hepatol. 2011;26:228-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 63. | Dumortier J, Guillaud O, Gagnieu MC, Janbon B, Juillard L, Morelon E, Leroy V. Anti-viral triple therapy with telaprevir in haemodialysed HCV patients: is it feasible? J Clin Virol. 2013;56:146-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 64. | Knapstein J, Galle PR, Zimmermann T. Antiviral triple therapy with boceprevir in a chronic hepatitis C haemodialysis patient awaiting kidney re-transplantation. Dig Liver Dis. 2014;46:88-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | American Association for the Study of Liver Diseases (AASLD)/Infectious Diseases Society of America (IDSA)/International Antiviral Society (IAS)-USA. Recommendations for testing, managing, and treating hepatitis C. 2015. Available from: http://www.hcvguidelines.org/. |
| 66. | European Association for Study of Liver. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63:199-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 911] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 67. | Myers RP, Shah H, Burak KW, Cooper C, Feld JJ. An update on the management of chronic hepatitis C: 2015 Consensus guidelines from the Canadian Association for the Study of the Liver. Can J Gastroenterol Hepatol. 2015;29:19-34. [PubMed] |
| 68. | Gordon FD, Kwo P, Vargas HE. Treatment of hepatitis C in liver transplant recipients. Liver Transpl. 2009;15:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Carbone M, Mutimer D, Neuberger J. Hepatitis C virus and nonliver solid organ transplantation. Transplantation. 2013;95:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Fattovich G, Giustina G, Favarato S, Ruol A. A survey of adverse events in 11,241 patients with chronic viral hepatitis treated with alfa interferon. J Hepatol. 1996;24:38-47. [PubMed] |
| 71. | Wang BY, Chang HH, Chen IM, Shih CC, Yang AH. Peginterferon alpha-2b and acute allograft failure in a heart transplant recipient. Ann Thorac Surg. 2010;89:1645-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 72. | Kim EY, Ko HH, Yoshida EM. A concise review of hepatitis C in heart and lung transplantation. Can J Gastroenterol. 2011;25:445-448. [PubMed] |
| 73. | Doucette KE, Weinkauf J, Sumner S, Ens K, Lien D. Treatment of hepatitis C in potential lung transplant candidates. Transplantation. 2007;83:1652-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 74. | Vispo E, Barreiro P, Soriano V. Pharmacokinetics of new oral hepatitis C antiviral drugs. Expert Opin Drug Metab Toxicol. 2013;9:5-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Janssen. Simeprevir (galexos(r)) product monograph. 2015. Available from: http://www.janssen.com/canada/sites/www_janssen_com_canada/files/product/pdf/gal01292015cpm_snds.pdf. |
| 76. | AbbVie. Viekira pak™, with or without ribavirin (rbv), is indicated for the treatment of adult patients with genotype 1 chronic hepatitis c virus infection, including those with compensated cirrhosis. 2015. Available from: http://www.abbvie.com/content/dam/abbviecorp/us/desktop/contentrooms/downloads/ProductFactsheet_ViekiraPak_US.pdf. |
| 77. | Gilead. Sovaldi (r) product monograph. 2015. Available from: http://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/harvoni/harvoni_pi.pdf. |
| 78. | Bristol-Myers Squibb. Prescribing information for daklinza (daclatasvir). 2014. Available from: http://www.medicines.org.uk/emc/medicine/29129. |
| 79. | AbbVie. A study to evaluate the efficacy, safety, and pharmacokinetics of co-administration of abt-493 and abt-530 with and without ribavirin in subjects with hcv genotype 1, 4, 5, and 6 infection. 2015. Available from: http://clinicaltrials.gov/ct2/show/NCT02243280. |
