Published online Jan 28, 2016. doi: 10.3748/wjg.v22.i4.1433
Peer-review started: May 15, 2015
First decision: August 31, 2015
Revised: October 11, 2015
Accepted: November 13, 2015
Article in press: November 13, 2015
Published online: January 28, 2016
Processing time: 251 Days and 15.9 Hours
Even in cases where viral replication has been controlled by antiretroviral therapy for long periods of time, human immunodeficiency virus (HIV)-infected patients have several non-acquired immunodeficiency syndrome (AIDS) related co-morbidities, including liver disease, cardiovascular disease and neurocognitive decline, which have a clear impact on survival. It has been considered that persistent innate and acquired immune activation contributes to the pathogenesis of these non-AIDS related diseases. Immune activation has been related with several conditions, remarkably with the bacterial translocation related with the intestinal barrier damage by the HIV or by hepatitis C virus (HCV)-related liver cirrhosis. Consequently, increased morbidity and mortality must be expected in HIV-HCV coinfected patients. Disrupted gut barrier lead to an increased passage of microbial products and to an activation of the mucosal immune system and secretion of inflammatory mediators, which in turn might increase barrier dysfunction. In the present review, the intestinal barrier structure, measures of intestinal barrier dysfunction and the modifications of them in HIV monoinfection and in HIV-HCV coinfection will be considered. Both pathogenesis and the consequences for the progression of liver disease secondary to gut microbial fragment leakage and immune activation will be assessed.
Core tip: Even in patients with a long-term controlled human immunodeficiency virus (HIV) replication by antiretroviral therapy, HIV-infected patients have several non-acquired immunodeficiency virus (AIDS) related co-morbidities, including liver disease. Persistent innate and acquired immune activation contributes to the pathogenesis of these non-AIDS related diseases. Immune activation has been related with bacterial translocation secondary to gut barrier damage by the HIV or the hepatitis C virus (HCV)-related liver cirrhosis. Modifications in gut barrier structure and function and immune activation in HIV-HCV coinfected patients will be reviewed.
- Citation: Márquez M, Fernández Gutiérrez del Álamo C, Girón-González JA. Gut epithelial barrier dysfunction in human immunodeficiency virus-hepatitis C virus coinfected patients: Influence on innate and acquired immunity. World J Gastroenterol 2016; 22(4): 1433-1448
- URL: https://www.wjgnet.com/1007-9327/full/v22/i4/1433.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i4.1433
Human immunodeficiency virus (HIV) infection has evolved from a relative rapidly fatal disease to a chronic entity as a consequence of antiretroviral treatment (ART). However, even in patients with a long-term controlled disease, HIV-infected patients have several non-acquired immunodeficiency syndrome (AIDS) related co-morbidities, including cardiovascular and liver diseases, malignancy and neurocognitive decline, with a clear impact on survival[1,2]. It has been considered that persistent innate and acquired immune activation contributes to the pathogenesis of these non-AIDS related diseases[3].
Immune activation has been related to several situations, such as the persistence of HIV replication in the lymph nodes (even when peripheral blood HIV replication has been controlled)[4], the existence of other coinfections, [such as cytomegalovirus[5] and hepatitis C virus (HCV)[6] infection] and bacterial translocation which is an effect of the intestinal barrier damage caused by the HIV itself[7]. Bacterial translocation is defined as the translocation of bacteria and/or bacterial products from the gut to the mesenteric lymph nodes and other extra-intestinal organs[8,9].
In the present review the intestinal barrier structure, measures of intestinal barrier dysfunction and the modifications of them in HIV monoinfection and HIV-HCV coinfection will be considered.
The intestinal mucosa is made up of the epithelium, the lamina propria and the muscularis mucosa[10]. Innate and adaptative immune cells are distributed in the intestinal mucosa and submucosa. Effector immune cells are located primarily in the epithelium and lamina propria. The epithelium contains T cells, whereas lamina propria contains T and B lymphocytes and several cellular elements implicated in innate response, such as dendritic cells, macrophages, eosinophils and mast cells. Organized structures of the gut-associated lymphoid tissue (GALT) lie in the mucosa and submucosa[11].
In an expanded sense, we define the intestinal barrier as the sum of commensal intraluminal bacteria, the epithelial lining of intestine and the immune system. There are three barriers against pathological bacterial translocation: firstly, structures and mediators that limit the direct contact between the epithelial surface and the intestinal bacteria; secondly, the integrity of intestinal epithelial lining; finally, immune protection, characterized by the rapid detection and killing of bacteria that manage to penetrate[12].
In healthy subjects, the composition of the gut microbiome is essential to maintain both local and systemic immunity[13]. The microbial density increases from 105 colony forming units (CFU)/mL in the jejunum to 108 in distal ileum and cecum, up to 1012 in the colon[14]. Most intestinal bacteria belong to two phylogenetic lineages, Firmicutes and Bacteroidetes, and in minor proportion, Actinobacteria and Proteobacteria[15].
Small intestinal bacterial overgrowth is defined as > 105 CFU/mL and/or the presence of colonic bacteria in upper jejunal aspirate[16].
Anaerobes are 100 times more abundant than aerobes. Anaerobic bacteria do not easily translocate[17]. In contrast, gram-negative aerobic bacteria [Escherichia coli (E. coli), Klebsiella pneumoniae, Pseudomonas aeruginosa (P. aeruginosa)], Enterococci and other Streptococci, translocate readily, even across a histologically intact intestinal epithelium[18,19] and are those mainly implicated in infections in conditions characterized by pathological bacterial translocation, such as liver cirrhosis[20]. Alterations in the intestinal ecosystem equilibrium (dysbiosis) has been correlated with several pathologies[21].
In the normal individual, the intestinal epithelium absorbs water and nutrients while effectively preventing translocation of intraluminal bacteria[22]. Intestinal defensive mechanisms include the following elements: (1) Bile[23]; (2) Mucin and antimicrobial peptides, secreted by goblet cells and Paneth cells respectively[24-26]. Intestinal epithelial cells directly transport secretory immunoglobulin A (IgA), synthesized by plasma cells in the lamina propria, across the epithelial barrier; and (3) Intestinal epithelium. The barrier is formed by individual epithelial cell membranes and junction proteins. Intestinal epithelial cells express pattern-recognition receptors (PRRs), such as toll-like receptors (TLRs), cell surface C-type lectin receptors, and intracytoplasmic nucleotide oligomerization domain-like receptors (NLR), that enable them to act as sensors of the microbial flora and as necessary elements to maintain the mutualism[27-31]. Pathogen-associated molecular patterns (PAMPs) are conserved molecular patterns that are recognized by these PRRs[32].
Transmembrane adhesion molecules organized into structures called tight junctions, adherens junctions and desmosomes, connected to the actin cytoskeleton, ensure the stability of the epithelial barrier[33,34].
Inflammation and immune-related cytokines might modify the epithelial integrity and the function of the tight junctions: (1) Interferon gamma (IFN-γ) modifies actin-myosin contractility, resulting in intestinal tight junctions disruption and increased paracellular permeability[35]; (2) Tumor necrosis factor alpha (TNF-α) induce an inflammatory response and apoptosis in intestinal epithelial cells[36]; (3) Interleukin (IL)-10 antagonizes the cellular functions induced by TNF-α and IFN-γ[37]; and (4) Likewise, transforming growth factor-β (TGF-β) has protective effects on intestinal barrier function[38].
The peaceful coexistence with the intestinal bacterial flora is demonstrated by the lack of inflammatory responses against commensal bacteria. In healthy people, bacteria present in the autochthonous flora translocate in low numbers, but are killed during their passage through the epithelial barrier or in the mesenteric lymph nodes[39]. In fact, mesenteric lymph nodes are normally sterile.
Microbial antigens access to innate system by various routes: (1) Microfold cells (M cells), located within epithelium or in the follicle-associated structures of the GALT, can sample microbial antigens and transport them from the lumen into the dendritic cell-rich region[40]; (2) Dendritic cells that underlie the epithelium may open tight junctions, sending processes into the lumen that directly sample microbes and present them to lymphoid cells[41]; and (3) When the intestinal integrity failed, dendritic cells recognize the antigenic material in lamina propria[42].
Myeloid and plasmocitoid dendritic cells (DCs) are two subsets of DCs with ability to recognize different PAMPs[43]. For instance, E. coli lipopolysaccharide (LPS) stimulates myeloid DCs through TLR4[44] and induces a Th1 differentiation via secretion of IL-12. Viral particles are recognized by plasmocytoid DCs via TLR-7, TLR-8 or TLR-9, which secrete interferon-alpha (INF-α) as response to them[45]. Once activated, intestinal DCs induce mucosal B and T cells[46].
Measures of gut barrier dysfunction has been recently revised[47]. They can be classified as histological (structural) or functional measures[48-52].
Methods of evaluating the intestinal function in vivo can be classified as follows: (1) Intestinal permeability can be assessed analyzing the urinary recovery of orally administered inert test markers (sugars, such as monosaccharides and disaccharides, polyethylene glycols or radiolabeled chelates)[47,53]; (2) Intestinal barrier dysfunction can be assessed by measuring intestinal fatty acid binding protein (I-FABP) in plasma or urine[54,55]. I-FABP is uniquely located in mature small-intestinal enterocytes. Its leakage into the circulation from enterocytes is detected when intestinal mucosal damage occurs[56]. Serum levels of zonulin, a protein linked to tight junctions, have been studied as markers of intestinal permeability, although further analyses are needed; and (3) Another method to analyze barrier permeability is the measurement in extraintestinal fluids, such as the systemic blood, of gut-derived microbial fragments levels: LPS[57], bacterial 16S ribosomal DNA[58] or bacterial flagellin[59].
