Published online Oct 21, 2016. doi: 10.3748/wjg.v22.i39.8790
Peer-review started: August 23, 2016
First decision: September 12, 2016
Revised: September 19, 2016
Accepted: September 28, 2016
Article in press: September 28, 2016
Published online: October 21, 2016
Processing time: 58 Days and 19.5 Hours
To evaluate the cytological diagnostic capacity and sample quality of the slow-pull technique and compare them with different suction techniques.
From July 2010 to December 2015, 102 patients with pancreatic solid lesions who underwent endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) with 22-gauge needles were retrospectively evaluated. EUS-FNA diagnosis was based on a cytological examination, and final diagnosis was based on a comprehensive standard of cytological diagnosis, surgical pathology and clinical or imaging follow-up. Cytological specimens were characterized for cellularity and blood contamination. The cytological diagnostic capacity and sample quality of the slow-pull technique and suction techniques with 5-mL/10-mL/20-mL syringes were analyzed.
Of all of the EUS-FNA procedures, the slow-pull technique and suction techniques with 5-mL/10-mL/20-mL syringes were used in 31, 19, 34 and 18 procedures, respectively. There were significant differences between these four suction techniques in terms of cytological diagnostic accuracy (90.3% vs 63.2% vs 58.8% vs 55.6%, P = 0.019), sensitivity (88.2% vs 41.7% vs 40.0% vs 36.4%, P = 0.009) and blood contamination (score ≥ 2 for 29.0% vs 52.6% vs 70.6% vs 72.2%, P = 0.003). The accuracy and sensitivity of the slow-pull technique were significantly higher than those of the suction techniques using 5-mL (P = 0.03, P = 0.014), 10-mL (P = 0.005; P = 0.006) and 20-mL syringes (P = 0.01, P = 0.01). Blood contamination was significantly lower in the slow-pull technique than in the suction techniques with 10-mL (P = 0.001) and 20-mL syringes (P = 0.007).
The slow-pull technique may increase the cytological diagnostic accuracy and sensitivity with slight blood contamination during EUS-FNA when using 22-gauge needles for solid pancreatic masses.
Core tip: Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is an essential technique for obtaining tissue diagnoses for pancreatic masses, and application of suction is one of the potential influencing factors of EUS-FNA. The slow-pull technique has recently emerged as a new sampling technique in EUS-FNA of pancreatic masses. We found that the slow-pull technique using 22-gauge needles may increase the cytological diagnostic accuracy and sensitivity and result in only slight blood contamination in EUS-FNA of pancreatic solid lesions.
- Citation: Chen JY, Ding QY, Lv Y, Guo W, Zhi FC, Liu SD, Cheng TM. Slow-pull and different conventional suction techniques in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid lesions using 22-gauge needles. World J Gastroenterol 2016; 22(39): 8790-8797
- URL: https://www.wjgnet.com/1007-9327/full/v22/i39/8790.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i39.8790
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) was first reported in 1992 and currently is applied as an essential technique to obtain tissue diagnoses for pancreatic masses[1-3]. A recent meta-analysis study concluded that the pooled sensibility and specificity of pancreatic EUS-FNA were 86.8% and 95.8%, respectively[4]. Although the diagnostic accuracy is generally high, the optimal method for EUS-FNA has not been established. Various factors are potential influencing factors, such as the experience of the endosonographer, the size and location of the target lesion, the size and type of needle, the presence of a stylet, the application of suction, and the availability of onsite cytopathology[5-7].
Application of suction during EUS-FNA has been controversial because it may result in damage to the cell structure and contamination of the blood while increasing cell quantity[8-10]. Different levels of negative pressure were applied to seek a balanced point between specimen quantity and quality. Some studies suggested that low or no suction reduced the contamination from blood and improved specimen quality[11-15]. Some suggested that high negative pressure obtained more tissue specimens for histological examination and improved diagnostic accuracy[16,17]. However, there is no consensus on the optimal suction technique. The slow-pull technique has been recently introduced as a new sampling technique in EUS-FNA of pancreatic solid lesions[18,19]. Different from conventional suction techniques using a syringe, the slow-pull technique provides minimum negative pressure by removing the stylet from the needle slowly and continuously[20]. Several studies have found that the slow-pull technique could obtain high-quality specimens with unsubstantial blood contamination when combined with a novel core biopsy needle (EchoTip ProCore, Cook Medical, Bloomington, IN, United States)[21-23]. These studies mainly focused on the biopsy needles; however, regular needles, such as 22-gauge needles, and cytological examinations are more frequently used in most institutions.