| 80. | Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS, Fried MW, Terrault NA, O’Leary JG, Vargas HE. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 638] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 81. | Bourlière M, Bronowicki JP, de Ledinghen V, Hézode C, Zoulim F, Mathurin P, Tran A, Larrey DG, Ratziu V, Alric L. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS). Lancet Infect Dis. 2015;15:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 228] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 82. | Curry MP, Forns X, Chung RT, Terrault NA, Brown R, Fenkel JM, Gordon F, O’Leary J, Kuo A, Schiano T. Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver transplantation: an open-label study. Gastroenterology. 2015;148:100-107.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 251] [Article Influence: 22.8] [Reference Citation Analysis (1)] |
| 83. | Available from: http://www.natap.org/2014/AASLD/AASLD_16.htm. |
| 84. | Ledipasvir/sofosbuvir with ribavirin is safe and efficacious in decompensated and post-liver transplant patients with hcv infection: Preliminary results of the solar-2 trial. Proceedings of the Program and abstracts of the 50th Annual Meeting of the European Association for the Study of the Liver; 2015 April 18-22. Available from: http://www.natap.org/2015/EASL/EASL_27.htm. |
| 85. | Leroy V, Dumortier J, Coilly A, Sebagh M, Fougerou-Leurent C, Radenne S, Botta D, Durand F, Silvain C, Lebray P. Efficacy of Sofosbuvir and Daclatasvir in Patients With Fibrosing Cholestatic Hepatitis C After Liver Transplantation. Clin Gastroenterol Hepatol. 2015;13:1993-2001.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 86. | Daclatasvir, sofosbuvir, and ribavirin combination for hcv patients with advanced cirrhosis or post-transplant recurrence: Ally-1 phase 3 study. Proceedings of the Program and abstracts of the 50th Annual Meeting of the European Association for the Study of the Liver; 2015 April 22-16. Available from: http://www.natap.org/2015/EASL/EASL_56.htm. |
| 87. | Available from: http://www.natap.org/2015/EASL/EASL_49.htm. |
| 88. | Stewart RK, Dangi A, Huang C, Murase N, Kimura S, Stolz DB, Wilson GC, Lentsch AB, Gandhi CR. A novel mouse model of depletion of stellate cells clarifies their role in ischemia/reperfusion- and endotoxin-induced acute liver injury. J Hepatol. 2014;60:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 89. | Available from: http://www.natap.org/2014/AASLD/AASLD_02.htm. |
| 90. | A phase 3, randomised, open-label study to evaluate the efficacy and safety of 12 and 8 weeks of simeprevir (smv) plus sofosbuvir (sof) in treatment-naive and -experienced patients with chronic HCV genotype 1 infection without cirrhosis: Optimist-1. Proceedings of the Program and abstracts of the 50th Annual Meeting of the European Association for the Study of the Liver; 2015 April 22-26. J Hepatol. 2015;62 Suppl 2:S269-S270. [DOI] [Full Text] |
| 91. | A phase 3, open-label, single-arm study to evaluate the efficacy and safety of 12 weeks of simeprevir (smv) plus sofosbuvir (sof) in treatment-naive or -experienced patients with chronic hcv genotype 1 infection and cirrhosis: Optimist-2. Proceedings of the Program and abstracts of the 50th Annual Meeting of the European Association for the Study of the Liver; 2015 April 22-26. J Hepatol. 2015;62 Suppl 2:S264-S265. [DOI] [Full Text] |
| 92. | Mdluli T, Pargett M, Buzzard GT, Rundell AE. Specifying informative experiment stimulation conditions for resolving dynamical uncertainty in biological systems. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:298-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 93. | Gane EJ, Agarwal K. Directly acting antivirals (DAAs) for the treatment of chronic hepatitis C virus infection in liver transplant patients: “a flood of opportunity”. Am J Transplant. 2014;14:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 94. | Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, Tam E, Marinho RT, Tsai N, Nyberg A. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 548] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 95. | Alqahtani SA, Afdhal N, Zeuzem S, Gordon SC, Mangia A, Kwo P, Fried M, Yang JC, Ding X, Pang PS. Safety and tolerability of ledipasvir/sofosbuvir with and without ribavirin in patients with chronic hepatitis C virus genotype 1 infection: Analysis of phase III ION trials. Hepatology. 2015;62:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 96. | Reddy KR, Bourlière M, Sulkowski M, Omata M, Zeuzem S, Feld JJ, Lawitz E, Marcellin P, Welzel TM, Hyland R. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: An integrated safety and efficacy analysis. Hepatology. 2015;62:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 97. | Huard G, Kim B, Patel A, Aljarallah B, Perumalswami P, Odin A, Geatrakas S, Ahmad J, Dieterich D, Nair V. E. Early safety and efficacy profiles of renal transplant recipients with chronic hepatitis c treated with sofosbuvir and ribavirin. Hepatology. 2014;60:117A. |