Markers of intestinal inflammation are the following: (1) Fecal calprotectin, a zinc-binding protein complex, is a sensitive marker of intestinal inflammation. It constitutes one of the cytosolic proteins in neutrophil granulocytes and in activated macrophages[60,61]; and (2) Alpha-1-antitrypsin is a protease inhibitor, highly resistant to proteolysis in the intestine. Alpha-1-antitrypsin can be extravasated from serum into the gut in the case of increased intestinal permeability, and finally be detected in the faeces[62].
LPS is a component of the outer membrane of Gram-negative bacteria, considered a major marker of microbial translocation. LPS elicit several responses in the innate immune system, after the interaction with the liver-derived LPS binding protein (LBP), which transfers LPS onto membrane CD14-TLR4 complex. TLR4 transduces the signal to the cell nucleus, leading to transcription factor nuclear factor κ-B (NF-κB) activation and cytokine production[63]. CD14 is shed during activation as soluble CD14 (sCD14). Both increased sCD14 and proinflammatory citokines (TNF-α, IL-6) are considered evidence of this proinflammatory state.
Continuous exposure to antigens is associated with lymphocyte activation[64], increase of “exhausted” cytotoxic populations (CD8+CD45RO+CD57+)[65] and an increase of lymphocytes that have evolved close to apoptosis[66]. To down-regulate the chronic activation, a modification of lymphocyte co-stimulatory molecules (monocyte CD80 and CD86 and lymphocyte CD28 molecules)[67] and an expansion of the population of suppressor or regulatory T lymphocytes (CD4+CD25highFoxP3+)[68], is expected.
Even in patients with a long-term controlled HIV replication by antiretroviral therapy, HIV-infected patients have several non-AIDS related co-morbidities, including liver disease. Persistent innate and acquired immune activation contributes to the pathogenesis of these non-AIDS related diseases. Immune activation has been related with bacterial translocation secondary to gut barrier damage by the HIV[68].
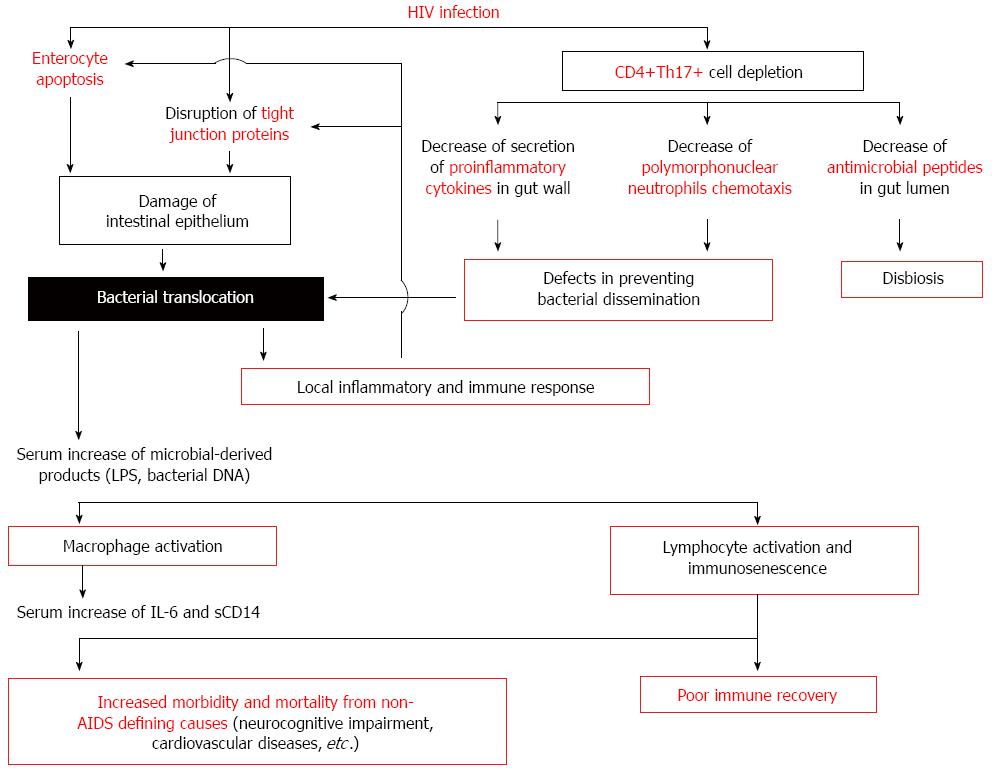
HIV-associated microbial translocation results from a series of events occurring at the gastrointestinal mucosa (Figure 1): (1) early mucosal CD4+ depletion; (2) immune hyperactivation/persistent inflammation; (3) damage to the integrity of the intestinal epithelium; and (4) modifications of the gut microbiome[69].
The simian immunodeficiency virus (SIV) model has been very valuable to define host-virus interactions and immunologic consequences in GALT[70]. During acute infection of SIV-infected macaques or HIV-infected patients, it has been clearly demonstrated the massive and rapid destruction of memory CD4+ T cells within the gastrointestinal tract[4,71,72], due to both direct virus cytopathicity and CD8+ T-cell-mediated killing of infected CD4+ T cells[69,73,74].
Th17 CD4+ T helper cells generate a rapid response to microbial pathogens at mucosal sites (including the intestinal mucosa), inducing chemokine expression for recruitment of neutrophils, monocytes and lymphocytes[75]. Also, Th17 stimulate the production of antimicrobial proteins and peptides[76-79]. Moreover, Th17 cells, through the expression of IL-17, regulate the synthesis of tight junction proteins[80].
Th17 cells express the CCR5 receptor and are severely depleted during acute HIV and SIV infections[81,82]. The consequences of gut Th17 depletion in SIV and HIV infections are the following: (1) The Th17-derived cytokines IL-17 and IL-22 induce the production of antimicrobial peptides that controls microbial replication at the luminal surface of the intestine. In a HIV or SIV infection, Th17 depletion could favors a disbiosis in the intestinal flora; (2) During SIV infection, gut Th17 cell depletion impairs the ability to secrete proinflammatory cytokines and to mount local acute inflammatory responses after gram negative bacilli challenge; the impaired Th17 response has been associated with increased bacterial translocation across the epithelial barrier[83]; and (3) Th17 depletion results in a reduced number and activity of neutrophils[84,85], which may contribute to defects in preventing bacterial dissemination.
A significant support for the importance of Th17 cell depletion in pathogenic HIV infection comes from investigations of HIV-infected elite controllers (those who maintained CD4+ T cell levels at healthy levels and control HIV replication without ART). Elite controllers retain the gut Th17 subset and do not exhibit systemic immune activation[86].
Regulatory T cells (FoxP3+ Treg) are essential to control inflammation and autoimmunity. The loss of Treg lymphocytes in GALT, demonstrated during primary SIV infection[87], might contribute to chronic immune activation in HIV and SIV infections[88].
Numbers and functions of other gut immune cell subsets are also altered during HIV infection. Increased turnover, cell activation, apoptosis, and altered function in cytotoxic CD8 T cells, natural killer cells, innate lymphoid cells and B cells have been reported[89,90].
Immunohistochemistry and confocal fluorescence microscopy have detected the presence of LPS in the gut mucosa since the earliest phases of infection, associated with epithelial barrier dysfunction[91], persisting even after ART-induced control of HIV replication[92]. The extent of damage to the epithelial barrier is correlated with the degree of microbial translocation[91], and of innate immune activation[92]. However, whereas an intense macrophage phagocytosis occurs during acute infection in SIV-infected animals, intestinal macrophages appear free from bacterial cells during chronic infection although accumulation of microbial components persists[93], suggesting a progressive exhaustion of macrophage phagocytic function.
Reciprocal interactions between immune activation and microbial translocation would be hypothesized in HIV infection: the sustained activation induces a cycle whereby new susceptible HIV targets (activated CD4+ T lymphocytes) are created and cytotoxic and inflammatory responses increase damage to the intestinal gut barrier and stimulate further translocation[69].
Damage of intestinal epithelium might occur as a consequence of the HIV exposure itself or by immune-induced enterocyte damage.
In vitro, a disruption of tight junction proteins has been observed after exposure to HIV glycoprotein gp120[94]. In addition, the HIV transactivator factor Tat alters microtubule and actin cytoskeleton and induce apoptosis[95]. These data have been supported by immunohistochemical studies of gut mucosa from HIV-infected individuals: an alteration of enterocyte microtubules and increased paracellular permeability has been observed[96].
Immune-induced enterocyte damage is also observed in HIV infection. In acute HIV and SIV infection, a noticeable perforin expression of mucosal CD8+ T cells has been detected and was associated with significant numbers of apoptotic epithelial cells[97]. In acute SIV infection, increased expression of Fas-ligand on lamina propria lymphocytes and Fas on enterocytes has been found[74]. Furthermore, proinflammatory cytokines, secreted by activated gut macrophages, can induce enterocyte apoptosis[98]. Finally, the induction of the kynurenine pathway of tryptophan catabolism by indoleamine 2,3-dioxygenase-1 in infiltrating activated myeloid cells contributes to suppress T-cell proliferation and Th17 development[99,100]. Taken together, these data suggest that altered tight junction composition and cellular apoptosis may contribute to the barrier defect.
Antiretroviral therapy improves the immune function in the periphery, but restoration of GALT is only partial[101,102]. ART-treated chronic HIV infected patients continue to show increased neutrophil infiltration in the gut compartment and epithelial cell apoptosis[92], as well as persistent pathological microbial translocation[103].
There is evidence of gastrointestinal dysbiosis in HIV-infected individuals[104]. Previous studies demonstrated an abundance of P. aeruginosa and Candida albicans and a reduction of bifidobacteria and lactobacilli in faecal samples of HIV-infected patients compared with healthy controls[105]. More recently, Dillon et al. have showed that HIV-infected individuals had increased proportion of Proteobacteria and decreased percentages of Firmicutes in the colonic mucosa. At the genus level, a significant outgrowth of Prevotella and a decrease of Bacteroides were detected[106]. In these patients, dysbiosis is associated with increased tryptophan catabolism and biomarkers of inflammation[107]. Dysbiosis is persistent in ART-treated HIV-infected patients[104,106]. It must be noted that those patients with virological response to ART (control of HIV replication) but poor immunological reconstitution (limited increase of peripheral blood CD4+ T cells, maintaining values lower than 200/mm3) exhibit a translocating bacterial microflora enriched in Enterobacteriaceae compared with those with a good immunological response[108], suggesting that changes in the intestinal microflora could affect the immune reconstitution via continued lymphocyte activation.
Increased plasma concentration of several markers indicative of pathological bacterial translocation or systemic inflammation has been detected in HIV-infected patients: LPS[109], bacterial DNA[110], bacterial flagellin[111], LBP[112], sCD14[113], IL-6[113] and EndocAb[114].
During acute and chronic HIV-infection, LPS is identified not only in mucosa, but also within systemic lymph nodes, liver and peripheral blood: LPS concentrations in gut mucosa, lymph nodes and liver are positively correlated, therefore supporting the systemic passage of gut-derived microbial fragments[91].
In acute HIV infection, serum levels of LPS are normal but EndoCAb (IgM, IgG and IgA antibodies directed against LPS core antigen) titers are increased, thus suggesting that the translocation of LPS is rapidly counteracted by the host Ig response. In chronic HIV infection, EndoCAb titers decrease progressively and higher plasma levels of LPS are detected[109]. Brenchley et al[109] reported that increased levels of circulating LPS in chronically HIV-infected individuals positively correlate with measures of immune activation. This finding has been corroborated by other groups[91,94,115,116].
Chronic immune activation is observed in HIV-infected patients. HIV- and SIV-associated chronic immune activation is characterized by high T-cell turnover of both CD4+ and CD8+ T cells, increased surface expression of HLA-DR and CD38 molecules, high levels of circulating proinflammatory cytokines and chemokines and polyclonal B-cell activation[117,118]. The HIV-related immunosenescence is another concept characteristic of both HIV infection and aging; it is defined by an expansion of CD28-/CD57+CD8+ T cells, shortened telomeres, reduced IL-2 production, elevated IL-6 levels, and resistance to apoptosis[119,120].
It is accepted that one of the most important forces inducing immunoactivation and immunosenescense in these individuals is gut bacterial translocation. In vitro stimulation by microbial TLR ligands induces T-cell activation in ART-naïve and ART-treated, HIV-infected patients[121]. In accordance with these findings, the concentration of bacterial-derived fragments has been correlated with systemic immune activation, mainly measured as circulating activated CD8+ lymphocytes (CD8+HLA-DR+CD38+ T cells)[109,110,122,123].
Notably, ART-induced complete suppression of HIV replication is not sufficient to fully turn off altered intestinal permeability or immune activation: circulating levels of intestinal permeability markers or immune activation parameters decrease after ART, even though they did not return to the levels observed in healthy individuals[109,110,124-128].
The prognostic importance of markers of intestinal permeability and immune activation has been analyzed. Whereas bacterial translocation markers, such as LPS, have been inconsistently associated with the progression of HIV infection[129,130], multiple studies have demonstrated that the main determinant of disease progression is the chronic immune activation, independent of the HIV load[131,132].
Two types of clinical consequences of the maintained intestinal permeability have been described: (1) A poor immune recovery in those patients with higher values of bacterial translocation parameters. An inverse correlation between serum concentrations of barrier damage or immune activation markers and the magnitude of recovery of peripheral blood CD4+ T cell count has been demonstrated in ART-treated individuals[109,110,126,133]; and (2) Increased morbidity and mortality from non-AIDS defining causes, such as neurocognitive impairment or cardiovascular diseases, in those patients with a more pathological bacterial translocation and immune activation[113].
In ART-treated patients, causes of death are different of those classically associated with AIDS: most of patients in the Hunt’s study[134] died by non-AIDS related causes, such as cardiovascular diseases (19%-27%), non-AIDS related cancer (11%-13%) and end-stage liver disease (8%-11%), among others. It has been demonstrated that chronic inflammation markers are independent prognostic factors of non-AIDS related morbidity (myocardial infarction, stroke, non-AIDS-defining cancer, non-AIDS-defining serious bacterial infection) or death in HIV-infected patients[135]. However, not every marker has the same prognostic value at each stage of HIV-infection. In a recently published nested case-control study of individuals with ART-suppressed HIV infection, Hunt et al[134] have assessed the relationship between intestinal barrier alteration, monocyte activation markers and immunologic factors with mortality. Both gut epithelial barrier function markers (serum levels of I-FABP) and parameters of innate immunity activation (serum levels of sCD14 or IL-6, kynurenine/tryptophan ratio) strongly predicted mortality in individuals with ART-suppressed HIV infection and a history of AIDS. However, T-cell activation (percentages of CD8+CD38+ cells) or T-cell senescence (proportion of CD28-CD57+ lymphocytes) failed to predict mortality in treated patients with an acceptable stage of immunocompetence. Other investigations have demonstrated the importance of T cell activation and senescence in untreated or immunodeficient-treated HIV-infected patients[131,136]. Thus, Hunt et al[134] stated that T-cell activation may predict mortality in situations in which persistent T-cell immunodeficiency may play an important role in susceptibility to opportunistic infections and AIDS-related malignancies, but not necessarily in treated patients with less advanced immunodeficiency; in these less advanced phases, gut barrier dysfunction or monocyte activation markers are the predominant prognostic factors.
Several therapeutic interventions aimed at reducing microbial translocation and its downstream effects have been proposed[137]:
Restoring the normal composition of the intestinal microbiome: Prebiotics and probiotics can be used to modify the altered intestinal microbioma. In a pilot, placebo-controlled study, untreated HIV-infected individuals received a prebiotic oligosaccharide mixture for 12 wk[138]. Microbiota composition improved substantially, increasing the proportion of bifidobacteria; also, there was a significant reduction in sCD14 levels and in activated CD4+ T cell lymphocytes.
In HIV-infected subjects during ART, non-absorbed antibiotics available for oral administration, such as rifaximin, have been also assayed to decrease the intestinal load of aerobic gram-negative bacilli and reduce gut microbial translocation and immune activation levels. However, results showed only minimal effect on serum levels of LPS and sCD14 or on the CD8+ T cell activation[139].
Decreasing the intestinal concentration of microbial products to be translocated: Studies in patients with renal insufficiency have demonstrated that blocking microbial translocation using sevelamer, a LPS-binding resin, decreased both systemic microbial translocation and systemic T cell activation and inflammation[140] Oral sevelamer has been assayed in HIV-infected individuals naïve to antiretrovirals as a proof-of-principle in this strategy. Sevelamer did not significantly change markers of microbial translocation, inflammation, or T-cell activation[141].
Improving intestinal barrier damage: IL-7 is a cytokine produced by non-marrow-derived stromal and epithelial cells, required for the development and persistence of T cells. The administration of recombinant IL-7 to a cohort of subjects with low-level CD4+ T-cell recovery on ART has demonstrated to be efficacious in increase the peripheral and, more importantly, gut T lymphocyte numbers, decreasing the inflammatory infiltrate of the gut lamina propria and reducing plasma levels of sCD14[142].
The anti-inflammatory drug mesalamine has been assayed to reduce the inflammation and consequently the intestinal barrier damage. After 12 wk of treatment, no significant changes in activated T lymphocytes counted at gut mucosa or at peripheral blood, or in the serum levels of sCD14 or IL-6 were observed in mesalamine-treated HIV-infected individuals[143].
Limiting immune activation: Chloroquine (which inhibits toll-like receptor signalling) has been also assayed in a pilot study. CD4 and CD8 T-cell counts, T-cell activation, and the kynurenine/tryptophan ratio did not change after 24 wk of chloroquine treatment[144].
Also, statins have been used to decrease the immune activation. After 24 wk of rosuvastatin, significant decreases in plasma levels of sCD14 but not in levels of T-cell activation were detected; these findings were independent of the lipid-lowering effect of rosuvastatin and the use of protease inhibitors[145].
Antagonizing molecules implicated in lymph node or liver lesions: TGF-β1 has been implicated in the lymph node fibrosis (which hinders CD4+ T cell reconstitution)[146] and in the progression of liver disease[147]. Angiotensin 2 is proinflammatory and induces fibrosis by increasing levels of TGF-β1[148]. Angiotensin-converting enzyme inhibitors have consistently proven beneficial in a number of clinical settings, but emerging data suggest that these drugs may also have anti-fibrotic properties[149,150]. We are waiting more data in HIV-infected patients.
In brief, some interventions (probiotics, IL-7, statins) have shown beneficial effects on gut barrier damage effects. However, until now these interventions have not been applied to the care of HIV-infected patients.