Therefore, to evaluate the diagnostic value of the slow-pull technique and to explore the optimal suction technique, we retrospectively analyzed the cytological diagnostic capacity and specimen quality of the slow-pull technique and different conventional suction techniques during EUS-FNA of pancreatic solid lesions using 22-gauge needles.
We retrospectively analyzed all patients who underwent EUS-FNA for solid pancreatic lesions at our institution between July 2010 and December 2015. Inclusion criteria were as follows: primary pancreatic solid lesions, usage of 22-gauge needles, application of cytological examination, and a ≥ 6-mo radiologic or clinical follow-up in patients diagnosed with benign lesions. Exclusion criteria were as follows: pancreatic cystic lesion or extra-pancreatic lesion, usage of 19-gauge or other needles, combination use of different suction techniques, and lack of follow-up data. This study was conducted in accordance with the Declaration of Helsinki.
All procedures were performed by one of two experienced endosonographers. Patients were conscious but sedated with intravenous propofol or a combination of intravenous meperidine and diazepam in the left lateral position. A curved linear array echoendoscope (EG-530UR; Fujinon Medical Systems, or GF-UE260; Olympus Medical Systems) was used to assess the pancreatic lesion. Once an optimal puncture route was determined, EUS-FNA was then performed with a curvilinear echoendoscope (EG-530UT; Fujinon Medical Systems, or GF-UCT260; Olympus Medical Systems) and a 22-gauge needle (Echotip Ultra; Wilson-Cook, Tokyo, Japan, or Expect; Boston Scientific, Natick, MA, United States); the stylet was inserted into the target lesion guided by real-time EUS imaging. In the slow-pull technique, the stylet was slowly withdrawn from the needle while 10-20 to-and-fro movements within the target lesion were performed. In conventional suction techniques, the stylet is completely removed before suction using a 5-mL/10-mL/20-mL syringe that is applied while 10-20 to-and-fro movements are performed within the target lesion.
After EUS-FNA, the aspirated material was completely expelled onto glass slides by reinsertion of the stylet and flushing with air. The aspirated materials were smeared on glass slides and fixed in an absolute alcohol solution for cytological examinations. Smeared slides were prepared by endosonographers trained in the appropriate slide preparation techniques. No on-site cytopathology examination was performed at our institution. EUS-FNA diagnosis was based on cytological examination.
All cytological reports were retrospectively reviewed. Diagnosis of cytological examination was recorded in reports and categorized into the following five groups: benign or negative for malignant, atypical, suspicious for malignant, malignant, and inadequate for diagnosis. Cytological specimens were characterized for cellularity and bloodiness, and the semiquantitative scores were routinely recorded in the pathological reports. Cellularity was graded into 4 levels: 0, none; 1, few aggregates; 2, fair cellularity; 3, abundant cellularity. All types of cells, including tumor cells, were calculated in the cellularity scores. The contamination from blood was also graded in 4 levels: 0, none; 1, little; 2, moderate; 3, abundant.
The composite standard for each lesion was based on the EUS-FNA diagnosis, surgical pathology and a clinical/imaging follow-up. Lesions were considered benign if surgical pathology confirmed a benign condition or if a lack of deterioration was noted on a ≥ 6-mo follow-up. Lesions were considered malignant if surgical pathological diagnosis or EUS-FNA diagnosis (based on cytological examination with a compatible clinical course) was positive for malignancy or if there was clinical progression or an increase in the lesion size (or both) during follow-up.