| 98. | Bonacci M, Londoño MC, Esforzado N, Forns X, Sotoca JM, Campistol JM. Antiviral treatment with sofosbuvir and simeprevir in a kidney transplant recipient with HCV-decompensated cirrhosis: viral eradication and removal from the liver transplant waiting list. Transpl Int. 2015;28:1345-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 99. | Gane E, Robson R, Bonacini M, Maliakkal B, Liu L, Sajwani K, Stamm LM, Brainard D, McHutchison J, Stedman C. Safety, anti-viral efficacy and pharmacokinetics (pk) of sofosbuvir (sof) in patients with severe renal impairment. Hepatology. 2014;60:133A. |
| 100. | Safety of ombitasvir/paritaprevir/ritonavir plus dasabuvir for treating hcv gt1 infection in patients with severe renal impairment or end-stage renal disease: The ruby-i study. Proceedings of the Program and abstracts of the 50th Annual Meeting of the European Association for the Study of the Liver; 2015 April 22-16. J Hepatol. 2015;62 Suppl 2:S257. [DOI] [Full Text] |
| 101. | C-surfer: Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis c virus genotype 1 infection and chronic kidney disease. Proceedings of the Program and abstracts of the 50th Annual Meeting of the European Association for the Study of the Liver; 2015 April 22-26. J Hepatol. 2015;62 Suppl 2:S263-S264. [DOI] [Full Text] |
| 102. | eMedFusion. Drug interaction chart. 2015. Available from: http://www.hep-druginteractions.org/. |
| 103. | Badri P, Dutta S, Coakley E, Cohen D, Ding B, Podsadecki T, Bernstein B, Awni W, Menon R. Pharmacokinetics and dose recommendations for cyclosporine and tacrolimus when coadministered with ABT-450, ombitasvir, and dasabuvir. Am J Transplant. 2015;15:1313-1322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 104. | Pereira BJ, Milford EL, Kirkman RL, Quan S, Sayre KR, Johnson PJ, Wilber JC, Levey AS. Prevalence of hepatitis C virus RNA in organ donors positive for hepatitis C antibody and in the recipients of their organs. N Engl J Med. 1992;327:910-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 176] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 105. | Pereira BJ, Wright TL, Schmid CH, Levey AS. A controlled study of hepatitis C transmission by organ transplantation. The New England Organ Bank Hepatitis C Study Group. Lancet. 1995;345:484-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 106. | Pfau PR, Rho R, DeNofrio D, Loh E, Blumberg EA, Acker MA, Lucey MR. Hepatitis C transmission and infection by orthotopic heart transplantation. J Heart Lung Transplant. 2000;19:350-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 107. | Delgado J, Muñoz de Bustillo E, Ibarrola C, Colina F, Morales JM, Rodriguez E, Aguado JM, Fuertes A, Gomez MA. Hepatitis C virus-related fibrosing cholestatic hepatitis after cardiac transplantation: is azathioprine a contributory factor? J Heart Lung Transplant. 1999;18:607-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 108. | Delladetsima I, Psichogiou M, Sypsa V, Psimenou E, Kostakis A, Hatzakis A, Boletis JN. The course of hepatitis C virus infection in pretransplantation anti-hepatitis C virus-negative renal transplant recipients: a retrospective follow-up study. Am J Kidney Dis. 2006;47:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 109. | Maluf DG, Archer KJ, Mas VR. Kidney grafts from HCV-positive donors: advantages and disadvantages. Transplant Proc. 2010;42:2436-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 110. | Uyar M, Sahin S, Dheir H, Gurkan A. The influence of hepatitis B and C virus infections on patient and allograft outcomes in kidney transplantation. Transplant Proc. 2011;43:850-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 111. | Abbott KC, Bucci JR, Matsumoto CS, Swanson SJ, Agodoa LY, Holtzmuller KC, Cruess DF, Peters TG. Hepatitis C and renal transplantation in the era of modern immunosuppression. J Am Soc Nephrol. 