COAGULATION SYSTEM IN LIVER DISEASE
Patients with end stage liver disease most often exhibit profound changes in hemostatic variables. An elevated international normalized ratio (INR) is common in cirrhosis and is used in conjunction with bilirubin and creatinine concentrations to determine the model of end-stage liver disease, which describes the severity of liver disease[1]. The liver synthesizes nearly all coagulation factors (FII, FV, FVII, FVIII, FIX, FX, FXI, FXII, FXIII), therefore, a reduction of factor activities can be regularly observed in end stage liver disease[2]. Von Willebrand factor, which plays an important role in hemostatic processes, is synthesized in endothelial cells and often exhibits increased activity in liver disease[3,4].
In addition, most inhibitors of coagulation (antithrombin, heparin cofactor II, protein C, protein S, and tissue factor pathway inhibitor), and components of the fibrinolytic sytem (plasminogen, α2-antiplasmin, plasmin inhibitor) are synthesized in the liver and thus decreased levels are correspondingly observed in liver disease. In contrast, tissue type and urokinase-type plasminogen activator, and thrombomodulin are not synthesized by the liver and thus not affected by liver diseases[2].
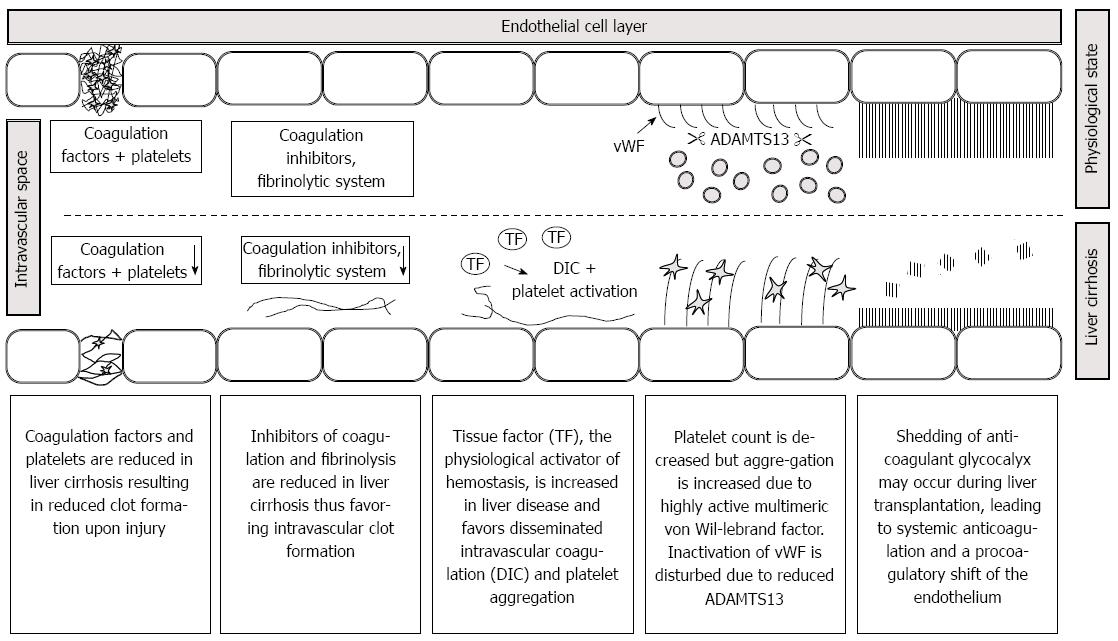
Tissue factor produced by hepatocytes is thought to contribute to the pathophysiology of liver diseases. Tissue factor is the principle physiological activator of coagulation. The fact, that hepatocytes express tissue factor and coagulation factors has been claimed as a “match in a dynamite factory” in a recent review[5]. In several models of liver injury, tissue factor deficiency was associated with reduced hepatic injury and inflammation. In accordance to the experimental findings, low molecular weight heparin reduced the frequency of hepatic decompensation in a prospective randomized study in 70 patients with cirrhosis[6]. Moreover, tissue factor showed a 38-fold increase in platelet poor plasma from patients with acute liver failure and was associated with mortality[7]. In another study, a 17-fold increase in circulating microparticle-bound tissue factor was observed in Child C patients[8]. For these reasons, increased tissue factor concentration may play a role in the hypercoagulable aspects of liver diseases. Some important mechanisms which may lead to hemostatic distubances in patients presenting for liver transplantation are given in Figure 1. Further details are given in the respective chapters of the review.
Figure 1 Pathophysiology of hemostasis in patients presenting for liver transplantation.
A reduction in coagulation factors due to end stage liver disease worsens clot quality. Decreases in anticoagulant factors, fibrinolysis and ADAMTS 13 as well as increases in tissue factor and von Willebrand factor multimeres may favor disseminated intravascular coagulation. Shedding of glycocalyx induces a systemic anticoagulation and contributes to the procoagulatory shift of the endothelium. Note that pathophysiology may vary upon the underlying liver pathophysiology.
PLATELETS IN LIVER DISEASE
Platelet count is often decreased in advanced liver disease and several contributing mechanisms have been described. Hypersplenism represents the most important cause of thrombocytopenia followed by reduced platelet production due to the reduced synthesis of thrombopoietin, a hematopoietic growth factor, in the liver (for review see[9]). Further causative factors may include consumption of platelets due to coagulopathy and bone marrow suppression for examples in cases of Hepatitis C, or the use of antiviral or anticancer agents.
Similarly, platelets have been demonstrated to be involved in inflammatory processes in hepatitis B and preclinical data of antiplatelet therapy in liver disease suggest that inhibition of platelet function might be an effective mean to limit inflammation, fibrosis and even hepatocellular carcinoma development[10]. Importantly, platelet activity is often increased in liver disease as von Willebrand factor, derived from endothelium, is often elevated and the inactivating metalloproteinase ADAMTS13, synthesized in the liver, is often decreased[3,4].
END STAGE LIVER DISEASE AND REBALANCED HEMOSTASIS
The above alterations in the hemostatic system were historically interpreted as indicators of bleeding risk in liver disease and patients were thought to be autoanticoagulated. There are many lines of evidence which contradict this point of view. Not only are procoagulant pathways reduced in liver disease but also anticoagulant and fibrinolytic mechanisms are impaired. Moreover, low platelet count can be counterbalanced by increased platelet activity. Several clinical studies on the risk of bleeding and thrombosis suggest that liver disease is not simply a bleeding disorder but also confirm the concept of Lisman that the hemostatic system in liver disease is rebalanced[11].
The most common cause of bleeding in patients with liver disease is the rupture of esophageal varices. Every third patient with liver disease is affected by this life threatening complication[12]. Portal hypertension and local abnormalities of esophageal veins are significant contributing factors in the rupture while hemostasis is thought to be of minor importance at best[13].
A further argument against a general bleeding risk is the fact that nowadays liver transplantations can often be performed without requirement of blood product replacement, suggesting that the observed derangements in hemostatic variables might not translate to diffuse bleeding risk[14,15].
There are numerous reports on pulmonary embolism and cardiac thrombosis in liver transplantation. In a review of the 74 cases described in literature, symptoms most often included a decrease in arterial pressure, an increase in pulmonary arterial pressure and often complete circulatory collapse. Mortality rate was 68% with most deaths occurring already in the operation theatre[16].
Søgaard et al[17] demonstrated in a nationwide population-based case-control study, that liver disease is associated with a worsening risk for thromboembolism, the odds ratio was 2.1.
Liver biopsies and placement of intracranial pressure monitoring devices rarely led to bleeding complications[18,19].
HEMOSTATIC SYSTEMS IN PATIENTS PRESENTING FOR LIVER TRANSPLANTATION VARY WITH THE UNDERLYING DISEASE
Many patients presenting for liver transplantation have end stage liver disease and thus present with reduced synthesis capacity and rebalanced hemostasis[11,20]. However, there is a minority of patients who are transplanted for other causes. Hemostasis system can be unaltered in patients presenting with hepatocellular carcinoma and intact liver function. Even hypercoagulability may be observed, particularly in cases of cholestatic liver disease and non-alcoholic fatty liver disease, due to inflammation, procoagulant shift of the endothelium, and the acute phase response.
DILUTION COAGULOPATHY IN LIVER TRANSPLANTATION
Without doubt, surgical experience and technique has largely improved over time thus allowing liver transplantations to be performed in many cases without the need of transfusion. In certain situations (e.g., retransplantation, previous intraabdominal surgery), however, massive surgical bleeding can still occur. The resultant dilution of coagulation factors and platelets may result in diffuse bleeding as the rebalanced hemostasis system is especially susceptible to further dilution of components. A combination of observed blood loss, platelet count and deterioration of both classical and bed-side hemostatic variables in conjunction with the onset of diffuse bleeding can easily lead to the appropriate diagnosis of a dilution coagulopathy.