All statistical analyses were processed using SPSS software (version 21.0; IBM SPSS Statistics for Windows, Armonk, NY, United States). Categorical variables are presented as frequencies, and continuous variables are presented as medians and ranges. We calculated descriptive statistics for sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV). We considered the diagnoses of malignant, atypical and suspicious as true positive, negative for malignant as true negative, and inadequate as false negative. Statistical analysis was undertaken using the χ2 test, Fisher’s exact test and the Kruskal-Wallis H test in univariate analyses. Logistic regression analysis was performed in a multivariate analysis, using lesion size (≤ 30 mm vs > 30 mm), endoscopist (endoscopist 2 vs endoscopist 1), lesion location (body or tail vs uncinate or head), needle passes (≤ 3 vs > 3) and suction techniques (slow-pull vs 5-mL vs 10-mL vs 20-mL) as potential predictive factors. Statistical tests were considered significant when the corresponding 2-sided P value was less than 0.05.
From July 2010 to December 2015, 139 patients underwent EUS-FNA for pancreatic lesions. A total of 37 patients were excluded: 9 patients with a pancreatic cystic lesion, 9 patients on whom a 19-gauge needle was used, 1 patient on whom a 25-gauge needle was used, 16 patients on whom combinations of different suction techniques were used, and 2 patients who were not followed-up. Finally, 102 patients with pancreatic solid lesions were included. The baseline characteristics and final diagnoses are listed in Table 1. The final diagnoses were malignant in 58 cases (56.9%) and benign in 44 cases (43.1%). Final diagnoses were confirmed from EUS-FNA specimens for 19 cases and surgically resected specimens for 30 cases; the remaining 53 cases were confirmed from the radiologic or clinical follow-up. The mean follow-up was 12 months (range: 1-24 mo). Of the 102 procedures, adverse events occurred in 6 cases (5.88%). Four patients developed mild pancreatitis, and 2 patients developed fever, but all of these adverse events were successfully treated with conservative therapy.
| Characteristic | Value |
| Median age (range), yr | 53 (19-82) |
| Sex, male:female, n | 67:35 |
| Median tumor size (range), mm | 34 (8-89) |
| Endoscopist, endoscopist 1:endoscopist 2, n | 45:57 |
| Location, uncinate or head:body or tail, n | 59:43 |
| Median number of passes, n | 3 (1-5) |
| Final diagnosis, n | |
| Malignant | 58 |
| Pancreatic cancer | 53 |
| Neuroendocrine tumor, malignant | 2 |
| Solid-pseudopapillary neoplasm, malignant | 3 |
| Benign | 44 |
| Chronic pancreatitis | 23 |
| Autoimmune pancreatitis | 7 |
| Nonspecific inflammation | 10 |
| Cystadenoma, benign | 2 |
| Neuroendocrine tumor, benign | 1 |
| Benign lymphangioma | 1 |
Cytological examinations were performed in all procedures. Of these cases, 61 were diagnosed as benign lesions, 19 as malignant lesions, 10 as atypical lesions, and 7 as suspicious lesions. The remaining 5 cases were inadequate for diagnosis: 3 of which were eventually confirmed as malignant masses from surgically resected specimens, and 2 were confirmed as benign masses from clinical follow-up. Of all of the EUS-FNA procedures, the slow-pull technique and suction techniques with 5-mL/10-mL/20-mL syringes were used in 31, 19, 34 and 18 procedures, respectively. There were no significant differences between these four suction techniques in terms of patient age, sex, endoscopists, lesion location, and number of passes; only tumor size showed a significant difference (P = 0.031) (Table 2).
| Slow-pull | 5-mL | 10-mL | 20-mL | P value1 | |
| (n = 31) | (n = 19) | (n = 34) | (n = 18) | ||
| Median age (range), yr | 56 (20-82) | 54 (38-71) | 51 (19-77) | 49 (26-73) | 0.949 |
| Sex, male:female, n | 17:14 | 11:8 | 25:9 | 14:4 | 0.238 |
| Median lesion size (range), mm | 25 (8-72) | 38 (10-65) | 36 (17-89) | 35 (16-62) | 0.0312 |
| Endoscopist, endoscopist 1:endoscopist 2, n | 13:18 | 11:8 | 11:23 | 10:8 | 0.223 |
| Location, uncinate or head:body or tail, n | 19:12 | 11:8 | 16:18 | 13:5 | 0.348 |
| Median number of passes, n | 3 (1-4) | 3 (2-5) | 3 (1-5) | 3 (2-5) | 0.280 |
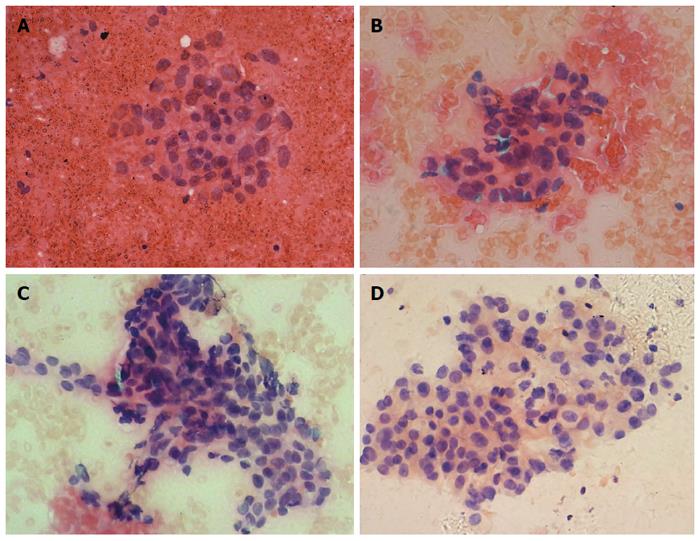
We compared the cytological diagnostic capacities of the four suction techniques. The cytological diagnostic capacities and cytological specimen qualities of the different suction methods are shown in Table 3. The cytological diagnostic accuracy (90.3% vs 63.2% vs 58.8% vs 55.6%, P = 0.019), sensitivity (88.2% vs 41.7% vs 40.0% vs 36.4%, P = 0.009) and blood contamination (score ≥ 2 for 29.0% vs 52.6% vs 70.6% vs 72.2%, P = 0.003) of the four suction methods were statistically significantly different (Figure 1). Thus, cytological diagnostic accuracy, sensitivity and blood contamination between the slow-pull technique and the conventional suction techniques were further compared (Table 4). The cytological diagnostic accuracy and sensitivity of the slow-pull technique were significantly higher than suction techniques with 5-mL (P = 0.03, P = 0.014), 10-mL (P = 0.005, P = 0.006) and 20-mL syringes (P = 0.01, P = 0.01), and the blood contamination with the slow-pull technique was lower than that in the suction technique with the 10-mL (P = 0.001) and 20-mL syringes (P = 0.007).
| Slow-pull (n = 31) | 5-mL (n = 19) | 10-mL (n = 34) | 20-mL (n = 18) | P value1 | |
| Cytological diagnostic capacity | |||||
| Accuracy | 28/31 (90.3%) | 12/19 (63.2%) | 20/34 (58.8%) | 10/18 (55.6%) | 0.0192 |
| Sensitivity | 15/17 (88.2%) | 5/12 (41.7%) | 8/20 (40.0%) | 4/11 (36.4%) | 0.0092 |
| Specificity | 13/14 (92.9%) | 7/7 (100%) | 12/14 (85.7%) | 6/7 (85.7%) | 0.914 |
| PPV | 15/16 (93.8%) | 5/5 (100%) | 8/10 (80.0%) | 4/5 (80.0%) | 0.542 |
| NPV | 13/15 (86.7%) | 7/14 (50.0%) | 12/24 (50.0%) | 6/13 (46.2%) | 0.079 |
| Cytological specimen quality | |||||
| Cellularity score ≥ 2 | 22/31 (71.0%) | 11/19 (57.9%) | 20/34 (58.8%) | 13/18 (72.2%) | 0.598 |
| Blood contamination score ≥ 2 | 9/31 (29.0%) | 10/19 (52.6%) | 24/34 (70.6%) | 13/18 (72.2%) | 0.0032 |
Both univariate and multivariate analyses were performed to define factors associated with the cytological diagnostic accuracy of EUS-FNA (Table 5). Suction techniques were significant factors in both the univariate (P = 0.019) and multivariate analyses [P = 0.005, odds ratio (OR) (95%CI) = 1.91 (1.21-3.00)].
| Variable | Accuracy | Univariate | Multivariate | ||
| P value1 | P value2 | OR (95%CI) | |||
| Lesion size | ≤ 30 mm | 74.4% (32/43) | 0.282 | 0.603 | 1.29 (0.50-3.36) |
| > 30 mm | 64.4% (38/59) | ||||
| Endoscopist | Endoscopist 2 | 71.9% (41/57) | 0.419 | 0.367 | 1.52 (0.61-3.74) |
| Endoscopist 1 | 64.4% (29/45) | ||||
| Location | Body or tail | 69.8% (30/43) | 0.832 | 0.687 | 1.21 (0.48-3.04) |
| Uncinate or head | 67.8% (40/59) | ||||
| Needle passes | ≤ 3 | 69.7% (46/66) | 0.753 | 0.233 | 1.81 (0.68-4.79) |
| > 3 | 66.7% (24/36) | ||||
| Suction techniques | Slow-pull | 90.3% (28/31) | 0.0193 | 0.0053 | 1.91 (1.21-3.00) |
| 5-mL | 63.2% (12/19) | ||||
| 10-mL | 58.8% (20/34) | ||||
| 20-mL | 55.6% (10/18) | ||||
There is still some dispute regarding the role of suction during EUS-FNA. Generally, the suction technique with a 10-mL syringe is used to increase the specimen cellularity. However, this procedure may also increase the risk of blood contamination and structural damage. The value of suction in EUS-FNA was first evaluated for lymph node sampling. In an experimental study, Bhutani et al[11] found that continuous suction with smaller syringes (5-10 mL) provided optimal cellularity and better specimen quality in EUS-FNA of mediastinal lymph nodes. Subsequently, Wallace et al[12] performed a randomized controlled trial comparing sampling techniques with or without suction in EUS-FNA of lymph nodes and concluded that the technique with suction increased the cellularity but worsened the specimen bloodiness. Different from lymph nodes, pancreatic lesions are rich in fibrous tissue, with fewer parenchymal cells, which increases the complexity and difficulty involved in obtaining a precise diagnosis for pancreatic lesions[24-26]. Therefore, the European Society of Gastrointestinal Endoscopy technical guideline recommended using suction for EUS-FNA of solid masses/cystic lesions but not for EUS-FNA of lymph nodes[27]. However, in a recent randomized controlled trial, Lee et al[28] found that using suction during EUS-FNA worsened specimen bloodiness, while the diagnostic yield and cellularity were improved.
In recent years, the slow-pull technique has been applied to EUS-FNA of pancreatic solid lesions, but its efficacy has not yet been clarified. In a retrospective study, Nakai et al[18] reported that the slow-pull technique was associated with less contamination from blood and potentially increased the diagnostic yield in comparison to the suction technique. Both 22- and 25-gauge needles were used in this study, and different needles may impact the diagnostic yield. Therefore, Kin et al[19] performed a prospective study to evaluate the value of the slow-pull technique with 22-gauge needles in EUS-FNA and found that this technique could obtain adequate, high-quality, and unsubstantially blood-contaminated samples. However, the sample size in this study was so limited that it could not compare the diagnostic efficacy or sample quality between the slow-pull technique and traditional suction techniques. To provide more evidence for endosonographers when choosing a suction technique, our study not only analyzed the cytological diagnostic capacity and sample quality of the slow-pull technique with 22-gauge needles but also continued the comparison with different traditional suction techniques.
When comparing the cytological diagnostic capacities and specimen qualities of the four different suction techniques, we found that the degree of negative pressure, the level of blood contamination and the diagnostic accuracy were closely related. With the rise of negative pressure, the cytological diagnostic accuracy tended to decrease, and the blood contamination increased, possibly because the needle is filled with blood tissue while using a higher degree of negative pressure, which increases the puncture difficulty and adds blood contamination. In a randomized controlled trial with 90 pancreatic lesions, Kudo et al[17] showed that EUS-FNA with high negative pressure obtained more contamination from blood than low negative pressure. Therefore, application of a lower degree of negative pressure during EUS-FNA may help decrease the blood contamination.
The slow-pull technique is a new suction technique with a very weak suction force. An experimental study showed that the suction force produced by the slow-pull technique with a 22-gauge needle was less than 2.0 kPa, which is significantly lower than those of the suction techniques with 10-mL and 20-mL syringes[29]. Such a slight suction force can help obtain enough specimens with minimal blood contamination. Our research showed that the cytological diagnostic accuracy and sensitivity of the slow-pull technique were 90.3% and 88.2%, respectively, which were significantly higher than the conventional suction techniques with 5-mL/10-mL/20-mL syringes. When evaluating the quality of cytological specimens, we found that blood contamination scores of 2 or 3 when using the slow-pull technique were noted 29.0% of the time, which was significantly lower than the blood contamination scores noted when using conventional suction techniques with 10-mL and 20-mL syringes. Therefore, the slow-pull technique has a unique advantage in improving the cytological diagnostic capacity and specimen quality of EUS-FNA for pancreatic masses.
There were some limitations in the present study. First, biases are inevitable in a retrospective analysis, and the lesion sizes were different in these four suction techniques. The lesion size associated with the slow-pull technique was smaller, which might affect the diagnostic accuracy. However, there was no significant difference in the cytological diagnostic accuracy between different lesion sizes. Moreover, suction techniques were still statistically significant factors in multivariate analysis after adjusting for tumor size. Second, on-site cytological evaluation was not available in our study, although it is beneficial for EUS-FNA[30,31]. On-site cytopathological evaluation could reduce the need for repeat punctures and could improve the specimen quality rate, but it could also prolong the procedure time and increase the working time of the pathologists. Therefore, many institutions, like ours, have not adopted on-site cytological evaluation[32-34].
In conclusion, our retrospective study showed that the slow-pull technique might increase the cytological diagnostic accuracy and sensitivity with slight blood contamination during EUS-FNA for solid pancreatic masses using 22-gauge needles. Additional studies should be performed with a prospective randomized design to better understand the efficacy of the slow-pull technique.
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) has become an essential technique to obtain tissue diagnoses for pancreatic masses. However, application of suction during EUS-FNA is controversial because it may result in damage to the cell structure and contamination of the blood while increasing cell quantity.
The slow-pull technique has recently been introduced as a new sampling technique in EUS-FNA of pancreatic solid lesions. In contrast to conventional suction techniques using a syringe, the slow-pull technique provides minimum negative pressure by slowly and continuously removing the stylet from the needle. Such a slight suction force may help obtain sufficient specimens with minimal blood contamination.
The cytological diagnostic accuracy and sensitivity of the slow-pull technique were 90.3% and 88.2%, respectively, which were significantly higher than those of the conventional suction techniques with 5-mL/10-mL/20-mL syringes. Blood contamination was lower in the slow-pull technique than in the suction techniques with 10-mL and 20-mL syringes.
The study supports the idea that the slow-pull technique using 22-gauge needles may increase the cytological diagnostic accuracy and sensitivity, and result in only slight blood contamination in EUS-FNA of pancreatic solid lesions.
Slow-pull technique: A new sampling technique that can provide minimum negative pressure by slowly and continuously removing the stylet from the needle.
This is an interesting study about the slow-pull and different conventional suction techniques in EUS-FNA of pancreatic solid lesions using 22-gauge needles. EUS-FNA using a slow-pull technique has recently emerged as a new method to obtain tissue diagnosis for pancreatic diseases. However, the optimal suction technique has not been clearly established, and the efficacy of the slow-pull technique remains unclear. In this study, the authors evaluated the cytological diagnostic capacity and sample quality of the slow-pull technique and compare it with different conventional suction techniques.
| 1. | Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 418] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 2. | Weston BR, Bhutani MS. Optimizing Diagnostic Yield for EUS-Guided Sampling of Solid Pancreatic Lesions: A Technical Review. Gastroenterol Hepatol (N Y). 2013;9:352-363. [PubMed] |
| 3. | Cosgrove ND, Yan L, Siddiqui A. Preoperative endoscopic ultrasound-guided fine needle aspiration for diagnosis of pancreatic cancer in potentially resectable patients: Is this safe? Endosc Ultrasound. 2015;4:81-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: A meta-analysis and systematic review. Pancreas. 2013;42:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 285] [Article Influence: 21.9] [Reference Citation Analysis (1)] |
| 5. | Hijioka S, Hara K, Mizuno N, Imaoka H, Bhatia V, Mekky MA, Yoshimura K, Yoshida T, Okuno N, Hieda N. Diagnostic performance and factors influencing the accuracy of EUS-FNA of pancreatic neuroendocrine neoplasms. J Gastroenterol. 2016;51:923-930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Berzosa M, Villa N, El-Serag HB, Sejpal DV, Patel KK. Comparison of endoscopic ultrasound guided 22-gauge core needle with standard 25-gauge fine-needle aspiration for diagnosing solid pancreatic lesions. Endosc Ultrasound. 2015;4:28-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Petrone MC, Arcidiacono PG. Basic technique in endoscopic ultrasound-guided fine needle aspiration for solid lesions: How many passes? Endosc Ultrasound. 2014;3:22-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Varadarajulu S, Fockens P, Hawes RH. Best practices in endoscopic ultrasound-guided fine-needle aspiration. Clin Gastroenterol Hepatol. 2012;10:697-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Wani S. Basic techniques in endoscopic ultrasound-guided fine-needle aspiration: Role of a stylet and suction. Endosc Ultrasound. 2014;3:17-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Tadic M, Stoos-Veic T, Kusec R. Endoscopic ultrasound guided fine needle aspiration and useful ancillary methods. World J Gastroenterol. 2014;20:14292-14300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Bhutani MS, Suryaprasad S, Moezzi J, Seabrook D. Improved technique for performing endoscopic ultrasound guided fine needle aspiration of lymph nodes. Endoscopy. 1999;31:550-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Wallace MB, Kennedy T, Durkalski V, Eloubeidi MA, Etamad R, Matsuda K, Lewin D, Van Velse A, Hennesey W, Hawes RH. Randomized controlled trial of EUS-guided fine needle aspiration techniques for the detection of malignant lymphadenopathy. Gastrointest Endosc. 2001;54:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 153] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Mohammad Alizadeh AH, Hadizadeh M, Padashi M, Shahbaazi S, Molaee M, Shariatpanahi ZV. Comparison of two techniques for endoscopic ultrasonography fine-needle aspiration in solid pancreatic mass. Endosc Ultrasound. 2014;3:174-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Gimeno-García AZ, Elwassief A, Paquin SC, Gariépy G, Sahai AV. Randomized controlled trial comparing stylet-free endoscopic ultrasound-guided fine-needle aspiration with 22-G and 25-G needles. Dig Endosc. 2014;26:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 15. | Paquin SC, Sahai AV. Techniques for EUS-guided FNA cytology. Gastrointest Endosc Clin N Am. 2014;24:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Larghi A, Noffsinger A, Dye CE, Hart J, Waxman I. EUS-guided fine needle tissue acquisition by using high negative pressure suction for the evaluation of solid masses: a pilot study. Gastrointest Endosc. 2005;62:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Kudo T, Kawakami H, Hayashi T, Yasuda I, Mukai T, Inoue H, Katanuma A, Kawakubo K, Ishiwatari H, Doi S. High and low negative pressure suction techniques in EUS-guided fine-needle tissue acquisition by using 25-gauge needles: a multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2014;80:1030-7.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Nakai Y, Isayama H, Chang KJ, Yamamoto N, Hamada T, Uchino R, Mizuno S, Miyabayashi K, Yamamoto K, Kawakubo K. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014;59:1578-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Kin T, Katanuma A, Yane K, Takahashi K, Osanai M, Takaki R, Matsumoto K, Gon K, Matsumori T, Tomonari A. Diagnostic ability of EUS-FNA for pancreatic solid lesions with conventional 22-gauge needle using the slow pull technique: a prospective study. Scand J Gastroenterol. 2015;50:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Matsubayashi H, Matsui T, Yabuuchi Y, Imai K, Tanaka M, Kakushima N, Sasaki K, Ono H. Endoscopic ultrasonography guided-fine needle aspiration for the diagnosis of solid pancreaticobiliary lesions: Clinical aspects to improve the diagnosis. World J Gastroenterol. 2016;22:628-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 21. | Wang J, Wu X, Yin P, Guo Q, Hou W, Li Y, Wang Y, Cheng B. Comparing endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) versus fine needle biopsy (FNB) in the diagnosis of solid lesions: study protocol for a randomized controlled trial. Trials. 2016;17:198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Iwashita T, Nakai Y, Samarasena JB, Park DH, Zhang Z, Gu M, Lee JG, Chang KJ. High single-pass diagnostic yield of a new 25-gauge core biopsy needle for EUS-guided FNA biopsy in solid pancreatic lesions. Gastrointest Endosc. 2013;77:909-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 23. | Iglesias-Garcia J, Poley JW, Larghi A, Giovannini M, Petrone MC, Abdulkader I, Monges G, Costamagna G, Arcidiacono P, Biermann K. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 24. | Bachem MG, Schünemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 517] [Article Influence: 24.6] [Reference Citation Analysis (5)] |
| 25. | Dumonceau JM, Polkowski M, Larghi A, Vilmann P, Giovannini M, Frossard JL, Heresbach D, Pujol B, Fernández-Esparrach G, Vazquez-Sequeiros E. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011;43:897-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 26. | Iglesias-Garcia J, Lariño-Noia J, Domínguez-Muñoz JE. When to puncture, when not to puncture: Pancreatic masses. Endosc Ultrasound. 2014;3:91-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Polkowski M, Larghi A, Weynand B, Boustière C, Giovannini M, Pujol B, Dumonceau JM; European Society of Gastrointestinal Endoscopy (ESGE). Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 28. | Lee JK, Choi JH, Lee KH, Kim KM, Shin JU, Lee JK, Lee KT, Jang KT. A prospective, comparative trial to optimize sampling techniques in EUS-guided FNA of solid pancreatic masses. Gastrointest Endosc. 2013;77:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 29. | Katanuma A, Itoi T, Baron TH, Yasuda I, Kin T, Yane K, Maguchi H, Yamazaki H, Sano I, Minami R. Bench-top testing of suction forces generated through endoscopic ultrasound-guided aspiration needles. J Hepatobiliary Pancreat Sci. 2015;22:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 361] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 31. | Iglesias-Garcia J, Lariño-Noia J, Abdulkader I, Domínguez-Muñoz JE. Rapid on-site evaluation of endoscopic-ultrasound-guided fine-needle aspiration diagnosis of pancreatic masses. World J Gastroenterol. 2014;20:9451-9457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 32. | Hébert-Magee S. Basic technique for solid lesions: Cytology, core, or both? Endosc Ultrasound. 2014;3:28-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | O’Connor K, Cheriyan DG, Li-Chang HH, Kalloger SE, Garrett J, Byrne MF, Weiss AA, Donnellan F, Schaeffer DF. Gastrointestinal Endoscopic Ultrasound-Guided Fine-Needle Aspiration Biopsy Specimens: Adequate Diagnostic Yield and Accuracy Can Be Achieved without On-Site Evaluation. Acta Cytol. 2015;59:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Harada R, Kato H, Fushimi S, Iwamuro M, Inoue H, Muro S, Sakakihara I, Noma Y, Yamamoto N, Horiguchi S. An expanded training program for endosonographers improved self-diagnosed accuracy of endoscopic ultrasound-guided fine-needle aspiration cytology of the pancreas. Scand J Gastroenterol. 2014;49:1119-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Hatta W, Zimmerman M S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Wang CH