2003;14:2908-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 112. | Bucci JR, Matsumoto CS, Swanson SJ, Agodoa LY, Holtzmuller KC, Peters TG, Abbott KC. Donor hepatitis C seropositivity: clinical correlates and effect on early graft and patient survival in adult cadaveric kidney transplantation. J Am Soc Nephrol. 2002;13:2974-2982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 113. | Abbott KC, Lentine KL, Bucci JR, Agodoa LY, Peters TG, Schnitzler MA. The impact of transplantation with deceased donor hepatitis c-positive kidneys on survival in wait-listed long-term dialysis patients. Am J Transplant. 2004;4:2032-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 114. | Kucirka LM, Singer AL, Ros RL, Montgomery RA, Dagher NN, Segev DL. Underutilization of hepatitis C-positive kidneys for hepatitis C-positive recipients. Am J Transplant. 2010;10:1238-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 115. | Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1066] [Article Influence: 88.8] [Reference Citation Analysis (1)] |
| 116. | Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1376] [Article Influence: 114.7] [Reference Citation Analysis (1)] |
| 117. | Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, Shiffman ML, Schiff E, Ghalib R, Ryan M. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 929] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 118. | Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, Aguilar H, Xiong J, Pilot-Matias T. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 659] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 119. | Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, DeJesus E, Pearlman B, Rabinovitz M, Gitlin N. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 601] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 120. | Sulkowski MS, Jacobson IM, Nelson DR. Daclatasvir plus sofosbuvir for HCV infection. N Engl J Med. 2014;370:1560-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 121. | Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, Freilich BF, Younes ZH, Harlan W, Ghalib R. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 513] [Article Influence: 46.6] [Reference Citation Analysis (1)] |
| 122. | Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, Shiffman ML, Lawitz E, Everson G, Bennett M. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 838] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 123. | Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, Illeperuma A, Svarovskaia E, Brainard DM, Symonds WT. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 638] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 124. | Kapoor R, Kohli A, Sidharthan S, Sims Z, Petersen TL, Osinusi A. All oral treatment for genotype 4 chronic hepatits c infection with sofosbuvir and ledipasvir: Interim results of the niaid synergy trial. Hepatology. 2014;60:321A. [DOI] [Full Text] |
| 125. | Gane E, Hyland R, An D, Svarovskaia E, Pang P, Symonds W. High efficacy of ldv/sof regimens for 12 weeks for patients with hcv genotypes 3 or 6. Hepatology. 2014;60:1274A. [DOI] [Full Text] |
| 126. | Ledipasvir/sofosbuvir treatment results in high svr rates in patients with chronic genotype 4 and 5 hcv infection Proceedings of the 2015 International Liver Congress: 50th Annual Meeting of the European Association for the Study of the Liver (EASL) 2015; 62 Suppl 2: S219-S220. Available from: http://onlinelibrary.wiley.com/doi/10.1002/hep.v62.S1/issuetoc. |
| 127. | Gilead. Prescribing information for harvoni (ledipasvir and sofosbuvir). 2014. Available from: http://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/harvoni/harvoni_pi.pdf. |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Boyacioglu AS, Grassi A, Song AT S- Editor: Gong ZM L- Editor: A E- Editor: Liu XM