TRAUMA INDUCED COAGULOPATHY IN LIVER TRANSPLANTATION?
Recently, it has been recognized that trauma can induce a disturbance of hemostasis which cannot be explained by dilutional coagulopathy. Instead, tissue damage and hemodynamic shock are supposed to cause the so called trauma induced coagulopathy. Liver transplantation shares important characteristics with the pathophysiology of trauma induced coagulopathy, due to the huge surgical trauma and eventual hemodynamic disturbances caused by crossclamping of vena cava, cardiac decompensation, and massive bleeding.
Comparable alterations in molecular mechanisms of hemostasis can be observed in trauma and liver transplantation patients.
Derangements in the thrombin-thrombomodulin-protein C system leads to anticoagulation in trauma induced coagulopathy[21]. Accordingly, consumption of activated protein C in the liver was measured during transplantation. Activation and thus consumption of the protein C pathway, however, seems to be beneficial for the organism in trauma and liver transplantation, as a blockade of this system was detrimental in experimental shock and low protein C levels were associated with thrombosis in liver transplantation[22-24].
A further mechanism in both trauma and liver transplantation which leads to anticoagulation is the shedding of the anticoagulant glycocalyx from endothelium[25,26].
In trauma, a decrease in fibrinogen levels as well as hyperfibrinolysis are common events and are independent predictors of mortality[27,28]. Hyperfibrinolysis is also common in liver transplantation and associated with bleeding[29]. During the anhepatic phase, the clearance of tissue plasminogen activator is inhibited and additionally in the reperfusion phase accelerated release can occur from graft endothelium[30,31].
REGIONAL HYPERCOAGULABILITY IN LIVER TRANSPLANTATIONS
The pathophysiology of portopulmonary hypertension and hepatopulmonary syndrome are nowadays thought to be related to hemostasis. Hepatopulmonary syndrome is characterized by a ventilation perfusion mismatch observed in fifteen to twenty percent of patients presenting for liver transplantation and is associated with increased mortality[32]. Portopulmonary hypertension occurs in three to eight percent of patients and mortality is increased especially in severe cases[33,34]. It is thought that the procoagulant shift of the pulmonary endothelium might be central to the pathophysiology of both diseases[35]. Accordingly, intrapulmonary microthrombi have been demonstrated in an autopsy study in patients with portopulmonary hypertension. During liver transplantation, reperfusion of the organ can lead to an entrapment of platelets[36]. Sinusoidal obstruction syndrome, formely called veno-occlusive disease occurs after liver transplantation, the incidence was described to be 1.9% in a large series and is thought to be associated with acute rejection, endothelialitis, and extravasation of fibrinogen and platelets finally resulting in fibrosis[37-39]. Although only tested in patients after stem cell transplantation, defibrotide and tissue plasminogen activator are claimed to improve the prognosis.
MONITORING OF HEMOSTASIS DURING LIVER TRANSPLANTATION
In patients presenting for liver surgery, hemostasis differs from patient to patient. Moreover, further alterations commonly occur during transplantation. Therefore, it seems plausible, that hemostasis monitoring might be beneficial. Plasma based laboratory tests and whole blood based bed-side monitoring are most commonly used.
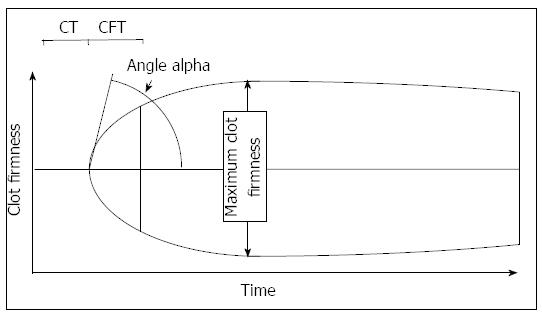
Conventional tests for the monitoring of hemostasis often include INR, aPTT, fibrinogen values, and platelet count. Bed-side devices often used in liver transplantation are often thrombelastography (TEG, Haemonetics, Niles, IL, United States) and thrombelastometry (TEM; ROTEM, TEM Innovations, Munich, Germany). Several recent reviews on different aspects of the methods are available[31,40-42]. Both bed-side methods measure the viscoelastic properties of a clot in a time dependent manner. Differences between the devices are related to the number of channels, technical differences, and costs. Both devices allow to determine the time to onset of clot formation, the kinetics of clot formation, the firmness of a clot and eventual clot lysis. TEG allows to determine platelet function by the “platelet mapping test”, TEM allows the use of activators of the extrinsic and intrinsic system (called EXTEM, INTEM), as well as inhibitors of platelet aggregation (FIBTEM) and fibrinolysis (APTEM). A typical thrombelastogram obtained by TEM and the variables are given in Figure 2. Characteristic changes due to hypofibrinogenemia, thrombocytopenia, hyperfibrinolysis, and heparin are shown in Figure 3. In a new version, platelet function can also be determined by an impedance aggregometry module.