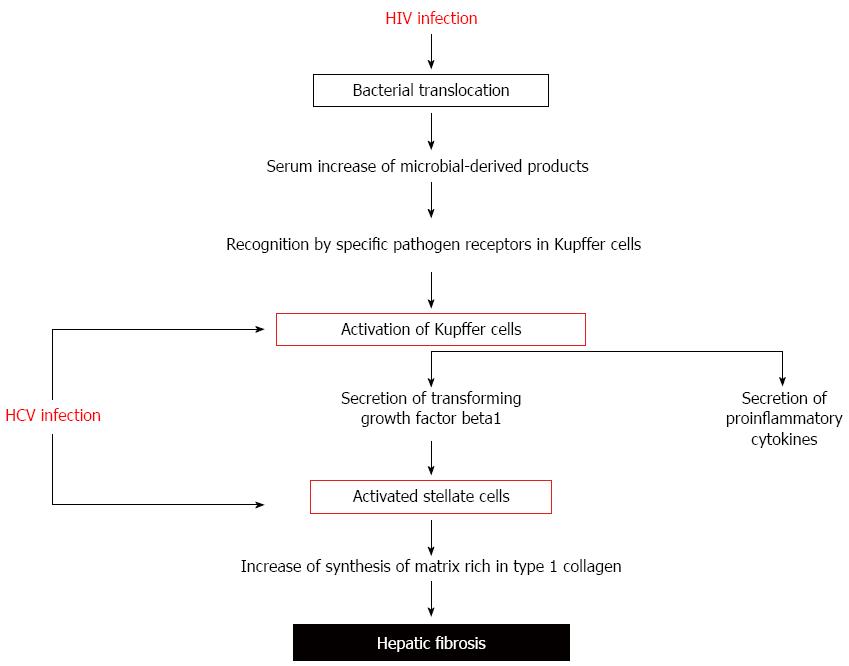
HCV-related liver cirrhosis is associated with gut barrier defects, thus increasing the bacterial permeability observed in individuals with HIV monoinfection. Also, bacterial translocation contributes to accelerating the process of liver fibrogenesis (Figure 2).
It has long been appreciated that liver disease is associated with increased intestinal barrier permeability[151]. When classical culture methods are used as measurement of intestinal permeability, the presence of enteric-derived bacteria in mesenteric lymph nodes occurs more frequently in patients with cirrhosis compared with controls, and bacterial translocation is more frequent in Child C compared to Child A and B[152-155]. In contrast, if we consider the translocation of non-viable organisms (bacterial DNA), translocation to mesenteric lymph node and to systemic circulation also occurs in non-ascitic cirrhosis and it is independent from the severity of liver disease[156]. Liver insufficiency[157] and portal hypertension[158] are the driving forces for bacterial translocation.
Several excellent reviews have been recently published on this subject[12,21,28,53,159]. As a summary, factors influencing pathological intestinal permeability and its consequences in cirrhotic patients include: (1) Advances stages of liver cirrhosis are frequently associated with malnutrition[160], which has been reported to contribute to decreased epithelial cell proliferation and synthesis of mucins and antimicrobial peptides[161,162]; (2) Significant decreases in intraluminal concentrations of bile acids[163,164]; (3) A deficit of Paneth cell-derived defensins, accompanied by a diminished in vitro antibacterial activity against various enterobacteria has been observed in experimental cirrhosis[165]. In cirrhosis, a reduced secretion of mucosal IgA into the jejunum have been detected[159,166]; (4) Higher gastric pH and autonomic neuropathy-related intestinal hypomotility, seen in patients with cirrhosis and exposition to health care structures and antibiotic therapy, may lead to failure in the control of bacterial intestinal growth with both qualitative (dysbiosis) and quantitative (overgrowth) differences[167-169]. A depletion of the beneficial Lachnospiraceae and Bacteroidetes (mainly the Bacteroidaceae family) and enrichment in Proteobacteria (mainly Gammaproteobacteria class and among those, particularly Enterobacteriaceae) has been observed[169], with differences more marked in patients with advanced cirrhosis[170]; (5) Alterations in tight junction proteins have been demonstrated[171]; (6) A mononuclear cell infiltrate in the lamina propria has been detected in cirrhotic patients[172,173], as well as increased faecal concentrations of polymorphonuclear elastase[166] and calprotectin[174]. Activated monocytes in the lamina propria disrupt epithelial tight junctions and perpetuate pathological bacterial translocation[175]; (7) Plasma markers of enterocyte necrosis (I-FABP), microbial translocation (LPS), and monocyte activation (sCD14) are increased in subjects with chronic hepatitis B or C infection, with higher values in those with advanced fibrosis[176]; (8) Immune activation and immunosenescence has been also demonstrated in cirrhotic patients[177]; and (9) Serum levels of inflammation markers are independently associated with cirrhosis complications and with mortality[178-182].
In HIV-HCV coinfected patients the additive effects of HIV and liver cirrhosis on intestinal permeability have been demonstrated. Increased sCD14 levels have been detected in HIV-HVC coinfected patients with liver cirrhosis compared with those with minimal or moderate fibrosis[176,183,184]. Elevated levels of barrier damage markers and proinflammatory cytokines have been observed in those HIV-HCV coinfected patients with more advanced forms of liver cirrhosis: significant higher concentrations of plasma LBP, sCD14 or IL-6 levels were observed in HIV-HCV coinfected patients with decompensated cirrhosis compared with those with compensated cirrhosis[185]. However, lymphocyte activation parameters show similar values than those observed in HIV-monoinfected patients[6], suggesting the existence of a maximal plateau in lymphocyte activation as result of gut bacterial fragment stimulus.
It has been demonstrated that the liver fibrosis progression is more rapid in HIV-HCV coinfected than in HCV-monoinfected patients, with a lower period of HCV infection being required for the development of liver cirrhosis[186,187]. Furthermore, the progression of liver cirrhosis towards death is accelerated in HIV-HCV coinfected patients, compared with HCV-monoinfected individuals[188-190]. Death occurs in these individuals by causes mainly related with liver disease[191]. Liver function indexes (Child-Pugh, MELD score), immunodepression and absence of ART have been considered prognostic factors in HIV-HCV coinfected patients[189,191].
Microbial translocation has been suggested to exert a major pathogenic role in the worsened liver disease in HIV-infected individuals[192]. Translocated bacterial products contribute to liver disease progression by binding to specific pathogen recognition receptors[193]. Several cells in the liver express significant levels of multiple Toll like receptors. TLR2, TLR3, and TLR4 are highly expressed in Kupffer cells. Free LPS binds to Kupffer cells via interaction with LBP and CD14[194]. The LPS-LBP-CD14 complex, via TLR4 and NFκB, lead to the rapid production of superoxide, TNF-α and IL-6[12]. Also, LPS sensitizes hepatic stellate cells to Kupffer-derived TGF-β[147]. Activated stellate cells produce a matrix rich in type 1 collagen, leading to liver fibrosis[195]. In support of this hypothesis, it has been observed that a polymorphism in the gene encoding TLR4, which attenuates the signaling downstream of the receptor in response to LPS stimulation, has been associated with a decreased risk of developing cirrhosis[196]. Furthermore, deficiency in TLR4 signalling reduces hepatic fibrosis after bile duct ligation[147]. Likewise, our group has demonstrated that a polymorphism in the TNF-α gene influences the rate of liver cirrhosis, probably due to a decreased synthesis of TGF-β[197].
Recently, French et al[198], in a 5-year longitudinal study of HIV-HCV coinfected patients, demonstrated that those individuals in whom liver disease progresses showed higher levels of intestinal mucosal lesion (I-FABP), macrophage activation (sCD14), and inflammation (IL-6) markers compared with non progressors. In progressors, I-FABP levels increased significantly with time. Studies carried out by our group have demonstrated that in these patients, proinflammatory cytokines levels were correlated with parameters indicative of haemodynamic alterations in cirrhotic patients, such as renin activity or the aldosterone concentration, as well as with the mortality[185].
Modification of the natural history of HCV-related liver disease in HIV-coinfected patients has been attempted in two main ways: (1) Treatment of HIV infection down-regulates the accelerated course of liver fibrosis in HIV-HCV coinfected patients: ART-treated individuals show a progression rate of HCV-related liver fibrosis similar to those patients without HIV coinfection[191,199]. As has been previously commented, circulating levels of intestinal permeability markers or immune activation parameters decrease after the initiation of ART, although they did not return to the levels observed in healthy individuals; and (2) Treatment of HCV infection. The attainment of a sustained virological response after HCV treatment is associated with a lower rate of the progression of liver disease[190], and lower mortality due to liver-related causes[200] and non-liver and non-AIDS related causes[201]. There is only limited data about modifications in gut barrier damage or proinflammatory cytokines related with the HCV treatment[202].
Both HIV and HCV infections could modify intestinal permeability, allowing the pass of gut bacterial fragments into the peripheral blood. Both markers of intestinal damage, increased gut permeability and immune activation, have been related to increased morbidity due to neurocognitive, cardiovascular or liver lesions. The combined effect of HIV infection and HCV-derived liver cirrhosis increases even more the levels of proinflammatory molecules and could be implicated in the elevated mortality observed in HIV-HCV coinfected patients compared with those with HIV- or HCV-monoinfection.
| 1. | Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, Berti A, Rossi E, Roverato A, Palella F. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 1041] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 2. | Vergara-Moragues E, de Campos AV, Girón-González JA. [Neurocognitive impairment related to acquired immunodeficiency syndrome in socially-excluded former intravenous drug abusers]. Enferm Infecc Microbiol Clin. 2010;28:294-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 335] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 4. | Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1317] [Cited by in RCA: 1396] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 5. | Hsu DC, Kerr SJ, Iampornsin T, Pett SL, Avihingsanon A, Thongpaeng P, Zaunders JJ, Ubolyam S, Ananworanich J, Kelleher AD. Restoration of CMV-specific-CD4 T cells with ART occurs early and is greater in those with more advanced immunodeficiency. PLoS One. 2013;8:e77479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Márquez M, Romero-Cores P, Montes-Oca M, Martín-Aspas A, Soto-Cárdenas MJ, Guerrero F, Fernández-Gutiérrez C, Girón-González JA. Immune activation response in chronic HIV-infected patients: influence of Hepatitis C virus coinfection. PLoS One. 2015;10:e0119568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10:655-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 378] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 8. | Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979;23:403-411. [PubMed] |
| 9. | Benten D, Wiest R. Gut microbiome and intestinal barrier failure--the “Achilles heel” in hepatology? J Hepatol. 2012;56:1221-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1381] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 11. | Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14:667-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 1255] [Article Influence: 104.6] [Reference Citation Analysis (0)] |
| 12. | Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 586] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 13. | Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu Rev Immunol. 2012;30:149-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 353] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 14. | Marteau P, Pochart P, Doré J, Béra-Maillet C, Bernalier A, Corthier G. Comparative study of bacterial groups within the human cecal and fecal microbiota. Appl Environ Microbiol. 2001;67:4939-4942. [PubMed] |
| 15. | Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM. Enterotypes of the human gut microbiome. Nature. 2011;473:174-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5822] [Cited by in RCA: 5215] [Article Influence: 347.7] [Reference Citation Analysis (3)] |
| 16. | Corazza GR, Menozzi MG, Strocchi A, Rasciti L, Vaira D, Lecchini R, Avanzini P, Chezzi C, Gasbarrini G. The diagnosis of small bowel bacterial overgrowth. Reliability of jejunal culture and inadequacy of breath hydrogen testing. Gastroenterology. 1990;98:302-309. [PubMed] |
| 17. | Wells CL, Maddaus MA, Reynolds CM, Jechorek RP, Simmons RL. Role of anaerobic flora in the translocation of aerobic and facultatively anaerobic intestinal bacteria. Infect Immun. 1987;55:2689-2694. [PubMed] |
| 18. | Steffen EK, Berg RD, Deitch EA. Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node. J Infect Dis. 1988;157:1032-1038. [PubMed] |
| 19. | Wiest R, Rath HC. Gastrointestinal disorders of the critically ill. Bacterial translocation in the gut. Best Pract Res Clin Gastroenterol. 2003;17:397-425. [PubMed] |
| 20. | Navasa M, Follo A, Llovet JM, Clemente G, Vargas V, Rimola A, Marco F, Guarner C, Forné M, Planas R. Randomized, comparative study of oral ofloxacin versus intravenous cefotaxime in spontaneous bacterial peritonitis. Gastroenterology. 1996;111:1011-1017. [PubMed] |
| 21. | Giannelli V, Di Gregorio V, Iebba V, Giusto M, Schippa S, Merli M, Thalheimer U. Microbiota and the gut-liver axis: bacterial translocation, inflammation and infection in cirrhosis. World J Gastroenterol. 2014;20:16795-16810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 156] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 22. | Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 483] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 23. | Wells CL, Jechorek RP, Erlandsen SL. Inhibitory effect of bile on bacterial invasion of enterocytes: possible mechanism for increased translocation associated with obstructive jaundice. Crit Care Med. 1995;23:301-307. [PubMed] |
| 24. | Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 821] [Cited by in RCA: 1009] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 25. | Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12:503-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 678] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 26. | Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1682] [Cited by in RCA: 2275] [Article Influence: 189.6] [Reference Citation Analysis (5)] |
| 27. | Johansen FE, Kaetzel CS. Regulation of the polymeric immunoglobulin receptor and IgA transport: new advances in environmental factors that stimulate pIgR expression and its role in mucosal immunity. Mucosal Immunol. 2011;4:598-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 269] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 28. | Henao-Mejia J, Elinav E, Thaiss CA, Licona-Limon P, Flavell RA. Role of the intestinal microbiome in liver disease. J Autoimmun. 2013;46:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 29. | Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004;126:1054-1070. [PubMed] |
| 30. | Chassin C, Kocur M, Pott J, Duerr CU, Gütle D, Lotz M, Hornef MW. miR-146a mediates protective innate immune tolerance in the neonate intestine. Cell Host Microbe. 2010;8:358-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 31. | Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507-514. [PubMed] |
| 32. | Anderson KV. Toll signaling pathways in the innate immune response. Curr Opin Immunol. 2000;12:13-19. [PubMed] |
| 33. | Citalán-Madrid AF, García-Ponce A, Vargas-Robles H, Betanzos A, Schnoor M. Small GTPases of the Ras superfamily regulate intestinal epithelial homeostasis and barrier function via common and unique mechanisms. Tissue Barriers. 2013;1:e26938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273:C1378-C1385. [PubMed] |
| 35. | Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923-933. [PubMed] |
| 36. | Schulzke JD, Bojarski C, Zeissig S, Heller F, Gitter AH, Fromm M. Disrupted barrier function through epithelial cell apoptosis. Ann N Y Acad Sci. 2006;1072:288-299. [PubMed] |
| 37. | Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5:262-270. [PubMed] |
| 38. | Howe KL, Reardon C, Wang A, Nazli A, McKay DM. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157: H7-induced increased permeability. Am J Pathol. 2005;167:1587-1597. [PubMed] |
| 39. | Berg RD. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 1995;3:149-154. [PubMed] |
| 40. | Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013;6:666-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 509] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 41. | Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1870] [Cited by in RCA: 1843] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 42. | Muñoz L, José Borrero M, Ubeda M, Lario M, Díaz D, Francés R, Monserrat J, Pastor O, Aguado-Fraile E, Such J. Interaction between intestinal dendritic cells and bacteria translocated from the gut in rats with cirrhosis. Hepatology. 2012;56:1861-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419-426. [PubMed] |
| 44. | Flacher V, Bouschbacher M, Verronèse E, Massacrier C, Sisirak V, Berthier-Vergnes O, de Saint-Vis B, Caux C, Dezutter-Dambuyant C, Lebecque S. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J Immunol. 2006;177:7959-7967. [PubMed] |
| 45. | Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2991] [Cited by in RCA: 3084] [Article Influence: 140.2] [Reference Citation Analysis (0)] |
| 46. | Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 631] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 47. | Wang L, Llorente C, Hartmann P, Yang AM, Chen P, Schnabl B. Methods to determine intestinal permeability and bacterial translocation during liver disease. J Immunol Methods. 2015;421:44-53. [PubMed] |
| 48. | Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375-412. [PubMed] |
| 49. | Tang VW. Proteomic and bioinformatic analysis of epithelial tight junction reveals an unexpected cluster of synaptic molecules. Biol Direct. 2006;1:37. [PubMed] |
| 50. | Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol. 2008;181:683-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 276] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 51. | Rao RK. Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res. 1998;22:1724-1730. [PubMed] |
| 52. | Ferrier L, Bérard F, Debrauwer L, Chabo C, Langella P, Buéno L, Fioramonti J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol. 2006;168:1148-1154. [PubMed] |
| 53. | Pijls KE, Jonkers DM, Elamin EE, Masclee AA, Koek GH. Intestinal epithelial barrier function in liver cirrhosis: an extensive review of the literature. Liver Int. 2013;33:1457-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 54. | Pelsers MM, Namiot Z, Kisielewski W, Namiot A, Januszkiewicz M, Hermens WT, Glatz JF. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem. 2003;36:529-535. [PubMed] |
| 55. | Grootjans J, Thuijls G, Verdam F, Derikx JP, Lenaerts K, Buurman WA. Non-invasive assessment of barrier integrity and function of the human gut. World J Gastrointest Surg. 2010;2:61-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 137] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 56. | Kanda T, Fujii H, Tani T, Murakami H, Suda T, Sakai Y, Ono T, Hatakeyama K. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology. 1996;110:339-343. [PubMed] |
| 57. | Caradonna L, Amati L, Magrone T, Pellegrino NM, Jirillo E, Caccavo D. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: biological and clinical significance. J Endotoxin Res. 2000;6:205-214. [PubMed] |
| 58. | Zapater P, Francés R, González-Navajas JM, de la Hoz MA, Moreu R, Pascual S, Monfort D, Montoliu S, Vila C, Escudero A. Serum and ascitic fluid bacterial DNA: a new independent prognostic factor in noninfected patients with cirrhosis. Hepatology. 2008;48:1924-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 59. | Targan SR, Landers CJ, Yang H, Lodes MJ, Cong Y, Papadakis KA, Vasiliauskas E, Elson CO, Hershberg RM. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005;128:2020-2028. [PubMed] |
| 60. | Johne B, Fagerhol MK, Lyberg T, Prydz H, Brandtzaeg P, Naess-Andresen CF, Dale I. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol Pathol. 1997;50:113-123. [PubMed] |
| 61. | Xiang JY, Ouyang Q, Li GD, Xiao NP. Clinical value of fecal calprotectin in determining disease activity of ulcerative colitis. World J Gastroenterol. 2008;14:53-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | Sharp HL. The current status of alpha-1-antityrpsin, a protease inhibitor, in gastrointestinal disease. Gastroenterology. 1976;70:611-621. [PubMed] |
| 63. | Schumann RR, Latz E. Lipopolysaccharide-binding protein. Chem Immunol. 2000;74:42-60. [PubMed] |
| 64. | Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169-1176. [PubMed] |
| 65. | Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711-2720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 844] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 66. | Bernard A, Lamy And L, Alberti I. The two-signal model of T-cell activation after 30 years. Transplantation. 2002;73:S31-S35. [PubMed] |
| 67. | Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 649] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 68. | Wang H, Kotler DP. HIV enteropathy and aging: gastrointestinal immunity, mucosal epithelial barrier, and microbial translocation. Curr Opin HIV AIDS. 2014;9:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev. 2013;26:2-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 391] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 70. | Daniel MD, Desrosiers RC, Letvin NL, King NW, Schmidt DK, Sehgal P, Hunt RD. Simian models for AIDS. Cancer Detect Prev Suppl. 1987;1:501-507. [PubMed] |
| 71. | Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427-431. [PubMed] |
| 72. | Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 828] [Cited by in RCA: 861] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 73. | Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093-1097. [PubMed] |
| 74. | Li Q, Estes JD, Duan L, Jessurun J, Pambuccian S, Forster C, Wietgrefe S, Zupancic M, Schacker T, Reilly C. Simian immunodeficiency virus-induced intestinal cell apoptosis is the underlying mechanism of the regenerative enteropathy of early infection. J Infect Dis. 2008;197:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 75. | Dandekar S, George MD, Bäumler AJ. Th17 cells, HIV and the gut mucosal barrier. Curr Opin HIV AIDS. 2010;5:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 76. | Godinez I, Raffatellu M, Chu H, Paixão TA, Haneda T, Santos RL, Bevins CL, Tsolis RM, Bäumler AJ. Interleukin-23 orchestrates mucosal responses to Salmonella enterica serotype Typhimurium in the intestine. Infect Immun. 2009;77:387-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 77. | Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271-2279. [PubMed] |
| 78. | Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, Butler BP, Chu H, Santos RL, Berger T. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe. 2009;5:476-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 447] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 79. | Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009;119:1899-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 276] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 80. | Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001-1011. [PubMed] |
| 81. | Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826-2835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 515] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 82. | Shi S, Seki S, Matano T, Yamamoto H. IL-21-producer CD4+ T cell kinetics during primary simian immunodeficiency virus infection. Microbes Infect. 2013;15:697-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 83. | Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 481] [Cited by in RCA: 468] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 84. | Cai XY, Gommoll CP, Justice L, Narula SK, Fine JS. Regulation of granulocyte colony-stimulating factor gene expression by interleukin-17. Immunol Lett. 1998;62:51-58. [PubMed] |
| 85. | Pitrak DL, Bak PM, DeMarais P, Novak RM, Andersen BR. Depressed neutrophil superoxide production in human immunodeficiency virus infection. J Infect Dis. 1993;167:1406-1410. [PubMed] |
| 86. | Ciccone EJ, Greenwald JH, Lee PI, Biancotto A, Read SW, Yao MA, Hodge JN, Thompson WL, Kovacs SB, Chairez CL. CD4+ T cells, including Th17 and cycling subsets, are intact in the gut mucosa of HIV-1-infected long-term nonprogressors. J Virol. 2011;85:5880-5888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 87. | Estes JD, Li Q, Reynolds MR, Wietgrefe S, Duan L, Schacker T, Picker LJ, Watkins DI, Lifson JD, Reilly C. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J Infect Dis. 2006;193:703-712. [PubMed] |
| 88. | Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, Mold J, Swainson L, Barbour JD, Baskin CR. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 89. | Taiwo B, Barcena L, Tressler R. Understanding and controlling chronic immune activation in the HIV-infected patients suppressed on combination antiretroviral therapy. Curr HIV/AIDS Rep. 2013;10:21-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 90. | Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, Connick E, Meditz A, Freeman GJ, Abesada-Terk G. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med. 2013;19:494-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 313] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 91. | Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, Barclay GR, Smedley J, Pung R, Oliveira KM. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 2010;6:e1001052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 407] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 92. | Somsouk M, Estes JD, Deleage C, Dunham RM, Albright R, Inadomi JM, Martin JN, Deeks SG, McCune JM, Hunt PW. Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS. 2015;29:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 93. | Estes JD, Gordon SN, Zeng M, Chahroudi AM, Dunham RM, Staprans SI, Reilly CS, Silvestri G, Haase AT. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J Immunol. 2008;180:6798-6807. [PubMed] |
| 94. | Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6:e1000852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 475] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 95. | Buccigrossi V, Laudiero G, Nicastro E, Miele E, Esposito F, Guarino A. The HIV-1 transactivator factor (Tat) induces enterocyte apoptosis through a redox-mediated mechanism. PLoS One. 2011;6:e29436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Clayton F, Kotler DP, Kuwada SK, Morgan T, Stepan C, Kuang J, Le J, Fantini J. Gp120-induced Bob/GPR15 activation: a possible cause of human immunodeficiency virus enteropathy. Am J Pathol. 2001;159:1933-1939. [PubMed] |
| 97. | Epple HJ, Schneider T, Troeger H, Kunkel D, Allers K, Moos V, Amasheh M, Loddenkemper C, Fromm M, Zeitz M. Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut. 2009;58:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 98. | Maingat F, Halloran B, Acharjee S, van Marle G, Church D, Gill MJ, Uwiera RR, Cohen EA, Meddings J, Madsen K. Inflammation and epithelial cell injury in AIDS enteropathy: involvement of endoplasmic reticulum stress. FASEB J. 2011;25:2211-2220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 99. | Boasso A, Herbeuval JP, Hardy AW, Anderson SA, Dolan MJ, Fuchs D, Shearer GM. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351-3359. [PubMed] |
| 100. | Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, Barbour JD, Lowe MM, Jayawardene A, Aweeka F. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32ra36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 433] [Cited by in RCA: 447] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 101. | Guadalupe M, Sankaran S, George MD, Reay E, Verhoeven D, Shacklett BL, Flamm J, Wegelin J, Prindiville T, Dandekar S. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol. 2006;80:8236-8247. [PubMed] |
| 102. | Estes J, Baker JV, Brenchley JM, Khoruts A, Barthold JL, Bantle A, Reilly CS, Beilman GJ, George ME, Douek DC. Collagen deposition limits immune reconstitution in the gut. J Infect Dis. 2008;198:456-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 103. | Douek DC. Immune activation, HIV persistence, and the cure. Top Antivir Med. 2013;21:128-132. [PubMed] |
| 104. | Lozupone CA, Li M, Campbell TB, Flores SC, Linderman D, Gebert MJ, Knight R, Fontenot AP, Palmer BE. Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe. 2013;14:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 379] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 105. | Gori A, Tincati C, Rizzardini G, Torti C, Quirino T, Haarman M, Ben Amor K, van Schaik J, Vriesema A, Knol J. Early impairment of gut function and gut flora supporting a role for alteration of gastrointestinal mucosa in human immunodeficiency virus pathogenesis. J Clin Microbiol. 2008;46:757-758. [PubMed] |
| 106. | Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, Gianella S, Siewe B, Smith DM, Landay AL. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014;7:983-994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 417] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 107. | Vujkovic-Cvijin I, Dunham RM, Iwai S, Maher MC, Albright RG, Broadhurst MJ, Hernandez RD, Lederman MM, Huang Y, Somsouk M. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med. 2013;5:193ra91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 560] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 108. | Merlini E, Bai F, Bellistrì GM, Tincati C, d’Arminio Monforte A, Marchetti G. Evidence for polymicrobic flora translocating in peripheral blood of HIV-infected patients with poor immune response to antiretroviral therapy. PLoS One. 2011;6:e18580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 109. | Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365-1371. [PubMed] |
| 110. | Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, Landay A, Martin J, Sinclair E, Asher AI. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177-1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 461] [Cited by in RCA: 481] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 111. | Abdurahman S, Barqasho B, Nowak P, Cuong do D, Amogné W, Larsson M, Lindquist L, Marrone G, Sönnerborg A. Pattern of microbial translocation in patients living with HIV-1 from Vietnam, Ethiopia and Sweden. J Int AIDS Soc. 2014;17:18841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 112. | Papasavvas E, Pistilli M, Reynolds G, Bucki R, Azzoni L, Chehimi J, Janmey PA, DiNubile MJ, Ondercin J, Kostman JR. Delayed loss of control of plasma lipopolysaccharide levels after therapy interruption in chronically HIV-1-infected patients. AIDS. 2009;23:369-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 113. | Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 926] [Cited by in RCA: 938] [Article Influence: 62.5] [Reference Citation Analysis (18)] |
| 114. | Bukh AR, Melchjorsen J, Offersen R, Jensen JM, Toft L, Støvring H, Ostergaard L, Tolstrup M, Søgaard OS. Endotoxemia is associated with altered innate and adaptive immune responses in untreated HIV-1 infected individuals. PLoS One. 2011;6:e21275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 115. | Romero-Sánchez M, González-Serna A, Pacheco YM, Ferrando-Martínez S, Machmach K, García-García M, Alvarez-Ríos AI, Vidal F, Leal M, Ruiz-Mateos E. Different biological significance of sCD14 and LPS in HIV-infection: importance of the immunovirology stage and association with HIV-disease progression markers. J Infect. 2012;65:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 116. | Canary LA, Vinton CL, Morcock DR, Pierce JB, Estes JD, Brenchley JM, Klatt NR. Rate of AIDS progression is associated with gastrointestinal dysfunction in simian immunodeficiency virus-infected pigtail macaques. J Immunol. 2013;190:2959-2965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 117. | Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1171] [Cited by in RCA: 1120] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 118. | Hellerstein M, Hanley MB, Cesar D, Siler S, Papageorgopoulos C, Wieder E, Schmidt D, Hoh R, Neese R, Macallan D. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 410] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 119. | High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, Deren S, Effros RB, Gebo K, Goronzy JJ. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;60 Suppl 1:S1-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 444] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 120. | Martin GE, Gouillou M, Hearps AC, Angelovich TA, Cheng AC, Lynch F, Cheng WJ, Paukovics G, Palmer CS, Novak RM. Age-associated changes in monocyte and innate immune activation markers occur more rapidly in HIV infected women. PLoS One. 2013;8:e55279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 121. | Tincati C, Bellistrì GM, Ancona G, Merlini E, d’Arminio Monforte A, Marchetti G. Role of in vitro stimulation with lipopolysaccharide on T-cell activation in HIV-infected antiretroviral-treated patients. Clin Dev Immunol. 2012;2012:935425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 122. | Ellis CL, Ma ZM, Mann SK, Li CS, Wu J, Knight TH, Yotter T, Hayes TL, Maniar AH, Troia-Cancio PV. Molecular characterization of stool microbiota in HIV-infected subjects by panbacterial and order-level 16S ribosomal DNA (rDNA) quantification and correlations with immune activation. J Acquir Immune Defic Syndr. 2011;57:363-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 123. | Gottlieb MS, Schroff R, Schanker HM, Weisman JD, Fan PT, Wolf RA, Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981;305:1425-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1768] [Cited by in RCA: 1529] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 124. | Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, Deeks SG. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534-1543. [PubMed] |
| 125. | Marchetti G, Gori A, Casabianca A, Magnani M, Franzetti F, Clerici M, Perno CF, Monforte Ad, Galli M, Meroni L. Comparative analysis of T-cell turnover and homeostatic parameters in HIV-infected patients with discordant immune-virological responses to HAART. AIDS. 2006;20:1727-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 126. | Cassol E, Malfeld S, Mahasha P, van der Merwe S, Cassol S, Seebregts C, Alfano M, Poli G, Rossouw T. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis. 2010;202:723-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 127. | Lederman MM, Calabrese L, Funderburg NT, Clagett B, Medvik K, Bonilla H, Gripshover B, Salata RA, Taege A, Lisgaris M. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204:1217-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 271] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 128. | Hileman CO, Kinley B, Scharen-Guivel V, Melbourne K, Szwarcberg J, Robinson J, Lederman MM, Mccomsey GA. Differential Reduction in Monocyte Activation and Vascular Inflammation With Integrase Inhibitor-Based Initial Antiretroviral Therapy Among HIV-Infected Individuals. J Infect Dis. 2015;212:345-354. [PubMed] |
| 129. | Redd AD, Dabitao D, Bream JH, Charvat B, Laeyendecker O, Kiwanuka N, Lutalo T, Kigozi G, Tobian AA, Gamiel J. Microbial translocation, the innate cytokine response, and HIV-1 disease progression in Africa. Proc Natl Acad Sci USA. 2009;106:6718-6723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 130. | Marchetti G, Cozzi-Lepri A, Merlini E, Bellistrì GM, Castagna A, Galli M, Verucchi G, Antinori A, Costantini A, Giacometti A. Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS. 2011;25:1385-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 131. | Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859-870. [PubMed] |
| 132. | Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narváez AB, Hunt P, Martin JN, Kahn JO, Levy J. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 601] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 133. | Marchetti G, Bellistrì GM, Borghi E, Tincati C, Ferramosca S, La Francesca M, Morace G, Gori A, Monforte AD. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008;22:2035-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 238] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 134. | Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, Robinson J, Huang Y, Epling L, Martin JN. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis. 2014;210:1228-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 424] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 135. | Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, Plants J, Seth A, Wilson CC, Deeks SG. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210:1248-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 466] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 136. | Giorgi JV, Lyles RH, Matud JL, Yamashita TE, Mellors JW, Hultin LE, Jamieson BD, Margolick JB, Rinaldo CR, Phair JP. Predictive value of immunologic and virologic markers after long or short duration of HIV-1 infection. J Acquir Immune Defic Syndr. 2002;29:346-355. [PubMed] |
| 137. | Hatano H. Immune activation and HIV persistence: considerations for novel therapeutic interventions. Curr Opin HIV AIDS. 2013;8:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 138. | Gori A, Rizzardini G, Van’t Land B, Amor KB, van Schaik J, Torti C, Quirino T, Tincati C, Bandera A, Knol J. Specific prebiotics modulate gut microbiota and immune activation in HAART-naive HIV-infected adults: results of the “COPA” pilot randomized trial. Mucosal Immunol. 2011;4:554-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 139. | Tenorio AR, Chan ES, Bosch RJ, Macatangay BJ, Read SW, Yesmin S, Taiwo B, Margolis DM, Jacobson JM, Landay AL. Rifaximin has a marginal impact on microbial translocation, T-cell activation and inflammation in HIV-positive immune non-responders to antiretroviral therapy - ACTG A5286. J Infect Dis. 2015;211:780-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 140. | Stinghen AE, Gonçalves SM, Bucharles S, Branco FS, Gruber B, Hauser AB, Pecoits-Filho R. Sevelamer decreases systemic inflammation in parallel to a reduction in endotoxemia. Blood Purif. 2010;29:352-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 141. | Sandler NG, Zhang X, Bosch RJ, Funderburg NT, Choi AI, Robinson JK, Fine DM, Coombs RW, Jacobson JM, Landay AL. Sevelamer does not decrease lipopolysaccharide or soluble CD14 levels but decreases soluble tissue factor, low-density lipoprotein (LDL) cholesterol, and oxidized LDL cholesterol levels in individuals with untreated HIV infection. J Infect Dis. 2014;210:1549-1554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 142. | Sereti I, Estes JD, Thompson WL, Morcock DR, Fischl MA, Croughs T, Beq S, Lafaye de Micheaux S, Yao MD, Ober A. Decreases in colonic and systemic inflammation in chronic HIV infection after IL-7 administration. PLoS Pathog. 2014;10:e1003890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 143. | Somsouk M, Dunham RM, Cohen M, Albright R, Abdel-Mohsen M, Liegler T, Lifson J, Piatak M, Gorelick R, Huang Y. The immunologic effects of mesalamine in treated HIV-infected individuals with incomplete CD4+ T cell recovery: a randomized crossover trial. PLoS One. 2014;9:e116306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 144. | Routy JP, Angel JB, Patel M, Kanagaratham C, Radzioch D, Kema I, Gilmore N, Ancuta P, Singer J, Jenabian MA. Assessment of chloroquine as a modulator of immune activation to improve CD4 recovery in immune nonresponding HIV-infected patients receiving antiretroviral therapy. HIV Med. 2015;16:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 145. | Funderburg NT, Jiang Y, Debanne SM, Storer N, Labbato D, Clagett B, Robinson J, Lederman MM, McComsey GA. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis. 2014;58:588-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 146. | Estes JD, Wietgrefe S, Schacker T, Southern P, Beilman G, Reilly C, Milush JM, Lifson JD, Sodora DL, Carlis JV. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis. 2007;195:551-561. [PubMed] |
| 147. | Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324-1332. [PubMed] |
| 148. | Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2357] [Cited by in RCA: 2405] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 149. | Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388-1393. [PubMed] |
| 150. | Baker JV, Huppler Hullsiek K, Prosser R, Duprez D, Grimm R, Tracy RP, Rhame F, Henry K, Neaton JD. Angiotensin converting enzyme inhibitor and HMG-CoA reductase inhibitor as adjunct treatment for persons with HIV infection: a feasibility randomized trial. PLoS One. 2012;7:e46894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 151. | Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165-172. [PubMed] |
| 152. | Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taurá P, Fuster J, García-Valdecasas JC, Lacy A, Suárez MJ. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32-37. [PubMed] |
| 153. | Pascual S, Such J, Esteban A, Zapater P, Casellas JA, Aparicio JR, Girona E, Gutiérrez A, Carnices F, Palazón JM. Intestinal permeability is increased in patients with advanced cirrhosis. Hepatogastroenterology. 2003;50:1482-1486. [PubMed] |
| 154. | Zuckerman MJ, Menzies IS, Ho H, Gregory GG, Casner NA, Crane RS, Hernandez JA. Assessment of intestinal permeability and absorption in cirrhotic patients with ascites using combined sugar probes. Dig Dis Sci. 2004;49:621-626. [PubMed] |
| 155. | Lee S, Son SC, Han MJ, Kim WJ, Kim SH, Kim HR, Jeon WK, Park KH, Shin MG. Increased intestinal macromolecular permeability and urine nitrite excretion associated with liver cirrhosis with ascites. World J Gastroenterol. 2008;14:3884-3890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 156. | Such J, Francés R, Muñoz C, Zapater P, Casellas JA, Cifuentes A, Rodríguez-Valera F, Pascual S, Sola-Vera J, Carnicer F. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 2002;36:135-141. [PubMed] |
| 157. | Andreu M, Sola R, Sitges-Serra A, Alia C, Gallen M, Vila MC, Coll S, Oliver MI. Risk factors for spontaneous bacterial peritonitis in cirrhotic patients with ascites. Gastroenterology. 1993;104:1133-1138. [PubMed] |
| 158. | Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, Heinisch BB, Hayden H, Lammert F, Trauner M, Peck-Radosavljevic M, Vogelsang H. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol. 2013;58:911-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 266] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 159. | Schnabl B. Linking intestinal homeostasis and liver disease. Curr Opin Gastroenterol. 2013;29:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 160. | Cheung K, Lee SS, Raman M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin Gastroenterol Hepatol. 2012;10:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 161. | Hodin CM, Lenaerts K, Grootjans J, de Haan JJ, Hadfoune M, Verheyen FK, Kiyama H, Heineman E, Buurman WA. Starvation compromises Paneth cells. Am J Pathol. 2011;179:2885-2893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 162. | Casafont F, Sánchez E, Martín L, Agüero J, Romero FP. Influence of malnutrition on the prevalence of bacterial translocation and spontaneous bacterial peritonitis in experimental cirrhosis in rats. Hepatology. 1997;25:1334-1337. [PubMed] |
| 163. | Clements WD, Parks R, Erwin P, Halliday MI, Barr J, Rowlands BJ. Role of the gut in the pathophysiology of extrahepatic biliary obstruction. Gut. 1996;39:587-593. [PubMed] |
| 164. | Lorenzo-Zúñiga V, Bartolí R, Planas R, Hofmann AF, Viñado B, Hagey LR, Hernández JM, Mañé J, Alvarez MA, Ausina V. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology. 2003;37:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 253] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 165. | Teltschik Z, Wiest R, Beisner J, Nuding S, Hofmann C, Schoelmerich J, Bevins CL, Stange EF, Wehkamp J. Intestinal bacterial translocation in rats with cirrhosis is related to compromised Paneth cell antimicrobial host defense. Hepatology. 2012;55:1154-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 166. | Saitoh O, Sugi K, Lojima K, Matsumoto H, Nakagawa K, Kayazawa M, Tanaka S, Teranishi T, Hirata I. Increased prevalence of intestinal inflammation in patients with liver cirrhosis. World J Gastroenterol. 1999;5:391-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 167. | Morencos FC, de las Heras Castaño G, Martín Ramos L, López Arias MJ, Ledesma F, Pons Romero F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci. 1995;40:1252-1256. [PubMed] |
| 168. | Bauer TM, Steinbrückner B, Brinkmann FE, Ditzen AK, Schwacha H, Aponte JJ, Pelz K, Kist M, Blum HE. Small intestinal bacterial overgrowth in patients with cirrhosis: prevalence and relation with spontaneous bacterial peritonitis. Am J Gastroenterol. 2001;96:2962-2967. [PubMed] |
| 169. | Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 823] [Article Influence: 54.9] [Reference Citation Analysis (3)] |
| 170. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 878] [Article Influence: 73.2] [Reference Citation Analysis (1)] |
| 171. | Assimakopoulos SF, Tsamandas AC, Tsiaoussis GI, Karatza E, Triantos C, Vagianos CE, Spiliopoulou I, Kaltezioti V, Charonis A, Nikolopoulou VN. Altered intestinal tight junctions’ expression in patients with liver cirrhosis: a pathogenetic mechanism of intestinal hyperpermeability. Eur J Clin Invest. 2012;42:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 172. | Misra V, Misra SP, Dwivedi M, Gupta SC. Histomorphometric study of portal hypertensive enteropathy. Am J Clin Pathol. 1997;108:652-657. [PubMed] |
| 173. | Garcia-Tsao G, Lee FY, Barden GE, Cartun R, West AB. Bacterial translocation to mesenteric lymph nodes is increased in cirrhotic rats with ascites. Gastroenterology. 1995;108:1835-1841. [PubMed] |
| 174. | Gundling F, Schmidtler F, Hapfelmeier A, Schulte B, Schmidt T, Pehl C, Schepp W, Seidl H. Fecal calprotectin is a useful screening parameter for hepatic encephalopathy and spontaneous bacterial peritonitis in cirrhosis. Liver Int. 2011;31:1406-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 175. | Hartmann P, Haimerl M, Mazagova M, Brenner DA, Schnabl B. Toll-like receptor 2-mediated intestinal injury and enteric tumor necrosis factor receptor I contribute to liver fibrosis in mice. Gastroenterology. 2012;143:1330-1340.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 176. | Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M, Kleiner DE, Deeks SG, Liang TJ, Heller T. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220-1230, 1230.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 177. | Márquez M, Fernández-Gutiérrez C, Montes-de-Oca M, Blanco MJ, Brun F, Rodríguez-Ramos C, Girón-González JA. Chronic antigenic stimuli as a possible explanation for the immunodepression caused by liver cirrhosis. Clin Exp Immunol. 2009;158:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 178. | Navasa M, Follo A, Filella X, Jiménez W, Francitorra A, Planas R, Rimola A, Arroyo V, Rodés J. Tumor necrosis factor and interleukin-6 in spontaneous bacterial peritonitis in cirrhosis: relationship with the development of renal impairment and mortality. Hepatology. 1998;27:1227-1232. [PubMed] |
| 179. | Rodríguez-Ramos C, Galan F, Díaz F, Elvira J, Martín-Herrera L, Girón-González JA. Expression of proinflammatory cytokines and their inhibitors during the course of spontaneous bacterial peritonitis. Dig Dis Sci. 2001;46:1668-1676. [PubMed] |
| 180. | Albillos A, de la Hera A, González M, Moya JL, Calleja JL, Monserrat J, Ruiz-del-Arbol L, Alvarez-Mon M. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 346] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 181. | Girón-González JA, Martínez-Sierra C, Rodriguez-Ramos C, Rendón P, Macías MA, Fernández-Gutiérrez C, Díaz F, Martín-Herrera L. Adhesion molecules as a prognostic marker of liver cirrhosis. Scand J Gastroenterol. 2005;40:217-224. [PubMed] |
| 182. | Francés R, González-Navajas JM, Zapater P, Muñoz C, Caño R, Pascual S, Santana F, Márquez D, Pérez-Mateo M, Such J. Translocation of bacterial DNA from Gram-positive microorganisms is associated with a species-specific inflammatory response in serum and ascitic fluid of patients with cirrhosis. Clin Exp Immunol. 2007;150:230-237. [PubMed] |
| 183. | Balagopal A, Philp FH, Astemborski J, Block TM, Mehta A, Long R, Kirk GD, Mehta SH, Cox AL, Thomas DL. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 220] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 184. | Marchetti G, Nasta P, Bai F, Gatti F, Bellistrì GM, Tincati C, Borghi F, Carosi G, Puoti M, Monforte Ad. Circulating sCD14 is associated with virological response to pegylated-interferon-alpha/ribavirin treatment in HIV/HCV co-infected patients. PLoS One. 2012;7:e32028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 185. | de Oca Arjona MM, Marquez M, Soto MJ, Rodriguez-Ramos C, Terron A, Vergara A, Arizcorreta A, Fernandez-Gutierrez C, Giron-González JA. Bacterial translocation in HIV-infected patients with HCV cirrhosis: implication in hemodynamic alterations and mortality. J Acquir Immune Defic Syndr. 2011;56:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 186. | Martinez-Sierra C, Arizcorreta A, Díaz F, Roldán R, Martín-Herrera L, Pérez-Guzmán E, Girón-González JA. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis. 2003;36:491-498. [PubMed] |
| 187. | Macías J, Berenguer J, Japón MA, Girón JA, Rivero A, López-Cortés LF, Moreno A, González-Serrano M, Iribarren JA, Ortega E. Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology. 2009;50:1056-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 210] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 188. | Pineda JA, Romero-Gómez M, Díaz-García F, Girón-González JA, Montero JL, Torre-Cisneros J, Andrade RJ, González-Serrano M, Aguilar J, Aguilar-Guisado M. HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology. 2005;41:779-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 211] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 189. | Girón-González JA, Brun F, Terrón A, Vergara A, Arizcorreta A. Natural history of compensated and decompensated HCV-related cirrhosis in HIV-infected patients: a prospective multicentre study. Antivir Ther. 2007;12:899-907. [PubMed] |
| 190. | Pineda JA, Aguilar-Guisado M, Rivero A, Girón-González JA, Ruiz-Morales J, Merino D, Ríos-Villegas MJ, Macías J, López-Cortés LF, Camacho A. Natural history of compensated hepatitis C virus-related cirrhosis in HIV-infected patients. Clin Infect Dis. 2009;49:1274-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 191. | Merchante N, Girón-González JA, González-Serrano M, Torre-Cisneros J, García-García JA, Arizcorreta A, Ruiz-Morales J, Cano-Lliteras P, Lozano F, Martínez-Sierra C. Survival and prognostic factors of HIV-infected patients with HCV-related end-stage liver disease. AIDS. 2006;20:49-57. [PubMed] |
| 192. | Scarpellini E, Valenza V, Gabrielli M, Lauritano EC, Perotti G, Merra G, Dal Lago A, Ojetti V, Ainora ME, Santoro M. Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: is the ring closed? Am J Gastroenterol. 2010;105:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 193. | Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 777] [Article Influence: 64.8] [Reference Citation Analysis (2)] |
| 194. | Jerala R. Structural biology of the LPS recognition. Int J Med Microbiol. 2007;297:353-363. [PubMed] |
| 195. | Page EE, Nelson M, Kelleher P. HIV and hepatitis C coinfection: pathogenesis and microbial translocation. Curr Opin HIV AIDS. 2011;6:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 196. | Figueroa L, Xiong Y, Song C, Piao W, Vogel SN, Medvedev AE. The Asp299Gly polymorphism alters TLR4 signaling by interfering with recruitment of MyD88 and TRIF. J Immunol. 2012;188:4506-4515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 197. | Corchado S, Márquez M, Montes de Oca M, Romero-Cores P, Fernández-Gutiérrez C, Girón-González JA. Influence of Genetic Polymorphisms of Tumor Necrosis Factor Alpha and Interleukin 10 Genes on the Risk of Liver Cirrhosis in HIV-HCV Coinfected Patients. PLoS One. 2013;8:e66619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 198. | French AL, Evans CT, Agniel DM, Cohen MH, Peters M, Landay AL, Desai SN. Microbial translocation and liver disease progression in women coinfected with HIV and hepatitis C virus. J Infect Dis. 2013;208:679-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 199. | Macías J, Mira JA, López-Cortés LF, Santos I, Girón-González JA, González-Serrano M, Merino D, Hernández-Quero J, Rivero A, Merchante N. Antiretroviral therapy based on protease inhibitors as a protective factor against liver fibrosis progression in patients with chronic hepatitis C. Antivir Ther. 2006;11:839-846. [PubMed] |
| 200. | Mira JA, Rivero-Juárez A, López-Cortés LF, Girón-González JA, Téllez F, de los Santos-Gil I, Macías J, Merino D, Márquez M, Ríos-Villegas MJ. Benefits from sustained virologic response to pegylated interferon plus ribavirin in HIV/hepatitis C virus-coinfected patients with compensated cirrhosis. Clin Infect Dis. 2013;56:1646-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 201. | Berenguer J, Rodríguez E, Miralles P, Von Wichmann MA, López-Aldeguer J, Mallolas J, Galindo MJ, Van Den Eynde E, Téllez MJ, Quereda C. Sustained virological response to interferon plus ribavirin reduces non-liver-related mortality in patients coinfected with HIV and Hepatitis C virus. Clin Infect Dis. 2012;55:728-736. [PubMed] |
| 202. | Chew KW, Hua L, Bhattacharya D, Butt AA, Bornfleth L, Chung RT, Andersen JW, Currier JS. The effect of hepatitis C virologic clearance on cardiovascular disease biomarkers in human immunodeficiency virus/hepatitis C virus coinfection. Open Forum Infect Dis. 2014;1:ofu104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Hu S S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN