Published online Jan 28, 2016. doi: 10.3748/wjg.v22.i4.1449
Peer-review started: May 6, 2015
First decision: August 31, 2015
Revised: September 30, 2015
Accepted: November 30, 2015
Article in press: December 1, 2015
Published online: January 28, 2016
Processing time: 267 Days and 5.6 Hours
Patients infected with the hepatitis C virus (HCV) are characterized by a high incidence of chronic infection, which results in chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. The functional impairment of HCV-specific T cells is associated with the evolution of an acute infection to chronic hepatitis. While T cells are the important effector cells in adaptive immunity, natural killer (NK) cells are the critical effector cells in innate immunity to virus infections. The findings of recent studies on NK cells in hepatitis C suggest that NK cell responses are indeed important in each phase of HCV infection. In the early phase, NK cells are involved in protective immunity to HCV. The immune evasion strategies used by HCV may target NK cells and might contribute to the progression to chronic hepatitis C. NK cells may control HCV replication and modulate hepatic fibrosis in the chronic phase. Further investigations are, however, needed, because a considerable number of studies observed functional impairment of NK cells in chronic HCV infection. Interestingly, the enhanced NK cell responses during interferon-α-based therapy of chronic hepatitis C indicate successful treatment. In spite of the advances in research on NK cells in hepatitis C, establishment of more physiological HCV infection model systems is needed to settle unsolved controversies over the role and functional status of NK cells in HCV infection.
Core tip: Natural killer (NK) cells protect our body from viral infections by killing infected cells and secreting cytokines that inhibit viral replication. Unlike T lymphocytes that require priming by antigen presenting cells, NK cells directly recognize virus-infected cells and immediately exert antiviral effector functions. Researchers who investigate immunity to hepatitis C virus (HCV), therefore, have been interested in NK cells. This review will give readers an overview of reports on NK cells in hepatitis C and insights into the role of NK cells in the defense against HCV infection, the immunopathogenesis of hepatitis C, and the prediction of the treatment response.
- Citation: Yoon JC, Yang CM, Song Y, Lee JM. Natural killer cells in hepatitis C: Current progress. World J Gastroenterol 2016; 22(4): 1449-1460
- URL: https://www.wjgnet.com/1007-9327/full/v22/i4/1449.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i4.1449
Hepatitis C virus (HCV) is a major cause of chronic viral hepatitis, liver cirrhosis, and hepatocellular carcinoma[1]. Chronic hepatitis C occurs in 60%-80% of individuals with acute HCV infection[2], liver cirrhosis arises in 20%-30% of individuals with chronic HCV infection, and hepatocellular carcinoma develops in 1%-4% of individuals with HCV-related liver cirrhosis[3]. HCV infection is, therefore, a leading cause of end-stage liver diseases throughout the world and the most common reason for liver transplantation in Western countries[4]. Although the characteristics of T lymphocyte and B lymphocyte responses against HCV have been revealed[2,5-8], natural killer (NK) cell responses are rather ill-defined and there are still many controversies to be resolved[9-14]. This review describes the current views on the role of NK cells in the defense against HCV infection, the relationship between NK cell responses and the immunopathogenesis of hepatitis C, the characteristics of NK cell responses in the coinfection of human immunodeficiency virus (HIV) and HCV, and the NK cell response as a predictor of treatment outcomes.
HCV belongs to the Flaviviridae family and has a single-stranded, positive sense RNA genome that is approximately 9.6 kb in length. The RNA genome has 5’ and 3’ untranslated regions (UTRs). An open reading frame (ORF) is flanked by the UTRs and translated into a polyprotein via an internal ribosome entry site (IRES) in the 5’ UTR. The polyprotein is cleaved by host and viral proteases to yield three structural proteins (core, E1, and E2) and seven nonstructural proteins (p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B). The core proteins form a capsid that surrounds the HCV RNA genome. E1 and E2 are envelope proteins that comprise a viral envelope. The nonstructural proteins are not incorporated into the viral particles but play important roles in viral protein processing and genome replication. The NS3-4A complex is a serine protease that cleaves the polyprotein into individual nonstructural proteins. NS5B is a viral RNA-dependent RNA polymerase that duplicates HCV RNA genome. NS5A is a regulator of viral replication and virion assembly[15,16]. Newly developed direct acting antivirals (DAAs) inhibit HCV replication by targeting NS3-4A (telaprevir, boceprevir, simeprevir, asunaprevir, etc.), NS5B (sofosbuvir, dasabuvir, and others), or NS5A (ledipasvir, ombitasvir, daclatasvir, etc.)[16].
Since their identification in 1975[17,18], NK cells have been considered lymphocytes because they originate from the common lymphoid progenitor cell in the bone marrow. Unlike other lymphoid cells in adaptive immunity, such as T lymphocytes and B lymphocytes, NK cells lack antigen-specific cell surface receptors and do not require antigen presentation by antigen-presenting cells or clonal expansion to initiate their effector functions[19].
NK cells participate in innate immunity to viruses[20,21] and tumors[22]. NK cells lyse virus-infected cells and tumor cells through degranulation of cytotoxic granules and the subsequent release of perforin and granzymes[23]. Another mechanism that NK cells utilize to exert cytotoxicity is the induction of target cell apoptosis through tumor necrosis factor-related apoptosis inducing ligand (TRAIL). In addition to their cytotoxic activity, NK cells are able to produce and secrete cytokines such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α, which have antiviral activity[24].
The antiviral activity of NK cells is tightly regulated by interactions between NK cell surface receptors and their cognate ligands[25,26]. NK cells express a wide variety of inhibitory and activating receptors on the plasma membrane[27]. Killer cell immunoglobulin-like receptors (KIRs), natural killer group 2A (NKG2A), and killer cell lectin-like receptor subfamily G member 1 (KLRG1) are examples of the inhibitory receptors, and NKG2D, NKG2C, and natural cytotoxicity receptors (NCRs), including NKp30, NKp44, and NKp46, are well-known activating receptors[19]. Normal nucleated cells express inhibitory ligands, such as class I major histocompatibility complex (MHC) molecules, on their surfaces and inhibit NK cell activation through interactions between the MHC molecules and the KIRs. Normal cells do not express activating ligands such as major histocompatibility complex class I-related chain A/B (MICA/B) and UL16-binding proteins (ULBPs). In contrast, virus-infected cells may reduce the class I MHC molecules and display activating ligands on their surfaces. NK cells that recognize the activating ligands become activated and exert their antiviral effector functions[19,27].
Human NK cells are CD3-CD56+ lymphocytes and are divided into two subsets based on the density of CD56 expression on their surfaces[28]. Approximately 10% of NK cells in the peripheral blood and nearly 100% of NK cells in the secondary lymphoid tissues, such as the lymph nodes, have high surface expression of CD56 (CD56bright NK cells). The CD56bright subset can produce a large amount of cytokines and chemokines upon activation but has little or no ability to kill the target cells[29]. The majority of NK cells in the peripheral blood have relatively low surface expression of CD56 (CD56dim NK cells). The CD56dim subset has a reduced ability to produce cytokines, but some of them can spontaneously kill the susceptible target cells[22].
NK cells comprise 30%-50% of liver-resident lymphocytes[30,31], whereas 5%-15% of peripheral blood mononuclear cells (PBMCs) are NK cells. Hepatitis further increases the number of NK cells in the liver[32-34]. Chemokines secreted by Kupffer cells recruit NK cells to the liver, and cytokines secreted by the Kupffer cells, liver sinusoidal endothelial cells, and T cells promote the survival of the recruited NK cells[35]. Because of their enrichment in the liver and their ability to eliminate viral infections, NK cells may play key roles in the immune response against HCV infection[9,14,36].
The importance of NK cells in antiviral immune responses has promoted studies on the interactions between NK cells and HCV. Noninfectious HCV model systems, including recombinant HCV proteins, expression vectors encoding HCV proteins, and HCV replicon-containing hepatoma cell lines have been employed to investigate the interplay between NK cells and HCV[37-41]. The conclusions of the studies are, however, often contradictory[39-41], because the different experimental systems yield dissimilar results. Because there is still a lack of efficient small animal models for investigating the immunopathogenesis of hepatitis C[42,43], it is difficult to resolve the controversies over the functional status of NK cells in HCV infection. In 2005, several research groups reported establishment of an HCV cell culture system[44-46], which produces infectious HCV virions. It provides researchers with an opportunity to simulate every step of the natural HCV life cycle in vitro. This revolutionary method has, therefore, encouraged in vitro studies on the interactions of NK cells with infectious HCV particles.
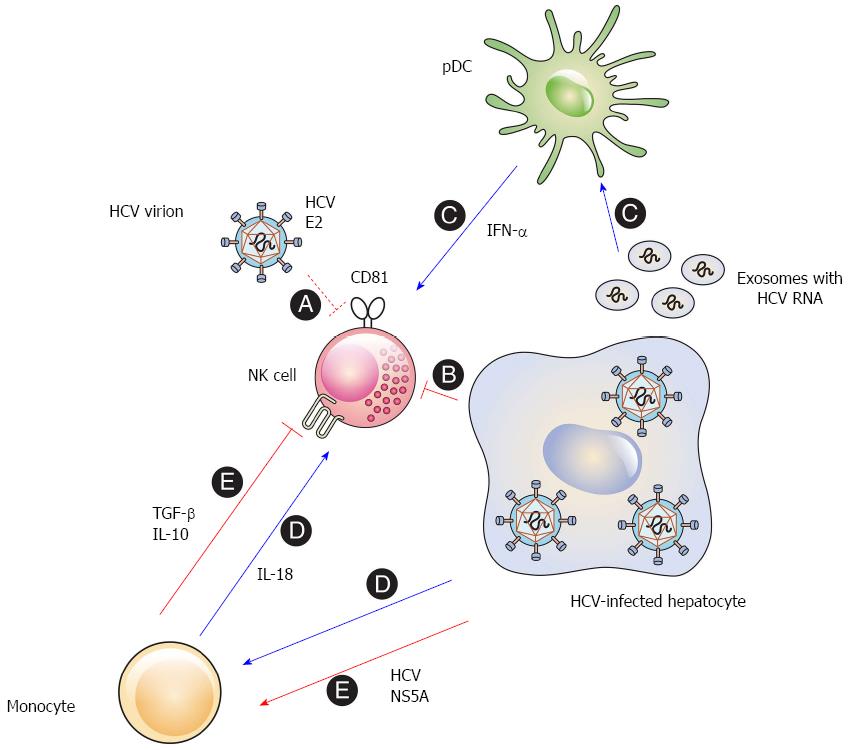
The early studies that observed interactions between HCV and NK cells employed recombinant HCV proteins. Plate-bound E2 inhibits the effector functions of primary human NK cells by cross-linking CD81 on the surface of NK cells[37,38]. Cell-culture-generated HCV virions are also able to inhibit NK cells when they are bound to a plate[47]. Nevertheless, HCV virions do not impair the effector functions of NK cells, if they are soluble and mobile[47,48] (Figure 1, A). These results suggest that HCV E2 can induce cross-linking of CD81 and inhibit NK cell function. However, this might be less likely in vivo, because CD81 cross-linking requires immobilization and a high concentration of cell-free HCV virions in the liver.
Direct contact of NK cells with HCV-infected hepatoma cells reduces the functional capacity of the NK cells[49]. The activating receptors NKG2D and NKp30 are downregulated on the surface of NK cells after cell-to-cell contact with the HCV-infected cells. Downregulation of the activating receptors results in the reduced functional capacity of the NK cells and the inhibition of the ex vivo NK cell functions[49,50] (Figure 1, B). It appears that this phenomenon is associated with the NS3-4A protease activity in the infected cells, because inhibition of the NS3-4A protease eliminated contact-dependent suppression of NK cells by the HCV-infected cells (unpublished data). The findings imply that HCV may impair the effector functions of NK cells in the infected liver.
Interestingly, NK cells that are prestimulated with exogenous IFN-α can kill HCV-infected hepatoma cells[51,52]. This implies that the effect of innate cytokines, such as type I IFNs including IFN-α and IFN-β, interleukin (IL)-8, IL-12, IL-15, and IL-18[12,53], should be considered when evaluating the interactions between NK cells and HCV-infected cells.
IFN-α and other innate cytokines secreted by accessory immune cells may modulate the interaction between NK cells and HCV-infected hepatocytes. The activation of NK cells by some pathogens indeed requires cytokines from accessory cells such as monocytes, macrophages, conventional dendritic cells (cDCs), and plasmacytoid dendritic cells (pDCs)[54-58]. In the liver, Kupffer cells can act as accessory cells that sense the viral RNA and indirectly activate NK cells by secreting innate cytokines[59,60].
Accessory cells regulate IFN-γ production by primary human NK cells after interacting with HCV-infected hepatoma cells. In particular, the IFN-α produced by pDCs activates NK cells to produce IFN-γ[61]. This suggests that the IFN-α secreted by the accessory cells may overcome the inhibitory effect induced by direct contact between NK cells and HCV-infected cells. Although HCV virions do not infect PBMCs[62], the interaction of pDCs with HCV-infected cells stimulates pDCs to secrete type I IFNs[63-66], because the pDCs sense the HCV RNA in exosomes derived from the infected hepatoma cells[66] (Figure 1, C). Therefore, close contact among HCV-infected hepatocytes, NK cells, and pDCs may simultaneously induce both inhibitory and activating signals, and the balance between the two signals may determine whether the NK cells are activated or inhibited.
In addition, monocytes sense HCV replicon-containing cells and secrete IL-18 which activates NK cells to produce IFN-γ[67] (Figure 1, D). Another study, however, reported somewhat different conclusions. The HCV NS5A protein stimulates monocytes through toll-like receptor (TLR)-4 and induces the secretion of IL-10, which is an immunosuppressive cytokine, while inhibiting the production of IL-12, which activates NK cells. IL-10 then stimulates the secretion of transforming growth factor (TGF)-β, which downregulates NKG2D on the NK cell surface, resulting in the subsequent functional impairment of the NK cells[68] (Figure 1, E). The discordance between the two studies may be due to the different experimental systems used. Further investigations are needed to clearly elucidate the role of accessory cells in the modulation of NK cells in HCV infection.
The natural course of HCV infection can be divided into three distinct phases: the incubation phase, in which exposure to HCV occurs, but hepatitis and viremia are not yet detected; the acute phase, in which hepatitis and viremia are detected; and the chronic phase, which begins six months after the onset of the acute phase and is characterized by persistent HCV infection and chronic liver inflammation. The early phase of HCV infection generally refers to the period from the beginning of the incubation phase to the end of the acute phase[12].
NK cell activation in the early phase is influenced by the interactions between KIRs on NK cells and class I MHC molecules, such as human leukocyte antigen (HLA)-A/B/C, on the HCV-infected cells. Genetic studies reveal that specific KIR and HLA-C pairs are associated with the spontaneous resolution of HCV infection[69,70], as host genetic factors, such as HLA genotypes, affect the T lymphocyte-related outcome of HCV infection[71]. NK cells from homozygous HLA-C group 1 (HLA-C1) individuals exert their effector functions more rapidly than those from homozygous HLA-C2 individuals[72]. This results from the reduced inhibition of NK cells by the KIR2DL3/HLA-C1 interaction than that observed with other KIR/HLA interactions. NK cell cytotoxicity is increased only in individuals with KIRs that interact with HLA-C1 and is maximal in the spontaneously resolved cases[73]. The results suggest that NK cells are indeed important in the antiviral immune responses in the early phase of HCV infection. Therefore, it may be feasible that NK cell responses are not optimal in the acute phase of a substantial number of HCV infection cases, because 60%-80% of individuals in the acute phase do not achieve spontaneous resolution and develop chronic hepatitis C[8].
Healthcare workers who have been accidentally exposed to HCV but do not develop acute hepatitis and viremia have strong NK cell responses against the very small amount of HCV in the incubation phase, which is within weeks after the exposure[74]. Increased NK cell degranulation in the early phase of HCV infection is also found in intravenous (IV) drug users who clear HCV spontaneously[75]. NK cells from individuals with spontaneous resolution show increased expression of the activating receptors, NKp46 and NKp44[74] and decreased expression of the inhibitory receptor NKG2A[75]. Moreover, increased NK cell cytotoxicity in the early phase is related to strong T cell responses in both healthcare workers and IV drug users[74,75]. Another study shows that NK cells from individuals who spontaneously eradicate HCV in the acute phase exhibit stronger IFN-γ secretion and express higher levels of NKG2D and NKp46 than NK cells from individuals who do not achieve spontaneous clearance of HCV infection[76]. These findings imply that sufficient and optimal NK cell responses in the early phase are associated with HCV clearance and prevent HCV infection from transitioning to the chronic phase. On the other hand, it is possible that individuals who do not spontaneously clear HCV and develop chronic hepatitis C cannot exert strong and optimal NK cell responses in the early phase. Weak and suboptimal activation of NK cells might be associated with the immune evasion strategies observed in the interactions of NK cells with HCV and accessory cells (Figure 1).
Ex vivo studies using NK cells in PBMCs or intrahepatic lymphocytes from individuals with chronic hepatitis C have provided insights into the functional status of NK cells in chronic HCV infection. Although there are discrepancies in the ex vivo studies[34,68,77-80], it appears that the role of NK cells in the chronic phase of HCV infection is different from that in the early phase.
NK cells may be persistently activated in the chronic phase[77]. However, chronic activation of NK cells does not necessarily mean that all aspects of NK cell function are enhanced. NK cell cytotoxicity is increased, but IFN-γ production by the NK cells is reduced in chronic HCV infections[34,77]. This functional polarization of NK cells is due to the persistent exposure to endogenous IFN-α, which upregulates the expression of signal transducer and activator of transcription 1 (STAT1) in NK cells and induces preferential phosphorylation of STAT1 over STAT4. Phosphorylated STAT1 (pSTAT1) then stimulates NK cell cytotoxicity, while the lack of STAT4 phosphorylation impairs IFN-γ production, which is stimulated by phosphorylated STAT4[81-84].
It is reported that NKp46 expression is upregulated on peripheral blood NK cells and intrahepatic NK cells in chronic HCV infection[77]. NK cells with high surface expression of NKp46 (NKp46high NK cells) exert strong cytotoxicity and produce more IFN-γ. NKp46high NK cells are enriched in the inflamed liver and show enhanced cytolytic activity against HCV-infected hepatocytes and the hepatic stellate cells that are involved in hepatic fibrosis[85]. Another report reveals that NK cells induce the apoptosis of activated hepatic stellate cells[86]. These observations suggest that intrahepatic NK cells may control HCV replication and modulate hepatic fibrosis. However, intrahepatic NK cells also contribute to the immunopathology of chronic hepatitis C by inducing inflammation in the liver[87]. In contrast, other studies report that the increased numbers of NKp46+ NK cells in the peripheral blood are associated with the production of IL-10 which is an immunosuppressive cytokine. These results imply a lack of virus control rather than virus clearance in chronic hepatitis C[88]. Moreover, degranulation and TRAIL expression by intrahepatic NK cells are quite reduced in chronic HCV infection, even though NKp46 expression increases, suggesting that the NK cell response is impaired in the HCV-infected liver[89]. Thus, both defensive and pathogenic roles of NK cells should be considered together to properly interpret the NK cell response in the chronic phase of HCV infection (Table 1).
| Phenotype/function | Ref. reported increase | Ref. reported decrease | Ref. reported no change |
| Activation marker | |||
| CD69 | [34] | [88] | |
| Activating receptor | |||
| NKG2D | [34] | [68] | |
| NKG2C | [77] | ||
| NKp46 | [77,88,89] | [80] | |
| NKp30 | [88] | [80] | |
| NKp44 | [77] | ||
| Inhibitory receptor | |||
| KIR3DL1 | [34] | ||
| NKG2A | [78-80] | ||
| KLRG1 | [90] | ||
| Cytotoxicity | |||
| Degranulation | [34,77] | [68,80,89] | [78] |
| TRAIL expression | [77] | [89] | |
| Immunostimulating cytokine | |||
| IFN-γ | [78] | [34,68] | [77,88] |
| Immunosuppressive cytokine | |||
| IL-10 | [88] |
Other activating receptors, such as NKG2D, NKG2C, NKp30, and NKp44, may also be highly expressed on the surface of NK cells from individuals with a chronic HCV infection[34,77,88]. A noticeable number of studies describe, however, a reduction in the expression of the activating receptors NKG2D, NKp30 and NKp46[68,80] and an increase in the expression of the inhibitory receptors NKG2A and KLRG1[78-80,90] (Table 1). Although the reason for this significant controversy is unclear, it may be due to the differences in patient numbers and selection methods[9].
The importance of NK cell responses for HCV clearance is also observed in HCV/HIV coinfection. The spontaneous resolution of acute hepatitis C is associated with an effective IFN-γ-mediated NK cell response, which inhibits HCV replication, in HCV/HIV-coinfected patients[76].
However, preexisting HIV infection may hinder NK cell responses against HCV infection[91,92]. HIV inhibits cDC activation, and this leads to the functional impairment of NK cells, because the crosstalk between activated cDCs and NK cells is important for the activation of the latter[21]. Therefore, the loss of cDC-NK cell interactions in HCV/HIV coinfection may result in defective NK cell responses to HCV[91]. In addition, NK cells are less sensitive to type I IFNs secreted by pDCs during HIV infection. This reduced responsiveness may attenuate NK cells’ ability to control HCV infection, because type I IFNs stimulate NK cell proliferation and cytotoxicity[21,91].
HCV/HIV coinfection results in an accelerated development of liver cirrhosis compared to HCV monoinfection. Because CD4+ T cells can stimulate NK cells to exert antifibrotic activity, the loss and functional impairment of CD4+ T cells by HIV may contribute to the faster progression to liver fibrosis[93].
The treatment response to the pegylated IFN-α (PEG-IFN-α)/ribavirin combination therapy in chronic HCV infection is related to the patients’ KIR and HLA-C genotypes[94,95]. NK cells are important not only in spontaneous resolution in the early phase of infection but also in HCV clearance by IFN-α-based therapy in the chronic phase. In the latter, the injection of exogenous IFN-α activates NK cells to increase cytotoxicity[53,96].
The levels of NK cell activating receptors NKp30 and NKp46 before PEG-IFN-α/ribavirin therapy may predict the treatment outcome[97]. In addition, higher IFN-α receptor (IFNAR) expression on the surface of CD16+CD56- NK cells before the combination therapy is associated with the treatment response[98].
Within hours of the first dose of IFN-α, the NK cells from the treated individuals are activated and express the NK cell activation marker CD69 and the activating receptors NKG2D and NKp30 on their surfaces. This phenomenon is more prominent in individuals who achieve early virological response[99]. Maximal NK cell cytotoxicity occurs at 24 h after treatment onset and is correlated with increased alanine aminotransferase in the serum, suggesting that the NK cells activated by exogenous IFN-α kill the HCV-infected cells in the liver. The functional polarization of NK cells observed in chronic HCV infection is intensified by IFN-α-based therapy[99] and mediated by STAT1 phosphorylation[81]. That is, both persistent exposure to endogenous IFN-α and acute exposure to large amount of exogenous recombinant IFN-α are able to induce preferential phosphorylation of STAT1 over STAT4 in intrahepatic NK cells.
NK cells from individuals with a rapid first-phase decrease in the HCV RNA level induce maximal STAT1 phosphorylation, whereas NK cells from individuals with slow first-phase decrease in the HCV RNA level induce lower levels of STAT1 phosphorylation. Interestingly, the NK cells from the former persons become refractory to stimulation by IFN-α within 72 h after the onset of the therapy. Therefore, the NK cell response in the first several days of PEG-IFN-α/ribavirin therapy may be important for successful treatment[81].
The increase in activated CD69+ NK cells during IFN-α-based therapy predicts the rapid virological response. The sustained virological response (SVR) is associated with a higher NK perforin content and higher proportion of CD56dimCD16- NK cells. Indeed, individuals achieving SVR exhibit higher NK cell cytotoxicity[100]. This implies that NK cell activation is required to achieve the early treatment response to HCV infection and that NK cell cytotoxicity is important to steadily suppress HCV replication in vivo. The NK cell response may be, therefore, used as a predictor of the treatment outcome for IFN-α-based therapy[12], and may supplement other predictors, such as the pretreatment level of interferon gamma-induced protein 10 (IP-10; CXCL10)[101,102] and the IL-28B (IFNL) genotype[103-106].
The IL-28B polymorphism is related to the altered expression of NK cell receptors, such as KIRs, NKG2A, and NKp30[106,107]. It is unclear, however, whether IL-28B (IFN-λ) directly affect NK cell responses in HCV infection[108-111]. The IL-28B-favorable (CC) genotype is unassociated with favorable KIR/HLA-C haplotypes[95]. Therefore, it would be better to utilize the NK cell response in hepatitis C as an additive predictor of the IFN-based treatment outcome.
It appears that TRAIL expression is also increased in NK cells from individuals achieving SVR after PEG-IFN-α/ribavirin treatment, suggesting that the NK cells activated by IFN-α kill the HCV-infected cells by employing not only perforin and granzymes but also TRAIL[52].
In HCV/HIV coinfection, the baseline frequency of the CD56-CD16+ NK cell subset, which is highly dysfunctional, is negatively correlated with response to IFN-α-based treatment[112].
Several DAAs, such as NS3-4A protease inhibitors, NS5B polymerase inhibitors, and NS5A inhibitors, have been developed to treat chronic hepatitis C. Highly efficient combinations of these DAAs allow IFN-free regimens[16]. Unlike IFN-α-based therapy, the clearance of HCV by the NS5A inhibitor (daclatasvir)/NS3-4A protease inhibitor (asunaprevir) combination therapy leads to the loss of intrahepatic immune activation by IFN-α and the normalization of functionally polarized intrahepatic NK cells[113]. Therefore, the restoration of the NK cells’ ability to produce IFN-γ during IFN-free DAA therapy may indicate the treatment response.
The lack of small animal models has greatly hampered research on the role of NK cells in hepatitis C. Although it supports the entire life cycle of HCV[114,115], the chimpanzee model is not readily available because of high costs and ethical concerns[42]. In vitro models and human ex vivo models are, therefore, the best currently available HCV model systems. These models have, however, several drawbacks.
In vitro infection of human hepatoma cell lines with cell-culture-generated HCV supports high-level HCV replication, but it cannot precisely mimic the natural HCV infection in normal hepatocytes. Moreover, in vitro HCV infection is supported by a limited number of Huh7-derived human hepatoma cell lines[44-46]. Although cultured primary human hepatocytes can be infected with cell-culture-generated HCV in vitro, they support only low levels of HCV replication, which requires the detection of the negative strand viral RNA by polymerase chain reaction (PCR)[116]. In terms of NK cell research, the major drawback of the in vitro HCV infection model is that both hepatoma cell lines and primary hepatocytes in a cell culture dish or a plate are unable to precisely reproduce the organization of the immune environment in the liver and in the adjacent lymph nodes.
Because T lymphocytes have antigen specificity, HCV-specific T cells are easily distinguished from other T cells ex vivo. However, NK cells do not possess antigen specificity[19]. It is, therefore, uncertain whether the majority of peripheral blood NK cells tested ex vivo have really interacted with the HCV-infected hepatocytes or HCV virions in vivo. The use of intrahepatic NK cells does not necessarily ensure that the NK cells have directly contacted HCV-infected hepatocytes, because the proportion of infected hepatocytes in the liver of individuals with chronic HCV infection could be as low as 1% and the infected cells only occur in clusters throughout the liver[117].
NK cells that actively exerted their effector functions on HCV in vivo and thereby have no remaining effector capacity may appear as functionally impaired cells ex vivo. In contrast, NK cells that could not exert any effector functions against HCV infection in vivo and thereby retain their effector capacity may appear as functionally competent cells ex vivo. Therefore, our ex vivo observations may not accurately reflect the real role and functional status of the NK cells in the HCV-infected liver.
Indeed, in vitro studies and human ex vivo studies have frequently produced contradictory results. Thus, advances in the experimental methods in the field of HCV research, such as the development of a genetically humanized, immunocompetent mouse model[118,119], are required to obtain clearer insights into the functional status of NK cells in HCV infection. Currently, a balanced interpretation of the results from various papers is needed to appropriately understand the role of NK cells in each phase of HCV infection.
While T cell responses are critical for adaptive immunity to HCV, NK cells are crucial players in innate immunity to HCV. In the early phase of HCV infection, the enhanced functional capacity of NK cells is correlated with spontaneous resolution and strong T cell responses. It appears that NK cells play an important role in preventing the conversion of the early phase of HCV infection to the chronic phase. The various strategies that are employed by HCV to evade NK cell responses may interfere with the protective functions of NK cells in the early phase and might contribute to the progression to chronic hepatitis C, which develops in about three fourths of individuals with an acute HCV infection. In the chronic phase, it appears that NK cells are important for controlling HCV replication and the resistance to hepatic fibrosis. A considerable number of studies, however, refute this possibility by showing that the NK cells’ function is impaired in chronic HCV infection. The NK cell response is also associated with the treatment response. Increased NK cell effector functions during IFN-α-based therapy may predict the treatment outcome. Although there has been significant progress in the research on NK cells in hepatitis C, physiological models, such as an immunocompetent mouse model, must be established to solve the problems of the current experimental methods and settle the controversies over the role and functional status of NK cells in each phase of HCV infection.
The authors would like to thank Dong-Su Jang, MFA, (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, South Korea) for his help with the illustration.
| 1. | Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 354] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 2. | Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 422] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 3. | Lee MH, Yang HI, Yuan Y, L’Italien G, Chen CJ. Epidemiology and natural history of hepatitis C virus infection. World J Gastroenterol. 2014;20:9270-9280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 66] [Reference Citation Analysis (1)] |
| 4. | Burra P. Hepatitis C. Semin Liver Dis. 2009;29:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Dustin LB, Rice CM. Flying under the radar: the immunobiology of hepatitis C. Annu Rev Immunol. 2007;25:71-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 235] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 6. | Ferrari C. T and B cells in hepatitis C virus control: what they do and when they fail. Gastroenterology. 2007;132:801-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Rehermann B. Chronic infections with hepatotropic viruses: mechanisms of impairment of cellular immune responses. Semin Liver Dis. 2007;27:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1202] [Cited by in RCA: 1225] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 9. | Ahlenstiel G. The natural killer cell response to HCV infection. Immune Netw. 2013;13:168-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Abdel-Hakeem MS, Shoukry NH. Protective immunity against hepatitis C: many shades of gray. Front Immunol. 2014;5:274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Park SH, Rehermann B. Immune responses to HCV and other hepatitis viruses. Immunity. 2014;40:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 12. | Rehermann B. Pathogenesis of chronic viral hepatitis: differential roles of T cells and NK cells. Nat Med. 2013;19:859-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 387] [Article Influence: 29.8] [Reference Citation Analysis (1)] |
| 13. | Mondelli MU, Varchetta S, Oliviero B. Natural killer cells in viral hepatitis: facts and controversies. Eur J Clin Invest. 2010;40:851-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Golden-Mason L, Rosen HR. Natural killer cells: multifaceted players with key roles in hepatitis C immunity. Immunol Rev. 2013;255:68-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453-463. [PubMed] |
| 16. | Scheel TK, Rice CM. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat Med. 2013;19:837-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 431] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 17. | Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16:216-229. [PubMed] |
| 18. | Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1208] [Cited by in RCA: 1225] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 19. | Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2103] [Cited by in RCA: 2080] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 20. | Lanier LL. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol. 2008;8:259-268. [PubMed] |
| 21. | Altfeld M, Fadda L, Frleta D, Bhardwaj N. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat Rev Immunol. 2011;11:176-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 23. | Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391-398. [PubMed] |
| 24. | Lee SH, Miyagi T, Biron CA. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007;28:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 25. | Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495-502. [PubMed] |
| 26. | Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2096] [Cited by in RCA: 2204] [Article Influence: 105.0] [Reference Citation Analysis (1)] |
| 27. | Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503-510. [PubMed] |
| 28. | Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633-640. [PubMed] |
| 29. | Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146-3151. [PubMed] |
| 30. | Doherty DG, O’Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol Rev. 2000;174:5-20. [PubMed] |
| 31. | Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513-528. [PubMed] |
| 32. | Bonorino P, Ramzan M, Camous X, Dufeu-Duchesne T, Thélu MA, Sturm N, Dariz A, Guillermet C, Pernollet M, Zarski JP. Fine characterization of intrahepatic NK cells expressing natural killer receptors in chronic hepatitis B and C. J Hepatol. 2009;51:458-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 33. | Morishima C, Paschal DM, Wang CC, Yoshihara CS, Wood BL, Yeo AE, Emerson SS, Shuhart MC, Gretch DR. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology. 2006;43:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 34. | Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, De Filippi F, Bruno S, Mondelli MU. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137:1151-160, 1151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 343] [Article Influence: 20.2] [Reference Citation Analysis (1)] |
| 35. | Grégoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 418] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 36. | Golden-Mason L, Rosen HR. Natural killer cells: primary target for hepatitis C virus immune evasion strategies? Liver Transpl. 2006;12:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Crotta S, Stilla A, Wack A, D’Andrea A, Nuti S, D’Oro U, Mosca M, Filliponi F, Brunetto RM, Bonino F. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J Exp Med. 2002;195:35-41. [PubMed] |
| 38. | Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002;195:43-49. [PubMed] |
| 39. | Herzer K, Falk CS, Encke J, Eichhorst ST, Ulsenheimer A, Seliger B, Krammer PH. Upregulation of major histocompatibility complex class I on liver cells by hepatitis C virus core protein via p53 and TAP1 impairs natural killer cell cytotoxicity. J Virol. 2003;77:8299-8309. [PubMed] |
| 40. | Tardif KD, Siddiqui A. Cell surface expression of major histocompatibility complex class I molecules is reduced in hepatitis C virus subgenomic replicon-expressing cells. J Virol. 2003;77:11644-11650. [PubMed] |
| 41. | Moradpour D, Grabscheid B, Kammer AR, Schmidtke G, Groettrup M, Blum HE, Cerny A. Expression of hepatitis C virus proteins does not interfere with major histocompatibility complex class I processing and presentation in vitro. Hepatology. 2001;33:1282-1287. [PubMed] |
| 42. | Yang DR, Zhu HZ. Hepatitis C virus and antiviral innate immunity: who wins at tug-of-war? World J Gastroenterol. 2015;21:3786-3800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Billerbeck E, de Jong Y, Dorner M, de la Fuente C, Ploss A. Animal models for hepatitis C. Curr Top Microbiol Immunol. 2013;369:49-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Lindenbach BD, Evans MJ, Syder AJ, Wölk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623-626. [PubMed] |
| 45. | Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Kräusslich HG, Mizokami M. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791-796. [PubMed] |
| 46. | Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T, Chisari FV. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci USA. 2005;102:9294-9299. [PubMed] |
| 47. | Crotta S, Brazzoli M, Piccioli D, Valiante NM, Wack A. Hepatitis C virions subvert natural killer cell activation to generate a cytokine environment permissive for infection. J Hepatol. 2010;52:183-190. [PubMed] |
| 48. | Yoon JC, Shiina M, Ahlenstiel G, Rehermann B. Natural killer cell function is intact after direct exposure to infectious hepatitis C virions. Hepatology. 2009;49:12-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 49. | Yoon JC, Lim JB, Park JH, Lee JM. Cell-to-cell contact with hepatitis C virus-infected cells reduces functional capacity of natural killer cells. J Virol. 2011;85:12557-12569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Holder KA, Stapleton SN, Gallant ME, Russell RS, Grant MD. Hepatitis C virus-infected cells downregulate NKp30 and inhibit ex vivo NK cell functions. J Immunol. 2013;191:3308-3318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Stegmann KA, Björkström NK, Ciesek S, Lunemann S, Jaroszewicz J, Wiegand J, Malinski P, Dustin LB, Rice CM, Manns MP. Interferon α-stimulated natural killer cells from patients with acute hepatitis C virus (HCV) infection recognize HCV-infected and uninfected hepatoma cells via DNAX accessory molecule-1. J Infect Dis. 2012;205:1351-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Stegmann KA, Björkström NK, Veber H, Ciesek S, Riese P, Wiegand J, Hadem J, Suneetha PV, Jaroszewicz J, Wang C. Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology. 2010;138:1885-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 53. | Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1593] [Cited by in RCA: 1579] [Article Influence: 58.5] [Reference Citation Analysis (7)] |
| 54. | Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175:1636-1642. [PubMed] |
| 55. | Newman KC, Riley EM. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat Rev Immunol. 2007;7:279-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 56. | Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25:47-52. [PubMed] |
| 57. | Velazquez VM, Hon H, Ibegbu C, Knechtle SJ, Kirk AD, Grakoui A. Hepatic enrichment and activation of myeloid dendritic cells during chronic hepatitis C virus infection. Hepatology. 2012;56:2071-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Michel T, Hentges F, Zimmer J. Consequences of the crosstalk between monocytes/macrophages and natural killer cells. Front Immunol. 2012;3:403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 59. | Lau DT, Negash A, Chen J, Crochet N, Sinha M, Zhang Y, Guedj J, Holder S, Saito T, Lemon SM. Innate immune tolerance and the role of kupffer cells in differential responses to interferon therapy among patients with HCV genotype 1 infection. Gastroenterology. 2013;144:402-413.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 60. | Tu Z, Bozorgzadeh A, Pierce RH, Kurtis J, Crispe IN, Orloff MS. TLR-dependent cross talk between human Kupffer cells and NK cells. J Exp Med. 2008;205:233-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 185] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 61. | Zhang S, Saha B, Kodys K, Szabo G. IFN-γ production by human natural killer cells in response to HCV-infected hepatoma cells is dependent on accessory cells. J Hepatol. 2013;59:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Marukian S, Jones CT, Andrus L, Evans MJ, Ritola KD, Charles ED, Rice CM, Dustin LB. Cell culture-produced hepatitis C virus does not infect peripheral blood mononuclear cells. Hepatology. 2008;48:1843-1850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 63. | Dental C, Florentin J, Aouar B, Gondois-Rey F, Durantel D, Baumert TF, Nunes JA, Olive D, Hirsch I, Stranska R. Hepatitis C virus fails to activate NF-κB signaling in plasmacytoid dendritic cells. J Virol. 2012;86:1090-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Takahashi K, Asabe S, Wieland S, Garaigorta U, Gastaminza P, Isogawa M, Chisari FV. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci USA. 2010;107:7431-7436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 222] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 65. | Zhang XA, Huang C. Tetraspanins and cell membrane tubular structures. Cell Mol Life Sci. 2012;69:2843-2852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | Dreux M, Garaigorta U, Boyd B, Décembre E, Chung J, Whitten-Bauer C, Wieland S, Chisari FV. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12:558-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 390] [Article Influence: 30.0] [Reference Citation Analysis (1)] |
| 67. | Serti E, Werner JM, Chattergoon M, Cox AL, Lohmann V, Rehermann B. Monocytes activate natural killer cells via inflammasome-induced interleukin 18 in response to hepatitis C virus replication. Gastroenterology. 2014;147:209-220.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 68. | Sène D, Levasseur F, Abel M, Lambert M, Camous X, Hernandez C, Pène V, Rosenberg AR, Jouvin-Marche E, Marche PN. Hepatitis C virus (HCV) evades NKG2D-dependent NK cell responses through NS5A-mediated imbalance of inflammatory cytokines. PLoS Pathog. 2010;6:e1001184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 69. | Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 930] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 70. | Cheent K, Khakoo SI. Natural killer cells and hepatitis C: action and reaction. Gut. 2011;60:268-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 71. | Bengsch B, Thimme R, Blum HE. Role of host genetic factors in the outcome of hepatitis C virus infection. Viruses. 2009;1:104-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Ahlenstiel G, Martin MP, Gao X, Carrington M, Rehermann B. Distinct KIR/HLA compound genotypes affect the kinetics of human antiviral natural killer cell responses. J Clin Invest. 2008;118:1017-1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 73. | Amadei B, Urbani S, Cazaly A, Fisicaro P, Zerbini A, Ahmed P, Missale G, Ferrari C, Khakoo SI. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology. 2010;138:1536-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 74. | Werner JM, Heller T, Gordon AM, Sheets A, Sherker AH, Kessler E, Bean KS, Stevens M, Schmitt J, Rehermann B. Innate immune responses in hepatitis C virus-exposed healthcare workers who do not develop acute infection. Hepatology. 2013;58:1621-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 75. | Pelletier S, Drouin C, Bédard N, Khakoo SI, Bruneau J, Shoukry NH. Increased degranulation of natural killer cells during acute HCV correlates with the magnitude of virus-specific T cell responses. J Hepatol. 2010;53:805-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 76. | Kokordelis P, Krämer B, Körner C, Boesecke C, Voigt E, Ingiliz P, Glässner A, Eisenhardt M, Wolter F, Kaczmarek D. An effective interferon-gamma-mediated inhibition of hepatitis C virus replication by natural killer cells is associated with spontaneous clearance of acute hepatitis C in human immunodeficiency virus-positive patients. Hepatology. 2014;59:814-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 77. | Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, Ghany MG, Hoofnagle JH, Liang TJ, Heller T. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138:325-35.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 217] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 78. | Golden-Mason L, Madrigal-Estebas L, McGrath E, Conroy MJ, Ryan EJ, Hegarty JE, O’Farrelly C, Doherty DG. Altered natural killer cell subset distributions in resolved and persistent hepatitis C virus infection following single source exposure. Gut. 2008;57:1121-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 79. | Jinushi M, Takehara T, Tatsumi T, Kanto T, Miyagi T, Suzuki T, Kanazawa Y, Hiramatsu N, Hayashi N. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J Immunol. 2004;173:6072-6081. [PubMed] |
| 80. | Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 81. | Edlich B, Ahlenstiel G, Zabaleta Azpiroz A, Stoltzfus J, Noureddin M, Serti E, Feld JJ, Liang TJ, Rotman Y, Rehermann B. Early changes in interferon signaling define natural killer cell response and refractoriness to interferon-based therapy of hepatitis C patients. Hepatology. 2012;55:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 82. | Miyagi T, Gil MP, Wang X, Louten J, Chu WM, Biron CA. High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. J Exp Med. 2007;204:2383-2396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 83. | Miyagi T, Takehara T, Nishio K, Shimizu S, Kohga K, Li W, Tatsumi T, Hiramatsu N, Kanto T, Hayashi N. Altered interferon-alpha-signaling in natural killer cells from patients with chronic hepatitis C virus infection. J Hepatol. 2010;53:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 84. | Nguyen KB, Cousens LP, Doughty LA, Pien GC, Durbin JE, Biron CA. Interferon alpha/beta-mediated inhibition and promotion of interferon gamma: STAT1 resolves a paradox. Nat Immunol. 2000;1:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 229] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 85. | Krämer B, Körner C, Kebschull M, Glässner A, Eisenhardt M, Nischalke HD, Alexander M, Sauerbruch T, Spengler U, Nattermann J. Natural killer p46High expression defines a natural killer cell subset that is potentially involved in control of hepatitis C virus replication and modulation of liver fibrosis. Hepatology. 2012;56:1201-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 86. | Glässner A, Eisenhardt M, Krämer B, Körner C, Coenen M, Sauerbruch T, Spengler U, Nattermann J. NK cells from HCV-infected patients effectively induce apoptosis of activated primary human hepatic stellate cells in a TRAIL-, FasL- and NKG2D-dependent manner. Lab Invest. 2012;92:967-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 87. | Pembroke T, Christian A, Jones E, Hills RK, Wang EC, Gallimore AM, Godkin A. The paradox of NKp46+ natural killer cells: drivers of severe hepatitis C virus-induced pathology but in-vivo resistance to interferon α treatment. Gut. 2014;63:515-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 88. | De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P, Congia S, Mingari MC, Moretta L. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37:445-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 89. | Varchetta S, Mele D, Mantovani S, Oliviero B, Cremonesi E, Ludovisi S, Michelone G, Alessiani M, Rosati R, Montorsi M. Impaired intrahepatic natural killer cell cytotoxic function in chronic hepatitis C virus infection. Hepatology. 2012;56:841-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 90. | Wang JM, Cheng YQ, Shi L, Ying RS, Wu XY, Li GY, Moorman JP, Yao ZQ. KLRG1 negatively regulates natural killer cell functions through the Akt pathway in individuals with chronic hepatitis C virus infection. J Virol. 2013;87:11626-11636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 91. | Gonzalez VD, Landay AL, Sandberg JK. Innate immunity and chronic immune activation in HCV/HIV-1 co-infection. Clin Immunol. 2010;135:12-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 92. | Hernandez MD, Sherman KE. HIV/hepatitis C coinfection natural history and disease progression. Curr Opin HIV AIDS. 2011;6:478-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 93. | Glässner A, Eisenhardt M, Kokordelis P, Krämer B, Wolter F, Nischalke HD, Boesecke C, Sauerbruch T, Rockstroh JK, Spengler U. Impaired CD4⁺ T cell stimulation of NK cell anti-fibrotic activity may contribute to accelerated liver fibrosis progression in HIV/HCV patients. J Hepatol. 2013;59:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 94. | Knapp S, Warshow U, Hegazy D, Brackenbury L, Guha IN, Fowell A, Little AM, Alexander GJ, Rosenberg WM, Cramp ME. Consistent beneficial effects of killer cell immunoglobulin-like receptor 2DL3 and group 1 human leukocyte antigen-C following exposure to hepatitis C virus. Hepatology. 2010;51:1168-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 95. | Suppiah V, Gaudieri S, Armstrong NJ, O’Connor KS, Berg T, Weltman M, Abate ML, Spengler U, Bassendine M, Dore GJ. IL28B, HLA-C, and KIR variants additively predict response to therapy in chronic hepatitis C virus infection in a European Cohort: a cross-sectional study. PLoS Med. 2011;8:e1001092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 96. | Kaser A, Enrich B, Ludwiczek O, Vogel W, Tilg H. Interferon-alpha (IFN-alpha) enhances cytotoxicity in healthy volunteers and chronic hepatitis C infection mainly by the perforin pathway. Clin Exp Immunol. 1999;118:71-77. [PubMed] |
| 97. | Bozzano F, Picciotto A, Costa P, Marras F, Fazio V, Hirsch I, Olive D, Moretta L, De Maria A. Activating NK cell receptor expression/function (NKp30, NKp46, DNAM-1) during chronic viraemic HCV infection is associated with the outcome of combined treatment. Eur J Immunol. 2011;41:2905-2914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 98. | Conry SJ, Meng Q, Hardy G, Yonkers NL, Sugalski JM, Hirsch A, Davitkov P, Compan A, Falck-Ytter Y, Blanton RE. Genetically associated CD16(+)56(-) natural killer cell interferon (IFN)-αR expression regulates signaling and is implicated in IFN-α-induced hepatitis C virus decline. J Infect Dis. 2012;205:1131-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 99. | Ahlenstiel G, Edlich B, Hogdal LJ, Rotman Y, Noureddin M, Feld JJ, Holz LE, Titerence RH, Liang TJ, Rehermann B. Early changes in natural killer cell function indicate virologic response to interferon therapy for hepatitis C. Gastroenterology. 2011;141:1231-1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 100. | Oliviero B, Mele D, Degasperi E, Aghemo A, Cremonesi E, Rumi MG, Tinelli C, Varchetta S, Mantovani S, Colombo M. Natural killer cell dynamic profile is associated with treatment outcome in patients with chronic HCV infection. J Hepatol. 2013;59:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 101. | He XS, Ji X, Hale MB, Cheung R, Ahmed A, Guo Y, Nolan GP, Pfeffer LM, Wright TL, Risch N. Global transcriptional response to interferon is a determinant of HCV treatment outcome and is modified by race. Hepatology. 2006;44:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 102. | Sarasin-Filipowicz M, Oakeley EJ, Duong FH, Christen V, Terracciano L, Filipowicz W, Heim MH. Interferon signaling and treatment outcome in chronic hepatitis C. Proc Natl Acad Sci USA. 2008;105:7034-7039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 547] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 103. | Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2776] [Cited by in RCA: 2735] [Article Influence: 160.9] [Reference Citation Analysis (1)] |
| 104. | Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1505] [Cited by in RCA: 1506] [Article Influence: 88.6] [Reference Citation Analysis (1)] |
| 105. | Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1777] [Article Influence: 104.5] [Reference Citation Analysis (1)] |
| 106. | Naggie S, Osinusi A, Katsounas A, Lempicki R, Herrmann E, Thompson AJ, Clark PJ, Patel K, Muir AJ, McHutchison JG. Dysregulation of innate immunity in hepatitis C virus genotype 1 IL28B-unfavorable genotype patients: impaired viral kinetics and therapeutic response. Hepatology. 2012;56:444-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 107. | Golden-Mason L, Bambha KM, Cheng L, Howell CD, Taylor MW, Clark PJ, Afdhal N, Rosen HR. Natural killer inhibitory receptor expression associated with treatment failure and interleukin-28B genotype in patients with chronic hepatitis C. Hepatology. 2011;54:1559-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 108. | Krämer B, Eisenhardt M, Glässner A, Körner C, Sauerbruch T, Spengler U, Nattermann J. Do lambda-IFNs IL28A and IL28B act on human natural killer cells? Proc Natl Acad Sci USA. 2011;108:E519-E520; author reply E521-E522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 109. | O’Connor KS, Ahlenstiel G, Suppiah V, Schibeci S, Ong A, Leung R, van der Poorten D, Douglas MW, Weltman MD, Stewart GJ. IFNL3 mediates interaction between innate immune cells: Implications for hepatitis C virus pathogenesis. Innate Immun. 2014;20:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 110. | Dring MM, Morrison MH, McSharry BP, Guinan KJ, Hagan R, O’Farrelly C, Gardiner CM. Innate immune genes synergize to predict increased risk of chronic disease in hepatitis C virus infection. Proc Natl Acad Sci USA. 2011;108:5736-5741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 111. | Shimoda S, Sumida K, Iwasaka S, Hisamoto S, Tanimoto H, Nomura H, Dohmen K, Takahashi K, Kawano A, Ogawa E. Interferon- α -Induced Changes to Natural Killer Cells Are Associated with the Treatment Outcomes in Patients with HCV Infections. Hepat Res Treat. 2013;2013:374196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 112. | Anthony DD, Conry SJ, Medvik K, Sandhya Rani MR, Falck-Ytter Y, Blanton RE, Lederman MM, Rodriguez B, Landay AL, Sandberg JK. Baseline levels of soluble CD14 and CD16+56- natural killer cells are negatively associated with response to interferon/ribavirin therapy during HCV-HIV-1 coinfection. J Infect Dis. 2012;206:969-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 113. | Serti E, Chepa-Lotrea X, Kim YJ, Keane M, Fryzek N, Liang TJ, Ghany M, Rehermann B. Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function. Gastroenterology. 2015;149:190-200.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 209] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 114. | Kolykhalov AA, Agapov EV, Blight KJ, Mihalik K, Feinstone SM, Rice CM. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science. 1997;277:570-574. [PubMed] |
| 115. | Lindenbach BD, Meuleman P, Ploss A, Vanwolleghem T, Syder AJ, McKeating JA, Lanford RE, Feinstone SM, Major ME, Leroux-Roels G. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc Natl Acad Sci USA. 2006;103:3805-3809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 345] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 116. | Farquhar MJ, McKeating JA. Primary hepatocytes as targets for hepatitis C virus replication. J Viral Hepat. 2008;15:849-854. [PubMed] |
| 117. | Wieland S, Makowska Z, Campana B, Calabrese D, Dill MT, Chung J, Chisari FV, Heim MH. Simultaneous detection of hepatitis C virus and interferon stimulated gene expression in infected human liver. Hepatology. 2014;59:2121-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 118. | Dorner M, Horwitz JA, Robbins JB, Barry WT, Feng Q, Mu K, Jones CT, Schoggins JW, Catanese MT, Burton DR. A genetically humanized mouse model for hepatitis C virus infection. Nature. 2011;474:208-211. [PubMed] |
| 119. | Dorner M, Ploss A. Deconstructing hepatitis C virus infection in humanized mice. Ann N Y Acad Sci. 2011;1245:59-62. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Liu J, Sener A S- Editor: Yu J L- Editor: A E- Editor: Zhang DN