Figure 2 Thrombelastometry variables most often used to interpretate a tracing.
The thrombelastogram shows the firmness of a clot during the measurement time. The clotting time is defined as the time from recalcification and activation of blood samples to the onset of coagulation. The maximum clot firmness describes the physical properties of a clot, which is defined by platelet count and fibrinogen concentration. The α angle and the clot formation time describe the kinetics of clot generation.
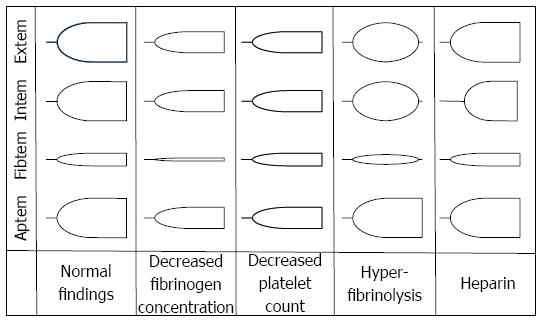
Figure 3 Typical changes in thrombelastometry tracings.
Shown are the effects of hypofibrinogenemia, thrombocytopenia, hyperfibrinolysis, and heparin.
Activated partial thromboplastin time, INR, fibrinogen, and platelet count are correlated with TEM findings. However, the hemochron signature device (ITC, NJ, United States), which is capable to measure aPTT and INR in whole blood samples, demonstrated far better correlation with laboratory findings[43].
The advantages of laboratory based assays are the simplicity and reliability for the anesthetist, however a practical drawback includes the turnaround time of more than one hour. This implies, that results are often too late to guide immediate treatment of bleeding patients. In contrast, bed-side assays can be determined within a relative short time, often 10 to 30 min, as transportation and centrifugation of samples are not necessary. However, time and technical expertise for the measurements and interpretation of results by the anesthetist are still necessary.
WHICH ALTERATIONS IN HEMOSTASIS CAN BE DETECTED BY TEM
Several alterations in hemostasis can be monitored by viscoelastic monitoring. Due to the fact, that our personal experiences are restricted to TEM, we limit our discussion to the typical findings with this device. It is, however, important to state that both devices are used in liver transplantations.
Dilutional coagulopathy can easily be detected by the reductions in clot firmness in both tissue factor and sialic acid activated assays. In these assays the clot firmness is dependent on both fibrinogen concentration and platelet count. More specifically, contribution of platelets to clot firmness can be eliminated by cytochalasin D resulting in a thrombelastogram, the amplitude of which is then solely dependent on fibrinogen concentration (and eventual disturbances of fibrin polymerization).
TEM is ideal to detect hyperfibrinolysis. Normally, clot firmness only marginally decreases with time. In the case of hyperfibrinolysis, however, the clot is often completely degraded. The time to lysis can vary, in extreme cases the absence of clot formation can also be observed. Measurement in the presence of aprotinin abolishes clot lysis and confirms the diagnosis of hyperfibrinolysis.
Autoheparinization caused by shedding of the glycocalyx results in a prolongation of sialic acid activated clotting time. Diagnosis of heparin or glycocalyx mediated anticoagulation can be obtained by addition of heparinase.
Hypercoagulability can be diagnosed by increases in fibrinogen concentration and platelet count resulting in an increased clot firmness. Moreover, shortening of clotting time may be indicative for hypercoagulability.
TEM cannot detect alterations in anticoagulant pathways, namely protein C, protein S, or antithrombin. Moreover, neither increased nor decreased platelet function can be distinguished (exceptions are eventually platelet mapping and impedance aggregometry module, respectively).
DOES GUIDANCE BY BED-SIDE MONITORING OR CONVENTIONAL COAGULATION ASSAYS REDUCE BLEEDING AND THROMBOSIS?
Concerning conventional coagulation assays, it is important to note that aPTT and INR were developed to monitor anticoagulation. Although often used as standard coagulation tests, there are no data to suggest that on the results of these, the risk of bleeding can be predicted or hemostasis therapy can be guided by these assays (for review see[44]). Conventional laboratory assays also failed to detect hypercoagulability[41]. Determination of thrombin-antithrombin-complexes, however, was predictive for portal vein thrombosis in patients with liver disease, but this method of monitoring is not readily available[45].
In contrast there is some evidence to indicate that viscoelastic methods can predict bleeding and thrombosis risk in patients presenting for liver transplantation. Potentially, whole blood viscoelastic methods containing both platelets and coagulation factors better simulate hemostasis alterations in vivo than highly artificial laboratory assays with platelet poor plasma. In a randomized controlled study, Wang et al[46] demonstrated that TEG in combination with a treatment algorithm reduced the transfusion of fresh frozen plasma but not 3-year survival in liver transplantation. Alamo et al[47] in a case-control study demonstrated that the use of TEM was associated with lower bleeding complications and mortality in high risk patients. Apart from liver transplantation, Wikkelsoe et al[40] concluded in their systemic review there is only weak evidence to support use of viscoelastic methods in patients at risk for massive bleeding. Intraoperative hypercoagulability was common in liver transplantation patient, especially in those presenting with cholestatic disease. Moreover thromboembolism was often associated with a hypercoagulable state as determined by viscoelastic methods[41].
VISCOELASTIC MONITORING TO GUIDE HEMOSTASIS INTERVENTIONS
Due to the complex alterations in hemostasis in liver transplantation, hemostasis treatment differs from patient to patient. Alterations in procoagulant pathways can best be detected by viscoelastic methods. However, there is no method to monitor all hemostasis alterations and anticoagulant pathways. Assuming a reduction of the procoagulant pathways is accompanied by comparable reductions in anticoagulant pathways, it seems logical (although not proven), that the thrombotic risk might be highest in those patients with the most pronounced decreases in procoagulant factors and platelet count. Therefore, in our opinion, it is of outmost importance to reduce procoagulant interventions in those patients. The need for transfusion can be guided by transfusion algorithms based on bed-side-viscoelastic methods[48]. In a Cochrane metaanalysis the authors came to the conclusion that use of viscoelastic methods might potentially decrease bleeding and transfusion demand in major surgery however further high quality studies were recommended[49].
PROPHYLAXIS AND TREATMENT OF COAGULOPATHY IN LIVER TRANSPLANTATION
Evidence for the benefits of blood products is low in perioperative medicine, while the supporting evidence for side effects like negative transfusion effect, transfusion associated lung injury, transfusion associated circulatory overload, and infectious complications is increasingly acknowledged. Increased morbidity and mortality have been demonstrated to be associated with transfusion of blood products in liver surgery and liver transplantation (fresh frozen plasma, platelets, red blood cells)[50-53]. Besides improvements in surgical experience and techniques in liver transplantation, strategies to reduce the use of blood products termed patient blood management are increasingly used. For monitoring of hemostasis disturbances, TEG/TEM are increasingly used and can guide the application of plasma components, platelets, and antifibrinolytics[54]. As bed-side-monitoring is increasingly used, the simple substitution of blood products by the 1:1:1 rule (red blood cells, fresh frozen plasma, platelets) is not encouraged in many liver transplantation centers.
Prophylaxis of coagulopathy
Concerning the use of a prophylactic intervention in hemostasis system, no benefit has been demonstrated so far for the use of fresh frozen plasma. Arguments against the use include the fact, that liver transplantations are nowadays increasingly done without blood products and fresh frozen plasma was associated with an increase in mortality in liver surgery[14,15,55]. In a multicenter randomized trial, use of recombinant activated factor VIIa failed to reduce bleeding but increased arterial thrombotic events by 70%[56]. Controversity persists with respect to the importance of prophylactic antifibrinolytics in liver transplantation, both detrimental effect and no effect on blood transfusions have been described after withdrawal of aprotinin[57,58]. In a recent meta-analysis, the authors come to the conclusion, that aprotinin and tranexamic acid reduce transfusion requirements in patients undergoing liver transplantation and that there was no evidence that antifibrinolytic drugs increase thromboembolic events[59]. However, as TEG/TEM can detect hyperfibrinolysis, a prophylactic antifibrinolytic therapy seems not to be necessary nowadays. Some basic measures during surgery are of outstanding importance to prevent coagulopathy: body temperature has to be monitored and decreases can be prevented by forced air-warming devices and warming of intravenous fluids[60,61]. Also of relevance risk for coagulopathy increases with ischemic time and the transplantation of dysfunctional and marginal grafts[14,62].
Fibrinogen in disturbances of hemostasis
Fibrinogen is the first factor to reach critical levels when haemodilution or massive bleeding occurs[63]. Advantages of fibrinogen use in comparison with fresh frozen plasma are the reduced rate of pathogen transmission as well as decreased risk of transfusion induced lung injury and transfusion induced circulatory overload. Fibrinogen concentration can easily be monitored by TEM and substitution therapy based on guidance according to the clot firmness reduced the rate of red blood cells, fresh frozen plasma and platelet transfusion by more than 50%. Additionally the rate of transplantation without transfusion of the above blood related products rose from 3.5% to 20%[64]. In a recent review on fibrinogen and fresh frozen plasma in surgery and trauma, the authors concluded, that fibrinogen level was generally associated with improved outcome measures, while fresh frozen plasma failed to show evidence for effectivity and had severe side effects[65].
TEM-guided treatment with fibrinogen concentrate and/or prothrombin complex concentrate (PCC) did not appear to increase the occurrence of thrombosis, pulmonary and ischemic events compared to patients who did not receive these concentrates[15].
Platelet transfusion
Besides their proven and essential role in hemostasis, platelets are involved in innate immunity and inflammation. Platelet transfusion, in general, may lead to complications such as febrile non-hemolytic transfusion reactions, allergic reactions, transfusion-associated sepsis, and transfusion associated lung injury[66]. Furthermore, evidence from a multicenter observational studies with 504208 patients demonstrates that platelet transfusion was associated with venous and arterial thromboembolism as well as death in patients with cancer[67]. Prophylactic use of platelets in internal medicine should be restricted to a platelet count below 10000/μL[68].Higher values are recommended for surgical procedures, however, platelet count in patients presenting for liver transplantation are often above these limits. Thrombopoeitin receptor agonists may in the future be used to increase platelet count in liver disease, however, the possible risks and benefits are at present unknown[9].
In patients presenting for liver transplantation, platelet count is often reduced but platelet function is increased due to highly active von Willebrand multimeres[4]. Platelets are supposed to be entrapped after reperfusion of the liver, and an involvement in sinusoidal obstruction syndrome, portopulmonary hypertension, and hepatopulmonary syndrome has been suggested[32-39]. Transfusion of platelets was associated with increased mortality in liver transplantations[51]. Contribution of platelet count to clot firmness can be assessed by TEG/TEM. The originally methods are not sensitive for alterations in platelet function, however, platelet mapping and impedance aggregometry module, respectively, may extend the diagnostic capabilities. In a recent study, we demonstrated, that platelet function, determined with whole blood impedance aggregometry, was markedly increased in patients presenting for liver transplantation (manuscript in preparation). It might thus be conceivable, that bed-side platelet function testing during liver transplantation can provide further information on the use of platelet transfusions and the thromboembolic risk.
Prothrombin complex concentrate
Besides fibrinogen, other coagulation factors may decrease during liver transplantation resulting in a reduction of thrombin generation and prolongation of clotting time. In these cases, prothrombin complex concentrate can correct coagulopathy. In principle, there are four factor PCC and three factor PCC. Four factor PCC contain the factors II, VII, IX, and X, and the anticoagulant factors protein S and protein C[69]. Infectious risk is reduced or eliminated by virus elimination and thromboembolic complication occurs in only 0.9%. In liver transplantation, TEM guided substitution of blood products including prothrombin complex concentrate in liver transplantation did not increase the occurrence of thrombosis and ischemic events[15]. The safety and effectivity of prothrombin complex concentrate in liver transplantation is investigated in a prospective randomized study[70].
CONCLUSION
Alterations of hemostasis of patients presenting for liver transplantation are complex and can differ according to the underlying liver disease. In patients with end stage liver disease, standard laboratory findings like INR and platelet count suggest hypocoagulability. However, both pro- and anticoagulant pathways are often reduced resulting in a rebalanced system. Thus, both diffuse bleeding and thrombosis can occur during liver transplantation. The most common used methods for the timely diagnosis of hemostatic disturbances currently available are TEG/TEM which with appropriate use can reduce the use of blood products. Substitution with coagulation factors, platelets, and antifibrinolytics can be guided by algorithms if diffuse bleeding occurs (or can be supposed to occur by the results of measurements). At time, no bed-side method is capable to measure the alterations in anticoagulant pathways, thus cautious substitution of coagulation factors is recommended.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kayaalp C, Marcos R S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH